Submitted:
29 March 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
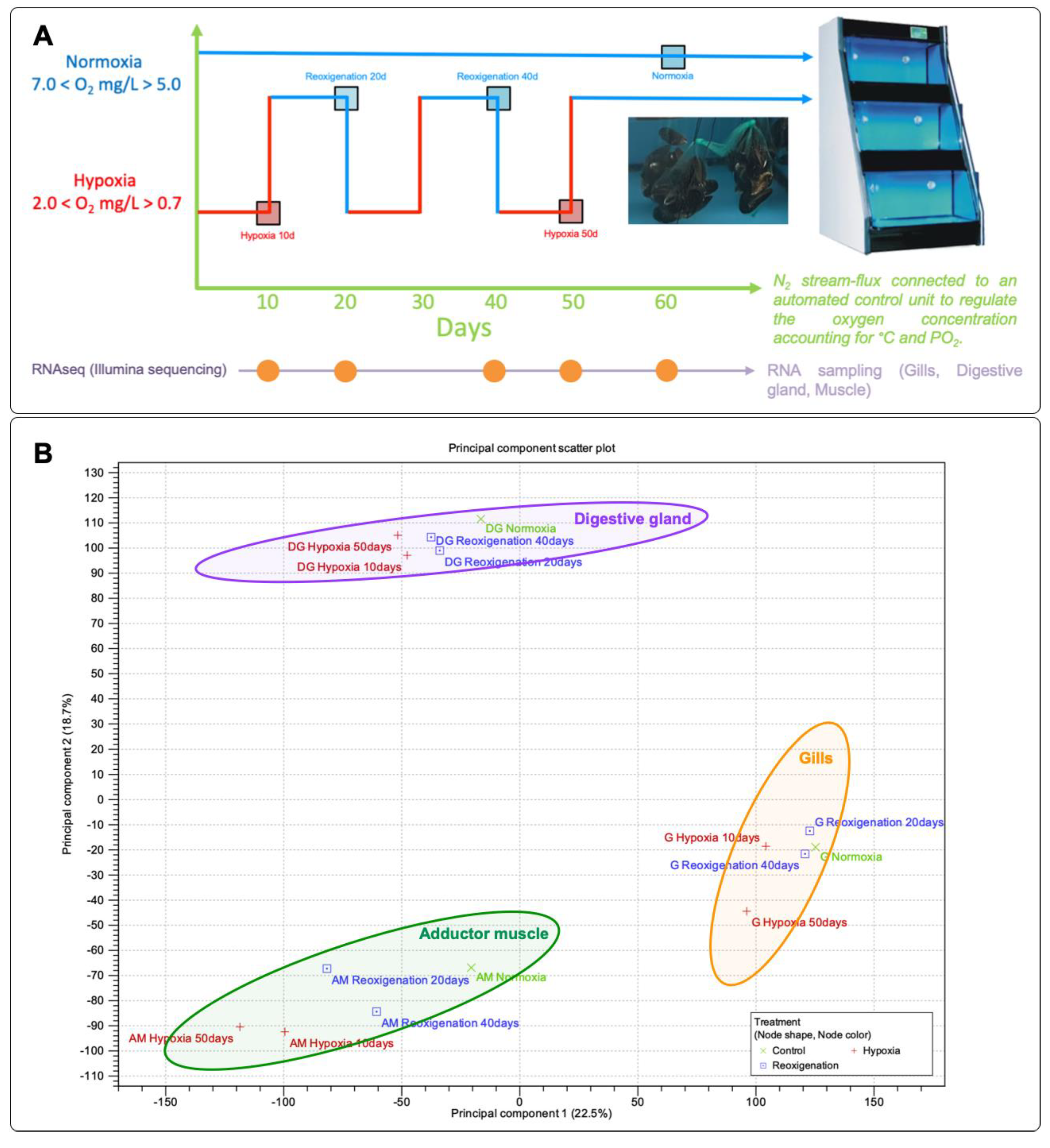
2.1. Mussel Acclimation, Hypoxia Challenge, and Sample Preparation
2.2. RNA Extraction and Library Preparation
2.3. Transcriptome Analysis and Gene Ontology Annotation
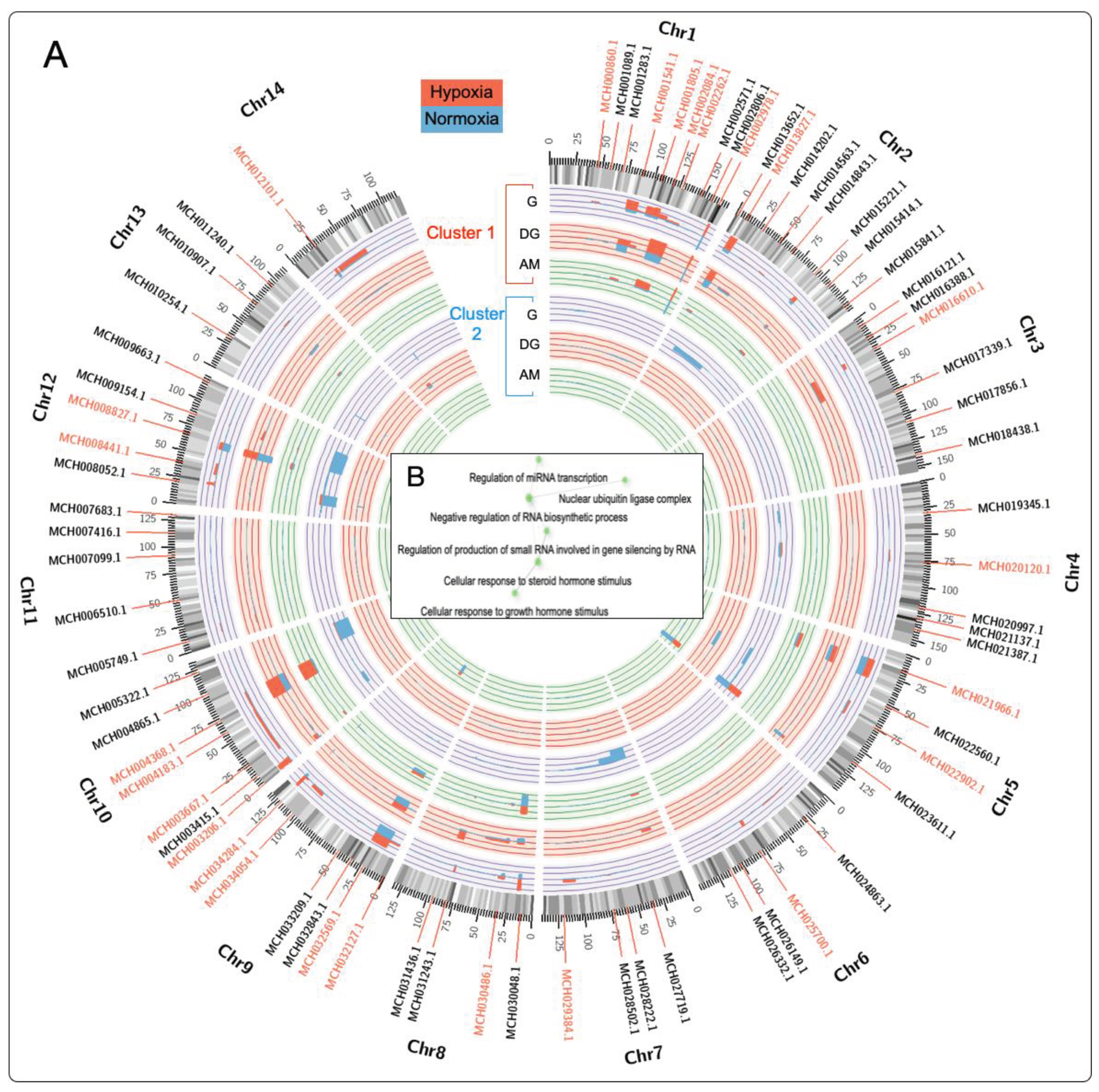
2.4. Chromosome Gene Expression (CGE) Analysis
2.5. Data Availability
3. Results
3.1. Principal Component Analysis (PCA) of Gene Expression Profiles in M. chilensis Tissues under Hypoxia and Reoxygenation
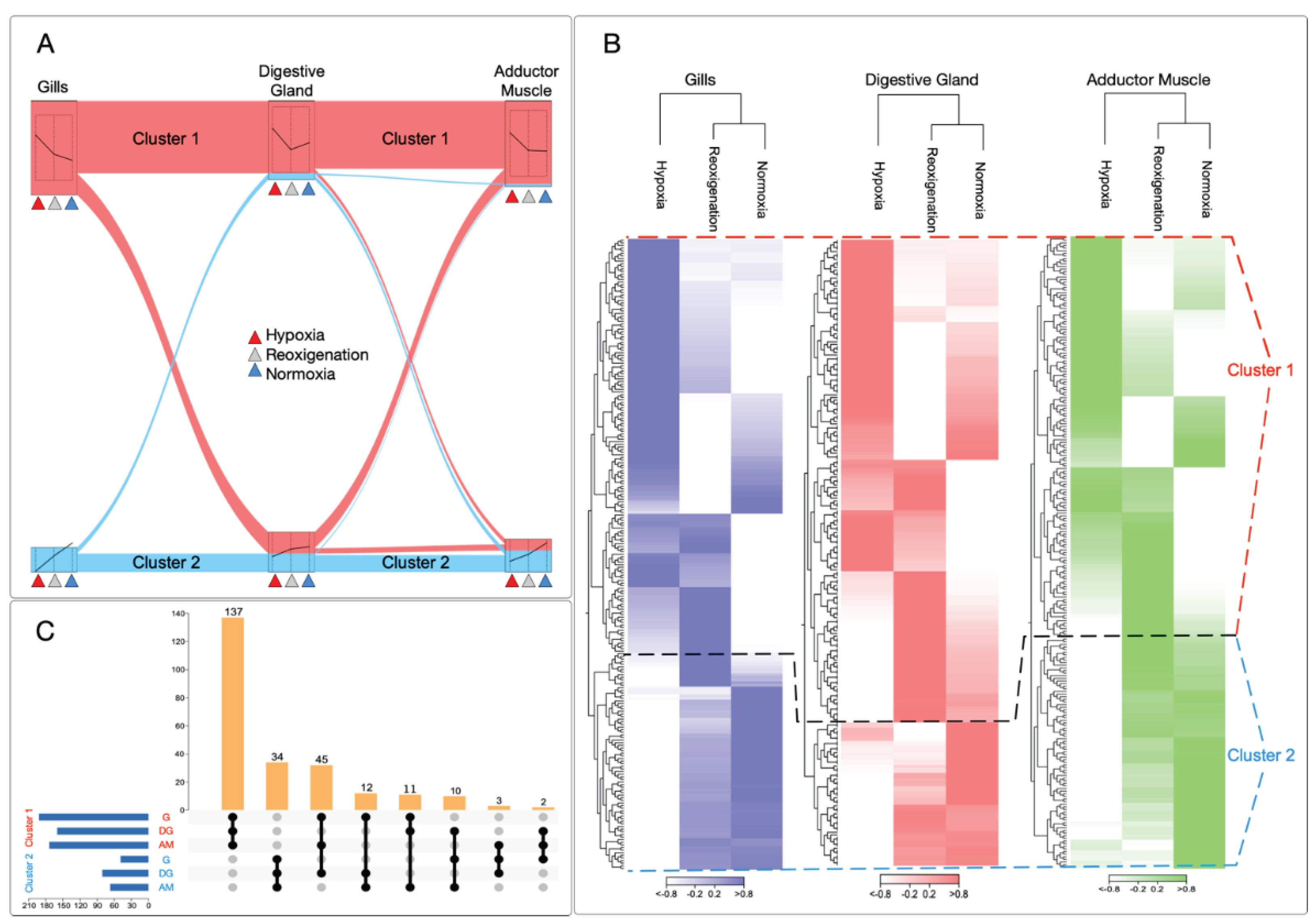
3.2. Differential Regulation of Transcripts under Normoxia and Hypoxia Conditions in Multiple Tissues of M. chilensis
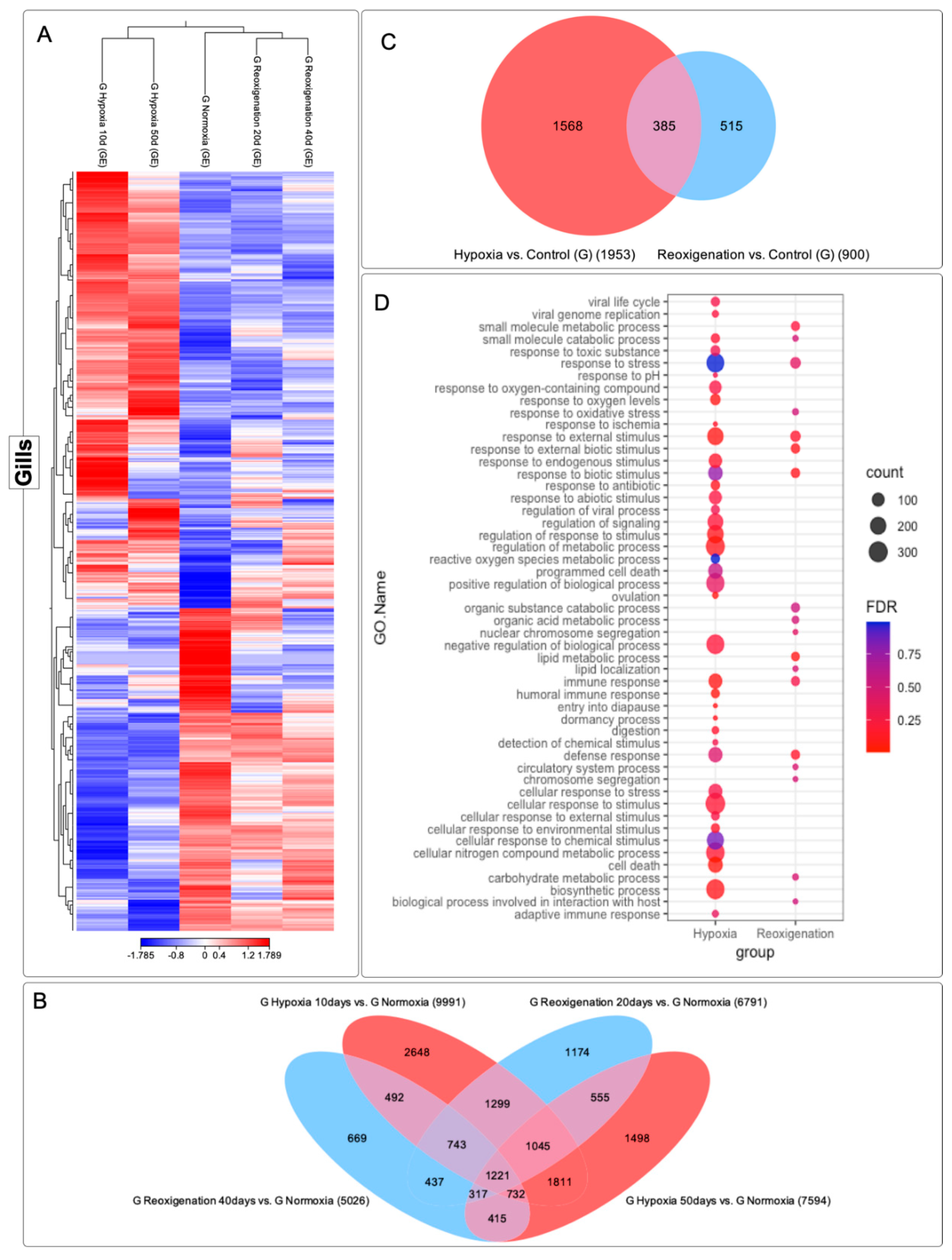
3.4. Differential Expression Analysis of Transcripts Expressed in M. chilensis Gills under Hypoxic and Reoxygenation Conditions
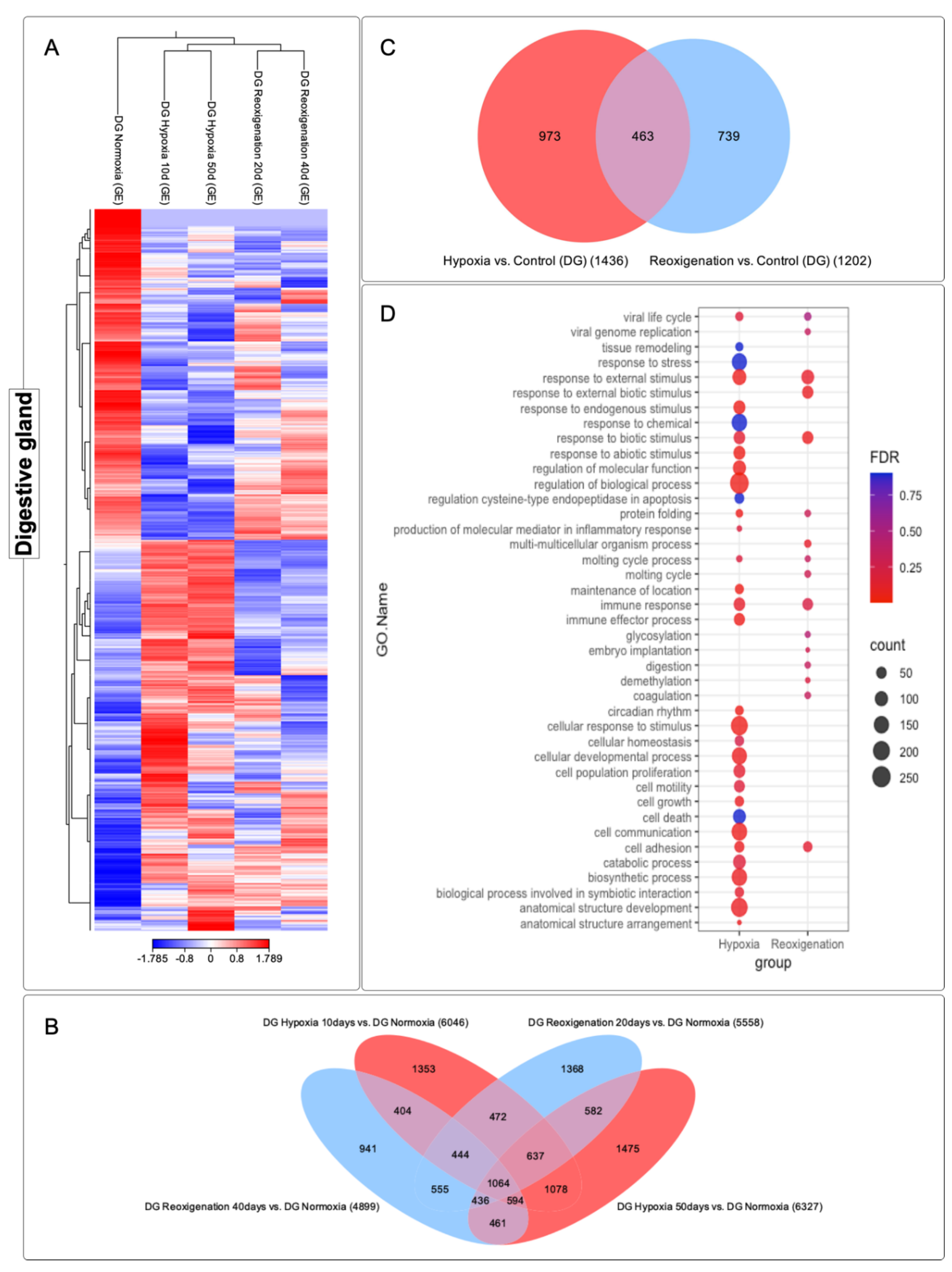
3.5. Differential Expression Analysis of Transcripts Observed in the Digestive Gland of M. chilensis under Hypoxic and Reoxygenation Conditions
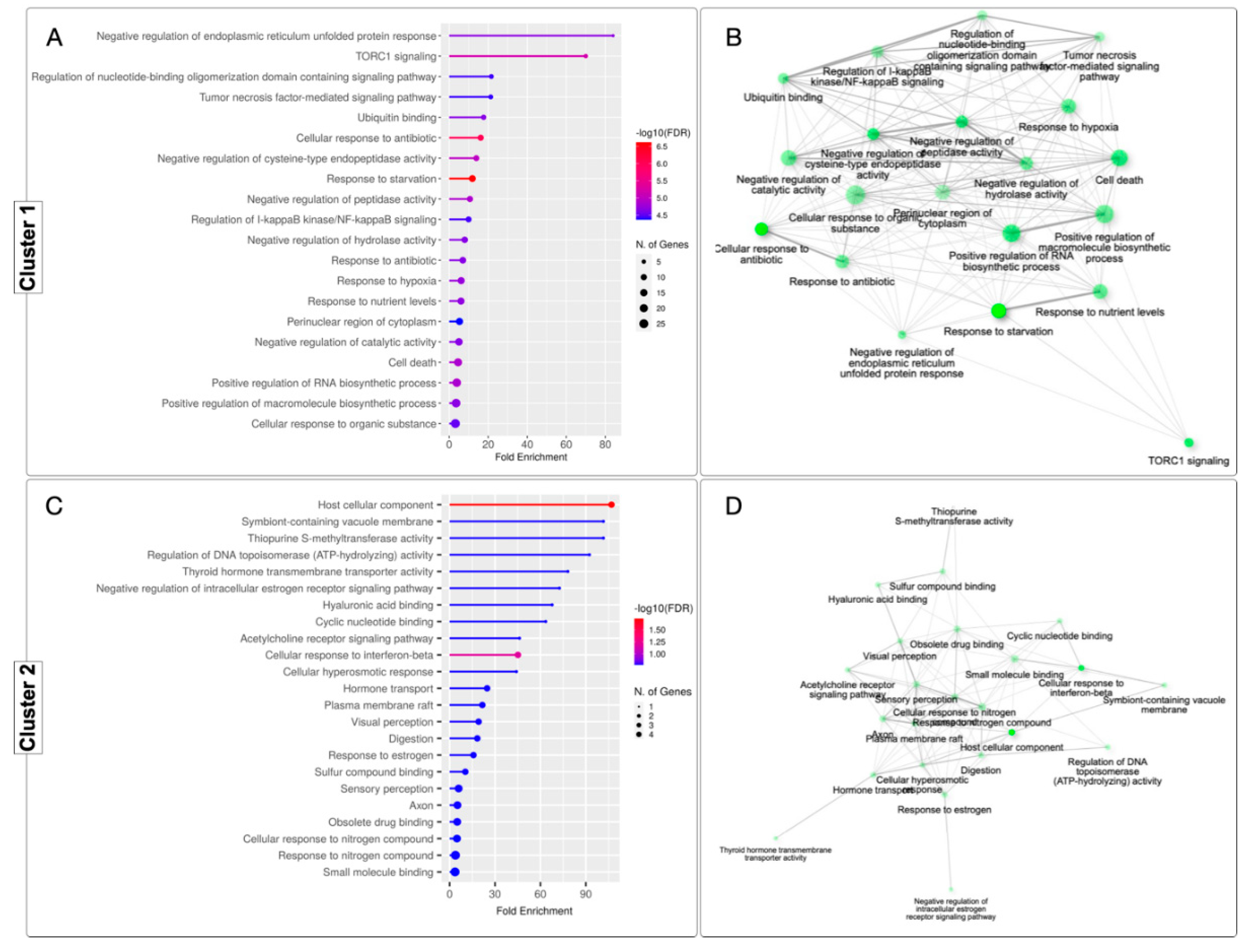
3.6. GO Enrichment Analysis in the Digestive Gland of M. chilensis under Hypoxia and Reoxygenation Conditions
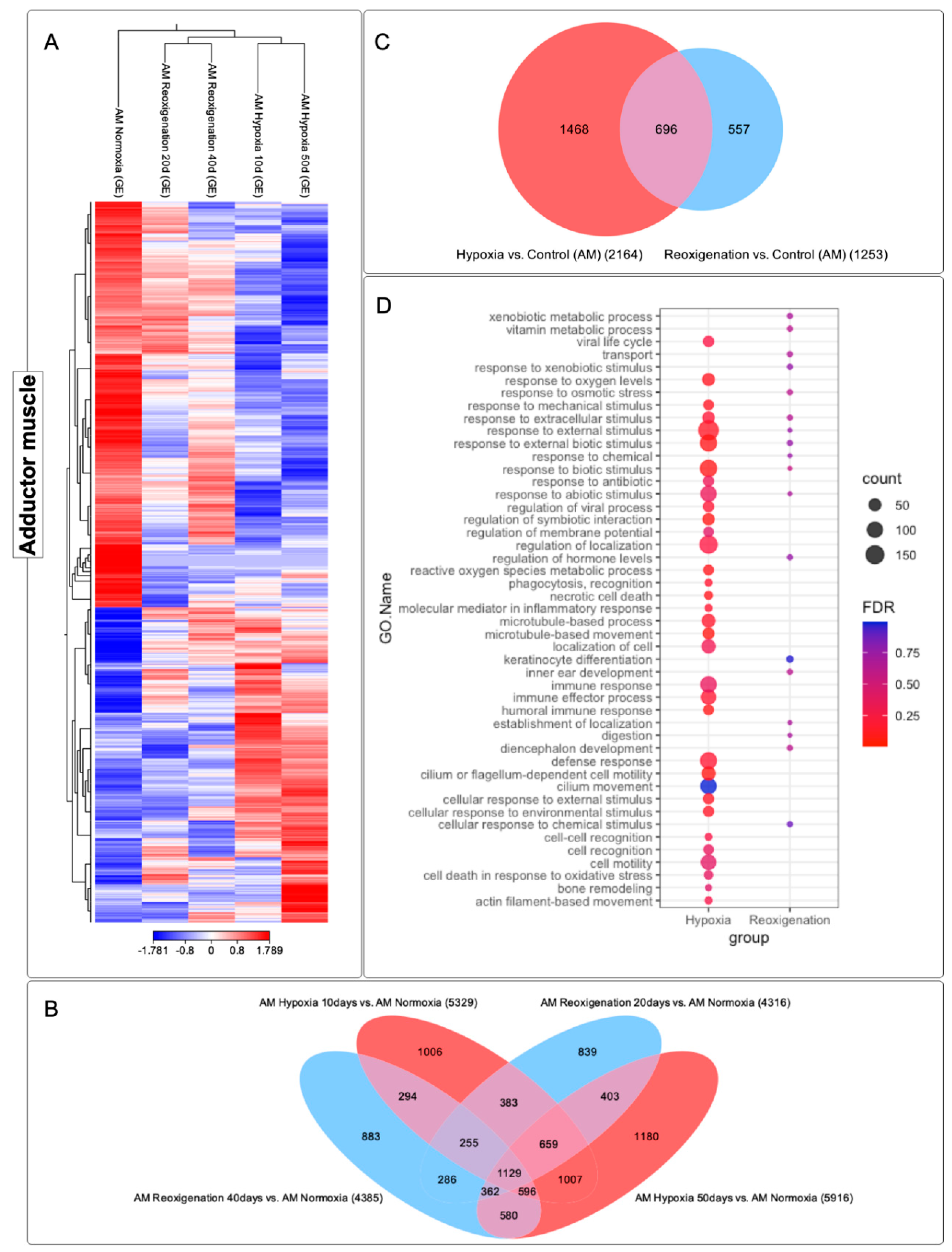
3.7. Differential Expression Analysis of Transcripts Observed in the Adductor Muscle of M. chilensis under Hypoxic and Reoxygenation Conditions
3.8. GO Enrichment Analysis in the Adductor Muscle of M. chilensis under Hypoxic and Reoxygenation Conditions
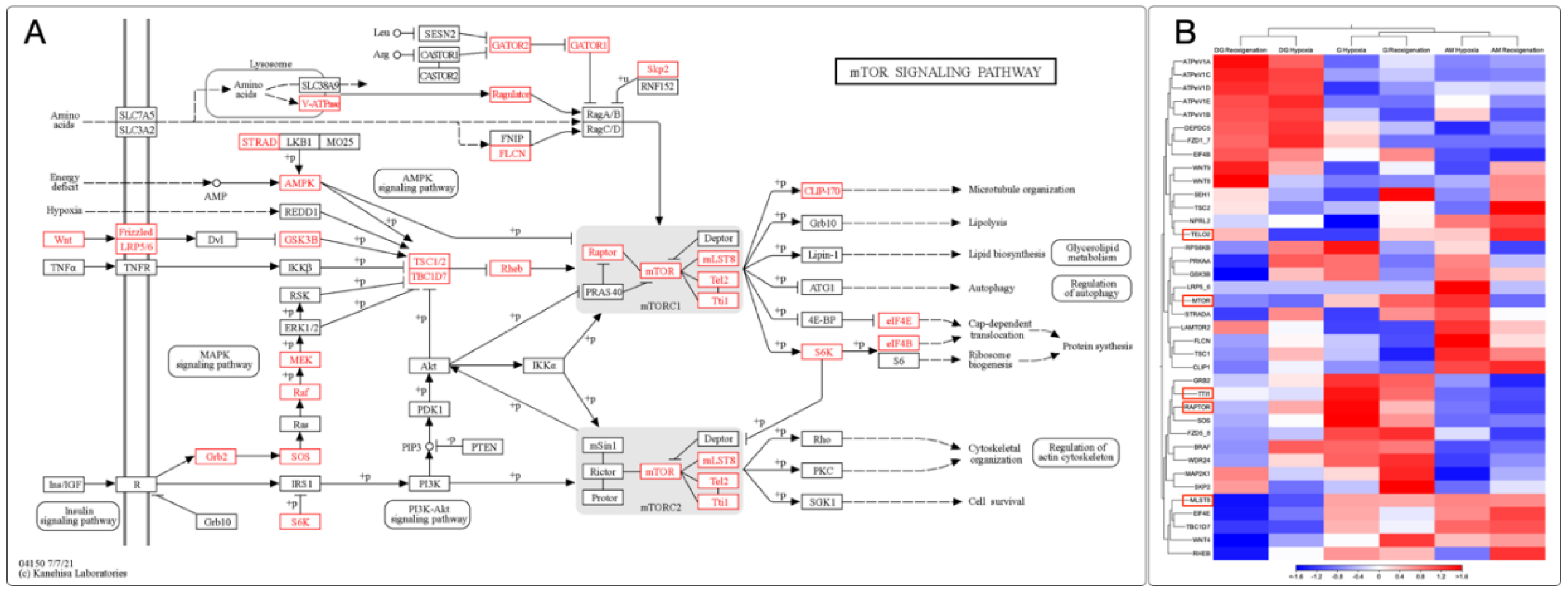
3.9. Identification and Expression of the mTOR Signaling Pathway in M. chilensis under Hypoxia
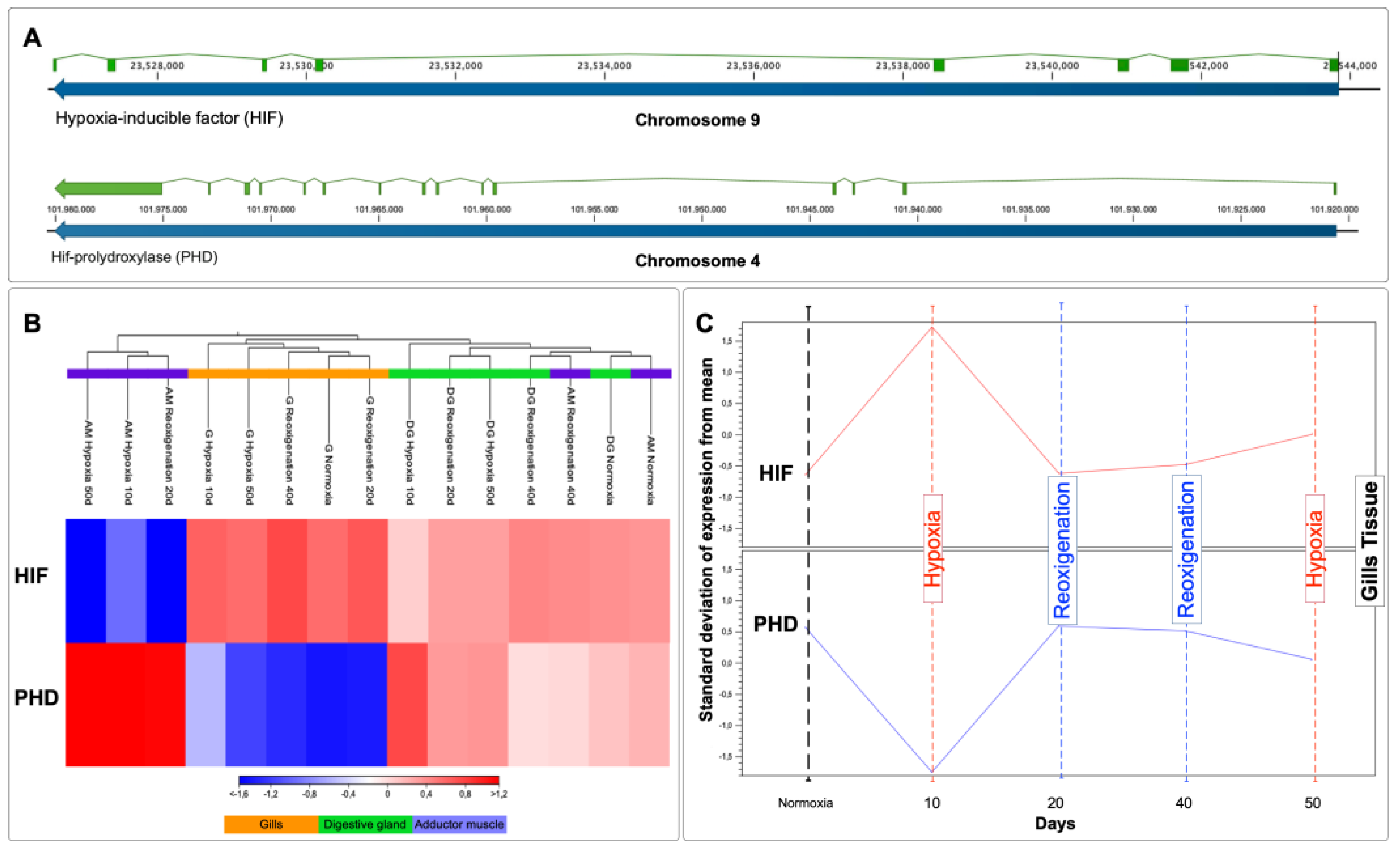
3.10. Transcriptional Response of HIF and PHD in Different Tissues of M. chilensis during Hypoxia and Reoxygenation Phases
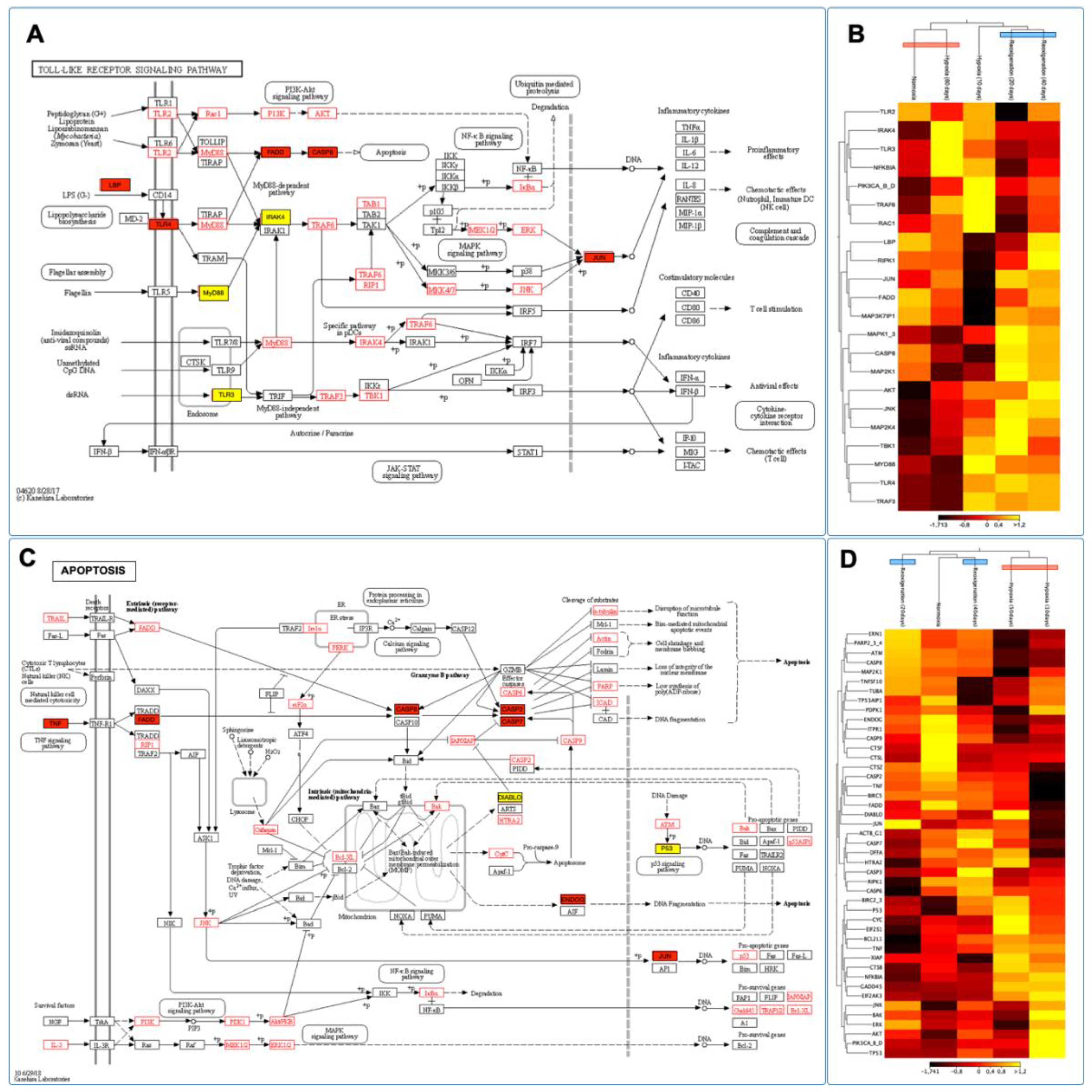
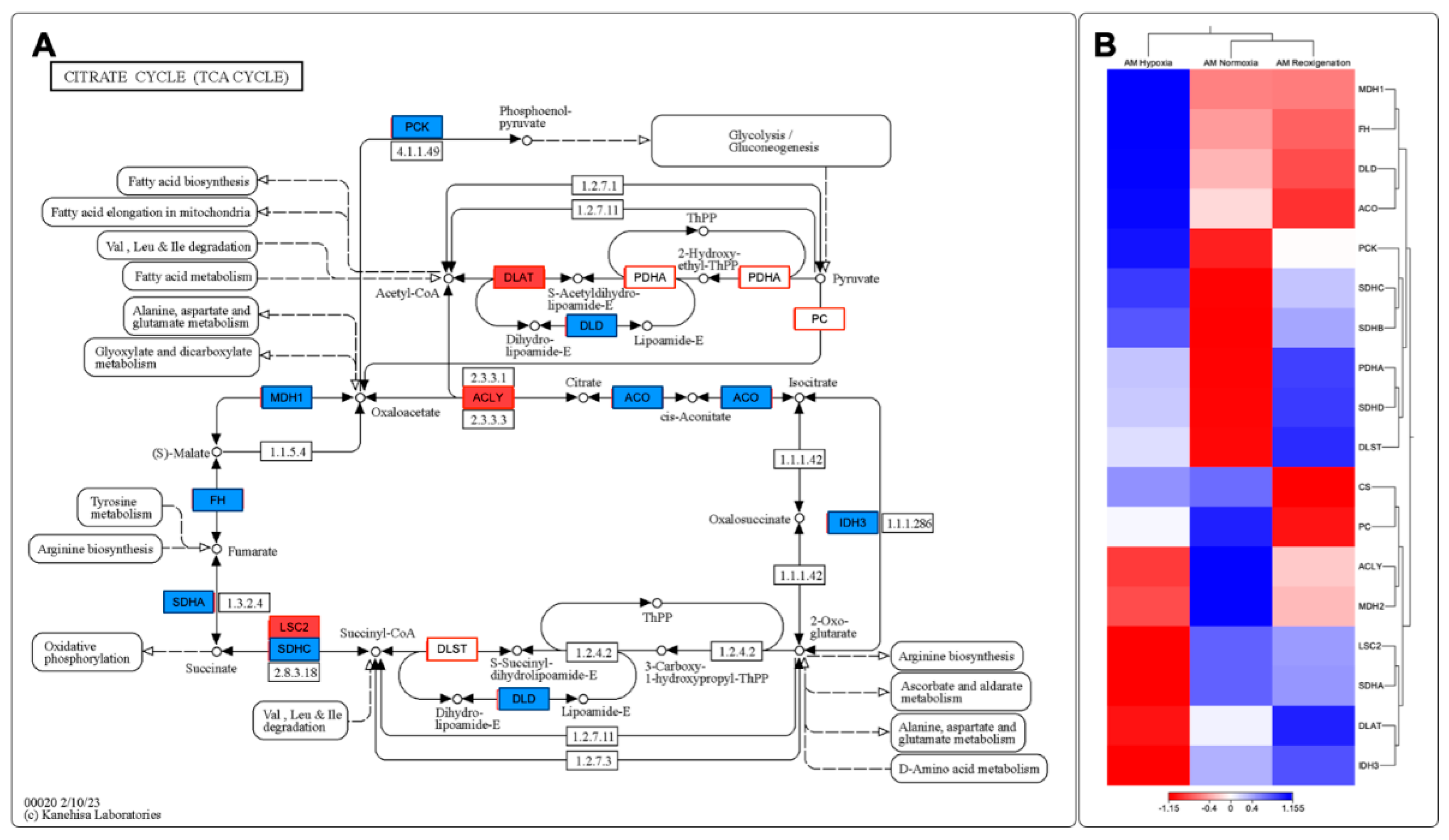
3.11. Identifying and Expressing Transcripts in the Toll-like Receptor, Citrate Cycle (TCA), and Apoptosis Signaling Pathways in the Gills of M. chilensis under Hypoxia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calle, X.; Jiménez-Gallegos, D.; Muñoz-Córdova, F.; Sánchez, P.; Lavandero, S. Mecanismo sensor y de adaptación a los niveles de oxígeno y su implicancia en las enfermedades cardiovasculares: a propósito del Premio Nobel de Fisiología-Medicina 2019. Revista chilena de cardiología 2019, 38, 225–235. [Google Scholar] [CrossRef]
- García, N.; Puentes, O.; Montalvo, J. Contaminación orgánica en el sector de la Bahía de Buena Vista cercano a la desembocadura de Río Guanó, Villa Clara, Cuba. Revista Cubana de Química 2008, 20, 39–46. [Google Scholar]
- Haider, F.; Falfushynska, H.; Timm, S.; Sokolova, I. Effects of hypoxia and reoxygenation on intermediary metabolite homeostasis of marine bivalves Mytilus edulis and Crassostrea gigas. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2020, 242, 1–16. [Google Scholar] [CrossRef]
- Ali, J.; Yang, Y.; Pan, G. Oxygen micro-nanobubbles for mitigating eutrophication induced sediment pollution in freshwater bodies. Journal of Environmental Management 2023, 331, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Breitburg, D.; Levin, L.; Oschlies, A.; M, G.; Chavez, F.; Conley, D.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Capet, A.; Beckers, J.; Grégoire, M. Drivers, mechanisms and long-term variability of seasonal hypoxia on the Black Sea northwestern shelf – is there any recovery after eutrophication? Biogeosciences 2013, 10, 3943–3962. [Google Scholar] [CrossRef]
- Conley, D.; Carstensen, J.; Vaquer-Sunyer, R.; Duarte, C. Ecosystem thresholds with hypoxia. Hydrobiologia 2009, 629, 21–29. [Google Scholar] [CrossRef]
- Sagasti, A.; Schaffner, L.; Duffy, J. Effects of periodic hypoxia on mortality, feeding and predation in an estuarine epifaunal community. Journal of Experimental Marine Biology and Ecology 2001, 258, 257–283. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Vargas, C. Hypoxia in Chilean Patagonian Fjords. Progress in Oceanography 2014, 129, 62–74. [Google Scholar] [CrossRef]
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.; Bange, H.; Hansen, H.; Körtzinger, A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Marine Biology 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- Scanes, E.; Scanes, P.; Ross, P. Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Scanes, E.; Parker, L.; Seymour, J.; Siboni, N.; King, W.; Danckert, N.; Wegner, K.; Dove, C.; O'Connor, A.; Ross, P. Climate change alters the haemolymph microbiome of oysters. Marine Pollution Bulletin 2021, 164, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, E.; Lagos, N.; Norambuena, R.; Silva, C.; Letelier, J.; Muck, K.; Martin, G.; Benítez, S.; Broitman, B.; Contreras, H.; et al. Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Phillips, B., Pérez-Ramírez, M., Eds.; John Wiley & Sons Ltd, 2017; Volume I, pp. 239–332. [Google Scholar]
- Bianchi, T.; Arndt, S.; Austin, W.; Benn, D.; Bertrand, S.; Cui, X.; Faust, J.; Koziorowska-Makuch, K.; Moy, C.; Savage, C.; et al. Fjords as Aquatic Critical Zones (ACZs). Earth-Science Reviews 2020, 203, 1–25. [Google Scholar] [CrossRef]
- Iriarte, J.; Pantoja, S.; Daneri, G. Oceanographic Processes in Chilean Fjords of Patagonia: From small to large-scale studies. Progress in Oceanography 2014, 129, 1–7. [Google Scholar] [CrossRef]
- Schmidtko, S.; Stramma, L.; Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ferraz, D.; Trevisan, R.; Garcia, D.; Da Silva, D.; Luiz, A.; Alves, E. Hypoxia effects on oxidative stress and immunocompetence biomarkers in the mussel Perna perna (Mytilidae, Bivalvia). Marine Environmental Research 2017, 126, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, C.; Kim, E.; Nam, Y. Transcriptional modulation patterns of abalone Haliotis discus hannai hypoxia inducible factor-1α (HIF-1α) in interdependent crosstalk between hypoxia, infection, and environmental stresses. Aquaculture Reports 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Levin, L.; Ekau, W.; Gooday, A.; Jorissen, F.; Middelburg, J.; Naqvi, S.; Neira, C.; Rabalais, N.; J., Z. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 2009, 6, 2063–2098. [Google Scholar] [CrossRef]
- Grieshaber, M.; Hardewig, I.; Kreutzer, U.; Pörtner, H. Physiological and metabolic responses to hypoxia in invertebrates. Reviews of Physiology, Biochemistry and Pharmacology. 1994, 125, 43–147. [Google Scholar] [CrossRef]
- Chu, J.; Curkan, C.; Tunnicliffe, V. Drivers of temporal beta diversity of a benthic community in a seasonally hypoxic fjord. Royal Society Open Science 2018, 5, 1–18. [Google Scholar] [CrossRef]
- Hernández-Miranda, E.; Veas, R.; Anabalón, V.; Quiñones, R. Short-term alteration of biotic and abiotic components of the pelagic system in a shallow bay produced by a strong natural hypoxia event. PLoS One 2017, 12, 1–25. [Google Scholar] [CrossRef]
- Tirpe, A.; Gulei, D.; Ciortea, S.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. International Journal of Molecular Sciences 2019, 20. [Google Scholar] [CrossRef]
- Kodama, K.; Horiguchi, T. Effects of hypoxia on benthic organisms in Tokyo Bay, Japan: A review. Marine Pollution Bulletin 2011, 63, 215–220. [Google Scholar] [CrossRef]
- Batie, M.; Del Peso, L.; Rocha, S. Hypoxia and Chromatin: A Focus on Transcriptional Repression Mechanisms. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, A.; Gostyukhina, O.; Golovina, I. Antioxidant enzyme complex of tissues of the bivalve Mytilus galloprovincialis Lam. under normal and oxidative-stress conditions: A review. Applied Biochemistry and Microbiology 2007, 43, 556–562. [Google Scholar] [CrossRef]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aquatic Toxicology 2016, 170, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Pytharopoulou, S.; Sazakli, E.; Grintzalis, K.; Georgiou, C.; Leotsinidis, M.; Kalpaxis, D. Translational responses of Mytilus galloprovincialis to environmental pollution: Integrating the responses to oxidative stress and other biomarker responses into a general stress index. Aquatic Toxicology 2008, 89, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Piontkivska, H.; Sokolova, I. Effects of intermittent hypoxia on cell survival and inflammatory responses in the intertidal marine bivalves Mytilus edulis and Crassostrea gigas. Journal of Experimental Biology 2020, 223, 1–13. [Google Scholar] [CrossRef]
- Sokolova, I. Mitochondrial Adaptations to Variable Environments and Their Role in Animals' Stress Tolerance. Integrative and Comparative Biology 2018, 58, 519–531. [Google Scholar] [CrossRef]
- Storey, K.; Storey, J. Metabolic rate depression in animals: transcriptional and translational controls. Biological Reviews of the Cambridge Philosophical Society 2004, 79, 207–233. [Google Scholar] [CrossRef]
- Tirpe, A.; Gulei, D.; Ciortea, S.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. International Journal of Molecular Sciences 2019, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P. HIF-1 signaling: A key orchestrator of cancer radioresistance. Radiation Medicine and Protection 2020, 1, 7–14. [Google Scholar] [CrossRef]
- Jung-whan, K.; Tchernyshyov, I.; Semenza, L.; Dang, V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Bailey, P.; Nathan, J. Metabolic Regulation of Hypoxia-Inducible Transcription Factors: The Role of Small Molecule Metabolites and Iron. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.; Sokolov, E.; Haider, F. Mitochondrial Mechanisms Underlying Tolerance to Fluctuating Oxygen Conditions: Lessons from Hypoxia-Tolerant Organisms. Integrative and Comparative Biology 2019, 59, 938–952. [Google Scholar] [CrossRef]
- Fava, L.; Bock, F.; Geley, S.; Villunger, A. Caspase-2 at a glance. Journal of Cell Science 2012, 125, 5911–5915. [Google Scholar] [CrossRef]
- Movassagh, M.; Foo, R. Simplified apoptotic cascades. Heart Failure Reviews 2008, 13, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Adli, M.; Merkhofer, E.; Cogswell, P.; Baldwin, A. IKKalpha and IKKbeta each function to regulate NF-kappaB activation in the TNF-induced/canonical pathway. PloS one 2010, 5, 1–7. [Google Scholar] [CrossRef]
- Angelo, A.; Rovere-Querini, P.; Clementi, S.; Clementi, B. Cell Death: Tipping the Balance of Autoimmunity and Tissue Repair. Current Pharmaceutical Design 2008, 14, 269–277. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Hofmann, T.; Hehner, S.; Dröge, W.; Schmitz, M. The pro- or anti-apoptotic function of NF-κB is determined by the nature of the apoptotic stimulus. European Journal of Biochemistry 2000, 267, 3828–3835. [Google Scholar] [CrossRef]
- Moret, I.; Cerrillo, E.; Navarro-Puche, A.; Iborra, M.; Rausell, F.; Tortosa, L.; Beltrán, B. Estrés oxidativo en la enfermedad de Crohn. Gastroenterología y Hepatología 2014, 37, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.; Leaw, B.; Mallard, C.; Nair, S.; Jinnai, M.; Hagberg, H. Cell Death in the Developing Brain after Hypoxia-Ischemia. Frontiers in Cellular Neuroscience 2017, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Feist, S.; Bignell, J.; De Vico, G. Comparative pathology in bivalves: Aetiological agents and disease processes. Journal of Invertebrate Pathology 2015, 131, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Connon, R.; D’Abronzo, L.; Hostetter, N.; Javidmehr, A.; Roby, D.D.; Evans, A.; Loge, F.; Werner, I. Transcription Profiling in Environmental Diagnostics: Health Assessments in Columbia River Basin Steelhead (Oncorhynchus mykiss). Environmental Science & Technology 2012, 46, 6081–6087. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: the teenage years. Nature Reviews Genetics 2019, 20, 631–656. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Wang, Y.; Day, R.; Yang, H.; Zhang, Z. Immunity-related genes and signaling pathways under hypoxic stresses in Haliotis diversicolor: a transcriptome analysis. Scientific Reports 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Escarate, C.; Valenzuela-Munoz, V.; Gustavo, N.; Valenzuela-Miranda, D.; Tapia, F.; Yevenes, M.; Gajardo, G.; Toro, J.; Oyarzun, P.; Arriagada, G.; et al. Chromosome-Level Genome Assembly of the Blue Mussel Mytilus chilensis Reveals Molecular Signatures Facing the Marine Environment. Genes 2023, 14, 1–27. [Google Scholar] [CrossRef]
- Yévenes, M.; Núñez-Acuña, G.; Gallardo-Escárate, C.; Gajardo, G. Adaptive mitochondrial genome functioning in ecologically different farm-impacted natural seedbeds of the endemic blue mussel Mytilus chilensis. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 2022, 42, 100955. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Gallardo-Escárate, C. Identification of immune-related SNPs in the transcriptome of Mytilus chilensis through high-throughput sequencing. Fish & Shellfish Immunology 2013, 35, 1899–1905. [Google Scholar] [CrossRef]
- Detree, C.; Nunez-Acuna, G.; Roberts, S.; Gallardo-Escarate, C. Uncovering the Complex Transcriptome Response of Mytilus chilensis against Saxitoxin: Implications of Harmful Algal Blooms on Mussel Populations. Plos One 2016, 11, e0165231. [Google Scholar] [CrossRef] [PubMed]
- Malachowicz, M.; Wenne, R. Mantle transcriptome sequencing of Mytilus spp. and identification of putative biomineralization genes. PeerJ 2019, 6, e6245. [Google Scholar] [CrossRef]
- Lohrmann, K.; Bustos, E.; Rojas, R.; Navarrete, F.; Robotham, H.; Bignell, J. Histopathological assessment of the health status of Mytilus chilensis (Hupé 1854) in southern Chile. Aquaculture 2019, 503, 40–50. [Google Scholar] [CrossRef]
- Osores, S.; Lagos, N.; San Martín, V.; Manríquez, P.; Vargas, C.; Torres, R.; Navarro, J.; Poupin, J.; Saldías, G.; Lardies, M. Plasticity and inter-population variability in physiological and life-history traits of the mussel Mytilus chilensis: A reciprocal transplant experiment. Journal of Experimental Marine Biology and Ecology 2017, 490, 1–12. [Google Scholar] [CrossRef]
- Ríos, V.; Ocampo, N.; Astorga, M. Proximal chemical composition and morphometry of the ribbed (Aulacomya ater, Molina 1782) and the blue (Mytilus chilensis, Hupé, 1854) mussels commercialized in the Region of Magallanes. Anales Instituto Patagonia (Chile) 2018, 46, 49–58. [Google Scholar] [CrossRef]
- Navarro, J.; Duarte, C.; Manríquez, P.; Lardies, M.; Torres, R.; Acuña, K.; Vargas, C.; Lagos, N. Ocean warming and elevated carbon dioxide: multiple stressor impacts on juvenile mussels from southern Chile. ICES Journal of Marine Science. 2016, 73, 764–771. [Google Scholar] [CrossRef]
- Subpesca. Mejillón. Available online: https://www.subpesca.cl/portal/616/w3-article-843.html#presentacion (accessed on 01/05/2023).
- Oyarzún, P.; Toro, E.; Jaramillo, R.; Guiñez, R.; Briones, C.; Astorga, M. Gonadal cycle of the mussel Mytilus chilensis (Bivalvia: Mytilidae) at two localities in southern of Chile. Latin american journal of aquatic research 2011, 39, 512–525. [Google Scholar] [CrossRef]
- Salas-Yanquin, L.; Navarro, J.; Pechenik, J.; Montory, J.; Chaparro, O. Volcanic ash in the water column: Physiological impact on the suspension-feeding bivalve Mytilus chilensis. Marine Pollution Bulletin 2018, 127, 342–351. [Google Scholar] [CrossRef]
- Molinet, C.; Diaz, M.; Marin, S.; Astorga, M.; Ojeda, M.; Cares, L.; Asencio, E. Relation of mussel spatfall on natural and artificial substrates: Analysis of ecological implications ensuring long-term success and sustainability for mussel farming. Aquaculture 2017, 467, 211–218. [Google Scholar] [CrossRef]
- IFOP. Innovaciones en la tecnología de cultivo de chorito (Mytilus chilensis), tendientes a mejorar la calidad y rentabilidad de la actividad mitícola en la X Región. Available online: https://www.ifop.cl/wp-content/contenidos/uploads/biblioteca/libros_digitales/Curso_Cultivo_de_choritos.pdf (accessed on 09/01/2023).
- Subpesca. Informe Sectorial de Pesca y Acuicultura Consolidado (2020-2021). Available online: https://www.subpesca.cl/portal/618/articles-114306_documento.pdf (accessed on 01/10/2023).
- ProChile. Chile se convierte en el mayor proveedor mundial de 28 productos liderados por cobre, cerezas y salmón. Available online: https://www.prochile.gob.cl/noticias/detalle-noticia/2021/08/12/chile-se-convierte-en-el-mayor-proveedor-mundial-de-28-productos-liderados-por-cobre-cerezas-y-salm%C3%B3n (accessed on 03/10/2023).
- Blanc, J.; Molinet, C.; Subiabre, R.; Díaz, P. Cadmium determination in Chilean blue mussels Mytilus chilensis: Implications for environmental and agronomic interest. Marine Pollution Bulletin 2018, 129, 913–917. [Google Scholar] [CrossRef]
- Yevenes, M.; Lagos, N.; Farías, L.; Vargas, C. Greenhouse gases, nutrients and the carbonate system in the Reloncaví Fjord (Northern Chilean Patagonia): Implications on aquaculture of the mussel, Mytilus chilensis, during an episodic volcanic eruption. Science of the Total Environment 2019, 669, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Beneden, R.; Muttray, A.; Böttger, A.; Kelley, M.; Tucker, A.; Kelley, T. Chapter One - p53 Superfamily Proteins in Marine Bivalve Cancer and Stress Biology; Michael, L., Ed.; Academic Press, 2011; Volume 59, pp. 1–36. [Google Scholar]
- Venier, P.; De Pittà, C.; Pallavicini, A.; Marsano, F.; Varotto, L.; Romualdi, C.; Dondero, F.; Viarengo, A.; Lanfranchi, G. Development of mussel mRNA profiling: Can gene expression trends reveal coastal water pollution? Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2006, 602, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Baqueiro–Cárdenas, E.; Borabe, L.; Goldaracena–Islas, C.; Rodríguez–Navarro, J. Mollusks and pollution. A review. Revista mexicana de biodiversidad 2007, 78, 1–7. [Google Scholar]
- Hernández-Miranda, E.; Quiñones, R.; Aedo, G.; Valenzuela, A.; Mermoud, N.; Román, C.; Yañez, F. A major fish stranding caused by a natural hypoxic event in a shallow bay of the eastern South Pacific Ocean. Journal of Fish Biology 2010, 76, 1543–1564. [Google Scholar] [CrossRef]
- Hernández-Miranda, E.; Veas, R.; Labra, F.; Salamanca, M.; Quiñones, R. Response of the epibenthic macrofaunal community to a strong upwelling-driven hypoxic event in a shallow bay of the southern Humboldt Current System. Marine Environmental Research 2012, 79, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Labra, F.; Hernández-Miranda, E.; Quiñones, R. Dynamic relationships between body size, species richness, abundance, and energy use in a shallow marine epibenthic faunal community. Ecology and Evolution 2015, 5, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Husmann, G.; Thorne, M.; Burns, G.; Truebano, M.; Peck, L.; Abele, D.; Philipp, E. Hypoxia impacts large adults first: consequences in a warming world. Global Change Biology 2013, 19, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Shang, Y.; Clements, J.; Dupont, S.; Wang, T.; Wei, S.; Wang, X.; Chen, J.; Huang, W.; Hu, M.; et al. Hypoxia aggravates the effects of ocean acidification on the physiological energetics of the blue mussel Mytilus edulis. Marine Pollution Bulletin 2019, 149, 1–7. [Google Scholar] [CrossRef]
- Piontkivska, H.; Chung, S.; Ivanina, A.; Sokolov, E.; Techa, S.; Sokolova, I. Molecular characterization and mRNA expression of two key enzymes of hypoxia-sensing pathways in eastern oysters Crassostrea virginica (Gmelin): hypoxia-inducible factor α (HIF-α) and HIF-prolyl hydroxylase (PHD). Comparative biochemistry and physiology. Part D, Genomics & proteomics 2011, 6, 103–114. [Google Scholar] [CrossRef]
- Coxe, N.; Casas, S.; Marshall, D.; La Peyre, M.; Kelly, M.; La Peyre, J. Differential hypoxia tolerance of eastern oysters from the northern Gulf of Mexico at elevated temperature. Journal of Experimental Marine Biology and Ecology 2023, 559. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhang, Q.; Xue, R.; Qu, Y.; Wang, Q.; Dong, Z.; Zhao, J. Different patterns of hypoxia aggravate the toxicity of polystyrene nanoplastics in the mussels Mytilus galloprovincialis: Environmental risk assessment of plastics under global climate change. Science of the Total Environment 2022, 818. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Sun, S. The survival and responses of blue mussel Mytilus edulis to 16-day sustained hypoxia stress. Marine Environmental Research 2022, 176. [Google Scholar] [CrossRef] [PubMed]
- Gostyukhina, O.; Yu, A.; Chelebieva, E.; Vodiasova, E.; Lantushenko, A.; Kladchenko, E. Adaptive potential of the Mediterranean mussel Mytilus galloprovincialis to short-term environmental hypoxia. Fish & Shellfish Immunology 2022, 131, 654–661. [Google Scholar] [CrossRef]
- Kotsyuba, E.; Dyachuk, V. Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef]
- Salmond, N.; Wing, S. Sub-lethal and lethal effects of chronic ammonia exposure and hypoxia on a New Zealand bivalve. Journal of Experimental Marine Biology and Ecology 2022, 549. [Google Scholar] [CrossRef]
- Andreyeva, A.; Gostyukhina, O.; Kladchenko, E.; Afonnikov, D.; Rasskazov, D.; Lantushenko, A.; Vodiasova, E. Hypoxia exerts oxidative stress and changes in expression of antioxidant enzyme genes in gills of Mytilus galloprovincialis (Lamarck, 1819). Marine Biology Research 2021, 17, 369–379. [Google Scholar] [CrossRef]
- Khan, F.; Chen, H.; Gu, H.; Wang, T.; Dupont, S.; Kong, H.; Shang, Y.; Wang, X.; Lu, W.; Hu, M.; et al. Antioxidant responses of the mussel Mytilus coruscus co-exposed to ocean acidification, hypoxia and warming. Marine Pollution Bulletin 2021, 162. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Kong, H.; Chang, X.; Dupont, S.; Chen, H.; Deng, Y.; Hu, M.; Wang, Y. Gonadal antioxidant responses to seawater acidification and hypoxia in the marine mussel Mytilus coruscus. Environmental Science and Pollution Research 2021, 28, 53847–53856. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E.; Adzigbli, L.; Markert, S.; Bundgaard, A.; Fago, A.; Becher, D.; Hirschfeld, C.; Sokolova, I. Intrinsic Mechanisms Underlying Hypoxia-Tolerant Mitochondrial Phenotype During Hypoxia-Reoxygenation Stress in a Marine Facultative Anaerobe, the Blue Mussel Mytilus edulis. Frontiers in Marine Science 2021, 8. [Google Scholar] [CrossRef]
- Altschul, S.; Madden, T.; Schaffer, A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research 1998, 12, 3389–3402. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.; Britto, R.; Cukura, A.; Denny, P.; et al. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Research 2022, 51, D523–D531. [Google Scholar] [CrossRef]
- Aleksander, S.; Balhoff, J.; Carbon, S.; Cherry, J.; Drabkin, H.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.; Hill, D.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, 1–14. [Google Scholar] [CrossRef]
- Tatusov, R.; Natale, D.; Garkavtsev, I.; Tatusova, T.; Shankavaram, U.; Rao, B.; Kiryutin, B.; Galperin, M.; Fedorova, N.; Koonin, E. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Research 2001, 29, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Science 2022, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.; Frank, M.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Molecular Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Ge, S.; Jung, D.; Yao, R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Muñoz, V.; Gallardo-Escárate, C.; Benavente, B.; Valenzuela-Miranda, D.; Núñez-Acuña, G.; Escobar-Sepulveda, H.; Váldes, J. Whole-Genome Transcript Expression Profiling Reveals Novel Insights into Transposon Genes and Non-Coding RNAs during Atlantic Salmon Seawater Adaptation. Biology-Basel 2022, 11, 1–21. [Google Scholar] [CrossRef]
- Cui, Z.; Cui, Y.; Zang, T.; Wang, Y. Genome analysis interacCircos: an R package based on JavaScript libraries for the generation of interactive circos plots. Bioinformatics 2021, 37, 3642–3644. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Kim, T.; Hwang, J.; Lee, J. AtHAD1, A haloacid dehalogenase-like phosphatase, is involved in repressing the ABA response. Biochemical and Biophysical Research Communications 2022, 587, 119–125. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Wu, Z.; Wang, C. Genome-wide analysis of haloacid dehalogenase genes reveals their function in phosphate starvation responses in rice. Plos One 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Pandey, B.; Mehra, P.; Verma, L.; Bhadouria, J.; Giri, J. OsHAD1, a Haloacid Dehalogenase-Like APase, Enhances Phosphate Accumulation. Plant Physiology 2017, 174, 2316–2332. [Google Scholar] [CrossRef] [PubMed]
- Levin, L. Oxygen Minimum Zone Benthos: Adaptation and Community Response to Hypoxia. In Oceanography and Marine Biology, An Annual Review, 1st Edition ed.; Gibson, R., Atkinson, A., Eds.; London, 2003; Volume 41, pp. 1–45. [Google Scholar]
- Rabalais, N.; Cai, W.; Carstensen, J.; Conley, D.; Fry, B.; Hu, X.; Quiñones-Rivera, Z.; Rosenberg, R.; Slomp, C.; Turner, R.; et al. Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 2014, 27, 172–183. [Google Scholar] [CrossRef]
- Nie, H.; Wang, H.; Jiang, K.; Yan, X. Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress: potential HIF and NF-κB crosstalk in immune responses in clam. BMC Genomics 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Nie, H.; Huo, Z.; Ding, J.; Li, Z.; Yan, L.; Jiang, L.; Mu, Z.; Wang, H.; Meng, X.; et al. Clam Genome Sequence Clarifies the Molecular Basis of Its Benthic Adaptation and Extraordinary Shell Color Diversity. iScience 2019, 19, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.; Wessels, W.; Gruber, H.; Strahl, J.; Wagner, A.; Ernst, I.; Rimbach, G.; Kraemer, L.; Schreiber, S.; Abele, D.; et al. Gene Expression and Physiological Changes of Different Populations of the Long-Lived Bivalve Arctica islandica under Low Oxygen Conditions. Plos One 2012, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Liu, T.; Chen, M.; Li, W.; Zhang, X. The transcriptomic responses of the ark shell, Anadara broughtonii, to sulfide and hypoxia exposure. Molecular Biology Reports 2019, 46, 4245–4257. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.; Torrealba, N.; Paredes, F.; Wang, Z.; Zorzano, A.; Hill, J.; Jaimovich, E.; et al. Endoplasmic Reticulum and the Unfolded Protein Response: Dynamics and Metabolic Integration. In International Review of Cell and Molecular Biology, Vol 301; Jeon, K., Ed.; International Review of Cell and Molecular Biology; Elsevier Academic Press Inc: San Diego, 2013; Volume 301, pp. 215–290. [Google Scholar]
- Steffen, J.; Falfushynska, H.; Piontkivska, H.; Sokolova, I. Molecular Biomarkers of the Mitochondrial Quality Control Are Differently Affected by Hypoxia-Reoxygenation Stress in Marine Bivalves Crassostrea gigas and Mytilus edulis. Frontiers in Marine Science 2020, 7, 19. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, G.; Jia, M.; Jiang, Q.; Li, S.; Wang, H.; Li, W.; Wang, Y.; Bian, X.; Zhao, Y.; et al. Stay in touch with the endoplasmic reticulum. Science China-Life Sciences 2024, 67, 230–257. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Ho, P.; Cubillos-Ruiz, J.; Huang, S. Control of immune cell function by the unfolded protein response. Nature Reviews Immunology 2023, 1–17. [Google Scholar] [CrossRef]
- Adams, C.; Kopp, M.; Larburu, N.; Nowak, P.; Ali, M. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Frontiers in Molecular Biosciences 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Noulsri, E.; Lerdwana, S. Reducing erythroblast apoptosis in β-thalassemia via unfolded protein response (UPR) signaling. Medical Hypotheses 2023, 177, 1–4. [Google Scholar] [CrossRef]
- Wang, L.; Alzayady, K.; Yule, D. Proteolytic fragmentation of inositol 1,4,5-trisphosphate receptors: a novel mechanism regulating channel activity? Journal of Physiology-London 2016, 594, 2867–2876. [Google Scholar] [CrossRef]
- Kokott-Vuong, A.; Jung, J.; Fehr, A.; Kirschfink, N.; Noristani, R.; Voigt, A.; Reich, A.; Schulz, J.; Huber, M.; Habib, P. Increased Post-Hypoxic Oxidative Stress and Activation of the PERK Branch of the UPR in Trap1-Deficient Drosophila melanogaster Is Abrogated by Metformin. International Journal of Molecular Sciences 2021, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.; Hashimi, A.; Austin, R. Endoplasmic reticulum stress and lipid dysregulation. Expert Reviews in Molecular Medicine 2011, 13, 1–14. [Google Scholar] [CrossRef]
- Stevenson, J.; Huang, E.; Olzmann, J. Endoplasmic Reticulum-Associated Degradation and Lipid Homeostasis. In Annual Review of Nutrition, Vol 36, Stover, P., Ed.; Annual Review of Nutrition; Annual Reviews: Palo Alto, 2016; Volume 36, pp. 511–542. [Google Scholar]
- Gerhardtova, I.; Jankech, T.; Majerova, P.; Piestansky, J.; Olesova, D.; Kovac, A.; Jampilek, J. Recent Analytical Methodologies in Lipid Analysis. International Journal of Molecular Sciences 2024, 25, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Storrie, B. Maintenance of Golgi apparatus structure in the face of continuous protein recycling to the endoplasmic reticulum: Making ends meet. In International Review of Cytology - a Survey of Cell Biology, Vol 244; Jeon, K., Ed.; International Review of Cytology-a Survey of Cell Biology; Elsevier Academic Press Inc: San Diego, 2005; Volume 244, pp. 69–94. [Google Scholar]
- Hetz, C.; Zhang, K.; Kaufman, R. Mechanisms, regulation and functions of the unfolded protein response. Nature Reviews Molecular Cell Biology 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, Y.; Sun, L. Emerging mechanisms of the unfolded protein response in therapeutic resistance: from chemotherapy to Immunotherapy. Cell Communication and Signaling 2024, 22, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Méthe, D.; Stewart-Clark, S.; Clark, F. Size and site specific transcriptomic responses of blue mussel (Mytilus edulis) to acute hypoxia. Marine Genomics 2023, 71, 1–9. [Google Scholar] [CrossRef]
- Soldatov, A.a.A.T.a.S.I.a.S.A. Tissue specificity of metabolism in bivalve mollusc Anadara inaequivalvis Br. under conditions of experimental anoxia. Comparative and Ontogenic Biochemistry 2009, 45, 284–289. [Google Scholar] [CrossRef]
- Dezwaan, A.; Cortesi, P.; Vandenthillart, G.; Roos, J.; Storey, K. Differential sensitivities to hypoxia by two anoxia-tolerant marine molluscs: a biochemical analysis. Marine Biology 1991, 111, 343–351. [Google Scholar] [CrossRef]
- Cook, K.; Shen, H.; McKelvey, K.; Gee, H.; Hau, E. Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas. International Journal of Molecular Sciences 2021, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Waldron, J.; Bushell, M. Codon optimality in cancer. Oncogene 2021, 40, 6309–6320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cubillos-Ruiz, J. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nature Reviews Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Gebert, M.; Slawski, J.; Kalinowski, L.; Collawn, J.; Bartoszewski, R. The Unfolded Protein Response: A Double-Edged Sword for Brain Health. Antioxidants 2023, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller -Herzog, C.; Hall, M. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends in Cell Biology 2012, 22, 274–282. [Google Scholar] [CrossRef]
- Marcondes-de-Castro, I.; Reis-Barbosa, P.; Marinho, T.; Aguila, M.; Mandarim-de-Lacerda, C. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. Journal of Gastroenterology and Hepatology 2023, 38, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-Inducible Factor α and Hif-prolyl Hydroxylase Characterization and Gene Expression in Short-Time Air-Exposed Mytilus galloprovincialis. Marine Biotechnology 2015, 17, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Flye-Sainte-Marie, J.; Chabot, D.; Aguirre-Velarde, A.; Marques, G.; Pecquerie, L. Effects of of hypoxia on metabolic functions in marine organisms: Observed patterns and modelling assumptions within the context of Dynamic Energy Budget (DEB) theory. Journal of Sea Research 2019, 143, 231–242. [Google Scholar] [CrossRef]
- Wu, R. Hypoxia: from molecular responses to ecosystem responses. Marine Pollution Bulletin 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Woo, S.; Denis, V.; Won, H.; Shin, K.; Lee, G.; Lee, T.; Yum, S. Expressions of oxidative stress-related genes and antioxidant enzyme activities in Mytilus galloprovincialis (Bivalvia, Mollusca) exposed to hypoxia. Zoological Studies 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Aguirre-Velarde, A.; Thouzeau, G.; Jean, F.; Mendo, J.; Cueto-Vega, R.; Kawazo-Delgado, M.; Vásquez-Spencer, J.; Herrera-Sanchez, D.; Vega-Espinoza, A.; Flye-Sainte-Marie, J. Chronic and severe hypoxic conditions in Paracas Bay, Pisco, Peru: Consequences on scallop growth, reproduction, and survival. Aquaculture 2019, 512, 1–14. [Google Scholar] [CrossRef]
- Sui, Y.; Hu, M.; Shang, Y.; Wu, F.; Huang, X.; Dupont, S.; Storch, D.; Pörtner, H.; Li, J.; Lu, W.; et al. Antioxidant response of the hard shelled mussel Mytilus coruscus exposed to reduced pH and oxygen concentration. Ecotoxicology and Environmental Safety 2017, 137, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. Journal of Comparative Physiology B-Biochemical Systems and Environmental Physiology 2017, 187, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




