Submitted:
20 January 2025
Posted:
20 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
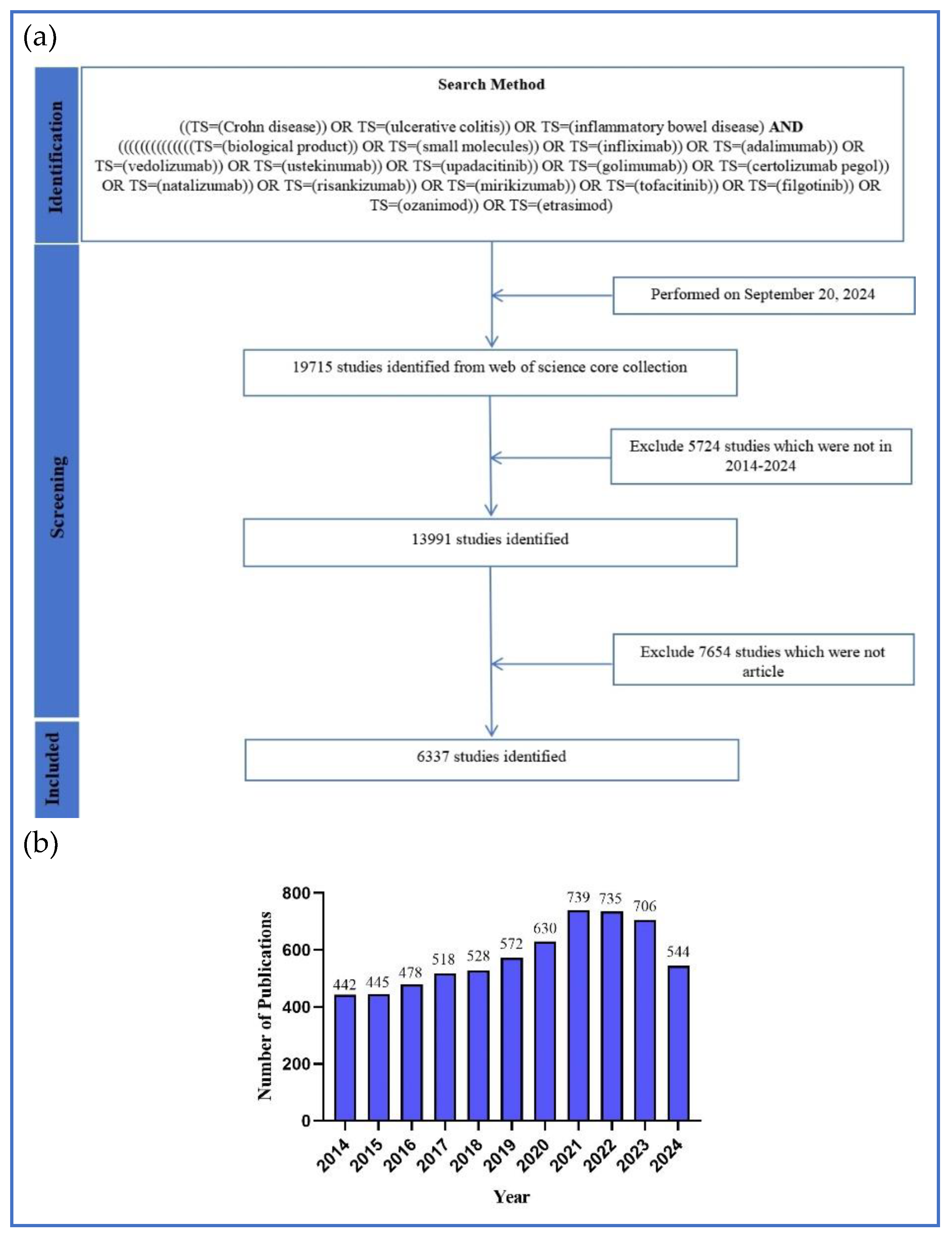
2.1. The Trends in Annual Global Publications
2.2. Distribution of Source Journals
2.3. Citation Analysis
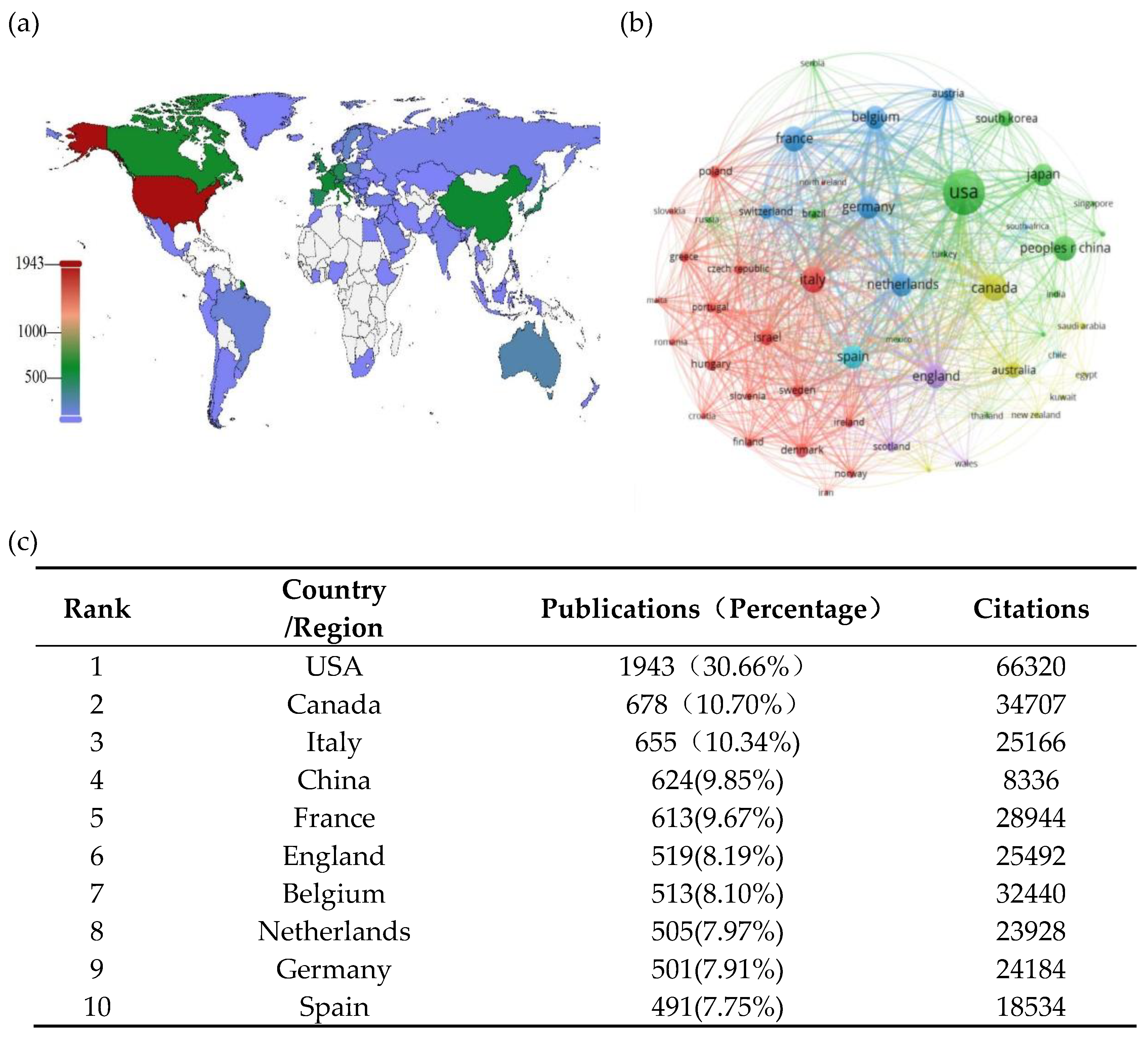
2.4. Distribution and Co-Authorship of Countries/Regions
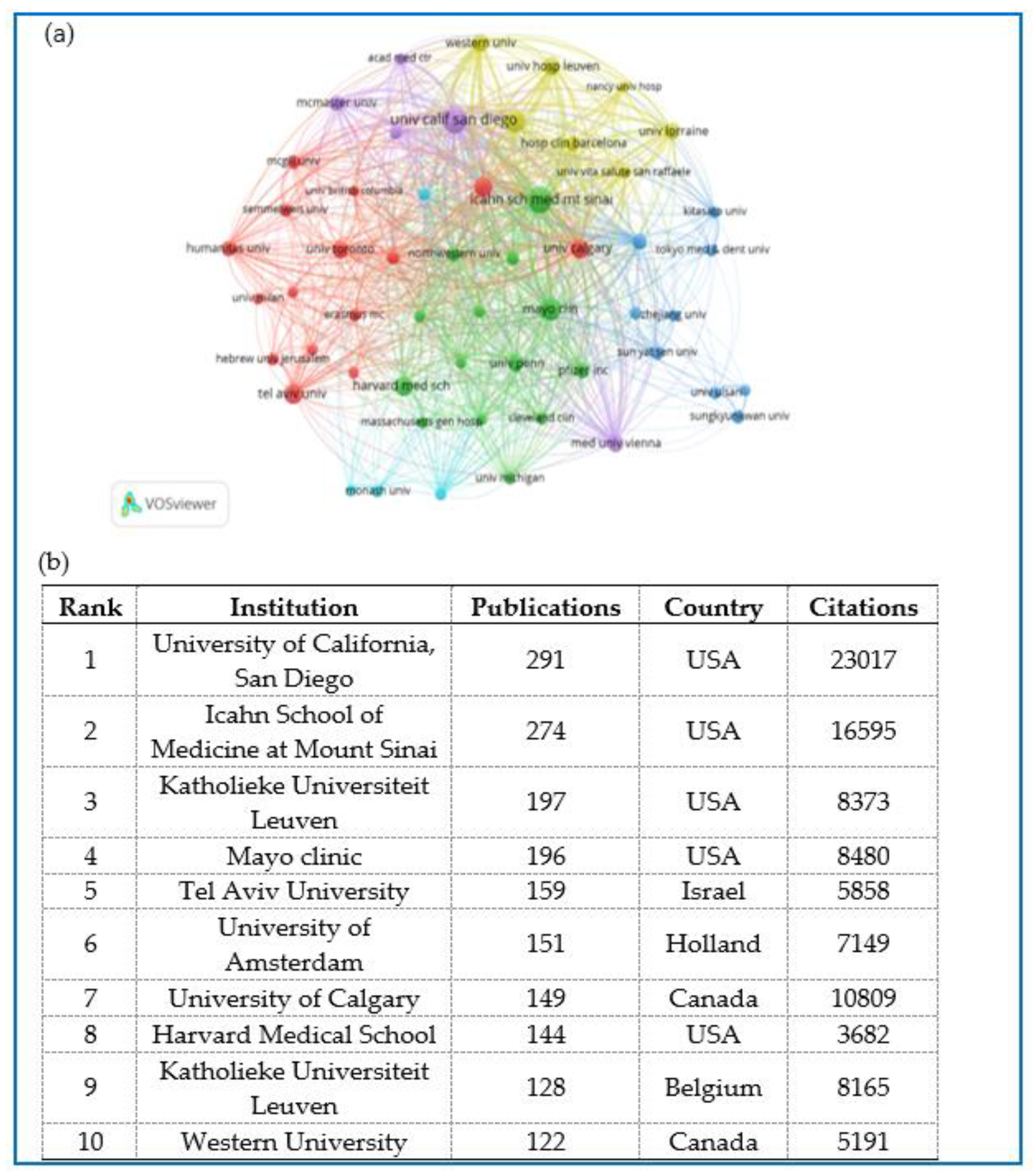
2.5. Distribution and Co-Authorship of Institutions
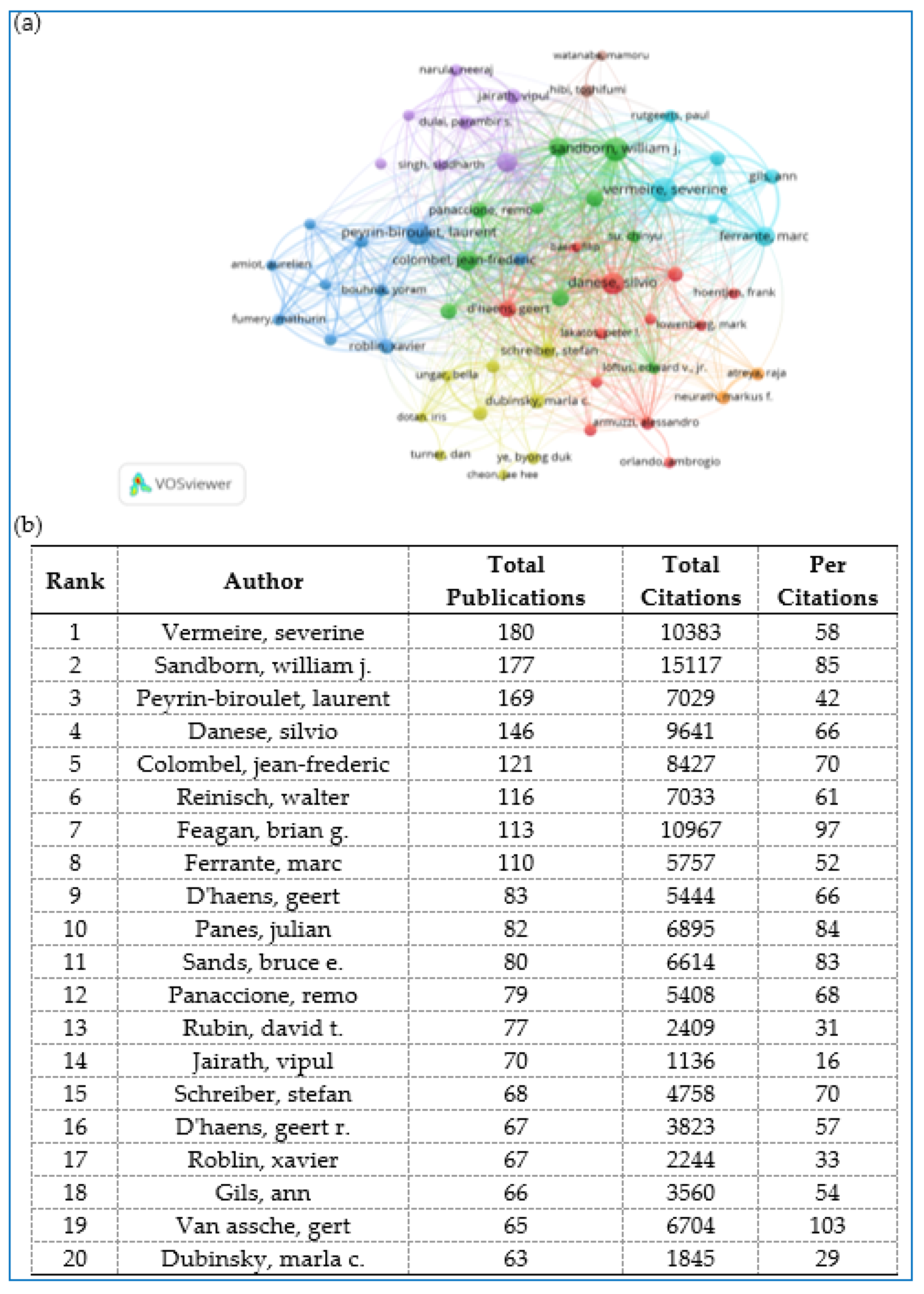
2.6. Distribution and Co-Authorship of Authors
2.7. Co-Citation Analysis
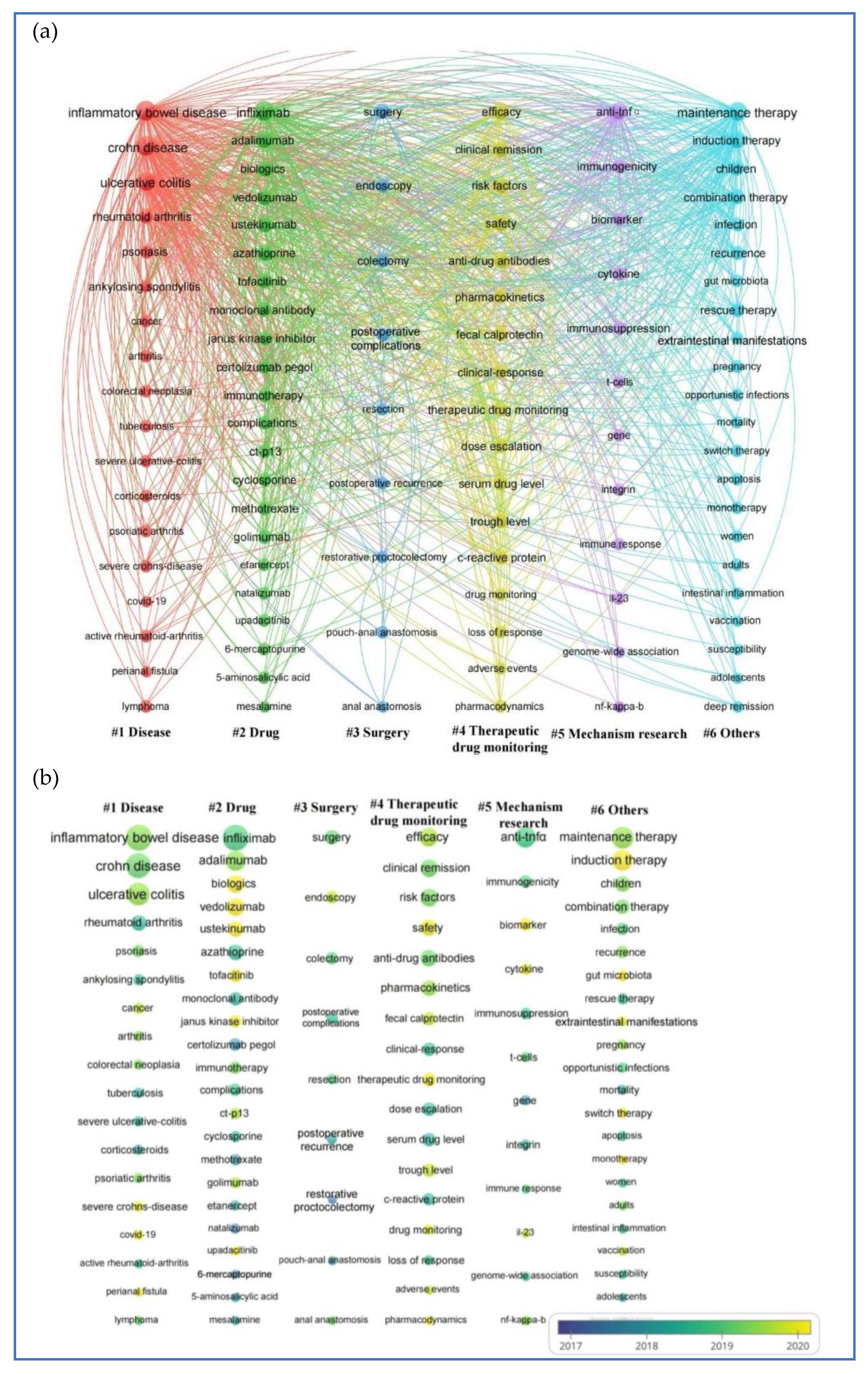
2.8. The Co-Occurrence Analysis of the Top 100 Keywords
3. Discussion
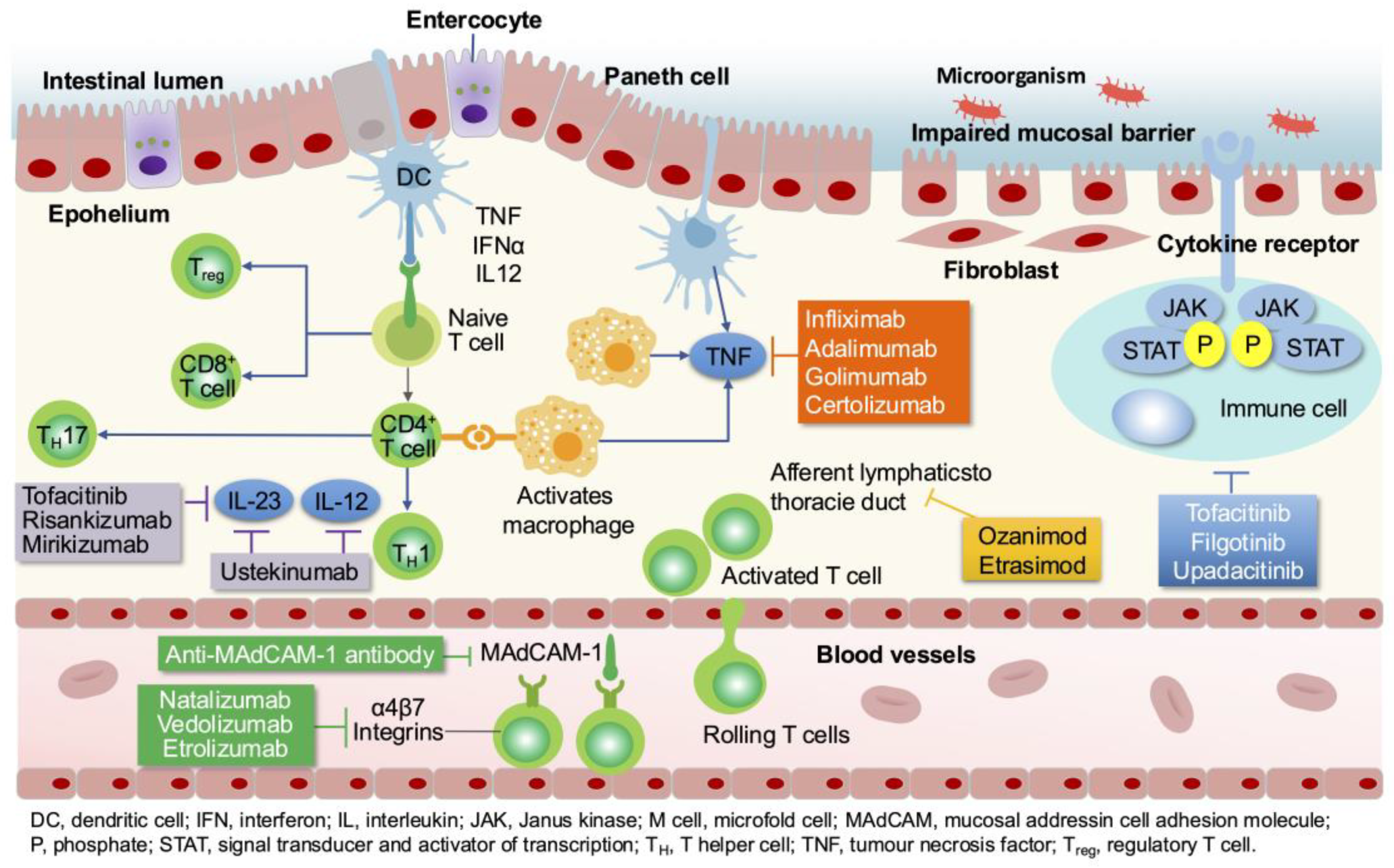
3.1. IBD and Immune Dysregulation
| Drug | Target | Indication | Induction therapy | Maintenance therapy | Trough level (ug/ml) | ||
| dose | by | dose | by | ||||
| infliximab | TNF-α | UC, CD |
5 mg/kg at 0, 2, 6wk | iv | 5 mg/kg q8w | iv | ≥5 [18]; 3-7 [24]; 5-10 [20]; 7-10 (I); 5-7(M) [22] |
| adalimumab | TNF-α | UC, CD |
160 mg day 1, 80 mg day 15 |
sc | 40 mg q2w | sc | ≥7.5 [18]; 4-8 [24]; 8-12 [20]; >10-12(I, M) [22] |
| golimumab | TNF-α | UC | 200 mg day 1, 100 mg day 15 |
sc | 100 mg q4 w; < 80 kg, 50 mg q4w; >80 kg, 100 mg q4w |
sc | >2.5 [19] |
| certolizumab | TNF-α |
CD | 400 mg 0, 2, 4 wk | sc | 400 mg q4w | sc | ≥20 [18] |
| vedolizumab | α4β7 Integrin | UC, CD |
300 mg at 0, 2, 6wk | iv | 300 mg q8w | iv | >33-37(6wk); >15-20(14wk); >10-15(M) [23] |
| natalizumab | α4 Integrin |
CD | 300 mg at 0, 4, 8, 12wk | iv | 300 mg, q4w | iv | |
| ustekinumab | IL-12/23 p40 | UC, CD |
<55 kg, 260 mg 55–85 kg, 390 mg >85 kg, 520 mg |
iv | 90 mg q8w | sc | >3-7(8wk); >1-3(maintenance) [23]; >11.1(I); >4.5(M) [22] |
| risankizumab | IL-23p19 | CD | 600 mg at 0, 4, 8 wk |
iv | 180 mg or 360 mg q8w | sc | NA |
| mirikizumab | IL-23p19 | UC | 300 mg at 0, 4, 8 wk |
iv | 200 mg at 12w, q4w | iv | |
| tofacitinib | JAK-1/2/3 | UC | 10 mg bid for 8 weeks |
po | 5 mg or 10 mg bid | po | |
| upadacitinib | JAK-1 | UC, CD |
45 mg daily for 8 weeks(UC) 12 weeks(CD) |
po | 15 mg or 30 mg qd | po | |
| filgotinib | JAK-1 | UC | 200 mg qd for 22 weeks |
po | 200 mg qd | po | |
| ozanimod | S1PR | UC | 0.23 mg qd, day 1–4 0.46 mg qd, day 5–7 |
po |
0.92 mg qd | po |
|
| etrasimod | S1PR | UC | 2mg qd | po | 2mg qd | po | |
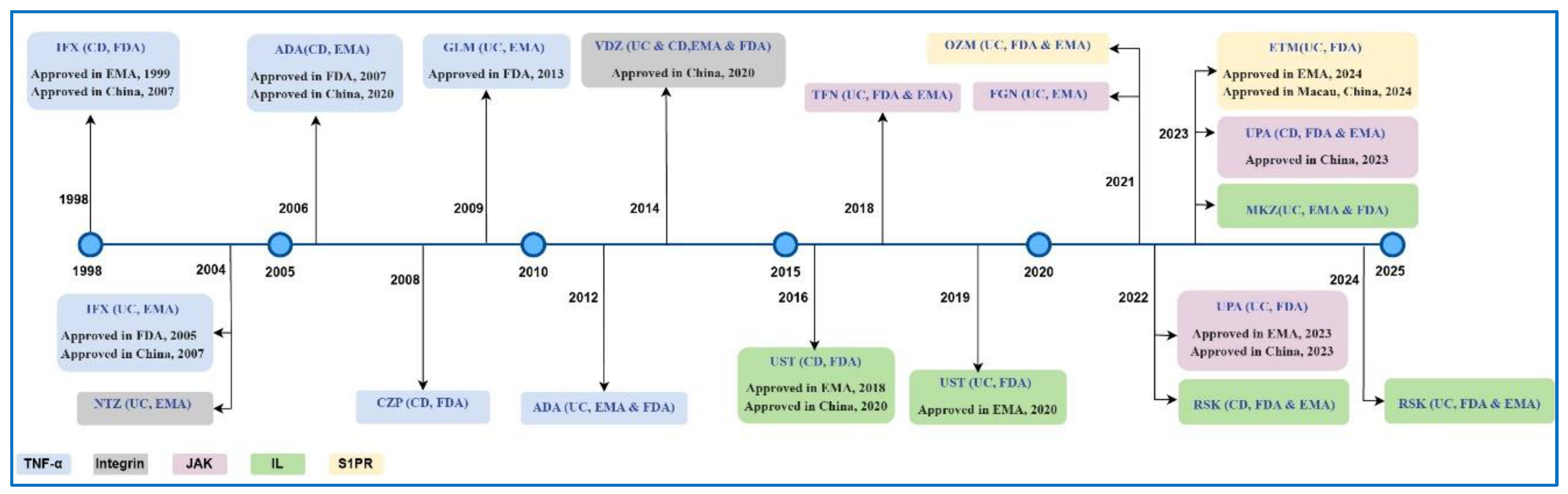
3.2. Biologics for IBD
3.3. Small Molecules for IBD
3.4. Natural Herbal Medicine
4. Materials and Methods
4.1. Data Source and Strategy for Retrieval
4.2. Bibliometric Analysis and Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr 2015, 169, 1053–60. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; Kunisaki, R.; Motoya, S.; Nagahori, M.; Nakase, H.; Omata, F.; Saruta, M.; Watanabe, T.; Tanaka, T.; Kanai, T.; Noguchi, Y.; Takahashi, K.I.; Watanabe, K.; Hibi, T.; Suzuki, Y.; Watanabe, M.; Sugano, K.; Shimosegawa, T. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018, 53, 305–353. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Murphy, M.; Malter, L. A Review of Available Medical Therapies to Treat Moderate-to-Severe Inflammatory Bowel Disease. Am J Gastroenterol 2024, 119, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- 5. Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; Bossuyt, P.; Burisch, J.; Collins, P.; El-Hussuna, A.; Ellul, P.; Frei-Lanter, C.; Furfaro, F.; Gingert, C.; Gionchetti, P.; Gomollon, F.; González-Lorenzo, M.; Gordon, H.; Hlavaty, T.; Juillerat, P.; Katsanos, K.; Kopylov, U.; Krustins, E.; Lytras, T.; Maaser, C.; Magro, F.; Marshall, J.K.; Myrelid, P.; Pellino, G.; Rosa, I.; Sabino, J.; Savarino, E.; Spinelli, A.; Stassen, L.; Uzzan, M.; Vavricka, S.; Verstockt, B.; Warusavitarne, J.; Zmora, O.; Fiorino, G. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; Eder, P.; Ellul, P.; Fidalgo, C.; Fiorino, G.; Gionchetti, P.; Gisbert, J.P.; Gordon, H.; Hedin, C.; Holubar, S.; Iacucci, M.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Lakatos, P.L.; Lytras, T.; Lyutakov, I.; Noor, N.; Pellino, G.; Piovani, D.; Savarino, E.; Selvaggi, F.; Verstockt, B.; Spinelli, A.; Panis, Y.; Doherty, G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; Ghosh, S.; Wiebe, S.; Kaplan, G.G. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Tsai, L.; Ma, C.; Dulai, P.S.; Prokop, L.J.; Eisenstein, S.; Ramamoorthy, S.L.; Feagan, B.G.; Jairath, V.; Sandborn, W.J.; Singh, S. Contemporary Risk of Surgery in Patients With Ulcerative Colitis and Crohn’s Disease: A Meta-Analysis of Population-Based Cohorts. Clin Gastroenterol Hepatol 2021, 19, 2031–2045.e11. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Cohen, B.L.; Velayos, F.S.; Weiss, J.M.; Sultan, S.; Siddique, S.M.; Adler, J.; Chachu, K.A. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- Ninkov, A.; Frank, J.R.; Maggio, L.A. Bibliometrics: Methods for studying academic publishing. Perspect Med Educ 2022, 11, 173–176. [Google Scholar] [CrossRef]
- Arruda, H.; Silva, E.R.; Lessa, M.; Proença, D., Jr.; Bartholo, R. VOSviewer and Bibliometrix. J Med Libr Assoc 2022, 110, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, D.; de Voogd, F.A.E.; Pruijt, M.J.; Grootjans, J.; van de Sande, M.G.; D’Haens, G.R. The Role of the Lymphatic System in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Vermeire, S.; Nielsen, O.H. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol Sci 2017, 38, 127–142. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw 2017, 17, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Harzallah, I.; Rigaill, J.; Williet, N.; Paul, S.; Roblin, X. Golimumab pharmacokinetics in ulcerative colitis: a literature review. Therap Adv Gastroenterol 2017, 10, 89–100. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Abreu, M.T.; Afif, W.; Cross, R.K.; Dubinsky, M.C.; Loftus, E.V., Jr.; Osterman, M.T.; Saroufim, A.; Siegel, C.A.; Yarur, A.J.; Melmed, G.Y.; Papamichael, K. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am J Gastroenterol 2021, 116, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.J.; Hilmi, I.; Banerjee, R.; Chuah, S.W.; Ng, S.C.; Wei, S.C.; Makharia, G.K.; Pisespongsa, P.; Chen, M.H.; Ran, Z.H.; Ye, B.D.; Park, D.I.; Ling, K.L.; Ong, D.; Ahuja, V.; Goh, K.L.; Sollano, J.; Lim, W.C.; Leung, W.K.; Ali, R.A.R.; Wu, D.C.; Ong, E.; Mustaffa, N.; Limsrivilai, J.; Hisamatsu, T.; Yang, S.K.; Ouyang, Q.; Geary, R.; De Silva, J.H.; Rerknimitr, R.; Simadibrata, M.; Abdullah, M.; Leong, R.W.L. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. J Gastroenterol Hepatol 2019, 34, 1296–1315. [Google Scholar] [CrossRef]
- Rodríguez-Moranta, F.; Argüelles-Arias, F.; Hinojosa Del Val, J.; Iborra Colomino, M.; Martín-Arranz, M.D.; Menchén Viso, L.; Muñoz Núñez, F.; Ricart Gómez, E.; Sánchez-Hernández, J.G.; Valdés-Delgado, T.; Guardiola Capón, J.; Barreiro-de Acosta, M.; Mañosa Ciria, M.; Zabana Abdo, Y.; Gutiérrez Casbas, A. Therapeutic drug monitoring in inflammatory bowel diseases. Position statement of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis. Gastroenterol Hepatol 2024, 47, 522–552. [Google Scholar] [CrossRef] [PubMed]
- Restellini, S.; Afif, W. Update on TDM (Therapeutic Drug Monitoring) with Ustekinumab, Vedolizumab and Tofacitinib in Inflammatory Bowel Disease. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.J.; Hilmi, I.; Banerjee, R.; Chuah, S.W.; Ng, S.C.; Wei, S.C.; Makharia, G.K.; Pisespongsa, P.; Chen, M.H.; Ran, Z.H.; Ye, B.D.; Park, D.I.; Ling, K.L.; Ong, D.; Ahuja, V.; Goh, K.L.; Sollano, J.; Lim, W.C.; Leung, W.K.; Ali, R.A.R.; Wu, D.C.; Ong, E.; Mustaffa, N.; Limsrivilai, J.; Hisamatsu, T.; Yang, S.K.; Ouyang, Q.; Geary, R.; De Silva, J.H.; Rerknimitr, R.; Simadibrata, M.; Abdullah, M.; Leong, R.W. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. Intest Res 2019, 17, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, M.P.; Papamichael, K.; Ward, M.G.; Riviere, P.; Laharie, D.; Paul, S.; Roblin, X. Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response. J Crohns Colitis 2020, 14, 542–556. [Google Scholar] [CrossRef] [PubMed]
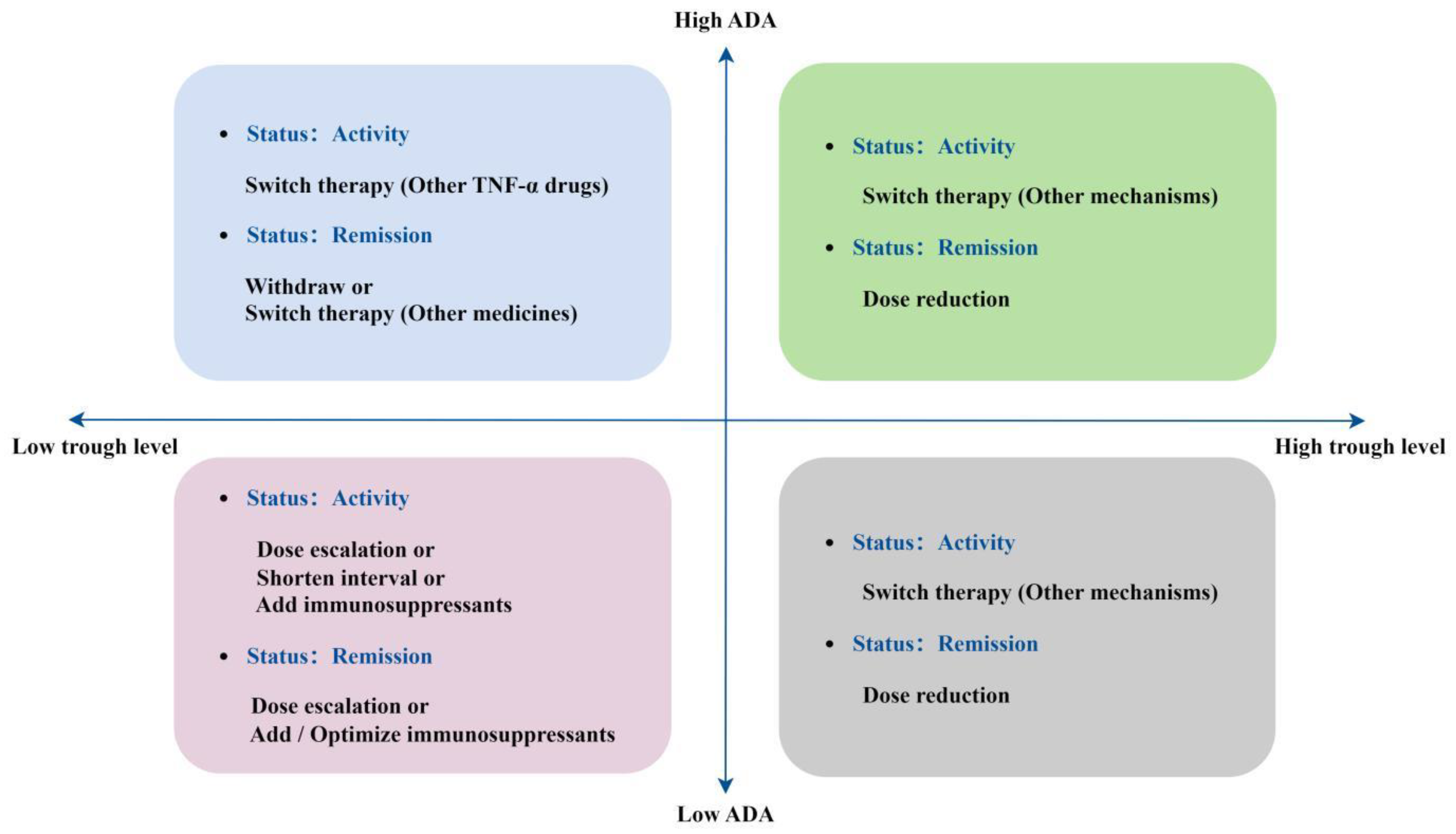
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Vande Casteele, N.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; Jones, J.; Kaplan, G.G.; Sparrow, M.P.; Velayos, F.S.; Ullman, T.; Siegel, C.A. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019, 17, 1655–1668.e3. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Lichtenstein, L.; Assa, A.; Mazor, Y.; Weiss, B.; Levine, A.; Ron, Y.; Kopylov, U.; Bujanover, Y.; Rosenbach, Y.; Ungar, B.; Eliakim, R.; Chowers, Y.; Shamir, R.; Fraser, G.; Dotan, I.; Ben-Horin, S. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015, 13, 522–530.e2. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.J.; Green, Z.; Kolimarala, V.; Beattie, R.M. Inflammatory bowel disease: long-term therapeutic challenges. Expert Rev Gastroenterol Hepatol 2019, 13, 1049–1063. [Google Scholar] [CrossRef]
- Vermeire, S.; Gils, A.; Accossato, P.; Lula, S.; Marren, A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018, 11, 1756283x17750355. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Basile, A.; Palleria, C.; Iannelli, C.; Vero, A.; Giubilei, L.; De Sarro, C.; Suraci, E.; Marasco, R.; Imeneo, M.; Russo, E.; Abenavoli, L.; De Sarro, G.; Luzza, F. Real-life burden of adverse reactions to biological therapy in inflammatory bowel disease: a single-centre prospective case series. Med Pharm Rep 2021, 94, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Solitano, V.; Vuyyuru, S.K.; MacDonald, J.K.; Zayadi, A.; Parker, C.E.; Narula, N.; Peyrin-Biroulet, L.; Danese, S.; Feagan, B.G.; Singh, S.; Ma, C.; Jairath, V. Efficacy and Safety of Advanced Oral Small Molecules for Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J Crohns Colitis 2023, 17, 800–1816. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Burisch, J.; Ellul, P.; Karmiris, K.; Katsanos, K.; Allocca, M.; Bamias, G.; Barreiro-de Acosta, M.; Braithwaite, T.; Greuter, T.; Harwood, C.; Juillerat, P.; Lobaton, T.; Müller-Ladner, U.; Noor, N.; Pellino, G.; Savarino, E.; Schramm, C.; Soriano, A.; Michael Stein, J.; Uzzan, M.; van Rheenen, P.F.; Vavricka, S.R.; Vecchi, M.; Zuily, S.; Kucharzik, T. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis 2024, 18, 1–37. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Mitrev, N.; Vande Casteele, N.; Seow, C.H.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; Corte, C.; Ghaly, S.; Lemberg, D.A.; Kariyawasam, V.; Lewindon, P.; Martin, J.; Mountifield, R.; Radford-Smith, G.; Slobodian, P.; Sparrow, M.; Toong, C.; van Langenberg, D.; Ward, M.G.; Leong, R.W. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017, 46, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Dutt, K.; Vasudevan, A. Therapeutic Drug Monitoring for Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. Medicina (Kaunas) 2024, 60. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Jairath, V.; Wong, E.; Narula, N. Integrating Evidence to Guide Use of Biologics and Small Molecules for Inflammatory Bowel Diseases. Gastroenterology 2024, 166, 396–408.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, C.; Lu, H.; Feng, C.; He, P.; Yang, X.; Xiang, C.; Zuo, J.; Tang, W. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B 2020, 10, 447–461. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; Shou, Z.; Li, J.; Zhu, R.; Fu, Y.; Sarapultsev, A.; Wang, H.; Luo, S.; Zhang, G.; Hu, D. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int J Biol Sci 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Li, M.X.; Li, M.Y.; Lei, J.X.; Wu, Y.Z.; Li, Z.H.; Chen, L.M.; Zhou, C.L.; Su, J.Y.; Huang, G.X.; Huang, X.Q.; Zheng, X.B. Huangqin decoction ameliorates DSS-induced ulcerative colitis: Role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine 2022, 100, 154052. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wei, L.; Wang, N.; Li, X.; Miao, M. Efficacy and safety of adjuvant curcumin therapy in ulcerative colitis: A systematic review and meta-analysis. J Ethnopharmacol 2022, 289, 115041. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, J.; Chen, S.; Chen, J.; Wang, C.; Li, J.; Shi, H.; Yin, X.; Wang, J.; Liu, J.; Song, H.; Zhou, Z.; Jiang, X.; Jiang, W.; Jiang, Y.; Cao, M.; Li, B.; Li, J.; Li, L.; Zhang, Y. Alleviation of colitis by honeysuckle MIR2911 via direct regulation of gut microbiota. J Control Release 2024, 376, 123–137. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.G.; Su, Y.H.; Zhao, R.X.; Song, P.; Li, H.; Cui, X.H.; Gao, H.M.; Zhai, R.X.; Fu, X.J.; Ren, X. Potential activity of Traditional Chinese Medicine against Ulcerative colitis: A review. J Ethnopharmacol 2022, 289, 115084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lin, S.; Feng, W.; Liu, Y.; Song, Z.; Pan, G.; Zhang, Y.; Dai, X.; Ding, X.; Chen, L.; Wang, Y. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization. Front Pharmacol 2022, 13, 999179. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, T.; Wang, B.; Guo, H.; Liu, Q. Chinese Medicine in the Treatment of Ulcerative Colitis: The Mechanisms of Signaling Pathway Regulations. Am J Chin Med 2022, 50, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Sedano, R.; Ma, C.; Jairath, V.; Feagan, B.G. Janus Kinase Inhibitors for the Management of Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2022, 18, 14–27. [Google Scholar]
- Parigi, T.L.; Solitano, V.; Peyrin-Biroulet, L.; Danese, S. Do JAK inhibitors have a realistic future in treating Crohn’s disease? Expert Rev Clin Immunol 2022, 18, 181–183. [Google Scholar] [CrossRef] [PubMed]
| Rank | Source | Count (Percentage) |
Citations(n) |
|---|---|---|---|
| 1 | Inflammatory Bowel Diseases | 579 (9.14%) | 13632 |
| 2 | Journal of Crohns & Colitis | 480 (7.57%) | 17553 |
| 3 | Alimentary Pharmacology & Therapeutics | 250 (3.95%) | 9681 |
| 4 | Clinical Gastroenterology and Hepatology | 178 (2.81%) | 9260 |
| 5 | Digestive Diseases and Sciences | 177 (2.79%) | 2202 |
| 6 | Journal of Pediatric Gastroenterology and Nutrition | 159 (2.51%) | 2168 |
| 7 | Scandinavian Journal of Gastroenterology | 145 (2.29%) | 1855 |
| 8 | Digestive and Liver Disease | 126 (1.99%) | 1916 |
| 9 | World Journal of Gastroenterology | 117 (1.85%) | 2439 |
| 10 | European Journal of Gastroenterology & Hepatology | 115 (1.81%) | 1189 |
| Rank | Title | Journal | Citations | PY | Doi |
|---|---|---|---|---|---|
| 1 | 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management | J Crohns Colitis | 1386 | 2017 | 10.1093/ecco-jcc/jjw168 |
| 2 | Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease | N Engl J Med | 1194 | 2016 | 10.1056/NEJMoa1602773 |
| 3 | Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis | N Engl J Med | 1121 | 2017 | 10.1056/NEJMc1707500 |
| 4 | ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment | J Crohns Colitis | 785 | 2020 | 10.1093/ecco-jcc/jjz180 |
| 5 | European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa | J Eur Acad Dermatol Venereol | 758 | 2015 | 10.1111/jdv.12966 |
| 6 | Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease | J Crohns Colitis | 737 | 2014 | 10.1016/j.crohns.2014.04.005 |
| 7 | Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial | Lancet | 706 | 2016 | 10.1016/S0140-6736(16)31203-X |
| 8 | Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis | N Engl J Med | 702 | 2019 | 10.1056/NEJMoa1900750 |
| 9 | Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis | Gastroenterology | 677 | 2014 | 10.1053/j.gastro.2013.10.052 |
| 10 | Through Concentrations of Infliximab Guide Dosing for Patients With Inflammatory Bowel Disease | Gastroenterology | 667 | 2015 | 10.1053/j.gastro.2015.02.031 |
| Rank | Year | Journal | Title | Citations | Doi |
|---|---|---|---|---|---|
| 1 | 2005 | N Engl J Med | Infliximab for induction and maintenance therapy for ulcerative colitis | 817 | 10.1056/nejmoa050516 |
| 2 | 2002 | Lancet | Maintenance infliximab for Crohn’s disease: the ACCENT I randomized trial | 801 | 10.1016/s0140-6736(02)08512-4 |
| 3 | 2010 | N Engl J Med | Infliximab, azathioprine, or combination therapy for Crohn’s disease | 785 | 10.1056/nejmoa0904492 |
| 4 | 2013 | N Engl J Med | Vedolizumab as induction and maintenance therapy for ulcerative colitis | 644 | 10.1056/nejmoa1215734 |
| 5 | 2013 | N Engl J Med | Vedolizumab as induction and maintenance therapy for Crohn’s disease | 596 | 10.1056/nejmoa1215739 |
| 6 | 2016 | Gastroenterology | Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial | 510 | 10.1053/j.gastro.2006.11.041 |
| 7 | 2016 | N Engl J Med | Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease | 437 | 10.1056/nejmoa1602773 |
| 8 | 2011 | Gastroenterology | Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis | 361 | 10.1053/j.gastro.2011.10.032 |
| 9 | 2005 | Gut | The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications | 357 | 10.1136/gut.2005.082909 |
| 10 | 1987 | N Engl J Med | Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study | 349 | 10.1056/nejm198712243172603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





