Submitted:
27 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
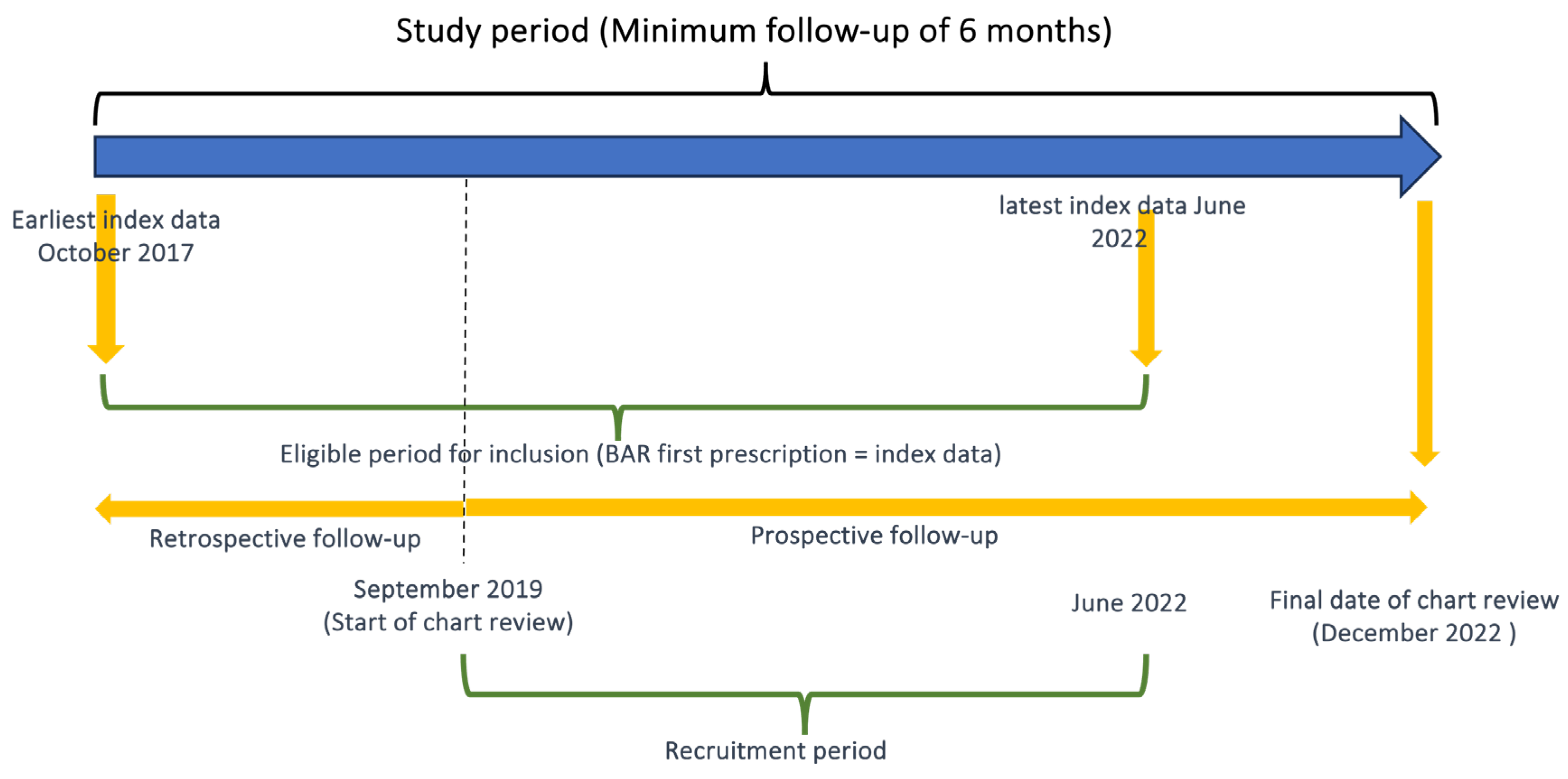
2.1. Study Design and Population
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Population Demographics and Treatments Patterns
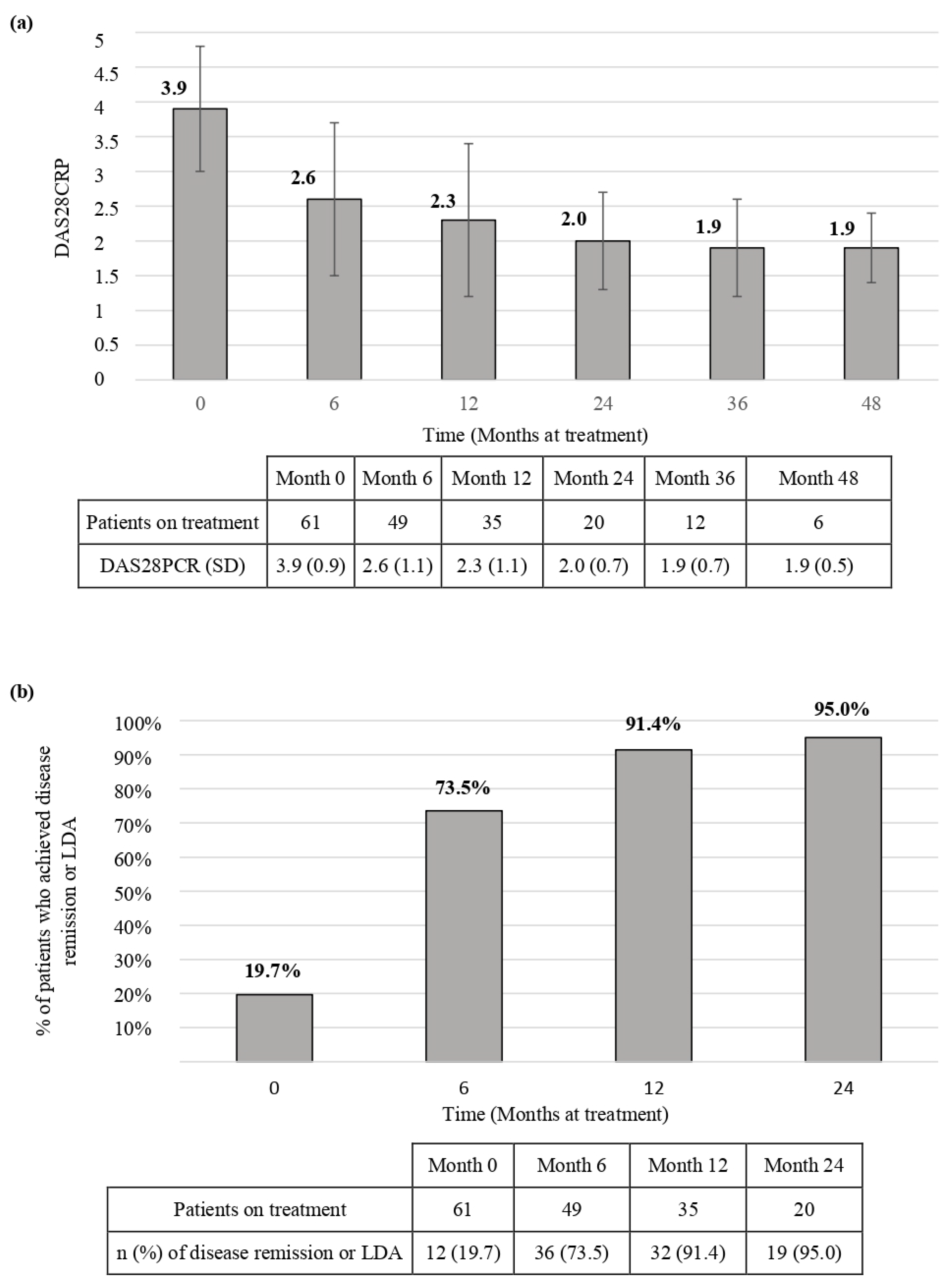
3.2. Effectiveness
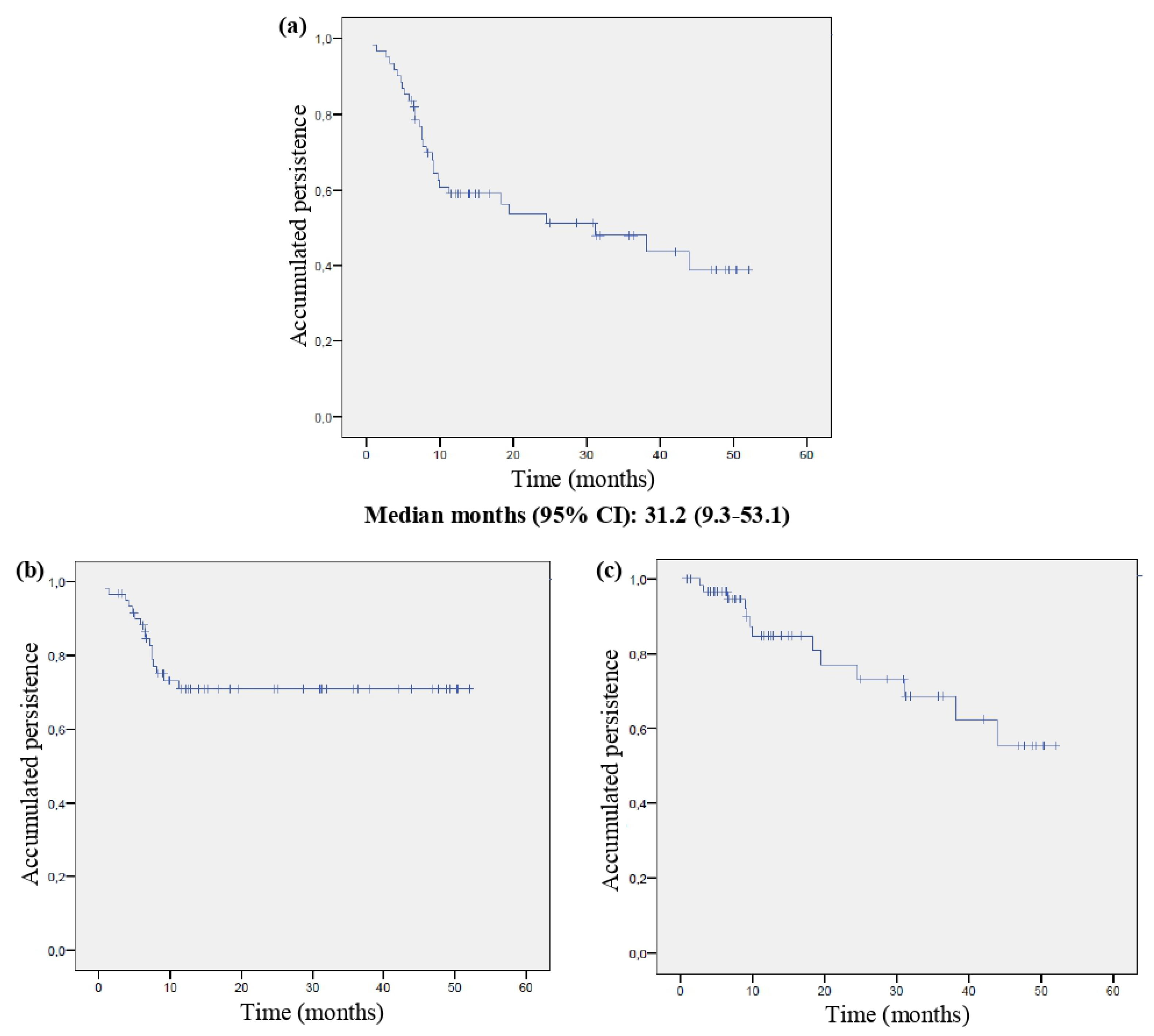
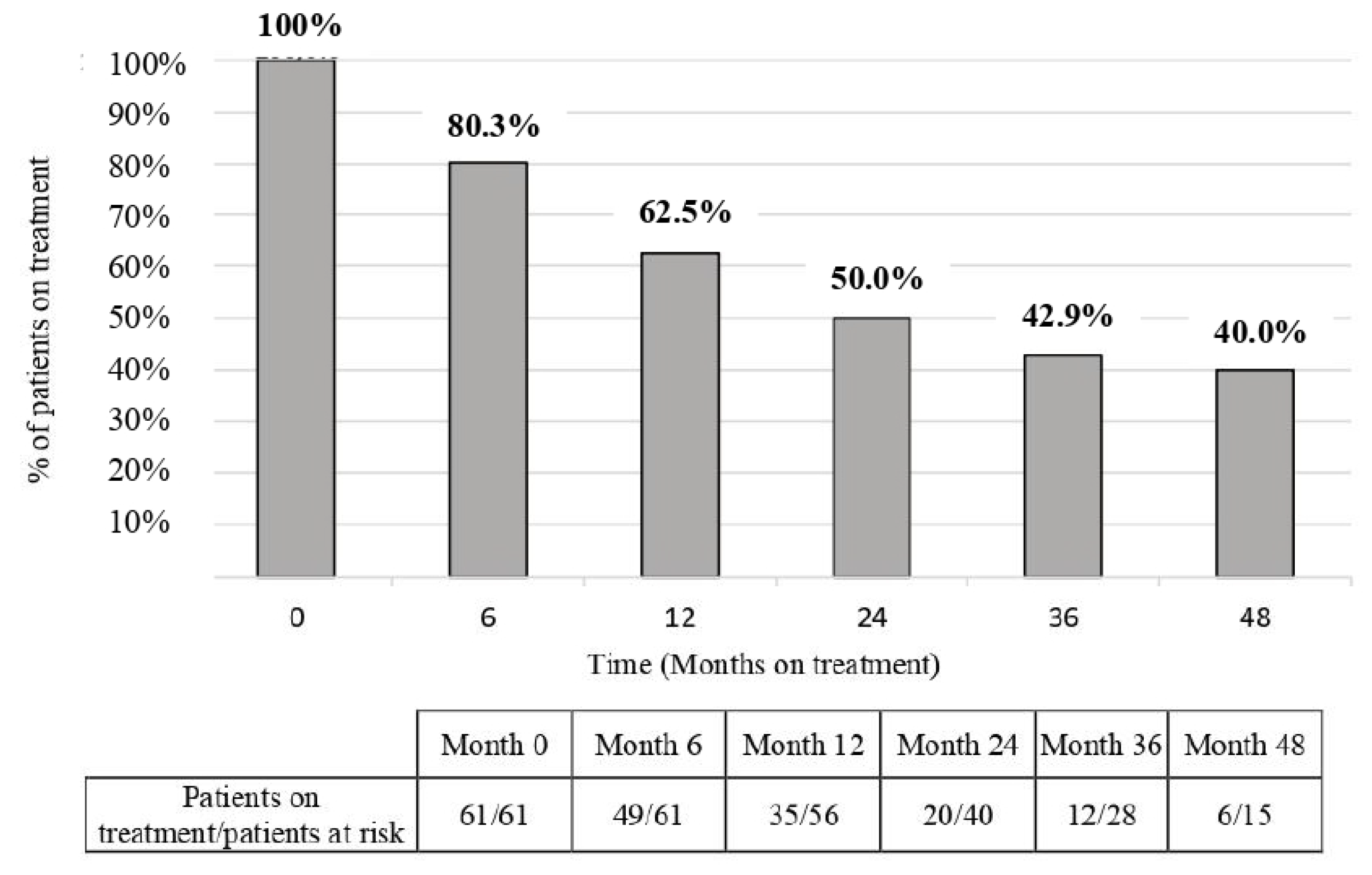
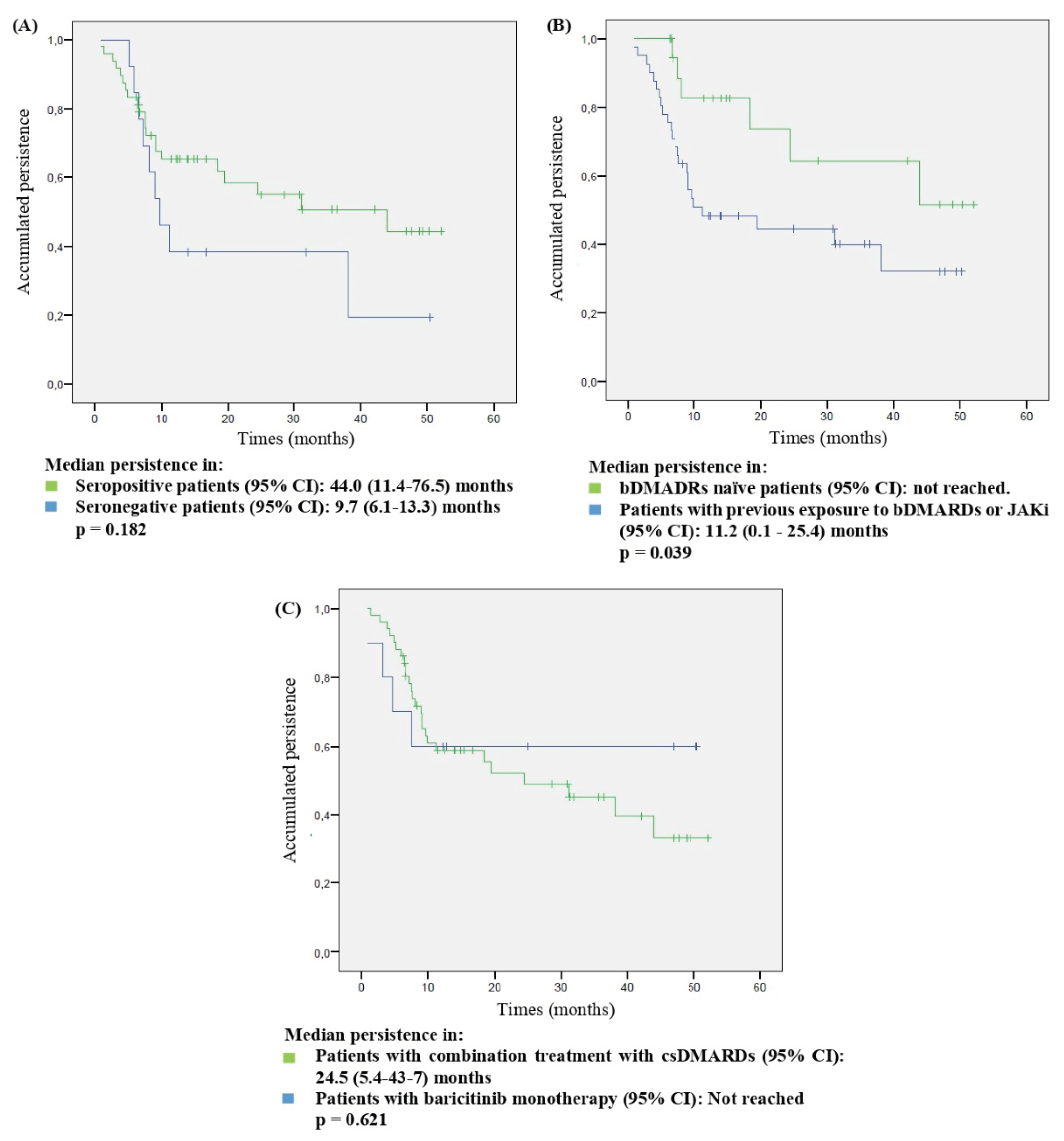
3.3. Persistence
3.4. Adherence
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castañeda, S.; González-Álvaro, I. Novedades en el panorama terapéutico de la Artritis Reumatoide. Reumatol Clin 2017, 13, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.; McInnes, I.B. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Schiff, M.; van der Heijde, D.; Ramos-Remus, C.; Spindler, A.; Stanislav, M.; Zerbini, C.A.; Gurbuz, S.; Dickson, C.; de Bono, S.; el, al.; el al. Baricitinib, Methotrexate, or Combination in Patients With RA and No or Limited Prior DMARDs Treatment. Arthritis Rheumatol 2017, 69, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; van der Heijde, D.; Chen, Y.C.; Greenwald, M.; Drescher, E.; Liu, J.; Beattie, S.; Witt, S.; de la Torre, I.; Gaich, C.; et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017, 76, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Kremer, J.; Zamani, O.; Ludivico, C.; Krogulec, M.; Xie, L.; Beattie, S.D.; Koch, A.E.; Cardillo, T.E.; Rooney, T.P.; et al. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med 2016, 374, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med 2017, 376, 652–662. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Taylor, P.C.; Bieber, T.; Alten, R.; Witte, T.; Galloway, J.; Deberdt, W.; Issa, M.; Haladyj, E.; De La Torre, I.; Grond, S.; et al. Baricitinib Safety for Events of Special Interest in Populations at Risk: Analysis from Randomised Trial Data Across Rheumatologic and Dermatologic Indications. Adv Ther 2023, 40, 1867–1883. [Google Scholar] [CrossRef]
- Sabaté, E. Adherence to long-term therapies: evidence for action. 2003. Available online: http://www.who.int/chronic_conditions/adherencereport/en/ (accessed on 15 July 2023).
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Europen Medicine Agency. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki (accessed on 06 March 2023).
- Fransen, J.; van Riel, P.L. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am 2009, 35, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Landewe, R.; Karonitsch, T.; Bathon, J.; Boers, M.; Bombardier, C.; Bombardieri, S.; Choi, H.; Combe, B.; Dougados, M.; et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis 2008, 67, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A.; Schmittdiel, J.; Karter, A.J.; Konieczny, J.L.; Steiner, J.F. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013, 51, S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López de Castro, N.; Álvarez-Payero, M.; Samartín-Ucha, M.; Martín-Vila, A.; Piñeiro-Corrales, G.; Pego Reigosa, J.M.; Rodríguez-Rodríguez, M.; Melero-González, R.B.; Maceiras-Pan, F.J. Adherence to biological therapies in patients with chronic inflammatory arthropathies. Farm Hosp 2019, 43, 134–139. [Google Scholar] [CrossRef]
- de Klerk, E.; van der Heijde, D.; Landewé, R.; van der Tempel, H.; van der Linden, S. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol 2003, 30, 2469–2475. [Google Scholar] [PubMed]
- Hughes, L.D.; Done, J.; Young, A. A 5 item version of the Compliance Questionnaire for Rheumatology (CQR5) successfully identifies low adherence to DMARDs. BMC Musculoskelet Disord 2013, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Avila, D.G.; Accini, M.; Tobón, M.; Moreno, S.; Rodriguez, V.; Matín Gutierrez, J. Validación y calibración al español del cuestionario CQR para la medición de adherencia a la terapia antirreumática en un grupo de pacientes colombianos con artritis reumatoide. Clin Exp Rheumatol 2019, 26, 105–110. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica Olumiant (Baricitinib). Available online: https://cima.aemps.es/cima/pdfs/ft/1161170010/FT_1161170010.pdf (accessed on 15 July 2022).
- United Stated Deparment of Health and Human Service. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 15 July 2022).
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death (accessed on 06 March 2023).
- Taylor, P.C.; Laedermann, C.; Alten, R.; Feist, E.; Choy, E.; Haladyj, E.; De La Torre, I.; Richette, P.; Finckh, A.; Tanaka, Y. A JAK Inhibitor for Treatment of Rheumatoid Arthritis: The Baricitinib Experience. J Clin Med 2023, 12, 4527. [Google Scholar] [CrossRef]
- Kearsley-Fleet, L.; Davies, R.; De Cock, D.; Watson, K.D.; Lunt, M.; Buch, M.H.; Isaacs, J.D.; Hyrich, K.L. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018, 77, 1405–1412. [Google Scholar] [CrossRef]
- Hernández-Cruz, B.; Kiltz, U.; Avouac, J.; Treuer, T.; Haladyj, E.; Gerwien, J.; Gupta, C.D.; Conti, F. Evidence on Baricitinib for the Treatment of Rheumatoid Arthritis. Rheumatol Ther 2023, 10, 1417–1457. [Google Scholar] [CrossRef]
- Takahashi, N.; Asai, S.; Kobayakawa, T.; Kaneko, A.; Watanabe, T.; Kato, T.; Nishiume, T.; Ishikawa, H.; Yoshioka, Y.; Kanayama, Y.; et al. Predictors for clinical effectiveness of baricitinib in rheumatoid arthritis patients in routine clinical practice: data from a Japanese multicenter registry. Sci Rep 2020, 10, 21907. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Sato, S.; Kurushima, S.; Michitsuji, T.; Nishihata, S.; Okamoto, M.; Tsuji, Y.; Endo, Y.; Shimizu, T.; Sumiyoshi, R. , et al. Real-world comparative effectiveness and safety of tofacitinib and baricitinib in patients with rheumatoid arthritis. Arthritis Res Ther 2021, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.; Melville, A.R.; Emery, P.; Nam, J.L.; Buch, M.H. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatology (Oxford) 2021, 60, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, A.; Askling, J.; Chatzidionysiou, K.; Forsblad-d’Elia, H.; Kastbom, A.; Lindström, U.; Turesson, C.; Frisell, T. Effectiveness of baricitinib and tofacitinib compared with bDMARDs in RA: results from a cohort study using nationwide Swedish register data. Rheumatology (Oxford) 2022, 61, 3952–3962. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, B.; Rosas, J.; Díaz-Torné, C.; Belzunegui, J.; García-Vicuña, R.; Inciarte-Mundo, J.; Pons, A.; Millán, A.M.; Jeria-Navarro, S.; Valero, J.A.; et al. Real-World Treatment Patterns and Clinical Outcomes of Baricitinib in Rheumatoid Arthritis Patients in Spain: Results of a Multicenter, Observational Study iRoutine Clinical Practice (The ORBIT-RA Study). Rheumatol Ther 2022, 9, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Ceccarelli, F.; Garufi, C.; Duca, I.; Mancuso, S.; Cipriano, E.; Dell’Unto, E.; Alessandri, C.; Di Franco, M.; Perricone, C.; et al. Effectiveness and safety of baricitinib in rheumatoid arthritis: a monocentric, longitudinal, real-life experience. Clin Exp Rheumatol 2021, 39, 39,525–31. [Google Scholar] [CrossRef] [PubMed]
- González-Freire, L.; Giménez-Candela, R.M.; Castro-Luaces, S.; Veiga-Villaverde, A.B.; Crespo-Diz, C. Baricitinib and tofacitinib in patients with rheumatoid arthritis: results of regular clinical practice. Farm Hosp 2021, 45, 165–169. [Google Scholar] [CrossRef]
- González Mazarío, R.; Fragío Gil, J.J.; Ivorra Cortés, J.; Grau García, E.; Cañada Martínez, A.J.; González Puig, L.; Negueroles Albuixech, R.M.; Román Ivorra, J.A. Efectividad y seguridad en el mundo real de los inhibidores de JAK en la artritis reumatoide: Estudio unicéntrico. Reumatol Clin (Barc.) 2022, 18, 523–530. [Google Scholar] [CrossRef]
- Takagi, M.; Atsumi, T.; Matsuno, H.; Tamura, N.; Fujii, T.; Okamoto, N.; Takahashi, N.; Nakajima, A.; Nakajima, A.; Tsujimoto, N.; et al. Safety and Effectiveness of Baricitinib for Rheumatoid Arthritis in Japanese Clinical Practice: 24-Week Results of All-Case Post-Marketing Surveillance. Mod Rheumatol. [CrossRef]
- Alten, R.; Burmester, G.R.; Matucci-Cerinic, M.; Salmon, J.H.; Lopez-Romero, P.; Fakhouri, W.; de la Torre, I.; Zaremba-Pechmann, L.; Holzkämper, T.; Fautrel, B. The RA-BE-REAL Multinational, Prospective, Observational Study in Patients with Rheumatoid Arthritis Receiving Baricitinib, Targeted Synthetic, or Biologic Disease-Modifying Therapies: a 6-Month Interim Analysis. Rheumatol Ther 2023, 10, 73–93. [Google Scholar] [CrossRef]
- Guidelli, G.M.; Viapiana, O.; Luciano, N.; De Santis, M.; Boffini, N.; Quartuccio, L.; Birra, D.; Conticini, E.; Chimenti, M.S.; Bazzani, C.; et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: a real-life multicentre study. Clin Exp Rheumatol 2021, 39, 868–873. [Google Scholar] [CrossRef]
- Rosas, J.; Senabre-Gallego, J.M.; Santos-Soler, G.; Antonio Bernal, J.; Pons Bas, A. Efficacy and Safety of Baricitinib in Patients with Rheumatoid Arthritis and Inadequate Response to Conventional Synthetic DMARDs and/or Biological DMARDs: Data from a Local Registry. Reumatol Clin (Engl Ed) 2022, 18, 188–189. [Google Scholar] [CrossRef]
- Retuerto, M.; Trujillo, E.; Valero, C.; Fernandez-Espartero, C.; Soleto, C.Y.; Garcia-Valle, A.; Aurrecoechea, E.; Garijo, M.; Lopez, A.; Loricera, J.; et al. Efficacy and safety of switching Jak inhibitors in rheumatoid arthritis: an observational study. Clin Exp Rheumatol 2021, 39, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Hirano, T.; Maeda, Y.; Yamamoto, W.; Hashimoto, M.; Murata, K.; Onishi, A.; Jinno, S.; Hara, R.; Son, Y.; et al. Drug retention of sarilumab, baricitinib, and tofacitinib in patients with rheumatoid arthritis: the ANSWER cohort study. Clin Rheumatol 2021, 40, 2673–2680. [Google Scholar] [CrossRef]
- Smolen, J.S.; Xie, L.; Jia, B.; Taylor, P.C.; Burmester, G.; Tanaka, Y.; Elias, A.; Cardoso, A.; Ortmann, R.; Walls, C.; et al. Efficacy of baricitinib in patients with moderate-to-severe rheumatoid arthritis with 3 years of treatment: results from a long-term study. Rheumatology (Oxford), 2021, 60, 2256–2266. [Google Scholar] [CrossRef]
- Baldi, C.; Berlengiero, V.; Falsetti, P.; Cartocci, A.; Conticini, E.; D’Alessandro, R.; D’Ignazio, E.; Bardelli, M.; Fabbroni, M.; Cantarini, L.; et al. Baricitinib retention rate: ‘real-life’ data from a mono-centric cohort of patients affected by rheumatoid arthritis. Front Med (Lausanne), 1176. [Google Scholar] [CrossRef]
- Marras, C.; Monteagudo, I.; Salvador, G.; de Toro, F.J.; Escudero, A.; Alegre-Sancho, J.J.; Raya, E.; Ortiz, A.; Carmona, L.; Mestre, Y.; et al. Identification of patients at risk of non-adherence to oral antirheumatic drugs in rheumatoid arthritis using the Compliance Questionnaire in Rheumatology: an ARCO sub-study. Rheumatol Int 2017, 37, 37,1195–02. [Google Scholar] [CrossRef]
- Ibarra Barrueta, O.; Morillo Verdugo, R.; Rudi Sola, N.; Ventura, J.M.; Navarro Aznárez, H. Adherencia en pacientes en tratamiento crónico: resultados del “Día de la Adherencia” del 2013. Farm Hosp 2015, 39, 109–113. [Google Scholar]
- Codes-Mendez, H.; Martinez-Molina, C.; Masip, M.; Riera, P.; Pagès Puigdemont, N.; Riera Magallón, A.; Lobo Prat, D.; Sainz Comas, L.; Corominas, H.; et al. Therapeutic adherence and persistence of tofacitinib and baricitinib in rheumatoid arthritis patients in daily clinical practice. Ann Rheum Dis 2022, 81, 1330. [Google Scholar] [CrossRef]
- Deprez, V.; Le Monnier, L.; Sobhy-Danial, J.M.; Grados, F.; Henry-Desailly, I.; Salomon-Goëb, S.; Rabin, T.; Ristic, S.; Fumery, M.; Fardellone, P.; et al. Therapeutic Maintenance of Baricitinib and Tofacitinib in Real Life. J Clin Med. 2020, 9, 3319. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xiao, K.; Ottaviani, S.; Stebbing, J.; Wang, Y.J. A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib. Expert Opin Drug Saf 2020, 19, 1505–1511. [Google Scholar] [CrossRef]
- Taylor, P.C.; Takeuchi, T.; Burmester, G.R.; Durez, P.; Smolen, J.S.; Deberdt, W.; Issa, M.; Terres, J.R.; Bello, N.; Winthrop, K.L. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 2022, 81, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Ahn, S.M.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Hong, S. Safety of JAK inhibitor use in patients with rheumatoid arthritis who developed herpes zoster after receiving JAK inhibitors. Clin Rheumatol 2022, 41, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Frisell, T.; Bower, H.; Morin, M.; Baecklund, E.; Di Giuseppe, D.; Delcoigne, B.; Feltelius, N.; Forsblad-d’Elia, H.; Lindqvist, E.; Lindström, U.; et al. Safety of biological and targeted synthetic disease-modifying antirheumatic drugs for rheumatoid arthritis as used in clinical practice: results from the ARTIS programme. Ann Rheum Dis 2023, 82, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Iwamoto, N.; Fukui, S.; Morimoto, S.; Aramaki, T.; Shomura, F.; Aratake, K.; Eguchi, K.; Ueki, Y.; Kawakami, A. Comparison of risks of cancer, infection, and MACEs associated with JAK inhibitor and TNF inhibitor treatment: a multicenter cohort study. Rheumatology (Oxford) 2023, 62, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
- Waldman, R.A.; Sharp, K.L.; Adalsteinsson, J.A.; Grant-Kels, J.M. Herpes zoster subunit vaccine for patients initiating a Janus kinase inhibitor. J Am Acad Dermatol 2022, 88, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Louder, A.; Polinski, J.; Zhang, T.C.; Bower, H.; Phillips, S.; Song, Y.; Rashidi, E.; Bosan, R.; Chang, H.C.; et al. Evaluation of VTE, MACE, and Serious Infections Among Patients with RA Treated with Baricitinib Compared to TNFi: A Multi-Database Study of Patients in Routine Care Using Disease Registries and Claims Databases. Rheumatol Ther 2023, 10, 201–223. [Google Scholar] [CrossRef]
- Gouverneur, A.; Avouac, J.; Prati, C.; Cracowski, J.L.; Schaeverbeke, T.; Pariente, A.; Truchetet, M.E. JAK inhibitors and risk of major cardiovascular events or venous thromboembolism: a self-controlled case series study. Eur J Clin Pharmacol 2022, 78, 1981–1990. [Google Scholar] [CrossRef]
- Hoisnard, L.; Pina Vegas, L.; Dray-Spira, R.; Weill, A.; Zureik, M.; Sbidian, E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis 2023, 82, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xue, X.; Shannon, J. Characteristics of adverse event reporting of Xeljanz/Xeljanz XR, Olumiant, and Rinvoq to the US Food and Drug Administration. J Manag Care Spec Pharm 2022, 28, 1046–1052. [Google Scholar] [CrossRef]
- Kiely, P.D. Biologic efficacy optimization--a step towards personalized medicine. Rheumatology (Oxford) 2016, 55, 780–788. [Google Scholar] [CrossRef]
| Gender (n, %, female) | 51 (83.6) |
| Age at initiation of BAR (years, mean, SD) | 58.1 (15.4) |
| Disease duration (years, mean, SD) | 13.9 (8.3) |
| RF positive (n, %) | 47 (77.0) |
| ACPAs positive (n, %) | 43 (70.5) |
| Erosive disease (n, %) | 34 (55.7) |
| Extra-articular disease (n, %) Rheumatic nodules Sjögren syndrome Interstitial pneumonitis Neuropathies Peripheral ulcerative keratitis Raynaud syndrome Felty syndrome |
26 (42.6) 10 (16.4) 7 (11.5) 4 (6.6) 2 (3.3) 1 (1.6) 1 (1.6) 1 (1.6) |
| Glucocorticoid treatment (n, %) | 30 (49.1) |
| Naïve to bDMARDs or JAKi treatment (n, %) | 20 (32.8) |
| Previous exposure to bDMARDs or JAKi (n, %) One previous bDMARD Two previous bDMARDs Three previous bDMARDs Four previous bDMARDs Five previous bDMARDs Six previous bDMARDs Seven previous bDMARDs Eight previous bDMARDs |
41 (67.2) 6 (9.8) 14 (23.0) 8 (13.1) 7 (11.5) 2 (3.3) 2 (3.3) 1 (1.6) 1 (1.6) |
| Previous exposure to one JAKi (tofacitinib) (n, %) | 7 (11.5) |
| BAR monotherapy (n, %) | 10 (16.4) |
| BAR in combination with csDMARDs Methotrexate Leflunomide Hydroxychloroquine Sulfasalazine Methotrexate plus leflunomide |
51 (83.6) 31 (50.8) 14 (23.0) 3 (4.9) 2 (3.3) 1 (1.6) |
| Baseline DAS28CRP (mean, SD) | 3.9 (0.9) |
| Baseline ESR (mml/h, mean, SD) | 27.8 (23.2) |
| Baseline CRP (mg/dl, mean, SD) | 2.0 (4.8) |
| Baseline | Final | p | |
|---|---|---|---|
| DAS28CRP (average, SD) | 3.9 (0.9) | 2.7 (1.3) | 0.000 |
| CRP (mg/dl, average, SD) | 2.0 (4.8) | 1.1 (1.7) | 0.105 |
| ESR (mml/h, average, SD) | 29.0 (23,2) | 25.7 (22.9) | 0.604 |
| Lymphocyte count (cells/mm3, mean, SD) | 2641 (1501) | 2482.6 (1505) | 0.154 |
| Neutrophil count (cells/mm3, mean, SD) | 4198 (2126) | 4157 (2132) | 0.865 |
| Haemoglobin (g/dl, mean, SD) | 13.5 (1.5) | 12.9 (1.4) | 0.000 |
| IR (n, %) | IR per 100 PY (95% CI) | |
|---|---|---|
| Patients with any AE (Total AEs=104) | 40/61 (65.6) | 15.2 (15.1-15.3) |
| Anaemia | 24/61 (39.3) | 9.1 (9.0-9.2) |
| Any infection Herpes Zoster URTI Skin and soft tissue infection Bacterial pneumonia Influenza A Oral herpes simple |
22/61 (36.1) 7/61 (11.5) 7/61 (11.5) 5/61 (8.2) 3/61 (4.9) 2/61 (3.3) 2/61 (3.3) |
8.4 (8.2-8.6) 2.7 (2.4-3.0) 2.7 (2.4-3.0) 1.9 (1.7-2.1) 1.1 (0.8-1.4) 0.8 (0.6-1.0) 0.8 (0.6-1.0) |
| Hypercholesterolemia | 20/61 (32.8) | 7.6 (7.4-7.8) |
| Abnormal liver enzymes (ALAT or ASAT) | 19/61 (31.1) | 7.2 (6.9-7.5) |
| Nausea and vomiting | 4/61 (6.6) | 1.5 (1.3-1.7) |
| Cancer | 3/61 (4.9) | 1.1 (0.8-1.4) |
| Alopecia | 2/61 (3.3) | 0.8 (0.6-1.0) |
| Skin disorders | 2/61 (3.3) | 0.8 (0.6-1.0) |
| Asthenia | 2/61 (3.3) | 0.8 (0.6-1.0) |
| Weight gain | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Venous thrombotic event | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Hypertriglyceridemia | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Rhabdomyolysis | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Platelet increase | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Patients with SAEs (Grade 3-4) (Total SAEs=11) | 9/61 (14.8) | 3.5 (3.3-3.7) |
| Bacterial pneumonia with intravenous treatment | 3/61 (4.9) | 1.1 (0.8-1.4) |
| Cancer | 3/61 (4.9) | 1.1 (0.8-1.4) |
| Abnormal liver enzymes (ALT or AST) | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Venous thrombotic event | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Hypertriglyceridemia | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Skin disorders (Urticarial) | 1/61 (1.6) | 0.4 (0.2-0.6) |
| Platelet increase | 1/61 (1.6) | 0.4 (0.2-0.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





