1. Introduction
First described in 1925, medulloblastoma remains as a formidable malignant brain neoplasm primarily affecting the pediatric population, with a peak age of diagnosis typically between 6 to 8 years [
1]. While the conventional therapeutic triad of surgery, chemotherapy (CT) and radiation therapy (RT) has historically formed the cornerstone of management, [
2,
3] recent decades have witnessed significant paradigm shifts in therapeutic strategies. These shifts have been propelled by an enhanced comprehension of molecular subgroups influencing both prognosis and treatment modalities [
4]. Nevertheless, the management of recurrent cases continues to present formidable clinical challenges, underscoring the aggressive nature of this disease and the imperative for effecting interventions [
5].
The intricate interplay between the infiltrative nature of medulloblastoma and the intricacies of neural architecture necessitates precise and efficacious therapeutic strategies [
6]. Clinical challenges associated with recurrent medulloblastoma, such as treatment resistance, neurocognitive impairments, and compromised quality of life for affected individuals and their families, highlight the urgent need for therapeutic approaches aimed at enhancing patient outcomes and mitigating long-term sequelae [
5].
In this context, stereotactic radiosurgery (SRS) has emerged as a promising treatment modality for managing recurrent medulloblastoma [
7]. By leveraging targeted ionizing radiation, SRS strategically exploits the biological vulnerabilities of the pathology, offering the advantage of delivering precisely targeted radiation to tumor sites while sparing surrounding healthy tissue, thereby minimizing treatment-related toxicities [
8]. Supported by advancements in radiological imaging, radiation delivery platforms, and an evolving understanding of radiobiology, SRS has assumed a pivotal role in combating this challenging adversary [
9]. The rationale for studying SRS in the context of recurrent medulloblastoma is rooted in its potential to address the limitations of conventional treatment approaches and provide durable control of disease progression.
Our study aim to investigate the long-term efficacy and safety of SRS for both pediatric and adult patients with recurrent medulloblastoma. Situated within a single institution, our investigation integrates clinical expertise with research acumen, furnishing a comprehensive perspective and evaluation on the multifaceted dimensions of SRS application and outcomes in this challenging patient population. By elucidating the clinical impact of SRS and identifying factors predictive of treatment response, our findings have the potential to inform treatment decision-making, contribute to the refinement of current management guidelines, and pave the way for future advancements in the management of recurrent medulloblastoma.
2. Methods
Patient Selection and Characteristics
Between 1998 and 2023, we identified and treated 15 cases of recurrent medulloblastomas in 10 patients at Stanford University Medical Center using CyberKnife SRS. Inclusion criteria comprised patients with recurrent medulloblastomas who underwent CyberKnife SRS and had a minimum follow-up period of 6 months. Clinical data were collected and securely stored in a database approved by the Institutional Review Board (IRB) #3019 at Stanford University, adhering to principles outlined in the Helsinki Declaration. All participants provided informed consent for the use of their data in research aimed at improving future patient care. Access to patient data is restricted to authorized personnel only. Data are available upon request from the first author, KHY, and are not publicly accessible to ensure compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations and safeguard patient privacy.
The study cohort comprised 8 (80%) pediatric patients (ages 3-18) and 2 (20%) adult patients (ages 19-75). The median age at the time of SRS was 13 years, with the pediatric group having a median age of 12 years (range: 9-16) and the adult group with a median age of 27 years (range: 25-29). Nine (90%) patients underwent prior surgical resection of prior to SRS, with histological confirmation of WHO grade IV tumors. Additionally, one (10%) adult patient received RT as primary treatment based on radiographic diagnosis. Symptoms observed in patients were correlated with lesion location and included headaches, ataxia, nausea, vomiting, seizures, visual impairment, and motor impairment. During the follow-up period, one patient deceased, while there have been no instances of patients lost to follow-up thus far (
Table 1).
Tumor Characteristics
The study included 10 patients with 15 recurrent cranial medulloblastomas. The median tumor volume was 1.9 cc (mean: 2.7; range: 0.02-8.7). In the pediatric group, the median tumor volume was 1.9 cc (range: 0.02-8.7), while in the adult group, it was 1.5 cc (range: 0.5-2.5). Most lesions (86.7%) occurred in pediatric patients, primarily localized in the cerebellum (n = 5, 33.3%), followed by ventricular (n=2, 13.3%), parietal (n=2, 13.3%), and frontal (n=2, 13.3%) regions (
Table 1).
Considering the protracted timeline of our study cohort, encompassing the period from 1998 to 2023, acquiring comprehensive molecular subgroup data, specifically pertaining to WNT and SHH subtypes, for all patients, particularly those diagnosed earlier in the timeline, posed significant challenges. Nonetheless, a thorough investigation of the histopathological characteristics of our recurrent medulloblastoma cohort was conducted. Notably, we noted a consistent trend towards features suggestive of anaplastic subtypes or focal anaplasia across our patient population. Furthermore, all cases were consistently categorized as high-risk and assigned a WHO grade IV classification.
Treatment
Cranial recurrent medulloblastomas were treated with CyberKnife SRS (Accuray, Inc., Sunnyvale, CA) utilizing the established method previously outlined by Shi et al. [
10]. The median time between initial diagnosis and SRS was 30 months (range: 12-108), with pediatric patients undergoing treatment at a median of 39 months (range: 12-108) and adult patients at a median of 24.5 months (range: 24-25). SRS was administered with a median single-fraction equivalent dose (SFED) of 18 Gy to the 75% median isodose line (range: 65-81). Treatment parameters, including marginal dose, maximum dose, isodose line, and the number of fractions, did not significantly differ between cohorts (
Table 2).
Biologically Effective Dose (BED), Single-Fraction Equivalent Dose (SFED), and Equivalent Total Doses in 2-Gy Fractions (EQD2)
The biologically effective dose (BED) was calculated using the linear-quadratic (LQ) model with a α/β ratio of 3 Gy, following established methods outlined in previous studies [
11,
12]. BED values were subsequently converted to SFED and equivalent total doses in 2-Gy fractions (EQD2) using LQ model [
13]. The median BED, SFED, and EQD2 for the entire cohort were calculated as 126 Gy (range: 66.7-153.3), 18 Gy (range: 12.7-20), and 75.6 Gy (range: 40-92), respectively. Importantly, no significant differences were observed in these parameters between patient groups (
Table 2).
Follow-Up
Following CyberKnife SRS for recurrent medulloblastomas, patients underwent clinical evaluation and MRI follow-up semi-annually during the first two years post-treatment, followed by annual assessments thereafter. Clinical evaluations included comprehensive neurological examinations to assess symptoms and treatment response.
MRI scans were performed using standardized imaging protocols, and tumor volume was measured in axial, sagittal, and coronal planes by experienced radiologists. The tumor volume was estimated as (D1 x D2 x D3)/2, where D represents the maximum diameter of the tumor in each plane. This approach ensured consistent and accurate assessment of tumor size over time.
Criteria for tumor response assessment were as follows: an increase of more than 10% of the initial treatment volume or tumor occurrence outside the planned treatment volume was considered tumor progression, while a decrease in volume greater than 10% was defined as tumor regression. Stable disease was characterized by tumor dimensions within 10% of the volume at the time of SRS treatment. Local tumor control was determined based on reduction or stability in tumor size.
Data from follow-up assessments were analyzed using Kaplan-Meier analysis to evaluate patient outcomes. Graphical representations were generated using standard statistical software (IBM SPSS Statistics 29.0, Chicago, IL).
3. Results
Patient Demographics and Characteristics
Following the exclusion of patients lost to follow-up, the study encompassed 15 lesions observed in 10 patients. Among these, 80% (n=8) were pediatric and 20% (n=2) were adults. Over the evaluation period, 50% (n=5) of the patients succumbed. The median follow-up duration post-SRS was 39 months (range: 6-78) (
Table 2).
Local Tumor Control
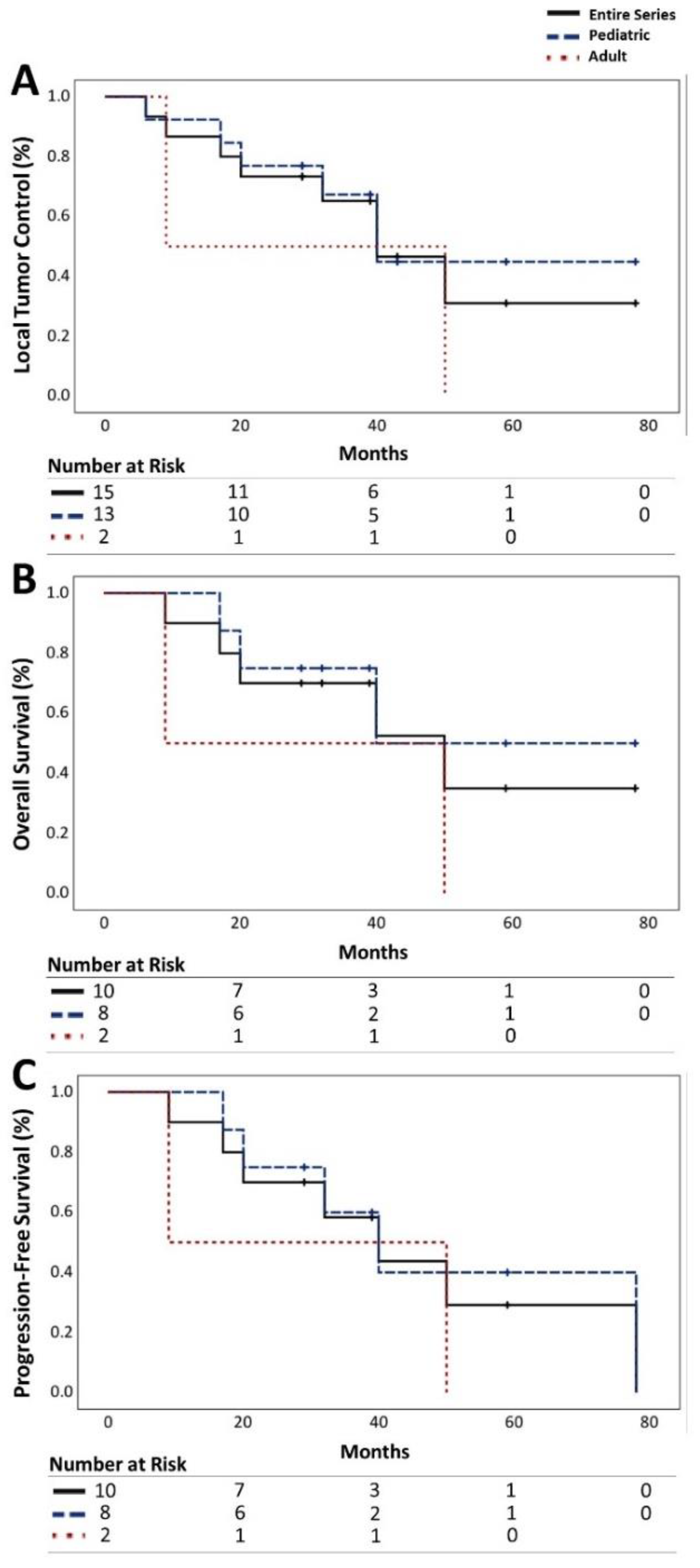
Among the 15 recurrent medulloblastomas, 53.3% (n=8) demonstrated progression, 13.3% (n=2) exhibited radiographic regression, and 33.3% (n=5) remained stable. The LTC rates for all medulloblastomas were 86%, 65%, and 31% at 1, 3, and 5 years, respectively. Notably, pediatric patients showed a higher LTC rate of 67% at 3 years compared to the adult cohort, which stood at 50% (
Table 3,
Figure 1A).
Further analysis of LTC rates revealed intriguing patterns associated with tumor location. Cerebellar lesions displayed a favorable response with a 3-year LTC rate of 78% compared to ventricular lesions, which exhibited a rate of 50%. Furthermore, LTC rates showed correlation with the number of prior surgeries, with a higher rate observed in patients who had undergone a single surgery (75%) compared to those with multiple surgeries (66.7%) (
Table 1 and
Table 3).
Patient Survival
OS rates for all patients stood at 90%, 70%, and 35% at 1, 3, and 5 years, respectively. Pediatric patients exhibited higher rates of OS (75%) compared to adult patients (50%) at 3-year mark (
Table 3,
Figure 1B).
Moreover, survival outcomes were influenced by clinical presentation. Asymptomatic patients demonstrated a 100% OS rate at 3 years, whereas symptomatic patients exhibited varied rates based on the location of the tumor (
Table 1 and
Table 3).
Progression-Free Survival
PFS rates were 90%, 58%, and 29% for all patients at 1, 3, and 5 years, respectively, with a higher rate observed in the pediatric cohort at 3 years (60%) (
Table 3,
Figure 1C).
Notably, PFS was significantly influenced by the age at treatment, with pediatric patients experiencing a 60% PFS rate at 3 years compared to 50% in adults. Additionally, a positive correlation was observed between PFS and target tumor volume, with larger volumes exhibiting a slightly higher PFS rate (
Table 2 and
Table 3).
Symptomatic Outcome
Among the entire cohort, one (10%) pediatric patient was asymptomatic, allowing the evaluation of symptomatic outcomes for 9 (90%) patients with 14 (93.3%) symptomatic medulloblastomas. Overall, 7 (77.8%) patients demonstrated symptomatic improvements following SRS (
Table 3).
Significantly, symptomatic improvement was more pronounced in the pediatric cohort (75%) compared to the adult cohort (50%) (p=0.05). These improvements encompassed relief from headaches, nausea, vomiting, ataxia, and visual impairment. However, one (12.5%) pediatric patient and one (50%) adult patient experienced worsening headaches, while one (50%) adult patient presented with new symptoms, including dizziness and vomiting post-SRS (
Table 3).
Symptomatic outcomes were notably associated with the location of tumors, with cerebellar lesions showing the highest rate of improvement (80%). Furthermore, improvements in symptoms were more evident in cases with a single prior surgery compared to multiple surgeries (81.3% vs. 57.1%) (
Table 1,
Table 2 and
Table 3).
Adverse Radiation Effect (ARE)
Among the entire cohort, 2 pediatric patients experienced radiation-induced edema, while 2 adult patients were diagnosed with radiation necrosis. Patients who experienced ARE exhibited similar dose parameters as the overall cohort, underscoring the importance of exploring individual factors that may increase susceptibility. Factors such as genetic predisposition, underlying medical conditions, and variations in tissue response to radiation may influence the likelihood and severity of ARE. Further research is warranted to elucidate the underlying mechanisms of ARE and identify strategies for risk stratification and prevention.
4. Discussion
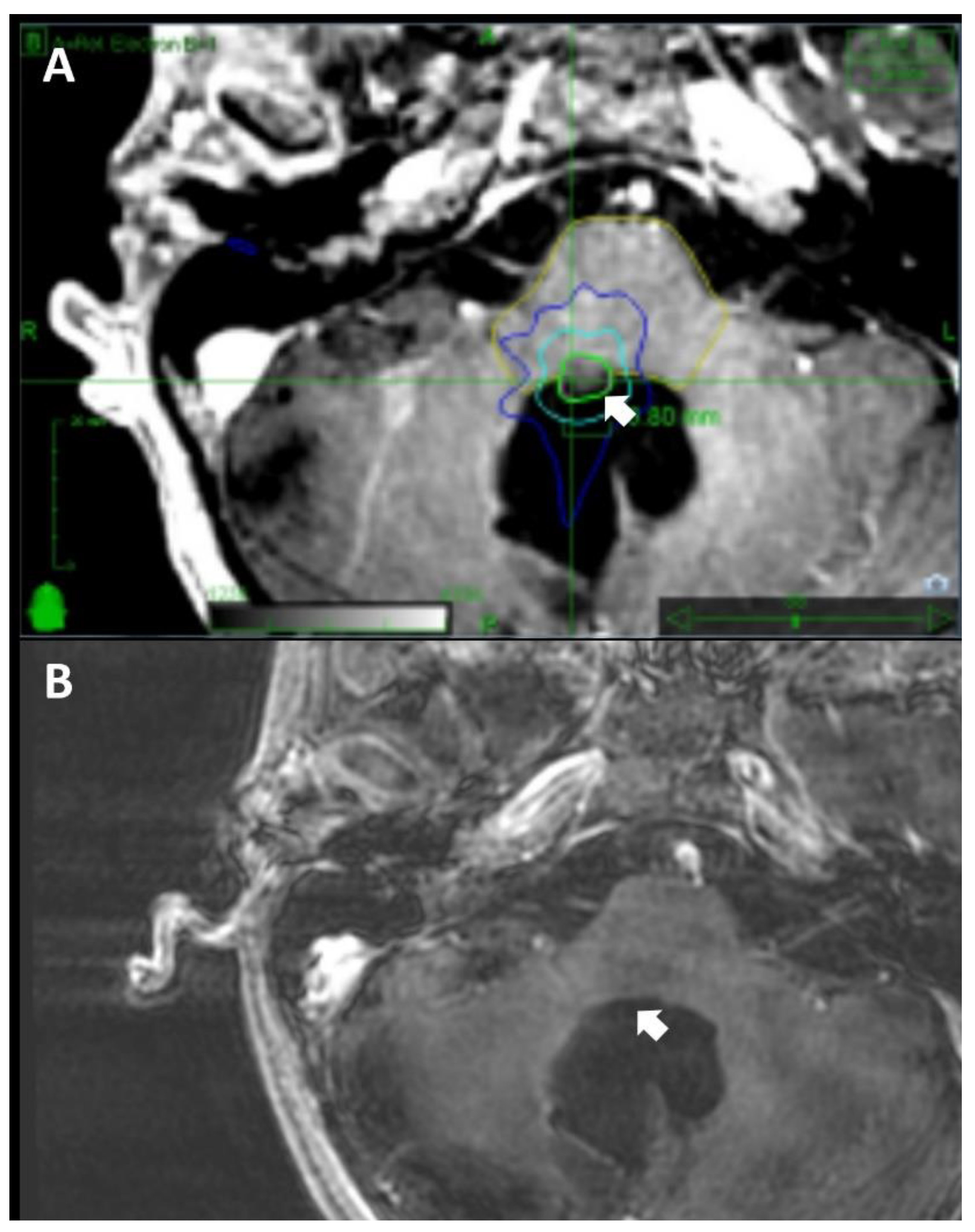
Our investigation into the outcomes of SRS for recurrent cranial medulloblastomas presents a thorough analysis of treatment efficacy and patient management (
Figure 2). The observed variations in treatment outcomes between pediatric and adult patients underscore the necessity for tailored treatment approaches. This divergence may be attributed to factors such as enhanced central nervous system (CNS) plasticity in pediatric patients or the presence of less aggressive tumor types [
14]. By integrating demographic, clinical, and treatment factors, our study provides a nuanced understanding of the intricate landscape associated with recurrent medulloblastomas.
Local Tumor Control
The observed LTC rates, especially the substantial 67% rate at 3 years in the pediatric cohort, emphasize the potential efficacy of SRS in managing recurrent medulloblastomas. These findings align with existing literature, emphasizing the significance of precision in targeting specific tumor locations, particularly cerebellar lesions, to optimize treatment outcomes [
7,
15,
16,
17,
18].
Pediatric patients, constituting a majority in our cohort, demonstrate a higher prevalence of cerebellar lesions, which may contribute to the enhanced LTC rates observed in this subgroup (
Table 1). Conversely, the higher prevalence of multiple prior surgeries in the adult cohort may be associated with comparatively lower LTC rates, suggesting a potential link between prior surgical intervention and LTC outcomes [
19,
20,
21]. Further exploration of associations between the number of fractions, BED, and LTC rates, considering factors such as age and tumor location, could enhance our understanding of the interplay influencing LTC outcomes.
Patient Survival and Progression-Free Survival
The OS rates of 90%, 70%, and 35% at 1, 3, and 5 years underscore the potential long-term benefits of SRS in managing recurrent cranial medulloblastomas, especially given their aggressive nature [
22]. The notably higher survival rates in pediatric patients compared to their adult counterparts emphasize age as a pertinent prognostic factor [
23,
24]. Moreover, the higher prevalence of multiple prior surgeries in the adult cohort may contribute to the lower OS rates, emphasizing the impact of prior treatment history on long-term survival outcomes [
25]. Shorter intervals between diagnosis and SRS may be associated with more favorable outcomes, suggesting that prompt initiation of SRS after diagnosis could contribute to improved survival rates. Smaller tumor volumes may correlate with greater OS and PFS outcomes, highlighting the importance of accurately defining and targeting tumor boundaries during SRS [
26]. Optimal margin doses play a crucial role in improving OS and PFS, with deviations potentially impacting outcomes [
27].
The nuanced interplay of these factors underscores the need for personalized treatment strategies, tailoring therapeutic approaches based on individual patient characteristics to optimize treatment outcomes.
Symptomatic Outcomes
Our investigation of symptomatic outcomes post-SRS reveals notable improvements, particularly in the pediatric patient cohort. With a higher percentage experiencing SI and lower rates of SW and NS, a potential correlation between clinical response and long-term survival outcomes is suggested [
28].
Pediatric patients, constituting 80% of our cohort, demonstrate a particularly encouraging trend in symptomatic outcomes [
29,
30]. The SI percentage of 75% in the pediatric group, compared to 50% in adults, underscores the positive impact of SRS on symptom relief in younger patients [
31]. Moreover, the correlation between tumor location and SI emphasizes the need for tailored treatment approaches, with targeting cerebellar lesions yielding favorable symptomatic outcomes.
The influence of the number of prior surgeries on symptomatic outcomes necessitates a critical examination of the optimal timing and extent of surgical interventions in managing recurrent medulloblastomas [
30,
32]. While SRS contributes to SI, understanding the interplay between prior surgeries and post-SRS symptoms is crucial for refining treatment strategies [
33]. Our findings suggest that a judicious approach to surgical interventions, considering factors such as timing and extent, may contribute to more favorable symptomatic outcomes in the context of recurrent medulloblastomas.
Adverse Radiation Effects
The identification of radiation-induced edema and necrosis, albeit in a subset of patients, emphasizes the critical role of meticulous dose planning [
29,
34]. These occurrences in both pediatric and adult patients underscore the imperative for personalized treatment strategies to mitigate ARE [
1].
Furthermore, the absence of myelopathy provides reassurance and aligns with the safety profile reported in the existing literature [
35,
36].
Integration with Previous Studies
Our findings align closely with prior studies investigating the role of SRS in recurrent medulloblastomas, reinforcing the essential role of a multidisciplinary approach in patient care [
4,
31]. The alignment with established literature enhances the generalizability of our results and significantly contributes to the growing body of evidence supporting the efficacy of SRS in managing recurrent cranial medulloblastomas.
Limitations and Future Directions
While our study offers promising insights into the efficacy and safety of CyberKnife SRS for recurrent medulloblastomas, several limitations merit consideration. The retrospective design and the relatively low sample size underscore the need for cautious interpretation of our findings. Prospective studies with larger cohorts are imperative to validate our results and strengthen the evidence base.
Moreover, despite the comprehensive nature of our follow-up protocol, the relatively short duration of follow up in some cases limit our ability to assess the long-term durability of treatment responses and potential late effects. Sustained long-term follow-up may be pivotal for elucidating the long-term efficacy and safety profile of CyberKnife SRS in this patient population.
In addition to these limitations, several areas warrant further investigation in future research endeavors. Firstly, comparative studies that evaluate the efficacy and safety of CyberKnife SRS against other treatment modalities commonly used for recurrent medulloblastomas, such as conventional radiotherapy and surgical resection, would provide valuable insights into optimal treatment strategies. Secondly, future research should explore novel treatment strategies, identify biomarkers for predicting treatment response in terms of a combination therapy, and investigate personalized medicine approaches in the management of recurrent medulloblastomas. These avenues hold the potential to further improve patient outcomes and advance our understanding of this challenging disease.
By acknowledging these limitations and delineating future research directions, we aim to lay the groundwork for ongoing investigations that build upon our initial insights and ultimately enhance the care of patients with recurrent medulloblastomas.
5. Conclusions
Our study provides a nuanced perspective on the efficacy and safety of CyberKnife SRS in addressing recurrent cranial medulloblastomas across pediatric and adult demographics. The low incidence of ARE accentuates the favorable safety profile associated with CyberKnife SRS, reaffirming its role in optimizing treatment outcomes. The intricacies of symptomatic responses, influenced by variables such as age, tumor localization, and prior surgical interventions, underscore the imperative for tailored therapeutic strategies. Our results underscore the ongoing research endeavors and the development of more refined and effective treatment approaches for recurrent medulloblastomas. Given the observed disparities in treatment outcomes between pediatric and adult cohorts, a meticulous customization of treatment modalities becomes crucial.
Conflicts of interest/Competing interests
Nothing to report.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Conference Presentations
This research has been presented as a poster at several prestigious conferences, including the American Association of Neurological Surgeons (AANS) Annual Meeting 2023 in Los Angeles, USA, the European Association of Neuro-Oncology (EANO) Annual Meeting 2023 in Rotterdam, Netherlands, the Congress of Neurological Surgeons (CNS) Annual Meeting 2023 in Washington, D.C., and the Radiosurgery Society (RSS) Scientific Meeting 2024 in Chicago, IL.
Acknowledgments
The authors express their gratitude for the valuable contributions of SDC, AM, DPJ, NJM, and KHY to the conception and design of this article. KHY played a pivotal role in data collection, analysis, manuscript composition, and the creation of figures and tables. SDC provided crucial resources and project supervision. The collaborative efforts of all authors were integral to shaping the article, and their approval has been obtained for the submitted version.
References
- Martin AM, Raabe E, Eberhart C, Cohen KJ. Management of pediatric and adult patients with medulloblastoma. Curr Treat Options Oncol. 2014;15(4):581-594. [CrossRef]
- Northcott PA, Robinson GW, Kratz CP; et al. Medulloblastoma. Nat Rev Dis Primers. 2019;5(1):11.
- Yoo KH, Park DJ, Choi JH; et al. Optimizing the synergy between stereotactic radiosurgery and immunotherapy for brain metastases. Front Oncol. 2023;13:1223599. [CrossRef]
- Thomas A, Noël G. Medulloblastoma: Optimizing care with a multidisciplinary approach. J Multidiscip Healthc. 2019;12:335-347. [CrossRef]
- Ramaswamy V, Remke M, Bouffet E; et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200-1207. [CrossRef]
- van Bree NFHN, Wilhelm M. The Tumor Microenvironment of Medulloblastoma: An Intricate Multicellular Network with Therapeutic Potential. Cancers . 2022;14(20). [CrossRef]
- Yoo KH, Park DJ, Marianayagam NJ; et al. Stereotactic Radiosurgery for Cranial and Spinal Hemangioblastomas: A Single-Institution Retrospective Series. Neurosurgery. Published online October 17, 2023. [CrossRef]
- Zeng M, Han LF. Stereotactic radiosurgery: A “targeted” therapy for cancer. Chin J Cancer. 2012;31(10):471-475.
- Choi CYH, Soltys SG, Gibbs IC; et al. Stereotactic radiosurgery of cranial nonvestibular schwannomas: Results of single- and multisession radiosurgery. Neurosurgery. 2011;68(5):1200-1208; discussion 1208. [CrossRef]
- Shi S, Jin MC, Koenig J; et al. Stereotactic Radiosurgery for Pediatric and Adult Intracranial and Spinal Ependymomas. Stereotact Funct Neurosurg. 2019;97(3):189-194. [CrossRef]
- Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554-568. [CrossRef]
- Verma V, Kulkarni RR, Bhirud AR, Bennion NR, McComb RD, Lin C. Metachronous medulloblastoma and glioblastoma: Implications for clinical and technical aspects of re-irradiation. Rep Pract Oncol Radiother. 2016;21(1):84-89. [CrossRef]
- Joiner MC, van der Kogel AJ. Basic Clinical Radiobiology. CRC Press; 2018.
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197-2221. [CrossRef]
- Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr Top Dev Biol. 2011;94:235-282.
- Peyrl A, Chocholous M, Sabel M; et al. Sustained Survival Benefit in Recurrent Medulloblastoma by a Metronomic Antiangiogenic Regimen: A Nonrandomized Controlled Trial. JAMA Oncol. 2023;9(12):1688-1695.
- Cooney T, Lindsay H, Leary S, Wechsler-Reya R. Current studies and future directions for medulloblastoma: A review from the pacific pediatric neuro-oncology consortium (PNOC) disease working group. Neoplasia. 2023;35:100861. [CrossRef]
- Suh JH, Barnett GH. Stereotactic radiosurgery for brain tumors in pediatric patients. Technol Cancer Res Treat. 2003;2(2):141-146. [CrossRef]
- Management and Long-term Outcomes of Adults With Medulloblastoma. Neurology. Accessed January 2, 2024. https://www.neurology.org/doi/full/10.1212/WNL.0000000000207631?casa_token=IwzRtTLffBMAAAAA:qD0QXqjsWZuJ20jlSRUZVTswiTOE_-_2NFRy3c-BrecfeltHpGRIby9NtCpkigblcAVwd-xS_zP3.
- Edelstein K, Spiegler BJ, Fung S; et al. Early aging in adult survivors of childhood medulloblastoma: Long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13(5):536-545. [CrossRef]
- Chan AW, Tarbell NJ, Black PM; et al. Adult medulloblastoma: Prognostic factors and patterns of relapse. Neurosurgery. 2000;47(3):623-631; discussion 631-632.
- King D, Connolly D, Zaki H, Lee V, Yeomanson D. Successful treatment of metastatic relapse of medulloblastoma in childhood with single session stereotactic radiosurgery: A report of 3 cases. J Pediatr Hematol Oncol. 2014;36(4):301-304.
- Yoo KH, Marianayagam NJ, Park DJ; et al. P11. 48. A STEREOTACTIC RADIOSURGERY FOR EPENDYMOMA IN PEDIATRIC AND ADULT PATIENTS: A SINGLE-INSTITUTION EXPERIENCE. Neuro Oncol. 2023;25(Supplement_2):ii84-ii85. [CrossRef]
- Wooley JR, Penas-Prado M. Pediatric versus Adult Medulloblastoma: Towards a Definition That Goes beyond Age. Cancers . 2021;13(24). [CrossRef]
- Gregory TA, Mastall M, Lin H; et al. Characterization of recurrence patterns and outcomes of medulloblastoma in adults: The University of Texas MD Anderson Cancer Center experience. Neurooncol Adv. 2023;5(1):vdad032. [CrossRef]
- Peña-Pino I, Chen CC. Stereotactic Radiosurgery as Treatment for Brain Metastases: An Update. Asian J Neurosurg. 2023;18(2):246-257. [CrossRef]
- Osuna-Marco MP, Martín-López LI, Tejera ÁM, López-Ibor B. Questions and answers in the management of children with medulloblastoma over the time. How did we get here? A systematic review. Front Oncol. 2023;13. [CrossRef]
- Fang FY, Rosenblum JS, Ho WS, Heiss JD. New Developments in the Pathogenesis, Therapeutic Targeting, and Treatment of Pediatric Medulloblastoma. Cancers . 2022;14(9). [CrossRef]
- Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE. Reirradiation of recurrent medulloblastoma: Does clinical benefit outweigh risk for toxicity? Cancer. 2014;120(23):3731-3737.
- Hill RM, Plasschaert SLA, Timmermann B; et al. Relapsed Medulloblastoma in Pre-Irradiated Patients: Current Practice for Diagnostics and Treatment. Cancers . 2021;14(1). [CrossRef]
- Napieralska A, Brąclik I, Radwan M, Mandera M, Blamek S. Radiosurgery or hypofractionated stereotactic radiotherapy after craniospinal irradiation in children and adults with medulloblastoma and ependymoma. Childs Nerv Syst. 2019;35(2):267-275. [CrossRef]
- Chin AL, Moding EJ, Donaldson SS; et al. Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro Oncol. 2018;20(8):1133-1141. [CrossRef]
- Hodgson DC, Goumnerova LC, Loeffler JS; et al. Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys. 2001;50(4):929-935. [CrossRef]
- Murphy ES, Merchant TE, Wu S; et al. Necrosis after craniospinal irradiation: Results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys. 2012;83(5):e655-e660. [CrossRef]
- Robinson GW, Rudneva VA, Buchhalter I; et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19(6):768-784. [CrossRef]
- Cohen ME, Duffner PK, Terplan KL. Myelopathy with severe structural derangement associated with combined modality therapy. Cancer. 1983;52(9):1590-1596. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).