Submitted:
02 April 2024
Posted:
03 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Adaptation of the Method for Unfracctioned Polysaccharides of Red Seaweed
2.1.1. Permethylation
2.1.2. Depolymerization and Reduction
2.1.3. Peracetylation and Final Cleanup
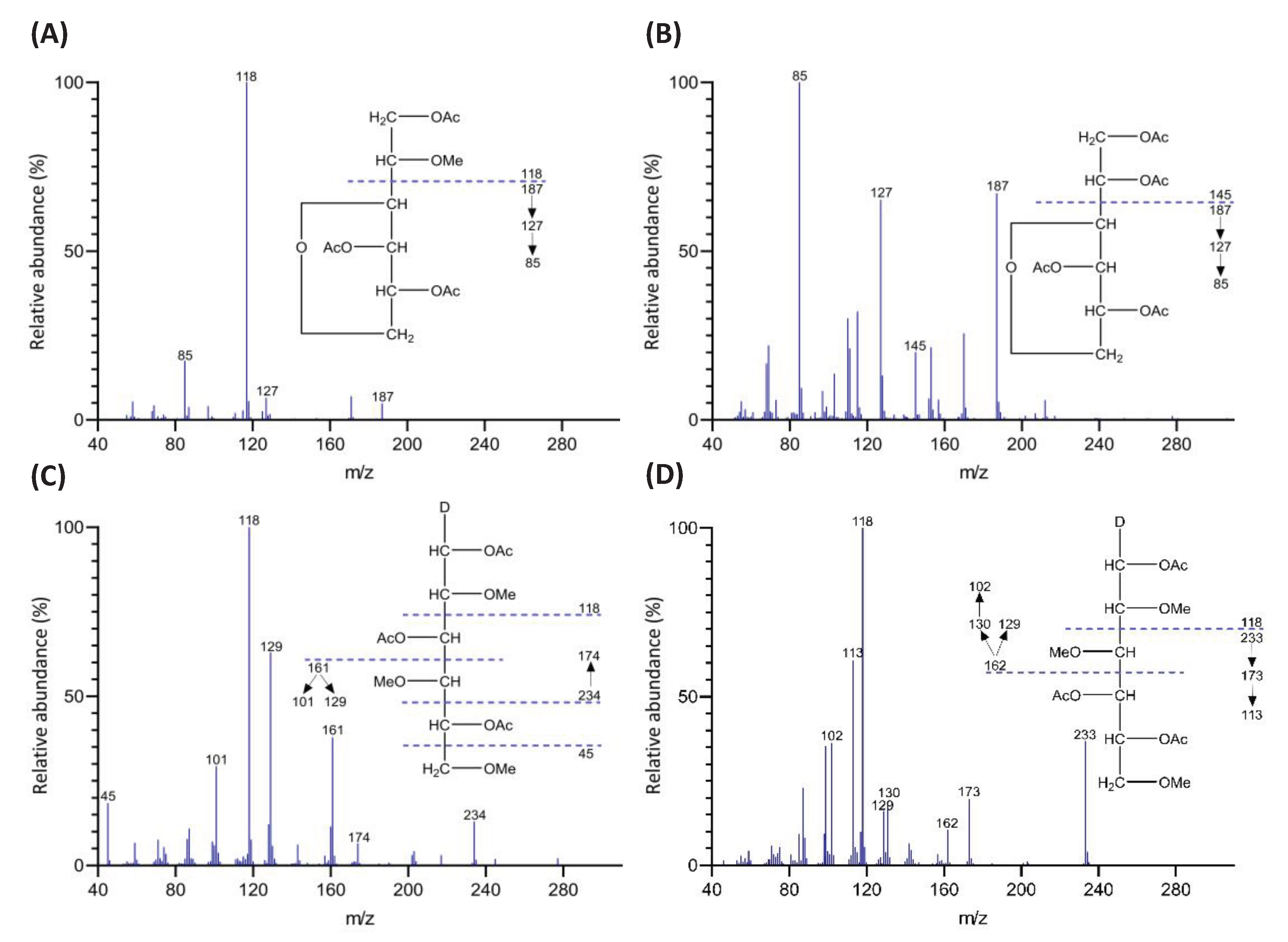
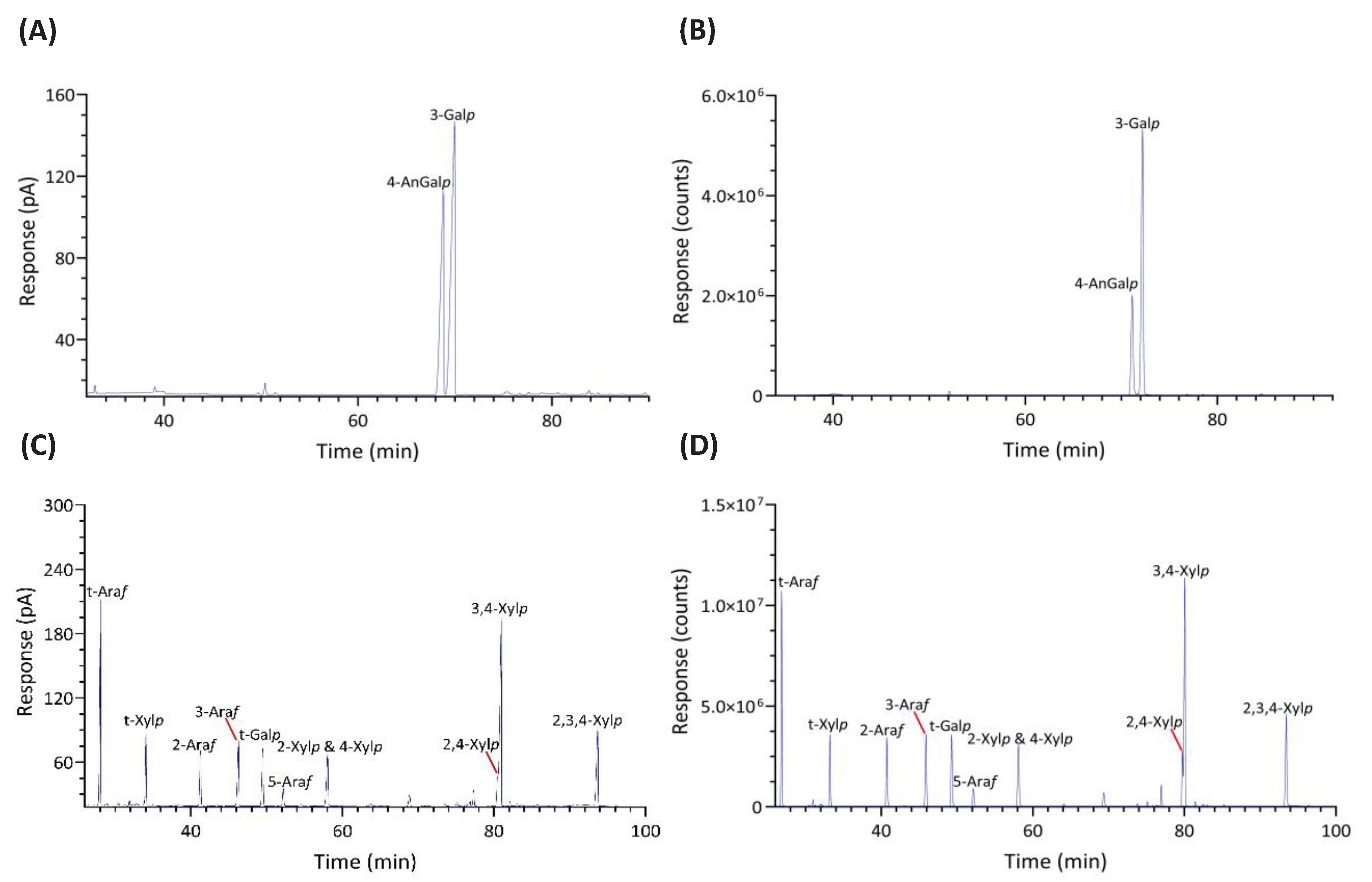
2.1.4. Identification and Quantitation of PMAAs
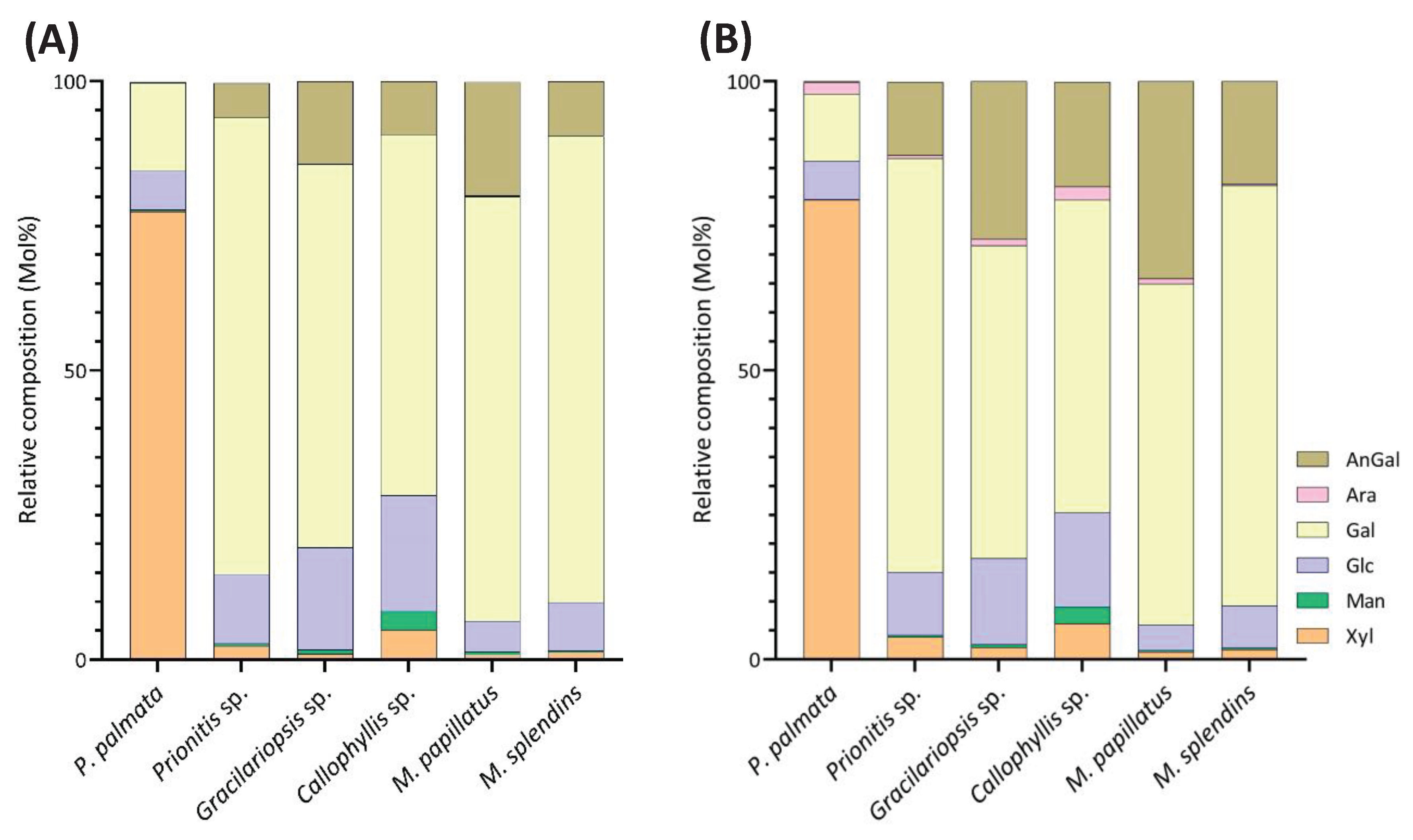
2.2. Results of Linkage Compositions of Unfractionatoed Polysaccharides in Six Red Seaweeds
2.2.1. Palmaria palmata
2.2.2. Gracilariopsis sp.
2.2.3. Prionitis sp.
2.2.4. Callophyllis sp.
2.2.5. Mastocarpus papillatus
2.2.6. Mazzaella splendins
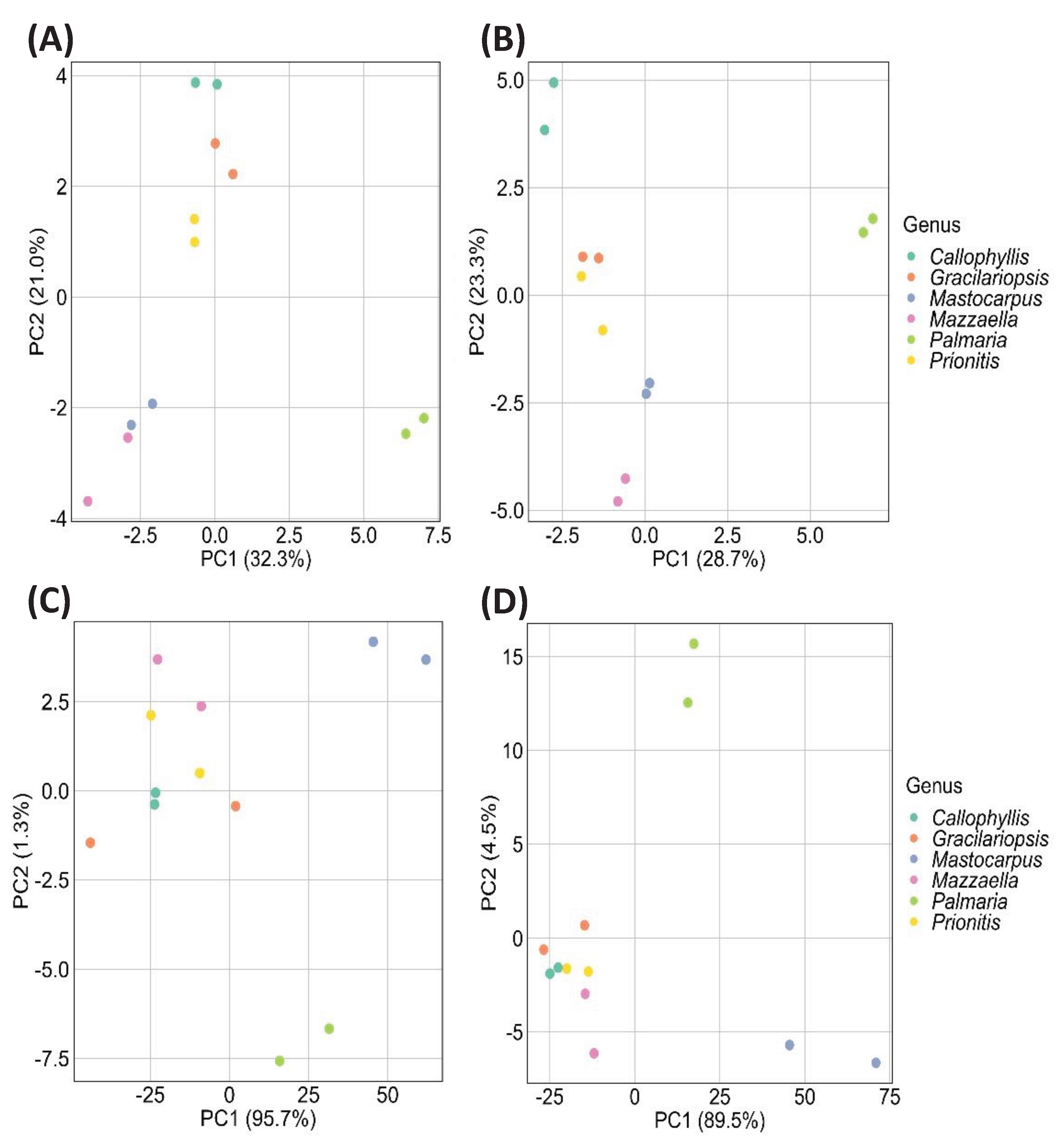
2.3. Chemometric Analysis by PCA
2.4. Further Discussions and Future Considerations
3. Materials and Methods
3.1. Materials and Reagents
3.1.1. Seaweed Materials
3.1.2. Chemicals, Reagents, and Consumables
3.1.3. Carbohydrate Standards
3.2. Preparation of Unfractionated Polysaccharides of Red Seaweeds
3.3. Preparation of PMAA Derivatives from Dry AIR Powder
3.3.1. Permethylation
3.3.2. Hydrolysis and Reduction
3.3.2.1. TFA Hydrolysis-NaBD4 Reduction
3.3.2.2. Reductive Hydrolysis
3.3.3. Peracetylation and Final Cleanup
3.4. Preparation of PMAA Standards
3.4.1. Preparation of PMAA Derivatives from Polysaccharide Standards
3.4.2. Preparation of PMAA Derivatives from Methyl Glycosides
3.5. GC-MS and GC-FID Analysis of PMAAs
3.6. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usov, A.I. , Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry, Horton, D., Ed. Academic Press: 2011; Vol. 65, pp 115-217.
- Cunha, L.; Grenha, A. , Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Marine Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.Y.; Harris, P.J. , Xylans of red and green algae: What is known about their structures and how they are synthesised? Polymers 2019, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. , Structural diversity in galactans from red seaweeds and its influence on rheological properties. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tan, H.; Nie, S. , Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chemistry 2022, 373, 131608. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M. , Food additive carrageenan: Part I: A critical review of carrageenan in vitro studies, potential pitfalls, and implications for human health and safety. Critical Reviews in Toxicology 2014, 44, 211–243. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. , Biological activities of carrageenan. In Advances in Food and Nutrition Research, Kim, S.-K., Ed. Academic Press: 2014; Vol. 72, pp 113-124.
- Weiner, M.L. , Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Critical Reviews in Toxicology 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Renn, D. , Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends in Biotechnology 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. , Carrageenan and its applications in drug delivery. Carbohydrate Polymers 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Kao, M.-R.; Saldivar, R.K.; Díaz-Moreno, S.M.; Xing, X.; Furlanetto, V.; Yayo, J.; Divne, C.; Vilaplana, F.; Abbott, D.W.; Hsieh, Y.S.Y. , The Gram-positive bacterium Romboutsia ilealis harbors a polysaccharide synthase that can produce (1,3;1,4)-β-d-glucans. Nature Communications 2023, 14, 4526. [Google Scholar] [CrossRef]
- Jæger, D.; Ndi, C.P.; Crocoll, C.; Simpson, B.S.; Khakimov, B.; Guzman-Genuino, R.M.; Hayball, J.D.; Xing, X.; Bulone, V.; Weinstein, P.; Møller, B.L.; Semple, S.J. , Isolation and structural characterization of echinocystic acid triterpenoid saponins from the Australian medicinal and food plant Acacia ligulata. Journal of Natural Products 2017, 80, 2692–2698. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. , Determining the polysaccharide composition of plant cell walls. Nature Protocols 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Xing, X.; Tingley, J.P.; Klassen, L.; King, M.L.; Alexander, T.W.; Abbott, D.W. , Analysis of active site architecture and reaction product linkage chemistry reveals a conserved cleavage substrate for an endo-alpha-mannanase within diverse yeast mannans. Journal of Molecular Biology 2020, 432, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Robb, C.S.; Hobbs, J.K.; Pluvinage, B.; Reintjes, G.; Klassen, L.; Monteith, S.; Giljan, G.; Amundsen, C.; Vickers, C.; Hettle, A.G.; Hills, R.; Nitin; Xing, X. ; Montina, T.; Zandberg, W.F.; Abbott, D.W.; Boraston, A.B., Metabolism of a hybrid algal galactan by members of the human gut microbiome. Nature Chemical Biology 2022, 18, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Lahnstein, J.; Hsieh, Y.S.Y.; Xing, X.; Yap, K.; Chaves, A.M.; Scavuzzo-Duggan, T.R.; Dimitroff, G.; Lonsdale, A.; Roberts, E.; Bulone, V.; Fincher, G.B.; Doblin, M.S.; Bacic, A.; Burton, R.A. , Functional characterization of a glycosyltransferase from the moss Physcomitrella patens involved in the biosynthesis of a novel cell wall arabinoglucan. The Plant Cell 2018, 30, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Little, A.; Lahnstein, J.; Jeffery, D.W.; Khor, S.F.; Schwerdt, J.G.; Shirley, N.J.; Hooi, M.; Xing, X.; Burton, R.A.; Bulone, V. , A novel (1,4)-β-Linked glucoxylan is synthesized by members of the cellulose synthase-like F gene family in land plants. ACS Central Science 2019, 5, 73–84. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. , A simple and rapid method for the permethylation of carbohydrates. Carbohydrate Research 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Stevenson, T.T.; Furneaux, R.H. , Chemical methods for the analysis of sulphated galactans from red algae. Carbohydr Res 1991, 210, 277–98. [Google Scholar] [CrossRef]
- Tingley, J.P.; Low, K.E.; Xing, X.; Abbott, D.W. , Combined whole cell wall analysis and streamlined in silico carbohydrate-active enzyme discovery to improve biocatalytic conversion of agricultural crop residues. Biotechnology for Biofuels 2021, 14, 16. [Google Scholar]
- Pham, T.A.T.; Kyriacou, B.A.; Schwerdt, J.G.; Shirley, N.J.; Xing, X.; Bulone, V.; Little, A. , Composition and biosynthetic machinery of the Blumeria graminis f. sp. hordei conidia cell wall. The Cell Surface 2019, 5, 100029. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Schwerdt, J.G.; Shirley, N.J.; Xing, X.; Hsieh, Y.S.Y.; Srivastava, V.; Bulone, V.; Little, A. , Analysis of cell wall synthesis and metabolism during early germination of Blumeria graminis f. sp. hordei conidial cells induced in vitro. The Cell Surface 2019, 5, 100030. [Google Scholar] [CrossRef]
- Wood, J.A.; Tan, H.-T.; Collins, H.M.; Yap, K.; Khor, S.F.; Lim, W.L.; Xing, X.; Bulone, V.; Burton, R.A.; Fincher, G.B.; Tucker, M.R. , Genetic and environmental factors contribute to variation in cell wall composition in mature desi chickpea (Cicer arietinum L.) cotyledons. Plant, Cell & Environment 2018, 41, 2195–2208. [Google Scholar]
- Li, J.; Hsiung, S.-Y.; Kao, M.-R.; Xing, X.; Chang, S.-C.; Wang, D.; Hsieh, P.-Y.; Liang, P.-H.; Zhu, Z.; Cheng, T.-J. R.; Shie, J.-J.; Liou, J.-P.; Abbott, D.W.; Kwon, S.W.; Hsieh, Y.S.Y. , Structural compositions and biological activities of cell wall polysaccharides in the rhizome, stem, and leaf of Polygonatum odoratum (Mill.) Druce. Carbohydrate Research 2022, 521, 108662. [Google Scholar] [CrossRef] [PubMed]
- Badhan, A.; Low, K.E.; Jones, D.R.; Xing, X.; Milani, M.R.M.; Polo, R.O.; Klassen, L.; Venketachalam, S.; Hahn, M.G.; Abbott, D.W.; McAllister, T.A. , Mechanistic insights into the digestion of complex dietary fibre by the rumen microbiota using combinatorial high-resolution glycomics and transcriptomic analyses. Computational and Structural Biotechnology Journal 2022, 20, 148–164. [Google Scholar] [CrossRef]
- Low, K.E.; Xing, X.; Moote, P.E.; Inglis, G.D.; Venketachalam, S.; Hahn, M.G.; King, M.L.; Tétard-Jones, C.Y.; Jones, D.R.; Willats, W.G.T.; Slominski, B.A.; Abbott, D.W. , Combinatorial glycomic analyses to direct CAZyme discovery for the tailored degradation of canola meal non-starch dietary polysaccharides. Microorganisms 2020, 8, 1888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Xing, X.; Cheng, T.-J. R.; Liang, P.-H.; Bulone, V.; Park, J.H.; Hsieh, Y.S.Y. , Structural analysis and biological activity of cell wall polysaccharides extracted from Panax ginseng marc. International Journal of Biological Macromolecules 2019, 135, 29–37. [Google Scholar] [CrossRef]
- Needs, P.W.; Selvendran, R.R. , An improved methylation procedure for the analysis of complex polysaccharides including resistant starch and a critique of the factors which lead to undermethylation. Phytochemical Analysis 1993, 4, 210–216. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. , Cell wall polysaccharides of marine algae. In Springer Handbook of Marine Biotechnology, Kim, S.-K., Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp 543-590.
- Hermouet, C.; Garnier, R.; Efthymiou, M.-L.; Fournier, P.-E. , Methyl iodide poisoning: Report of two cases. American Journal of Industrial Medicine 1996, 30, 759–764. [Google Scholar] [CrossRef]
- Heiss, C.; Wang, Z.; Azadi, P. , Sodium hydroxide permethylation of heparin disaccharides. Rapid Communications in Mass Spectrometry 2011, 25, 774–778. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. , A revised structure for fucoidan may explain some of its biological activities. Journal of Biological Chemistry 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Ciucanu, I.; Costello, C.E. , Elimination of oxidative degradation during the per-O-methylation of carbohydrates. Journal of the American Chemical Society 2003, 125, 16213–16219. [Google Scholar] [CrossRef]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. , Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydrate Polymers 2017, 165, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. , A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Emaga, T.; Rabetafika, H.; Blecker, C.; Paquot, M. , Kinetics of the hydrolysis of polysaccharide galacturonic acid and neutral sugars chains from flaxseed mucilage. Biotechnologie Agronomie Société et Environnement 2012, 16, 139–147. [Google Scholar]
- Ndukwe, I.E.; Black, I.; Heiss, C.; Azadi, P. , Evaluating the utility of permethylated polysaccharide solution NMR data for characterization of insoluble plant cell wall polysaccharides. Analytical Chemistry 2020, 92, 13221–13228. [Google Scholar] [CrossRef] [PubMed]
- Black, I.; Heiss, C.; Azadi, P. , Comprehensive monosaccharide composition analysis of insoluble polysaccharides by permethylation to produce methyl alditol derivatives for gas chromatography/mass spectrometry. Analytical Chemistry 2019, 91, 13787–13793. [Google Scholar] [CrossRef] [PubMed]
- Oades, J.M. , Gas-liquid chromatography of alditol acetates and its application to the analysis of sugars in complex hydrolysates. Journal of Chromatography A 1967, 28, 246–252. [Google Scholar] [CrossRef]
- Gunner, S.; Jones, J.; Perry, M. Analysis of sugar mixtures by gas-liquid partition chromatography. Chemistry and Industry 255–256.
- Voiges, K.; Adden, R.; Rinken, M.; Mischnick, P. , Critical re-investigation of the alditol acetate method for analysis of substituent distribution in methyl cellulose. Cellulose 2012, 19, 993–1004. [Google Scholar] [CrossRef]
- Yu, L.; Yakubov, G.E.; Zeng, W.; Xing, X.; Stenson, J.; Bulone, V.; Stokes, J.R. , Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydr Polym 2017, 165, 132–141. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Jiang, C.; Shi, J. , Preventive and therapeutic effects of an exopolysaccharide produced by Lacticaseibacillus rhamnosus on alcoholic gastric ulcers. International Journal of Biological Macromolecules 2023, 235, 123845. [Google Scholar] [CrossRef]
- Cayot, N.; Lafarge, C.; Bou-Maroun, E.; Cayot, P. , Substitution of carcinogenic solvent dichloromethane for the extraction of volatile compounds in a fat-free model food system. Journal of Chromatography A 2016, 1456, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, B.; van Beek, T.A. , Alternative solvents can make preparative liquid chromatography greener. Green Chemistry 2015, 17, 4073–4081. [Google Scholar] [CrossRef]
- Bartle, K.D.; Myers, P. , History of gas chromatography. TrAC Trends in Analytical Chemistry 2002, 21, 547–557. [Google Scholar] [CrossRef]
- Sweet, D.P.; Shapiro, R.H.; Albersheim, P. , Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydrate Research 1975, 40, 217–225. [Google Scholar] [CrossRef]
- Ciucanu, I. , Per-O-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Analytica Chimica Acta 2006, 576, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jay, A. , The methylation reaction in carbohydrate analysis. Journal of Carbohydrate Chemistry 1996, 15, 897–923. [Google Scholar] [CrossRef]
- Ciucanu, I.; Luca, C. , Avoidance of degradation during the methylation of uronic acids. Carbohydrate Research 1990, 206, 71–77. [Google Scholar] [CrossRef]
- Falshaw, R.; Furneaux, R.H.; Sims, I.M.; Hinkley, S.F.R.; Kidgell, J.T.; Bell, T.J. , Novel 4-O-β-d-xylopyranosyl-3,6-anhydro-l-galactopyranosyl disaccharide units in a polysaccharide from the red alga Pyrophyllon subtumens. Carbohydrate Polymers 2023, 318, 121066. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.G.; Noseda, M.D.; Gonçalves, A.G.; Duarte, M.E.R.; Yokoya, N.; Matulewicz, M.C.; Cerezo, A.S. , β-d-(1→4), β-d-(1→3) ‘mixed linkage’ xylans from red seaweeds of the order Nemaliales and Palmariales. Carbohydrate Research 2011, 346, 1023–1028. [Google Scholar] [CrossRef]
- Lahaye, M.; Michel, C.; Barry, J.L. , Chemical, physicochemical and in-vitro fermentation characteristics of dietary fibres from Palmaria palmata (L.) Kuntze. Food Chemistry 1993, 47, 29–36. [Google Scholar] [CrossRef]
- Turvey, J.R.; Williams, E.L. , The structures of some xylans from red algae. Phytochemistry 1970, 9, 2383–2388. [Google Scholar] [CrossRef]
- Deniaud, E.; Fleurence, J.; Lahaye, M. Preparation and chemical characterization of cell wall fractions enriched in structural proteins from Palmaria palmata (Rhodophyta). 2003, 46, 366–377.
- Deniaud, E.; Quemener, B.; Fleurence, J.; Lahaye, M. Structural studies of the mix-linked β-(1 → 3)/β-(1 → 4)-d-xylans from the cell wall of Palmaria palmata (Rhodophyta). International Journal of Biological Macromolecules 2003, 33, 9–18. [Google Scholar] [CrossRef]
- Painter, T.J. , Algal polysaccharides. In The Polysaccharides, Aspinall, G.O., Ed. Academic Press: 1983; pp 195-285.
- Joubert, Y.; Fleurence, J. , Simultaneous extraction of protein and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). Journal of Applied Phycology 2008, 20, 55–61. [Google Scholar] [CrossRef]
- Long, X.; Hu, X.; Liu, S.; Pan, C.; Chen, S.; Li, L.; Qi, B.; Yang, X. , Insights on preparation, structure and activities of Gracilaria lemaneiformis polysaccharide. Food Chem X 2021, 12, 100153. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, R.A.; Canelón, D.J.; Cosenza, V.A.; Fissore, E.N.; Gerschenson, L.N.; Matulewicz, M.C.; Ciancia, M. , Gracilariopsis hommersandii, a red seaweed, source of agar and sulfated polysaccharides with unusual structures. Carbohydrate Polymers 2019, 213, 138–146. [Google Scholar] [CrossRef]
- Rees, D.A. , Enzymic synthesis of 3:6-anhydro-l-galactose within porphyran from l-galactose 6-sulphate units. Biochem J 1961, 81, 347–52. [Google Scholar] [CrossRef]
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. , In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. Journal of Functional Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, X.; Hu, S.; Yu, Y.; Sun, X.; Xu, N. , Enzymatic degradation of Gracilariopsis lemaneiformis polysaccharide and the antioxidant activity of its degradation products. Mar Drugs 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.C.; Merino, E.R.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M.C. , Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales). Carbohydrate Research 2005, 340, 2742–2751. [Google Scholar] [CrossRef]
- Chopin, T.; Kerin, B.F.; Mazerolle, R. , Phycocolloid chemistry as a taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae: A review and current developments using Fourier transform infrared diffuse reflectance spectroscopy. Phycological Research 1999, 47, 167–188. [Google Scholar] [CrossRef]
- Usov, A.I.; Klochkova, N.G. , Polysaccharides of algae 45. Polysaccharide composition of red seaweeds from Kamchatka coastal waters (Northwestern Pacific) studied by reductive hydrolysis of biomass. Botanica Marina 1992, 35, 371–378. [Google Scholar] [CrossRef]
- McCandless, E.L.; Gretz, M.R. , Biochemical and immunochemical analysis of carrageenans of the Gigartinaceae and Phyllophoraceae. Hydrobiologia 1984, 116, 175–178. [Google Scholar] [CrossRef]
- Tasende, M.G.; Cid, M.; Fraga, M.I. , Qualitative and quantitative analysis of carrageenan content in gametophytes of Mastocarpus stellatus (Stackhouse) Guiry along Galician coast (NW Spain). Journal of Applied Phycology 2013, 25, 587–596. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Glazunov, V.P.; Sokolova, E.V.; Isakov, V.V.; Yermak, I.M. , Structural characteristics of carrageenans of red alga Mastocarpus pacificus from sea of Japan. Carbohydrate Polymers 2020, 229, 115518. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. , Antiviral activity of carrageenans and processing implications. Marine Drugs 2021, 19. [Google Scholar] [CrossRef]
- Kravchenko, A.; Anastyuk, S.; Glazunov, V.; Sokolova, E.; Isakov, V.; Yermak, I. , Structural peculiarities of carrageenans from Far Eastern red seaweed Mazzaella parksii (Gigartinaceae). International Journal of Biological Macromolecules 2023, 228, 346–357. [Google Scholar] [CrossRef]
- Kumar, K. , Principal component analysis: Most favourite tool in chemometrics. Resonance 2017, 22, 747–759. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. , Principal component analysis: A review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Sloneker, J.H.; Orentas, D.G. , Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature 1962, 194, 478–479. [Google Scholar] [CrossRef]
- Takano, R. , Desulfation of sulfated polysaccharides. Trends in Glycoscience and Glycotechnology 2002, 14, 343–351. [Google Scholar] [CrossRef]
- Wood, J.; Tan, H.-T.; Collins, H.; Yap, K.; Khor, S.; Lim, W.L.; Xing, X.; Bulone, V.; Burton, R.; Fincher, G.; Tucker, M. , Genetic and environmental factors contribute to variation in cell wall composition in mature desi chickpea (Cicer arietinum L.) cotyledons. Plant, cell & environment 2018, 41. [Google Scholar]
- Klassen, L.; Reintjes, G.; Li, M.; Jin, L.; Amundsen, C.; Xing, X.; Dridi, L.; Castagner, B.; Alexander, T.W.; Abbott, D.W. , Fluorescence activated cell sorting and fermentation analysis to study rumen microbiome responses to administered live microbials and yeast cell wall derived prebiotics. Frontiers in Microbiology 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.H.; Cleary, M.T.; Dokoozlian, N.; Ford, C.M.; Fincher, G.B. , Soluble cell wall carbohydrates and their relationship with sensory attributes in Cabernet Sauvignon wine. Food Chemistry 2019, 298, 124745. [Google Scholar] [CrossRef] [PubMed]
- Muhidinov, Z.K.; Bobokalonov, J.T.; Ismoilov, I.B.; Strahan, G.D.; Chau, H.K.; Hotchkiss, A.T.; Liu, L. , Characterization of two types of polysaccharides from Eremurus hissaricus roots growing in Tajikistan. Food Hydrocolloids 2020, 105, 105768. [Google Scholar] [CrossRef]
- Hosain, N.A.; Ghosh, R.; Bryant, D.L.; Arivett, B.A.; Farone, A.L.; Kline, P.C. , Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. International Journal of Biological Macromolecules 2019, 133, 76–85. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, Y.; Zhang, L.; Huang, L. , A facile method for the synthesis of partially O-methylated alditol acetate standards for GC–MS analysis of galactofuranose-containing structures. Carbohydrate Research 2013, 379, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Shea, E.M. Linkage structure of carbohydrates by gas chromatography-mass spectrometry (GC-MS) of partially methylated alditol acetates. In Analysis of Carbohydrates by GLC and MS, C.J., B.; McGinnis, G.D., Eds. CRC Press, Inc.: Boca Raton, Florida, 1989; pp 157-216.
- Yu, S.; Blennow, A.; Bojko, M.; Madsen, F.; Olsen, C.E.; Engelsen, S.B. , Physico-chemical characterization of floridean starch of red algae. Starch - Stärke 2002, 54, 66–74. [Google Scholar] [CrossRef]
- Wickham, H. , Ggplot2: Elegant Gaphics for Data Analysis. Springer-Verlag New York: 2016.
- Wickham, H. , Reshaping data with the reshape package. Journal of Statistical Software 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H.F.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: a grammar of data manipulation. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr.
- Wickham, H. Forcats: tools for working with categorical variables (factors). https://forcats.tidyverse.org/.
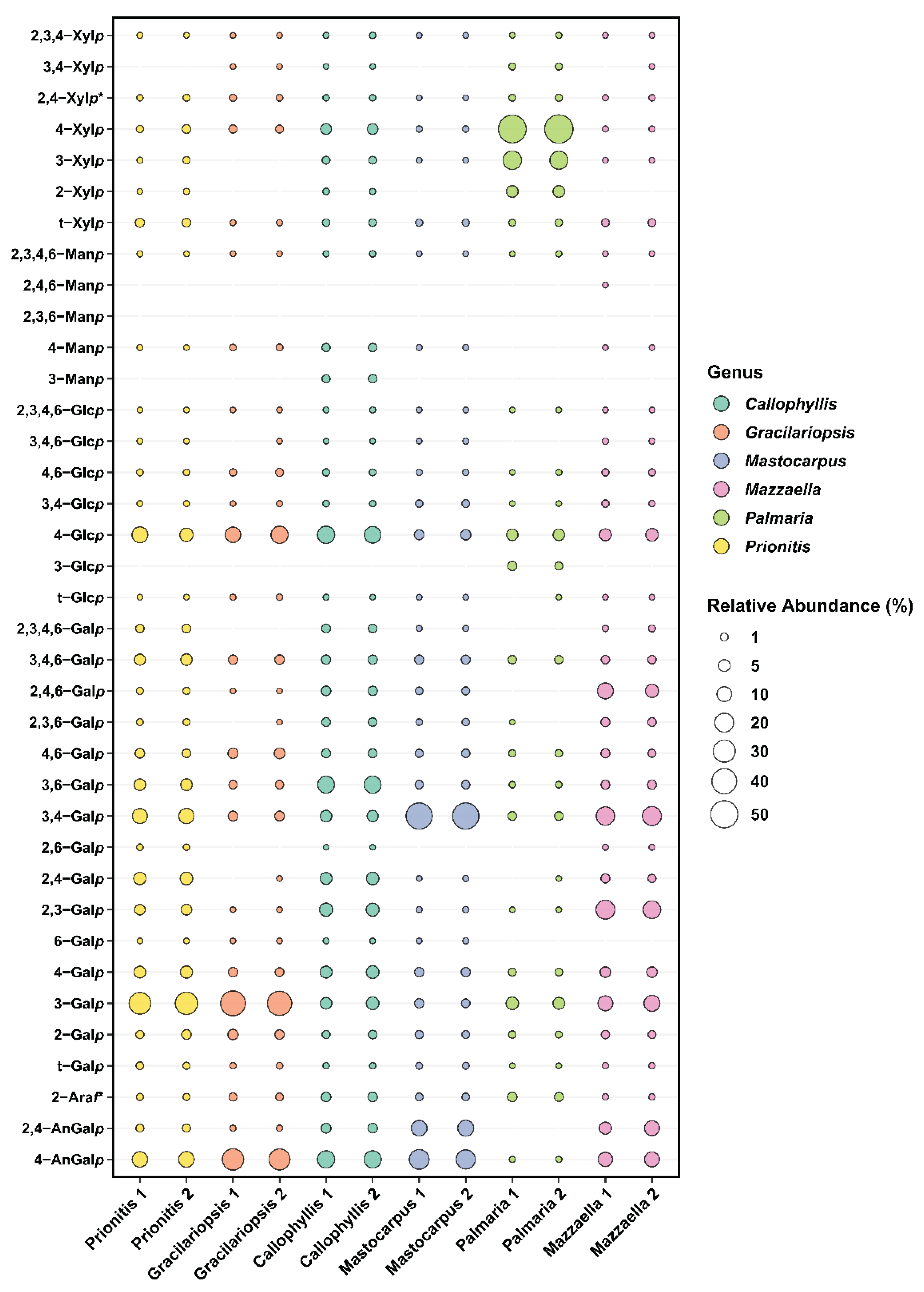
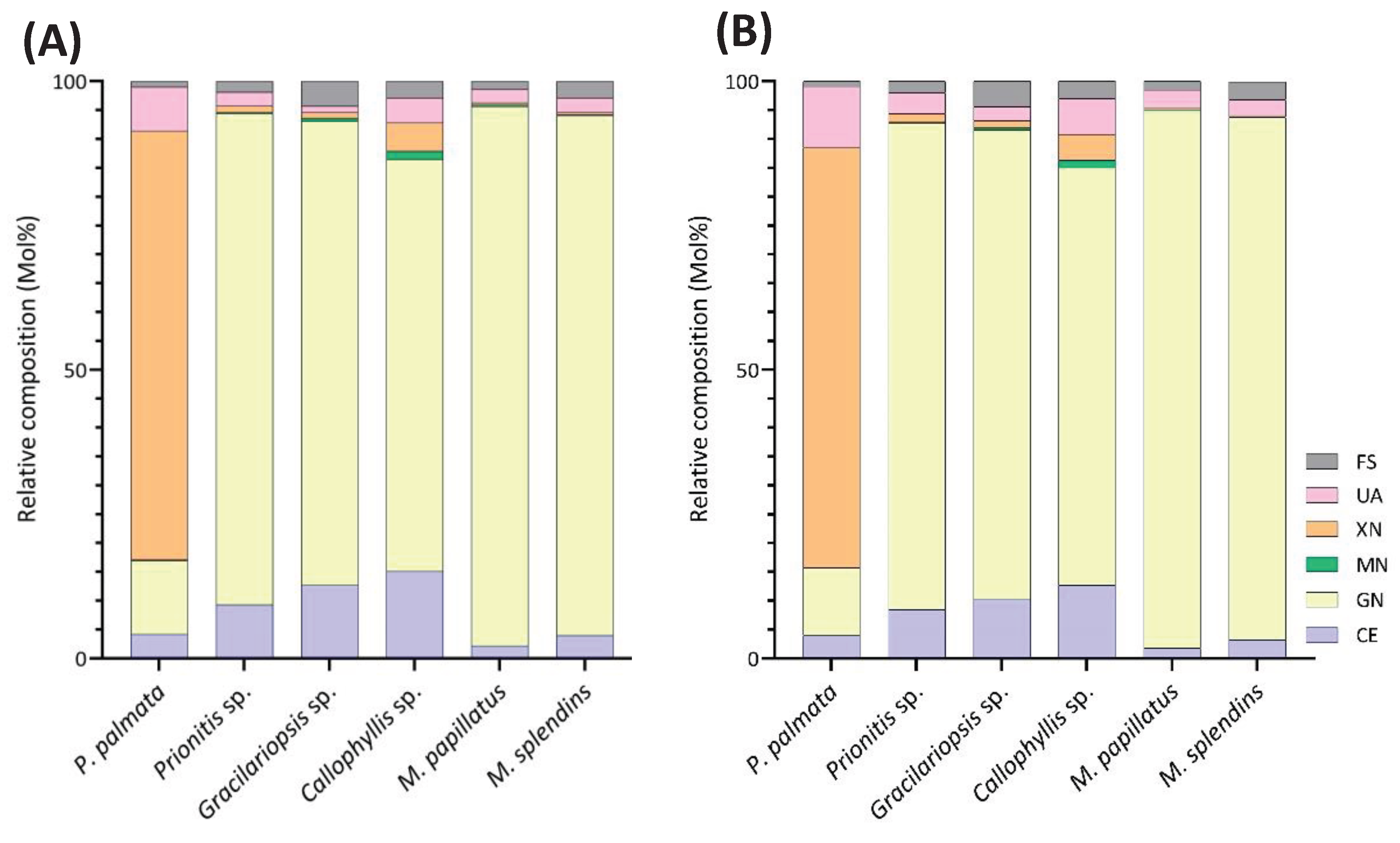
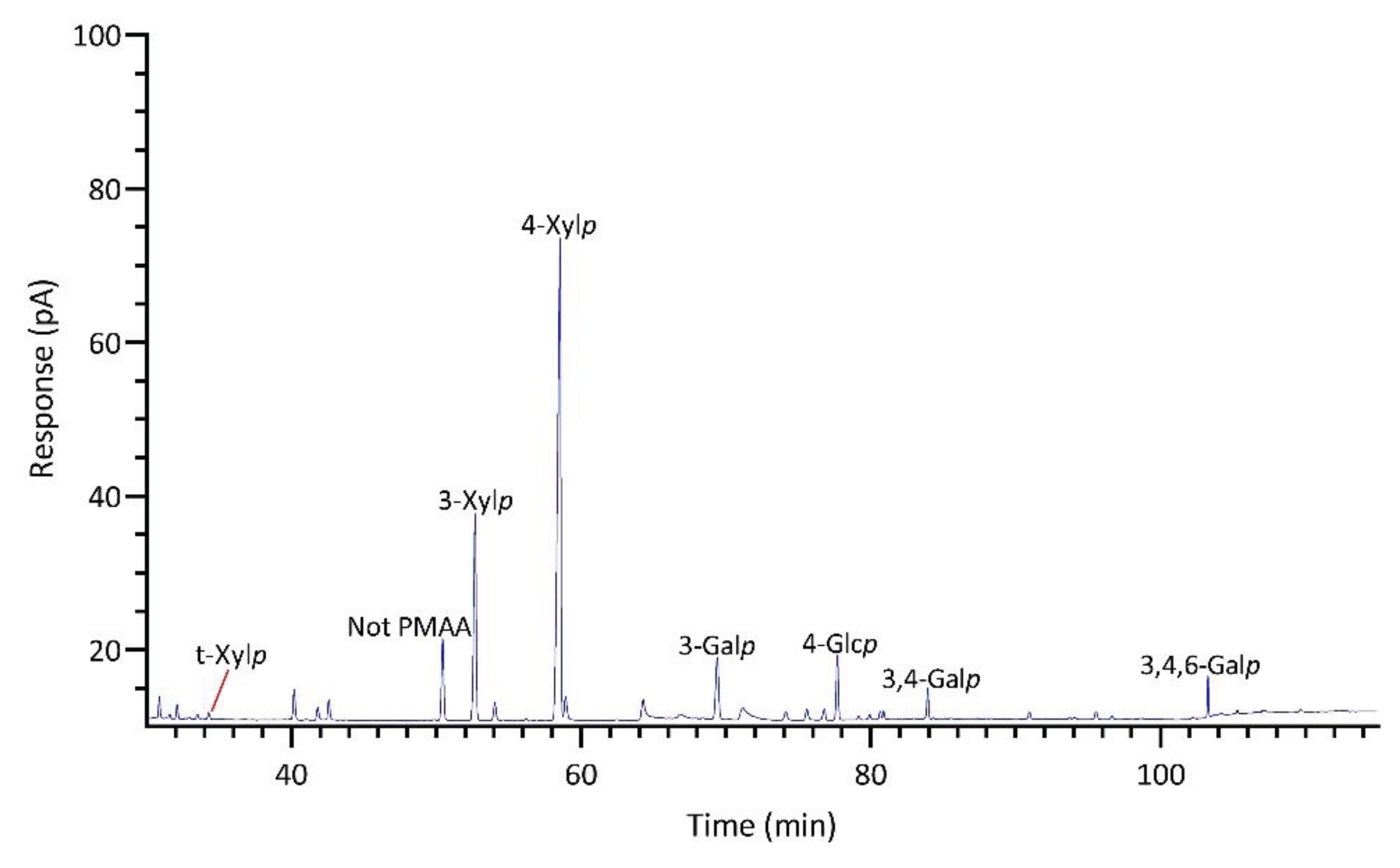
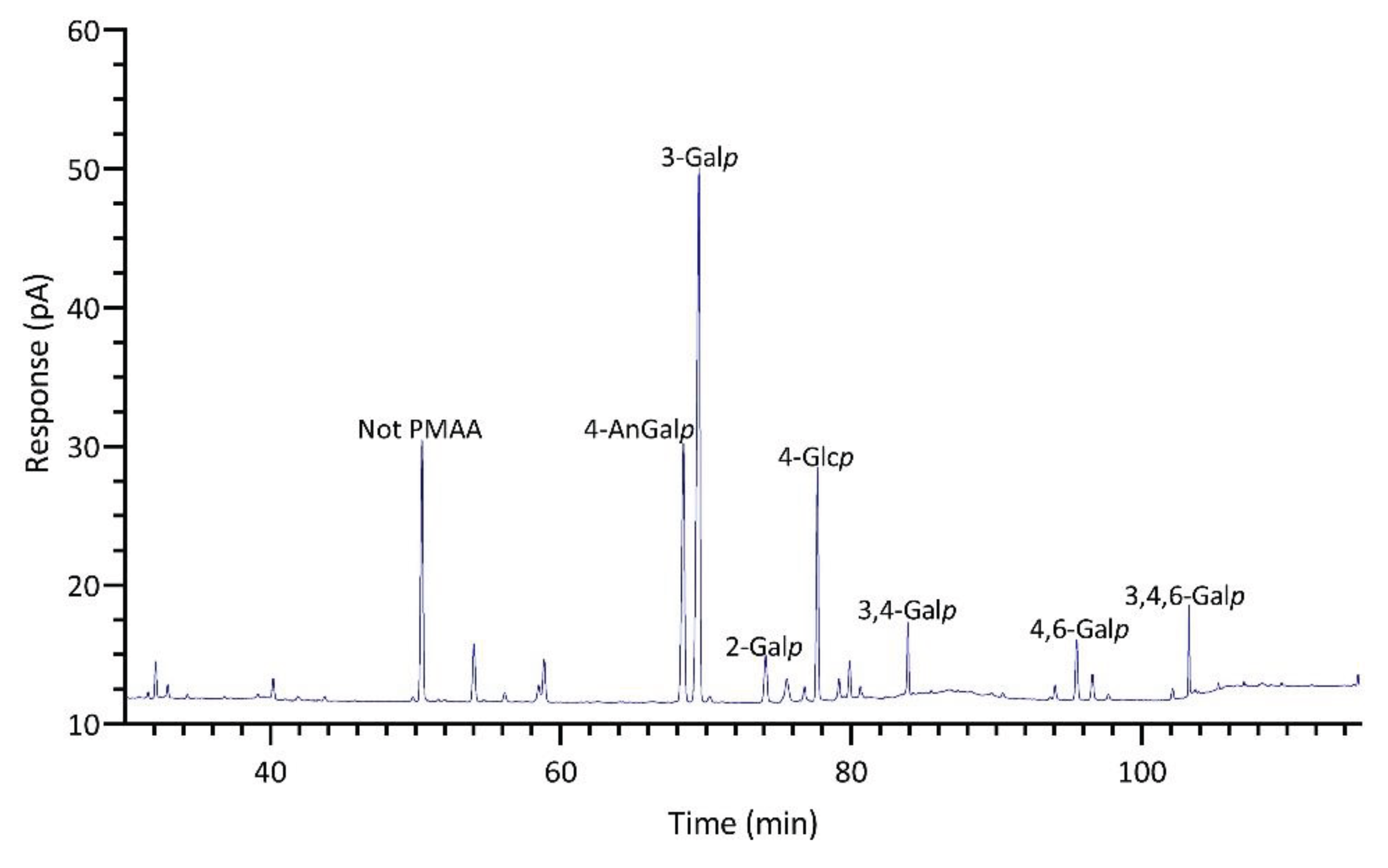
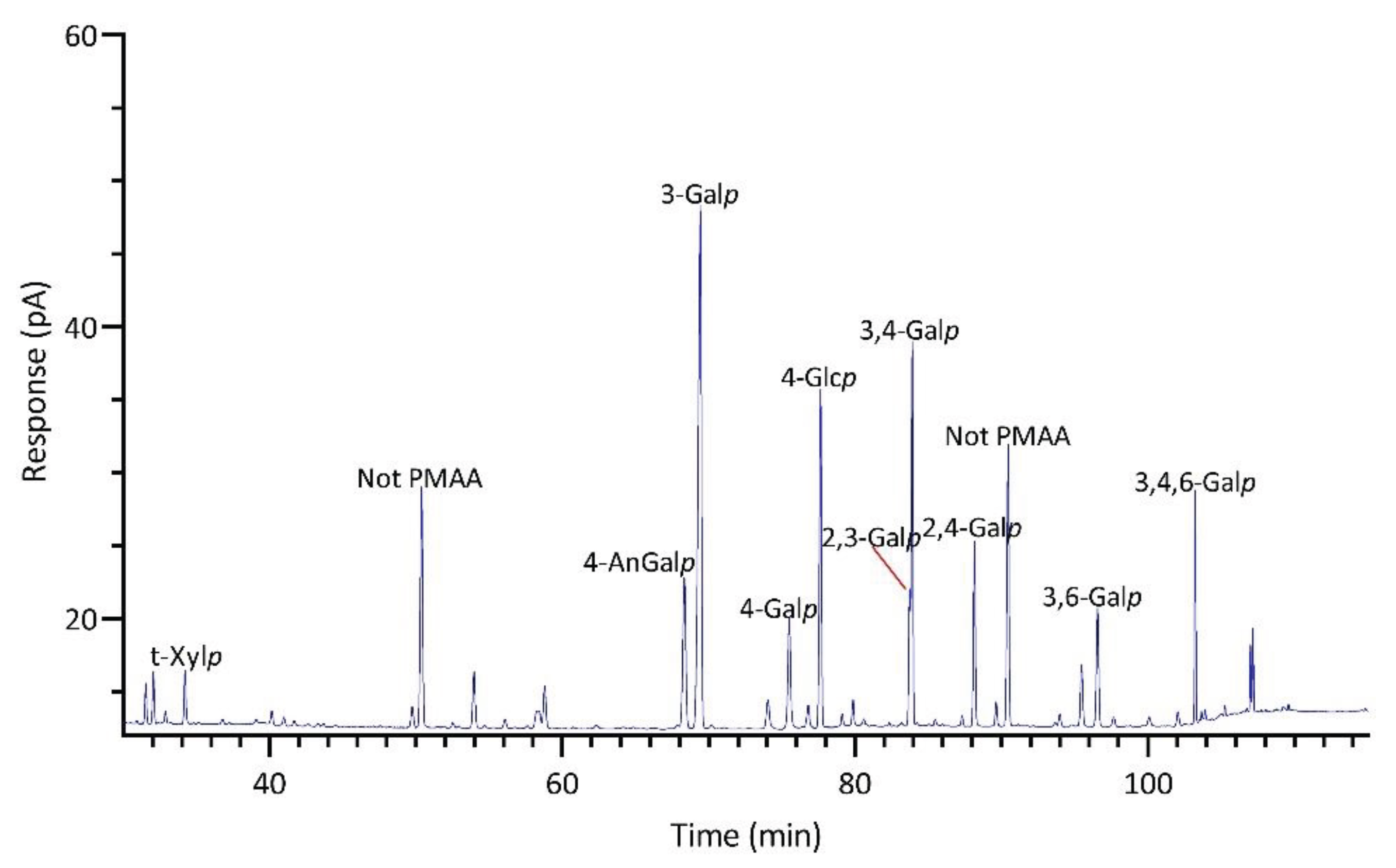
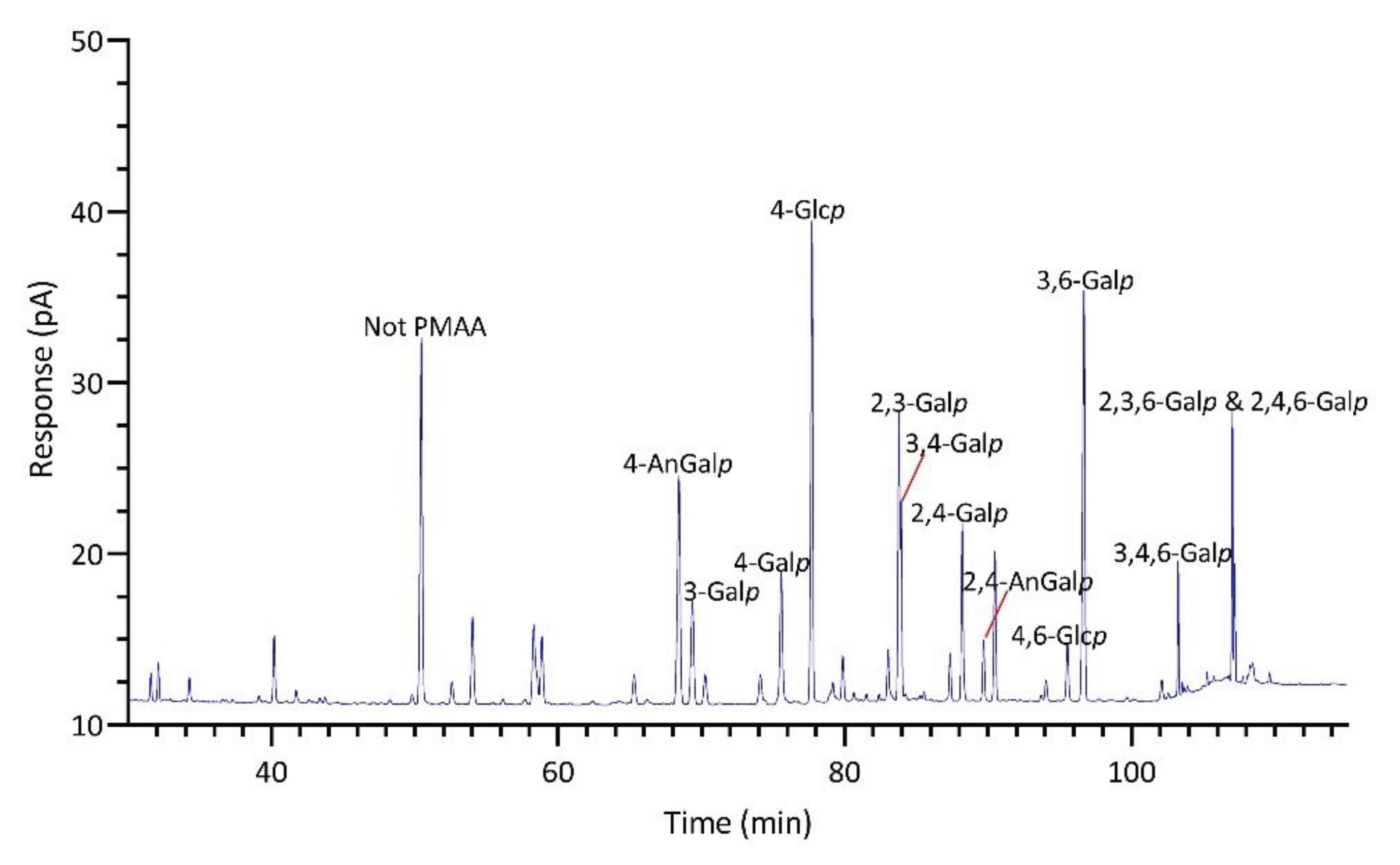
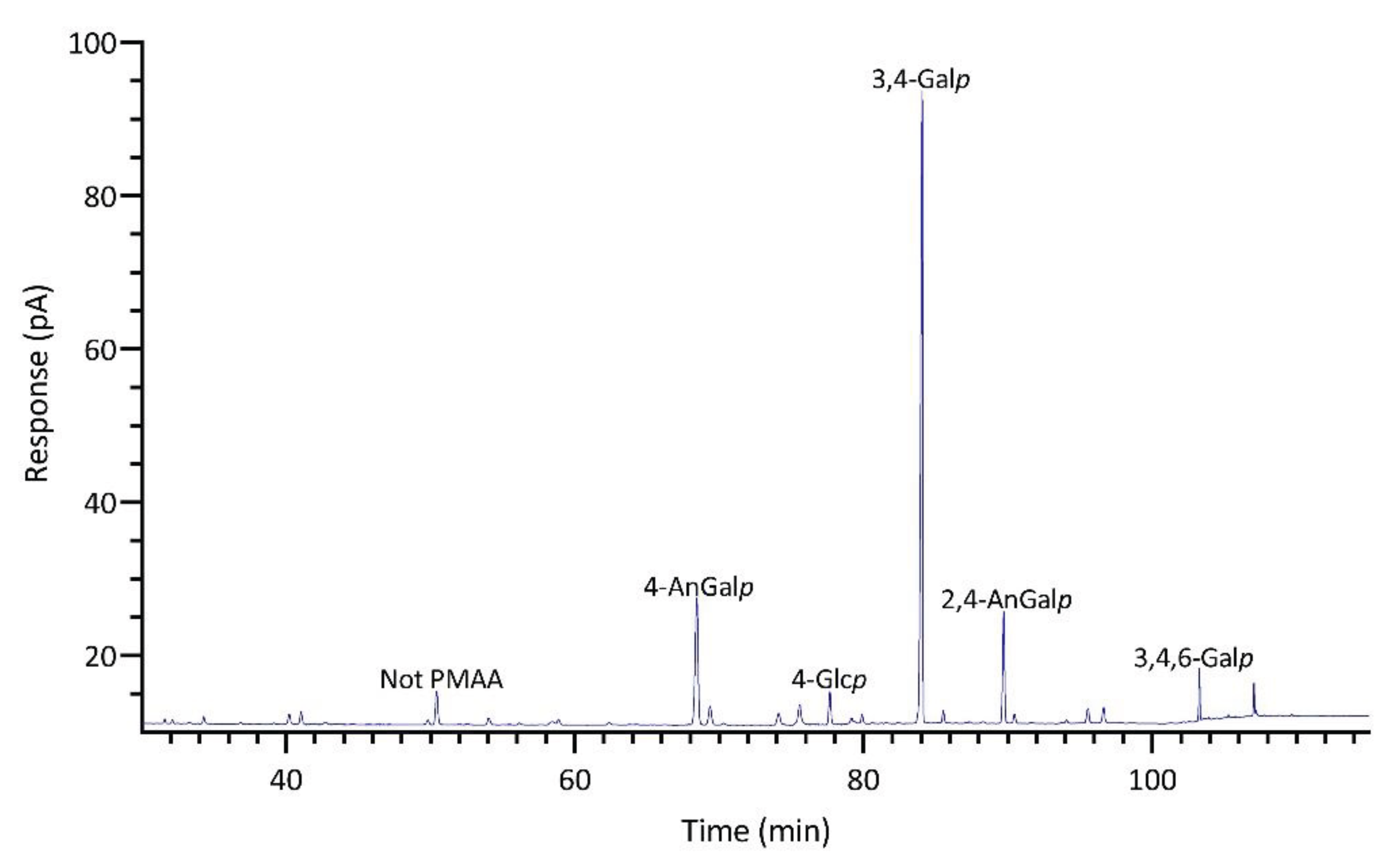
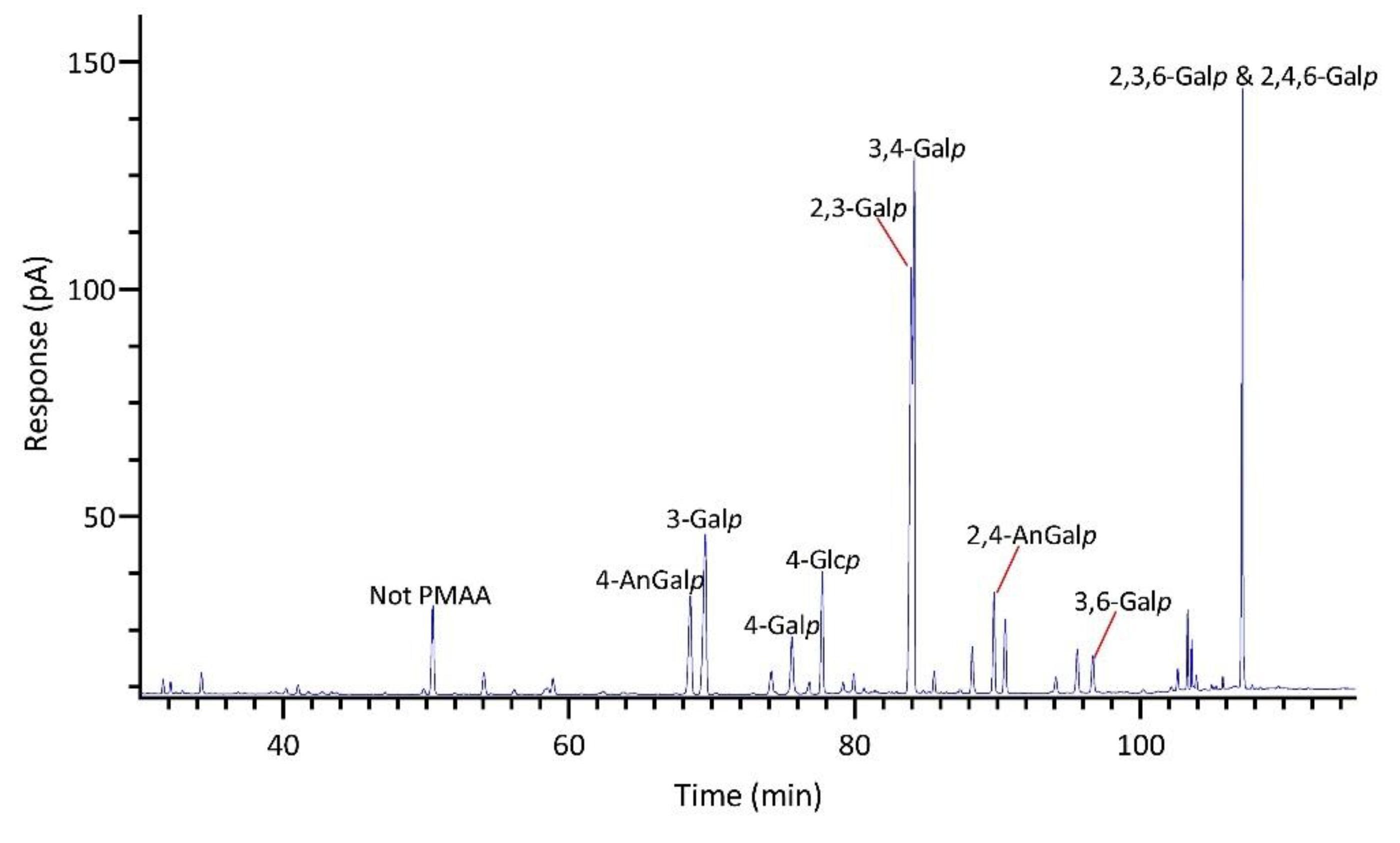
| Linkage | Prionitis | Gracilariopsis | Callophyllis | Mastocarpus | Palmaria | Mazzaella |
|---|---|---|---|---|---|---|
| 4-AnGalp | 11.6 | 27.1 | 15.7 | 21.8 | Tr. | 9.7 |
| 2,4-AnGalp | 1.1 | Tr. | 2.4 | 12.3 | Tr. | 8.0 |
| 2-Araf | 0.5 | 1.1 | 2.3 | 0.9 | 2.0 | Tr. |
| t-Galp | 0.7 | Tr. | Tr. | 0.5 | Tr. | Tr. |
| 2-Galp | 1.8 | 2.7 | 1.3 | 1.1 | 0.7 | 1.3 |
| 3-Galp | 29.8 | 39.5 | 5.6 | 1.9 | 5.6 | 11.5 |
| 4-Galp | 5.3 | 1.9 | 5.8 | 2.1 | 0.9 | 3.4 |
| 6-Galp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 2,3-Galp | 3.5 | Tr. | 7.0 | Tr. | Tr. | 18.4 |
| 2,4-Galp | 6.0 | Tr. | 5.6 | Tr. | Tr. | 1.5 |
| 2,6-Galp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 3,4-Galp | 10.8 | 2.5 | 4.4 | 46.2 | 1.6 | 19.4 |
| 3,6-Galp | 4.5 | 1.3 | 14.6 | 1.3 | Tr. | 1.7 |
| 4,6-Galp | 1.8 | 3.3 | 1.8 | 1.3 | 0.7 | 1.5 |
| 2,3,6-Galp | 0.5 | Tr. | 1.6 | 0.6 | Tr. | 1.8 |
| 2,4,6-Galp | 0.6 | Tr. | 2.2 | 1.0 | Tr. | 9.9 |
| 3,4,6-Galp | 4.3 | 2.2 | 2.0 | 2.0 | 1.4 | 1.5 |
| 2,3,4,6-Galp | 1.5 | Tr. | 1.6 | Tr. | Tr. | Tr. |
| t-Glcp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 3-Glcp | Tr. | Tr. | Tr. | Tr. | 1.6 | Tr. |
| 4-Glcp | 9.9 | 13.4 | 14.9 | 2.9 | 4.6 | 5.7 |
| 3,4-Glcp | Tr. | Tr. | Tr. | 0.9 | Tr. | 0.5 |
| 4,6-Glcp | Tr. | 0.9 | 0.6 | Tr. | Tr. | 0.7 |
| 3,4,6-Glcp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 2,3,4,6-Glcp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| t-Manp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 3-Manp | Tr. | Tr. | 1.3 | Tr. | Tr. | Tr. |
| 4-Manp | Tr. | Tr. | 1.3 | Tr. | Tr. | Tr. |
| 2,3,6-Manp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 2,4,6-Manp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| 2,3,4,6-Manp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
| t-Xylp | 1.8 | Tr. | 0.8 | 0.7 | 0.6 | 1.0 |
| 2-Xylp | Tr. | Tr. | Tr. | Tr. | 4.9 | Tr. |
| 4-Xylp | 1.1 | 1.2 | 3.6 | Tr. | 54.4 | Tr. |
| 3-Xylp | Tr. | Tr. | 0.9 | Tr. | 18.4 | Tr. |
| 2,4-Xylp | Tr. | 0.5 | Tr. | Tr. | 0.6 | Tr. |
| 3,4-Xylp | Tr. | Tr. | Tr. | Tr. | 0.5 | Tr. |
| 2,3,4-Xylp | Tr. | Tr. | Tr. | Tr. | Tr. | Tr. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





