Submitted:
22 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Semen Samples
2.2. Experimental Groups
2.3. Motility Analysis
2.4. ProAKAP4 ELISA
2.5. Mitochondrial Membrane Potential
2.6. Statistical Analysis
3. Results
3.1. Correlation between proAKAP4, Motility Descriptors and Mitochondrial Membrane Potential
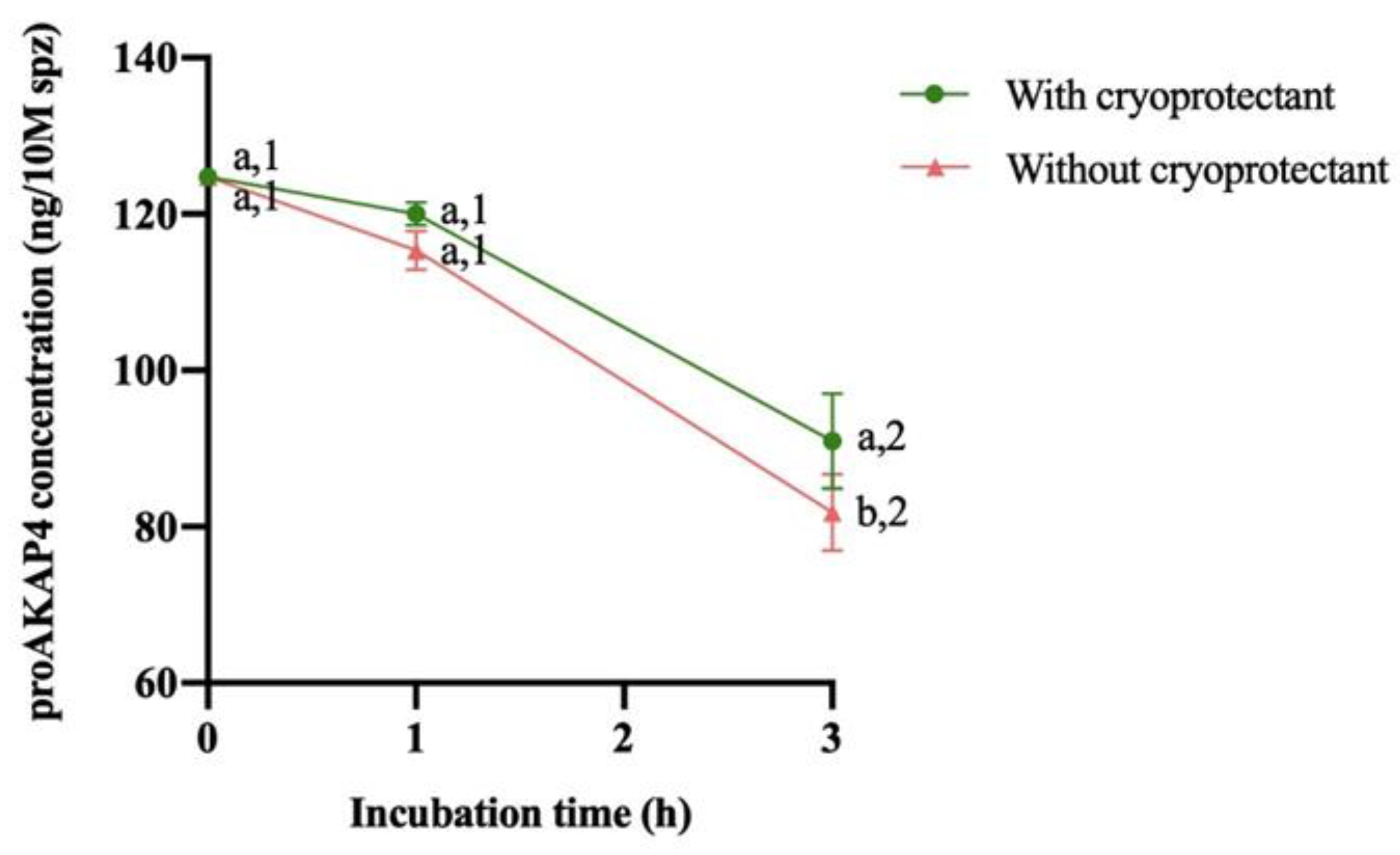
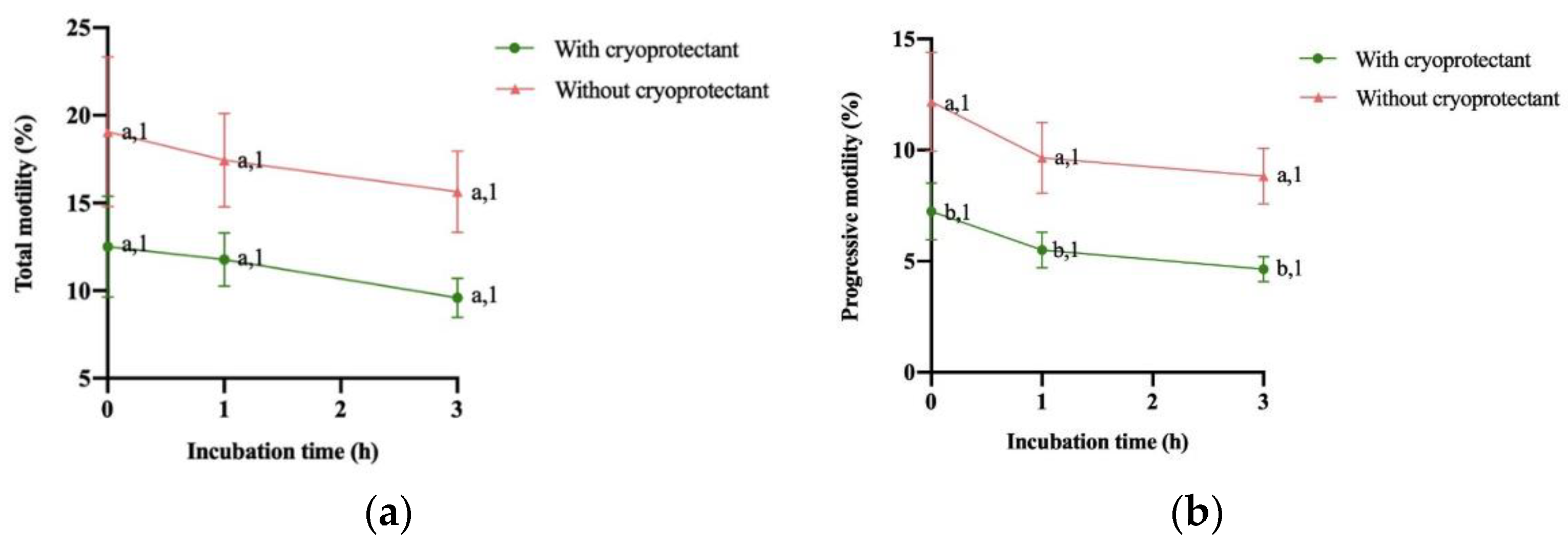
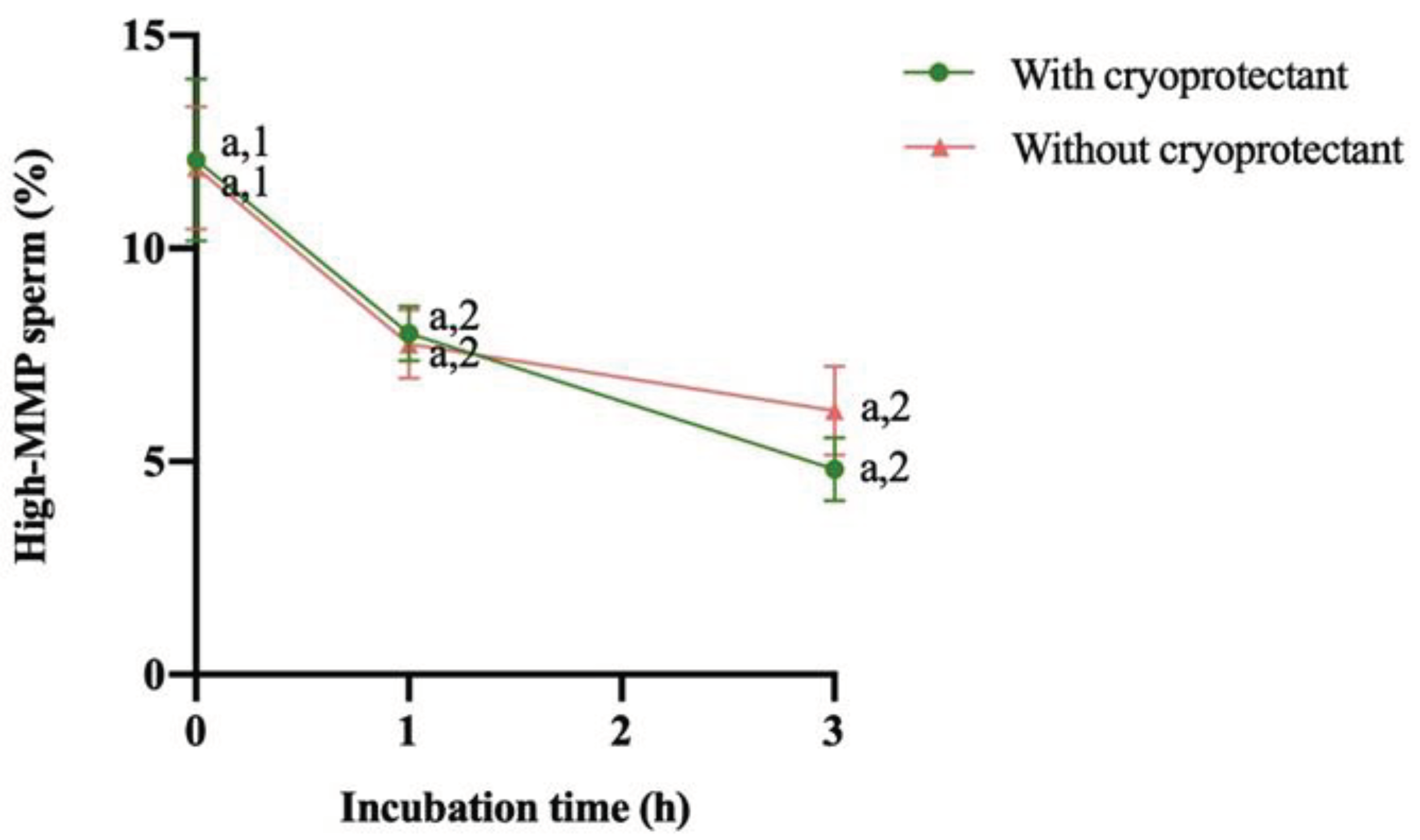
3.2. Evolution of proAKAP4, Motility Descriptors and Mitochondrial Membrane Potential over 3 Hours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson:, P.F. The causes of reduced fertility with cryopreserved semen. Animal Reproduction Science 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.D. Optimizing the use of frozen–thawed equine semen. Theriogenology 2008, 70, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Loomis PR, The equine frozen semen industry. Animal Reproduction Science 2001, 68, 191–200. [CrossRef] [PubMed]
- Barrier Battut, I.; Kempfer, A.; Lemasson, N.; Chevrier, L.; Camugli, S. Prediction of the fertility of stallion frozen-thawed semen using a combination of computer-assisted motility analysis microscopical observation and flow cytometry. Theriogenology 2017, 97, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Kuisma, P.; Andersson, M.; Koskinen, E.; Katila, T. Fertility of frozen-thawed stallion semen cannot be predicted by the currently used laboratory methods. Acta Veterinaria Scandinavica 2006, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Colenbrander, B.; Gadella, B.; Stout, T. The Predictive Value of Semen Analysis in the Evaluation of Stallion Fertility. Reproduction in Domestic Animals 2003, 38, 305–311. [Google Scholar] [CrossRef]
- Vidament, M.; Dupere, A.M.; Julienne, P.; Evian, A.; Noue, P.; Palmer, E. Equine frozen semen: Freezability and fertility field results. Theriogenology 1997, 48, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.D.; Brooks, R.M.; Carrington, J.L.; Cheng, L.; Carrington, A.C.; Porr, C.A.; Splan, R.K. Effects of Pentoxifylline, Caffeine, and Taurine on Post-Thaw Motility and Longevity of Equine Frozen Semen. Journal of Equine Veterinary Science. 2013, 33, 615–621. [Google Scholar] [CrossRef]
- Sanchez, R.; Herrera, C.; Blanco, M.; Rosati, I.; Vlek, J.; Sieme, H. Effect of the dose using equine frozen semen and deep horn insemination in a large scale commercial equine program. Journal of Equine Veterinary Science 2016, 43, S78. [Google Scholar] [CrossRef]
- Giaretta, E.,; Munerato, M., Yeste, M., Galeati, G., Spinaci, M., Tamanini, C., Mari, G., Bucci, D. Implementing an open-access CASA software for the assessment of stallion sperm motility: Relationship with other sperm quality parameters. Anim. Reprod. Sci. 2017, 176, 11–19. [CrossRef]
- Kowalczyk, A.; Czerniawska-Piatkowska, E.; Kuczaj, M. Factors influencing the popularity of artificial insemination of mares in Europe. Animals 2019, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, S.; Briand-Amirat, L.; Dordas-Perpinyà, M.; Ramos Escuredo, Y.; Delcombel, R.; Sergeant, N.; Delehedde, M. ProAKAP4 protein marker: Towards a functional approach to male fertility. Animal Reproduction Science 2022, 247, 107074. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Donnay, I.; Lejeune, J.P.; Serteyn, D. Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine semen: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.A.; Swegen, A.; Baker, M.; Aitken, R.J.; Skerrett-Byrne, D.A.; Silva Rodriguez, A.; Martín-Cano, F.E.; Nixon, B.; Peña, F.J.; Delehedde, M.; Sergeant, N.; Gibb, Z. Mass spectrometry reveals distinct proteomic profiles in high- and low-quality stallion spermatozoa. Reproduction 2020, 160, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Donnay, I.; Lejeune, J.P.; Franck, T.; Serteyn, D. First results about ProAKAP4 concentration in stallion semen after cryopreservation in two different freezing media. Cryobiology 2021, 102, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Yanez-Ortiz, I.; Sergeant, N.; Mevel, V.; Bruyas, J.F.; Catalán, J.; Delehedde, M.; Briand-Amirat, L.; Miró, J. ProAKAP4 Concentration Is Related to Sperm Motility and Motile Sperm Subpopulations in Frozen–Thawed Horse Semen. Animals 2022, 12, 3417. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.Z.Y.; Dilger, A.; Bryan, E.; Bytnar, G.; Choi, S.; Cook, K.; Kline, K. Preliminary Study of Effects of Centrifugation of Stallion Semen on Motility and Pro-AKAP4 Concentration over 72 Hours Post-collection. Journal of Agricultural Science and Technology 2022, 12, 65–72. [Google Scholar]
- Delehedde, M.; Carracedo, S.; Selleslagh, M.; Eddarkaoui, S.; Amirat-Briand, L.; Sergeant, N. ProAKAP4 polypeptide as a biomarker of sperm functionality and male fertility disorders. Int J Gynecol and Reprod Sci 2019, 2, 13–19. [Google Scholar]
- Johnson, L.R.; Foster, J.A.; Haig-Ladewig, L.; Vanscoy, H.; Rubin, C.S.; Moss, S.B.; Gerton, G.L. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Developmental biology 1997, 192, 340–350. [Google Scholar] [CrossRef]
- Turner, R.M.; Johnson, L.R.; Haig-Ladewig, L.; Gerton, G.L.; Moss, S.B. An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82: Genomic organization, protein kinase A-RII binding, and distribution of the precursor in the sperm tail. Journal of Biological Chemistry 1998, 273, 32135–32141. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, H.; Pask, A.J.; O'Brien, D.A.; Shaw, G.; Renfree, M.B. A-kinase anchoring protein 4 has a conserved role in mammalian spermatogenesis. Reproduction 2009, 137, 645. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Bernstein, I.R.; Cafe, S.L.; Delehedde, M.; Sergeant, N.; Anderson, A.L.; Bromfield, E.G. A kinase anchor protein 4 is vulnerable to oxidative adduction in male germ cells. Frontiers in Cell and Developmental Biology 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Sergeant, N.; Ruelle, I.; Bruyas, J.F.; Charreaux, F.; Michaud, S.; Carracedo, S.; Catalán, J.; Miró, J.; Delehedde, M.; Briand-Amirat, L. ProAKAP4 Semen Concentrations as a Valuable Marker Protein of Post-Thawed Semen Quality and Bull Fertility: A Retrospective Study. Vet. Sci 2022, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinyà, M.; Sergeant, N. , Yánez-Ortiz, I., Mevel, V., Catalán, J., Bruyas, J.F., Briand-Amirat, L., Miro, J. ProAKAP4 as a motility long-lasting marker in Catalan donkey spermatozoa. Anim Reprod Sci 2024, 262, 107427. [Google Scholar] [CrossRef] [PubMed]
- Le Couazer, D.; Delehedde, M.; Ruelle, I.; Sergeant, N.; Michaud, S.; Briand, L.; Bencharif, D. ProAKAP4 as a valuable marker to assess sperm quality in dogs. Reprod. Domest. Anim 2019, 54, 9192. [Google Scholar]
- Gardela, J.; Ruiz-Conca, M.; Palomares, A.; Olvera-Maneu, S.; García-Calvo, L.; López-Béjar, M.; Martínez-Pastor, F.; Álvarez-Rodríguez, M. Effect of Honey, Coenzyme Q10, and-Carotene-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals 2023, 13, 2392. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Carracedo, S.; Delehedde, M.; Sergeant, N.; Skidmore, L. Identification of proAKAP4 in Dromedary sperm and their correlation with monthly semen parameters. Reprod. Fertil 2021, 2, 268–279. [Google Scholar] [CrossRef]
- Bastan, I.; Akcay, E. Quality assessment of frozen bull semen with the precursor A-kinase anchor protein 4 biomarker. Andrologia 2021, 53, e14164. [Google Scholar] [CrossRef]
- Marques de Almeida, A.B.; Hidalgo, M.M.T.; de Moraes, F.L.Z.; Trautwein, L.G.C.; de Fátima Schnitzer, J.; dos Santos Silva, L.A.; Rizzoto, G.; Pinheiro Ferreira, J.C.; Mello Martins, M.I. The proAKAP4 concentrations in Nelore bull sperm and their relation to FTAI conception rate results. Anim. Reprod. Sci. 2022, 247, 107156. [Google Scholar] [CrossRef]
- Fatet, A.; Sergeant, N.; Dordas-Perpinyà, M.; Drouet, B.; Ponthoreau, O.; Carracedo, S.; Bruyas, J.F.; Thorin, C.; Dlehedde, M.; Briand-Amirat, L. Sperm-specific protein proAKAP4 as a marker to evaluate sperm quality and fertility of Alpine and Saanen Bucks. Proceedings of the 25th Annual Conference of the European Society for Domestic Animal Reproduction (ESDAR). Reprod. Domest. Anim 2022, 57, 79–79. [Google Scholar]
- Sergeant, N.; Briand-Amirat, L.; Bencharif, D.; Delehedde, M. The Sperm Specific Protein Proakap4 as an Innovative Marker to Evaluate Sperm Quality and Fertility. J. Dairy Vet. Sci. 2019, 11, 555803. [Google Scholar] [CrossRef]
- Boersma, A.; Primus, J.; Wagner, B.; Broukal, V.; Andersen, L.; Pachner, B.; Dahlhoff, M.; Rülicke, T.; Auer, K.E. Influence of sperm cryopreservation on sperm motility and proAKAP4 concentration in mice. Reprod Med Biol 2022, 21, e12480. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.Y.; Chandanee, M.R.; Dissanayake, W.M.N.; Lee, S.M.; Yi, Y.J. Comparison of fertility competence in toll-like receptor 4 (TLR4)-knock out male mice fed a high-fat diet. J Biomed Transl Res 2023, 24, 41–52. [Google Scholar] [CrossRef]
- Sigala, J. Qualité du protéome du spermatozoïde humain et infertilité (Doctoral thesis) 2016, Université du Droit et de la Santé-Lille II, France. http://www.theses.fr/2016LIL2S039/document.
- Jumeau, F.; Sigala, J.; Dossou-Gbete, F.; Frimat, K.; Barbotin, A.L.; Buée, L.; Béhal, H.; Sergeant, N.; Mitchell, V. A-kinase anchor protein 4 precursors (pro-AKAP4) in human sperm. Andrology 2018, 6, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Gacem S, Valverde A, Catalan J, Yánez-Ortiz I, Soler C, Miró J. A new approach of sperm motility subpopulation structure in donkey and horse. Frontiers in Veterinary Science 2021, 8, 651477. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Gałeska, E.; Bubel, A. The concentration of proAKAP4 and other indicators of cryopotential of spermatozoa cryopreserved in extender with holothuroidea extract addition. Animals 2022, 12, 521. [Google Scholar] [CrossRef]
- Love, C.C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology 2011, 76, 547–557. [Google Scholar] [CrossRef]
- Johannisson, A.; Cojkić, A.; Morrell, J.M. The relationship between sperm quality parameters and stallion fertility. Journal of Equine Veterinary Science 2023, 125, 10459. [Google Scholar] [CrossRef]
- Puglisi, R.; Pozzi, A.; Foglio, L.; Spanò, M.; Grollino, M.G.; Bongioni, G. The usefulness of combining traditional sperm assessments with in vitro heterospermic insemination to identify bulls of low fertility as estimated in vivo. Anim. Reprod. Sci 2012, 132, 17–28. [Google Scholar] [CrossRef]
- Farrell, P.B.; Presicce, G.A.; Brockett, C.C.; Foote, R.H. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology 1998, 49, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Polichronopoulos, T.; Gáspárdy, A.; Solti, L. Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Vet. Hung 2015, 63, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Len, J.A.; Jenkins, J.A.; Eilts, B.E.; Paccamonti, D.L.; Lyle, S.K.; Hosgood, G. Centrifugation has minimal effects on motility, viability, and acrosome integrity of equine sperm. Theriogenology 2008, 70, 582–583. [Google Scholar] [CrossRef]
- Cheng, F-P. ; Wu, J-T.; Chan, J-P.; Wang, J-S.; Fung, H-P.; Colenbrander, B.; Tung, K-C. The effect of different extenders on post-thaw sperm survival, acrosomal integrity and longevity in cryopreserved semen of Formosan Sika deer and Formosan Sambar deer. Theriogenology 2004, 61, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Shaliutina-Kolešová, A.; Dietrich, M.; Xian, M.; Nian, R. Seminal plasma transferrin effects on cryopreserved common carp Cyprinus carpio sperm and comparison with bovine serum albumin and antifreeze proteins. Animal Reproduction Science. 2019, 204, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, U.; Axnér, E. Epididymal and ejaculated cat spermatozoa are resistant to cold shock but egg yolk promotes sperm longevity during cold storage at 4°C. Theriogenology 2007, 67, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, F.; Paris, M.; Brent Briggs, M.; Farstad, W.K.; Paris, D. A two-step dilution tris-egg yolk extender containing Equex STM significantly improves sperm cryopreservation in the African wild dog (Lycaon pictus). Cryobiology 2018, 80, 18–25. [Google Scholar] [CrossRef]
- Catalán, J.; Llavanera, M.; Bonilla-Correal, S.S.; Papas, M.; Gacem, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. Irradiating frozen-thawed stallion sperm with red-light increases their resilience to withstand post-thaw incubation at 38ºC. Theriogenology 2020, 157, 85–05. [Google Scholar] [CrossRef]
- Catalán, J.; Papas, M.; Gacem, S.; Mateo-Orero, Y.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Red-light irradiation of horse spermatozoa increases mitochondrial activity and motility through changes in the motile sperm subpopulation structure. Biology 2020, 9, 254. [Google Scholar] [CrossRef]
| 0 hours | 1 hour | 3 hours | ||||
| r | p | r | p | r | p | |
| TM | 0.421 | 0.018* | 0.435 | 0.027* | 0.496 | 0.024* |
| PM | 0.352 | 0.041* | 0.367 | 0.039* | 0.402 | 0.027* |
| VCL | 0.666 | 0.000* | 0.211 | 0.247 | 0.222 | 0.223 |
| VSL | 0.440 | 0.012* | 0.139 | 0.449 | 0.120 | 0.511 |
| VAP | 0.527 | 0.002* | 0.160 | 0.382 | 0.105 | 0.567 |
| LIN | -0.273 | 0.131 | -0.069 | 0.707 | -0.243 | 0.181 |
| STR | -0.151 | 0.408 | -0.168 | 0.359 | -0.020 | 0.914 |
| ALH | 0.570 | 0.001* | 0.288 | 0.110 | 0.213 | 0.241 |
| BCF | 0.060 | 0.744 | -0.205 | 0.260 | 0.122 | 0.505 |
| JC1 | 0.190 | 0.298 | 0.135 | 0.461 | 0.338 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





