Submitted:
21 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
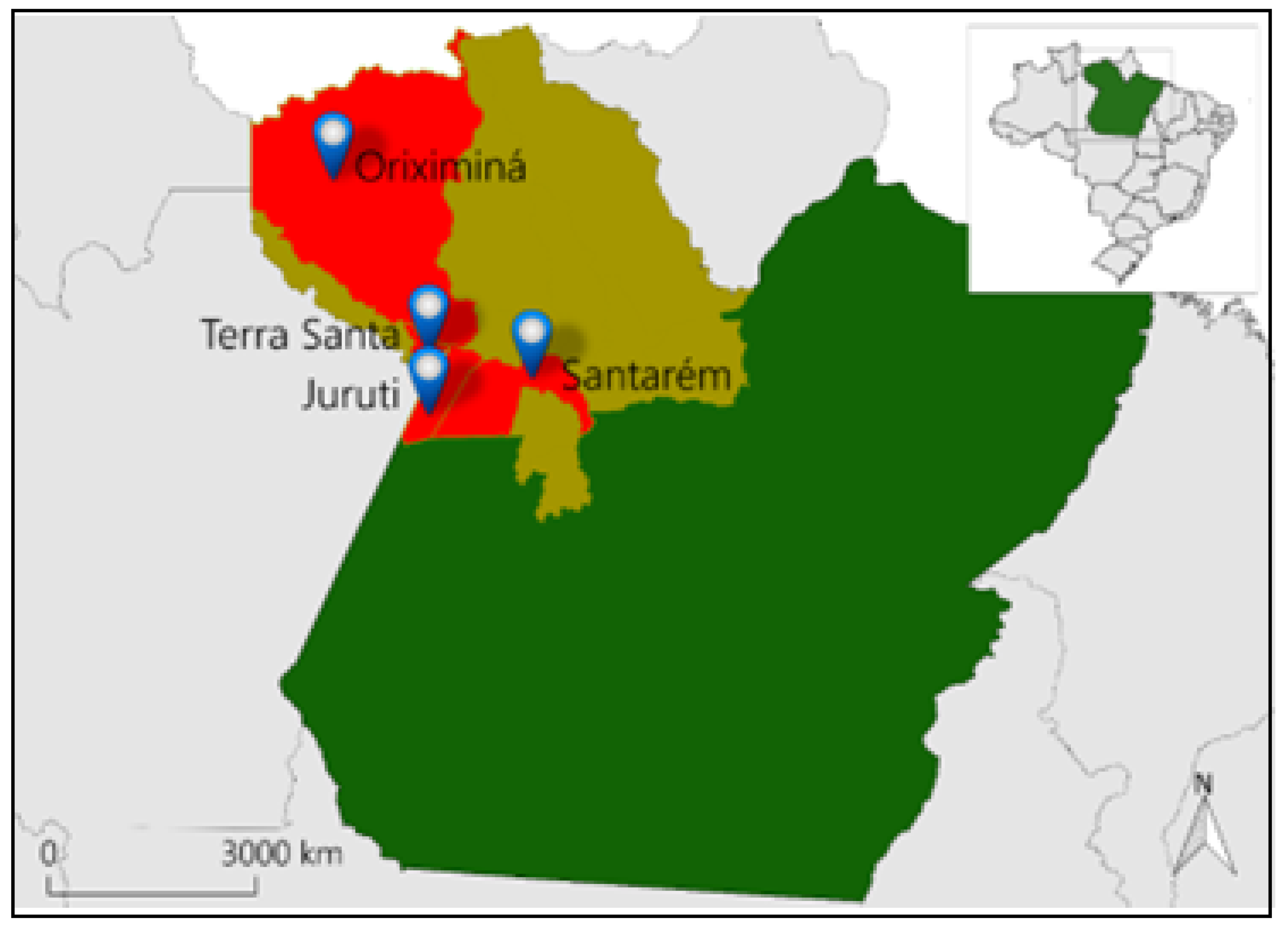
2.1. Plant Material
2.2. Obtaining and Analyzing Volatile Concentrates
2.3. Multivariate Statistical Analysis
3. Results and Discussion
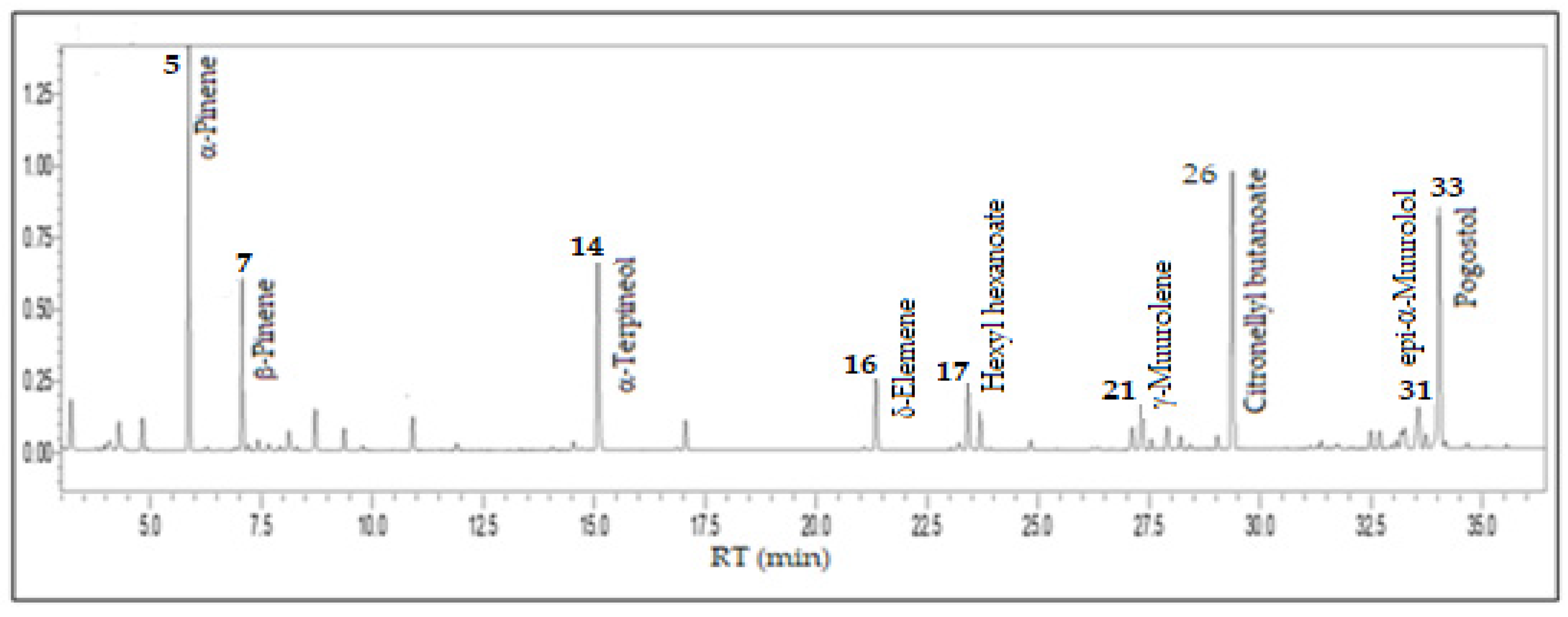
3.1. Eugenia stipitata McVaugh - Myrtaceae

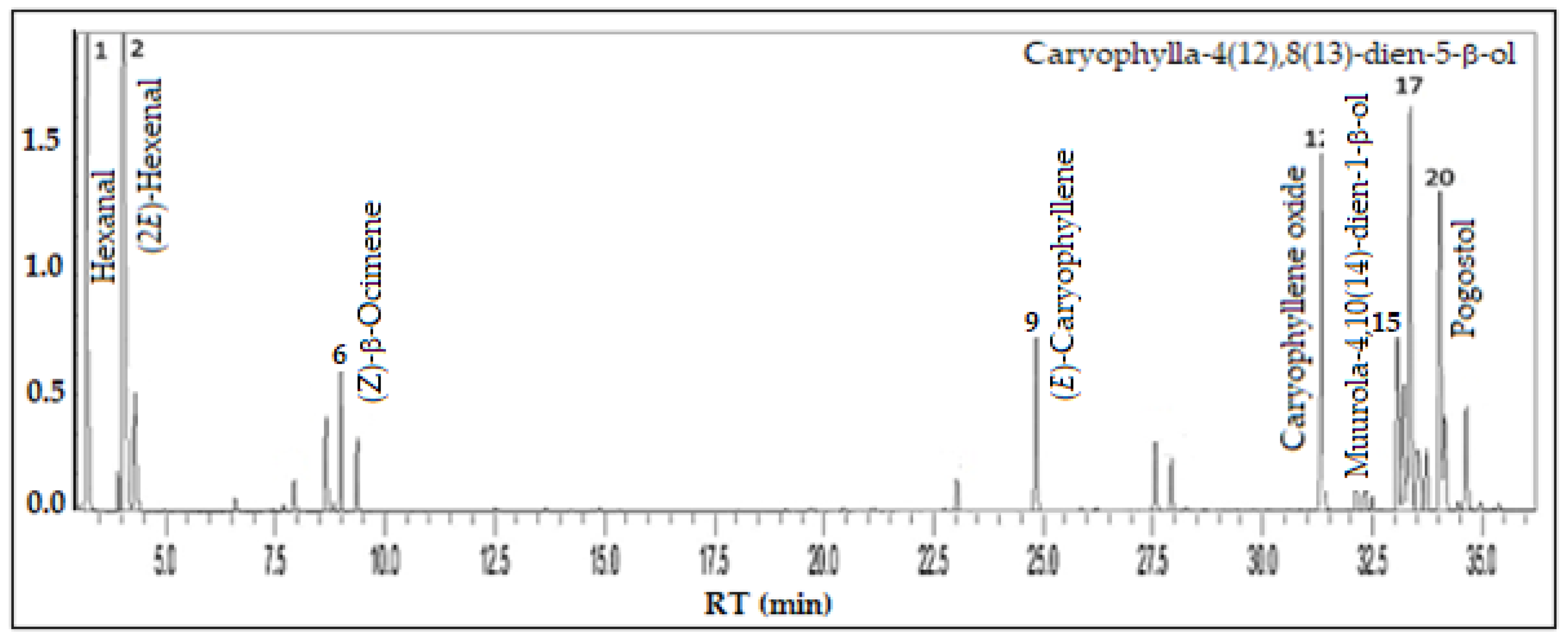
3.2. Eugenia uniflora L. - Myrtaceae

3.3. Myrciaria dubia (Kunth) McVaugh - Myrtaceae

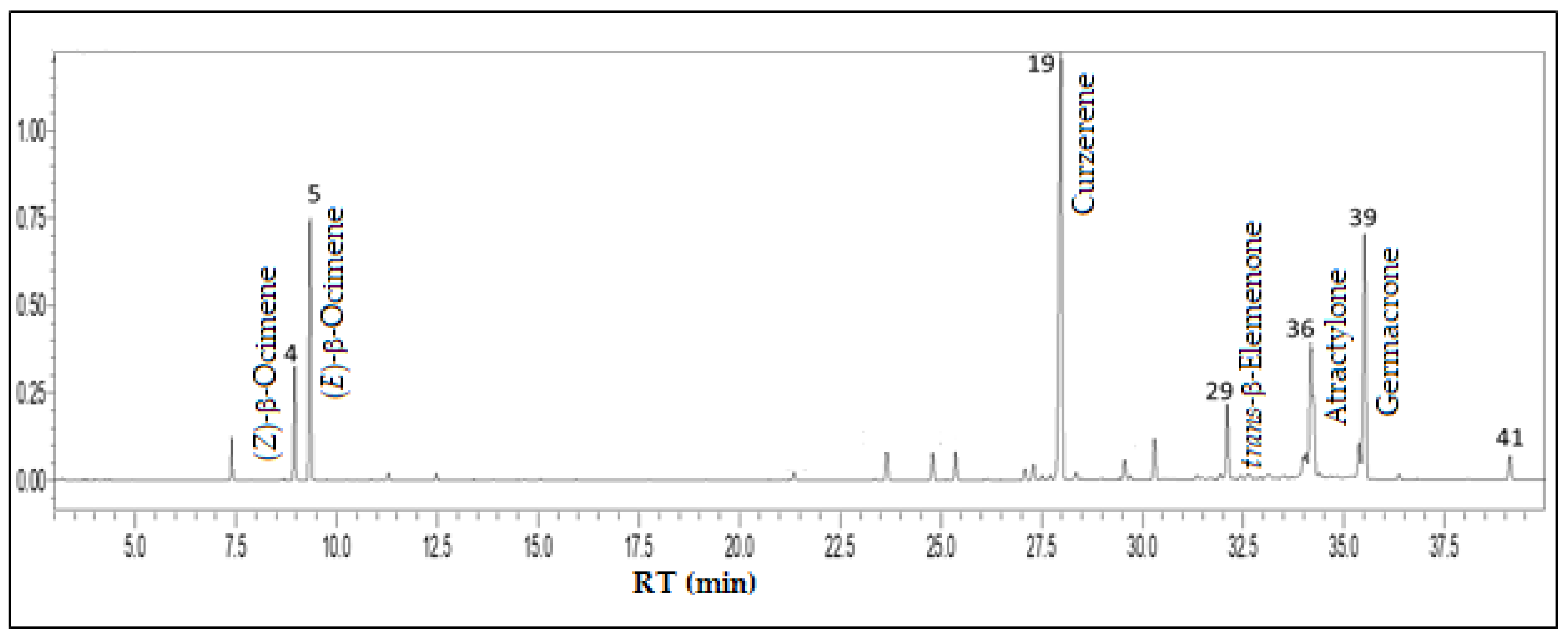
3.4. Psidium guajava L. - Myrtaceae

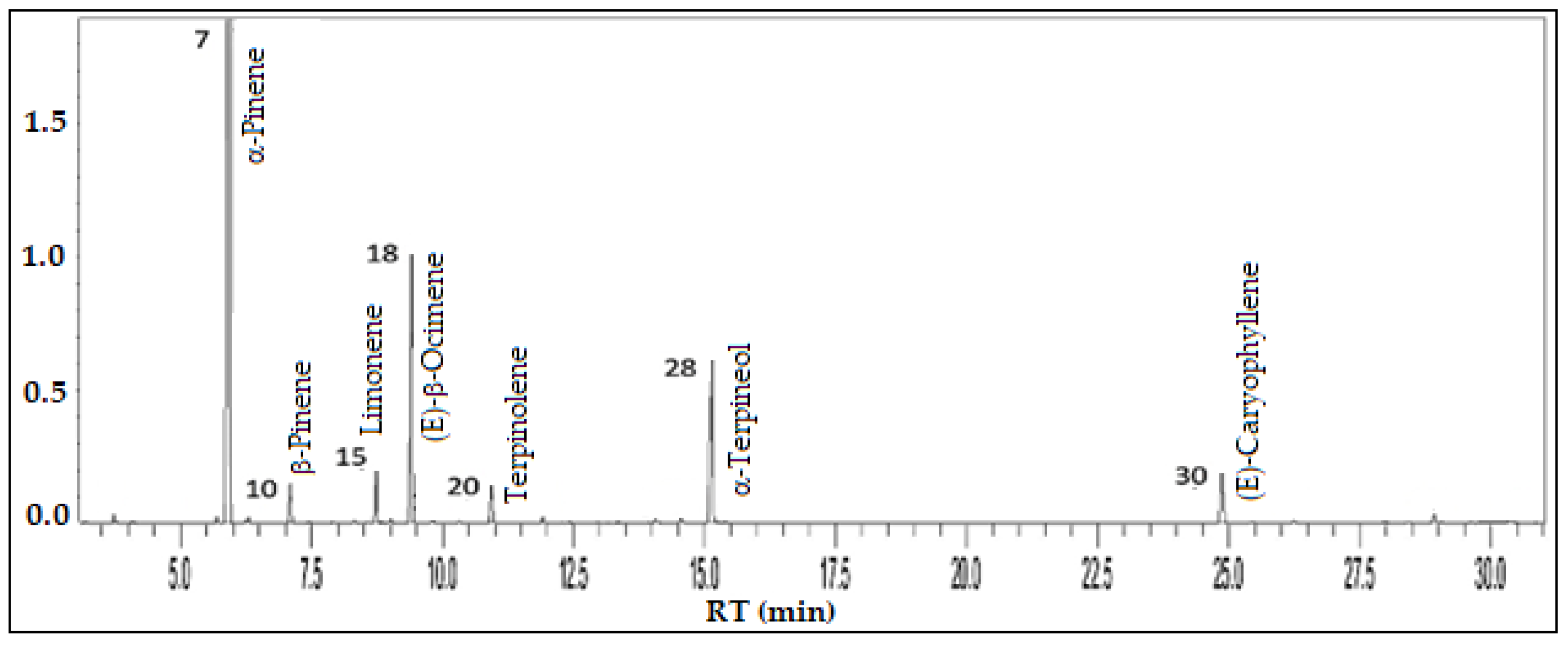
3.5. Psidium guineense Sw. - Myrtaceae

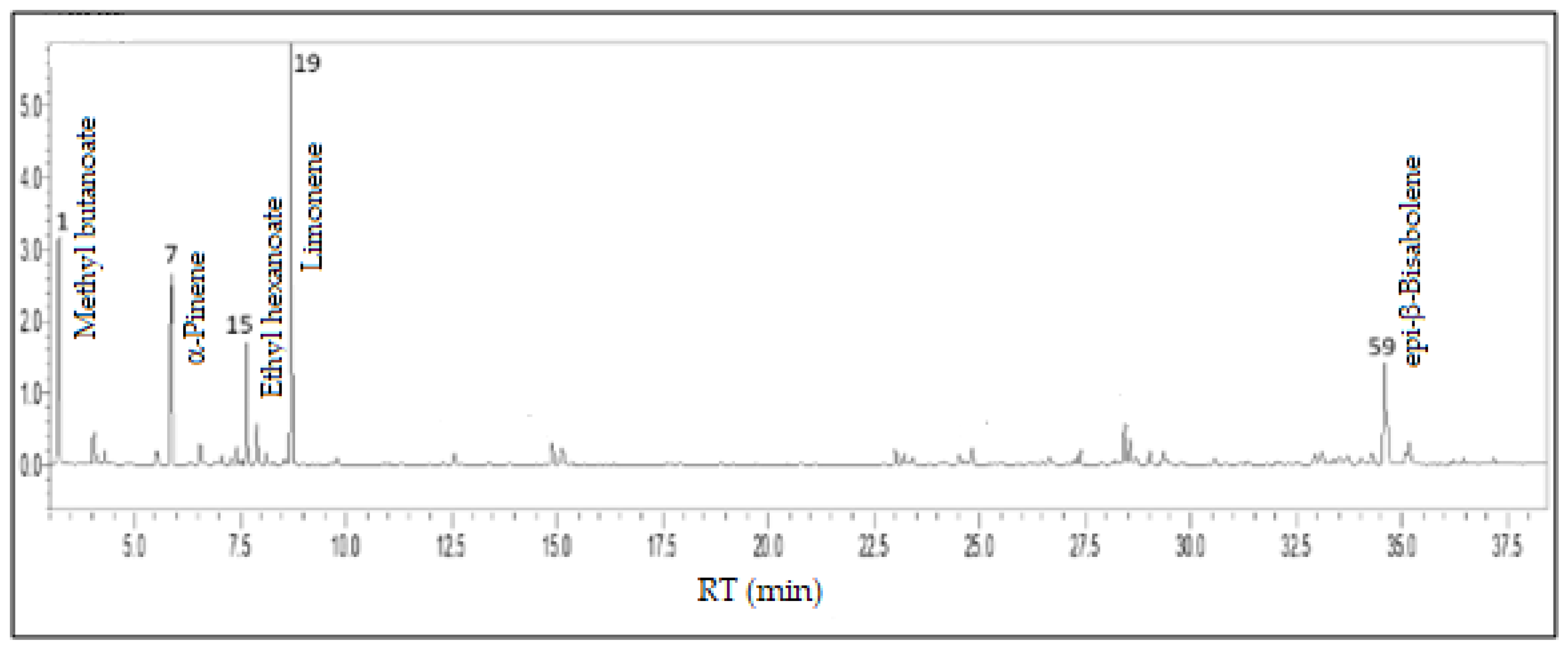
3.6. Fruit Scent: Chemistry and Ecological Function
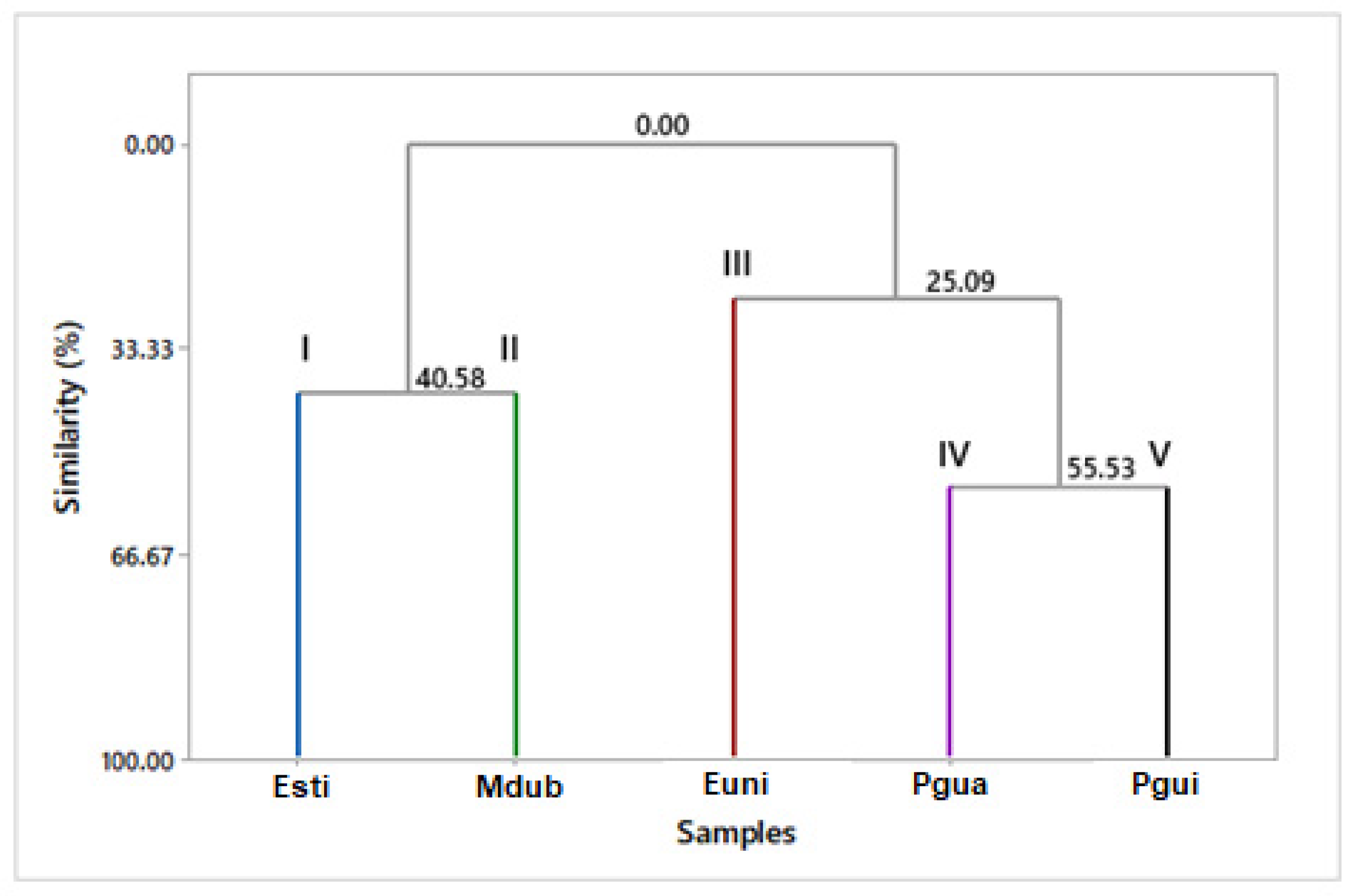
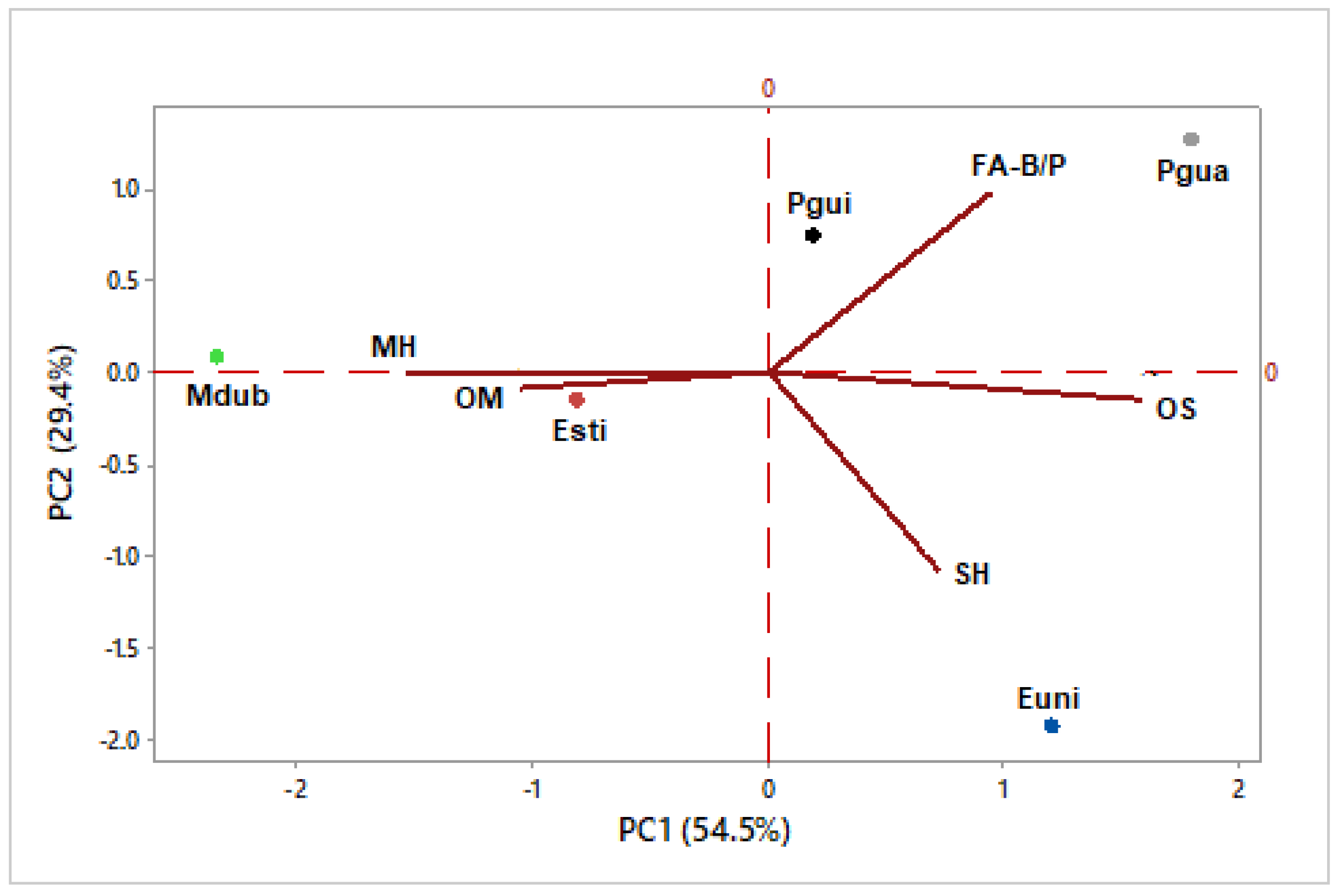
3.7. Multivariate Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homma, A.K.O. Extrativismo vegetal ou plantio: Qual a opção para a Amazônia? In Extrativismo vegetal na Amazônia: História, ecologia, economia e domesticação; Homma, A.K.O., Ed.; Embrapa: Brasília, Brazil, 2014.
- Freitas, D.G.C.; Mattietto, R.A. Ideal sweetness of mixed juices from Amazon fruits. Cienc. Tecnol. Aliment. 2013, 33, 148-154. [CrossRef]
- Fachinello, J. C.; Nachtigal, J. C. Introdução à fruticultura. In Fruticultura: Fundamentos e práticas; Fachinello, J. C.; Nachtigal, J. C.; Kersten, E., Eds., 2. ed.; Embrapa Clima Temperado: Pelotas, Brazil, 2009.
- Clerici, M.T.P.S; Carvalho-Silva, L.B. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Res. Int. 2011, 44, 1658-1670. [CrossRef]
- Cavalcante, P.B. Frutas comestíveis da Amazônia, 7th ed.; Museu Paraense Emílio Goeldi, Coleção Adolpho Ducke: Belém, Brazil, 2010.
- Bicas, J.L.; Molina, G.; Dionísio, A.P.; Barros, F.F.C.; Wagner, R.; Marostica Jr., M.R.; Pastore, G.M. Volatile constituent of exotic fruits from Brazil, Food Res. Int. 2011, 44, 1843-1855. [CrossRef]
- Neves, L.C.; de Campos, A.J.; Benedette, R.M.; Tosin, J.M.; Chagas, E.A. Characterization of the antioxidant capacity of native fruits from the Brazilian Amazon Region. Rev. Bras. Frutic. 2002, 34, 1165-1173. [CrossRef]
- IBGE 2019. https://biblioteca.ibge.gov.br/index.php/biblioteca-catalogo?id=22269&view=detalhes, accessed February 18, 2024.
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200-8229. [CrossRef]
- Likens, S.T.; Nickerson, G.B. Detection of certain hop oil constituents in brewing products. Am. Soc. Brew. Chem. Proc. 1964, 22, 5-13. [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation, Carol Stream, IL, USA, 2007.
- Mondello, L. FFNSC 2 - Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley and Sons Inc, New York, NY, USA, 2011.
- Van de Doll, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [CrossRef]
- Minitab Statistical Software. https://minitab.com/en-us/, accessed February 2024.
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: A tree reference and selection guide, version 4.0., 2009. http://www.worldagroforestry.org/treedb2/aftpdfs/eugenia_stipitata.pdf. Accessed February 2024.
- Franco, M.R.B.; Shibamoto, T. Volatile composition of some Brazilian fruits: Umbu-caja (Spondias citherea), Camu-camu (Myrciaria dubia), Araçá-boi (Eugenia stipitata), and Cupuaçu (Theobroma grandiflorum). J. Agric. Food Chem. 2000, 48, 1263-1265. [CrossRef]
- Pino, J.A.; Quijano, C.E. Volatile compounds of Arazá fruit (Eugenia stipitata McVaught). Rev. Cenic Cienc. Quim. 2007, 38, 363-366. http://www.redalyc.org/articulo.oa?id=181621616001.
- Medeiros, J.R.; Medeiros, N.; Medeiros, H.; Davin. L.B.; Lewis, N.G. Composition of the bioactive essential oils from the leaves of Eugenia stipitata McVaugh ssp. sororia from the Azores. J. Essent. Oil Res. 2003, 15, 293-295. [CrossRef]
- dos Santos, C.R.B.; Sampaio, M.G.V.; Vandesmet, L.C.S.; dos Santos, B.S.; de Menezes, S.A.; Portela, B.Y.M.; Gomes, D.W.R.; Correia, M.T.S.; Gomez, M.C.V.; Menezes, I.R.A.; da Silva, M.V. Chemical composition and biological activities of the essential oil from Eugenia stipitata McVaught leaves. Nat. Prod. Res. 2023, 37, 3844-3850. [CrossRef]
- Missouri Botanical Garden. https://www.tropicos.org/name/22101634, accessed February 2024.
- Pino, J.A.; Bello, A.; Urquiola, A.; Aguero, J.; Marbot, R. Fruit volatiles of Cayena Cherry (Eugenia uniflora L.) from Cuba. J. Essent. Oil Res. 2003, 15, 70-71. [CrossRef]
- Oliveira, A.L.; Lopes, R.B.; Cabral, F.A.; Eberlin, M.N. Volatile compounds from Pitanga fruit (Eugenia uniflora L.). Food.Chem. 2006, 99, 1-5. [CrossRef]
- Marin, R.; Apel, M.A.; Limberger, R.P.; Raseira, M.C.B.; Pereira, J.F.M.; Zuanazzi, J.A.S.; Henriques, A.T. Volatile Components and Antioxidant Activity from some Myrtaceous Fruits cultivated in Southern Brazil. Lat. Am. J. Pharm. 2008, 27, 172-177.
- Ogunwande, I.A.; Olawore, N.O.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on essential oil composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147-152. [CrossRef]
- Maia. J.G.S.; Andrade, E.H.A.; da Silva, M.H.L.; Zoghbi, M.G.B. a new chemotype of Eugenia uniflora L. from North Brazil. J. Essent. Oil Res. 1999, 11, 727-729. [CrossRef]
- Costa, D.P.; Alves Filho, L.G.; Silva, L.M.A.; Santos, S.C.; Passos, X.S.; Silva, M.R.R.; Seraphin, J.C.; Ferri, P.H. Influence of fruit biotypes on the chemical composition and antifungal activity of the essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2010, 21, 851-858. [CrossRef]
- Flora do Brasil. Myrciaria, 2020. Jardim Botânico do Rio de Janeiro. Available in: http://floradoBrasil,jbrj.gov.br/reflora/floradobrasil/FB24032, access in January 2024.
- Quijano, C.E.; Pino, J.A. Analysis of volatile compounds of camu-camu [Myrciaria dubia (HBK) McVaught] fruit isolated by different methods. J. Essent. Oil Res. 2007, 19, 527-533. [CrossRef]
- García-Chacón, J.M.; Forero, D.P.; Peterson, D.G.; Osorio, C. Aroma characterization and in vitro antihypertensive activity of Amazonian Camu-camu (Myrciaria dubia) fruit. J. Food Bioact. 2023, 21, 55-61. [CrossRef]
- da Costa, J.S.; Andrade, W.M.S.; de Figueiredo, R.O.; Santos, P.V.L.: Freitas, J.J.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Chemical composition and variability of the volatile components of Myrciaria species growing in the Amazon region. Molecules 2022, 27, 2234. [CrossRef]
- Missouri Botanical Garden. https://www.tropicos.org/name/22101794, accessed February 2024.
- Mahattanatawee, K.; Goodner, K.L.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc. Fla. State Hort. Soc. 2005, 118, 414-418.
- Chen, H.-C.; Sheu, M.-J.; Lin, L.-Y.; Wu, C.-M. Chemical composition of the leaf essential oil of Psidium guajava L. from Taiwan. J. Essent. Oil Res. 2006, 19, 345-347. [CrossRef]
- Pino, J.A.; Bent, L. Odour-active compounds in guava (Psidium guajava L. cv. Red Suprema). J. Sci. Food Agric. 2013, 93, 3114-3120. [CrossRef]
- Soares, F.D.; Pereira, T.; Marques, M.O.M.; Monteiro, A.R. Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007, 100, 15-21. [CrossRef]
- El-Ahmady, S.H.; Ashour, M.L.; Wink, M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013, 25, 475-481. [CrossRef]
- Missouri Botanical Garden. https://www.tropicos.org/name/22102032, accessed February 2024.
- Peralta-Bohorquezo, A.F.; Parada, F.; Quijano, C.E.; Pino, J.A. Analysis of volatile compounds of Sour Guava (Psidium guineense Swartz) fruit. J. Essent. Oil Res. 2011, 22, 493-498. [CrossRef]
- Abrao, F.Y.; da Costa, H.M.; Fiuza, T.S.; Romano, C.A.; Ferreira, H.D.; da Cunha, L.C.; Oliveira Neto, J.R.; de Paula, J.R. Anatomical study of the leaves and evaluation of the chemical composition of the volatile oils from Psidium guineense Swartz leaves and fruits. Res., Soc. Dev. 2021, 10, e49110615929. [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile leaf oils of American Myrtaceae. III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., P. guineense Sw., and P. sartorianum (Berg) Niedenzu. J. Essent. Oil Res. 1995, 7, 187-190. [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; Formagio, A.S.N. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2017, 210, 351-358. [CrossRef]
- da Silva, J.D.; Luz, A.I.R.; da Silva, M.H.L.; Andrade, E.H.A.; Zoghbi, M.G.B.; Maia, J.G.S. Essential oils of the leaves and stems of four Psidium spp. Flav. Frag. J. 2003, 18, 240-243. [CrossRef]
- Figueiredo. P.L.B.; Silva, R.C.; da Silva, J.K.R.; Suemitsu, C.; Mourão, R.H.V.; Maia, J.G.S. Chemical variability in the essential oil of leaves of Araçá (Psidium guineense Sw.), with occurrence in the Amazon. Chem. Cent. J. 2018, 12, 42. [CrossRef]
- Silva, R.C.; da Costa, J.S.; Figueiredo, R.O.; Setzer, W.N.; da Silva, J.K.R.; Mmaia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and sesquiterpenes of essential oils from Psidium species and their biological properties. Molecules 2021, 26, 965. [CrossRef]
- Nevo, O.; Aysse, M. Fruit scent: Biochemistry, ecological function, and evolution. In Co-evolution of secondary metabolites; Mérillon, J.-M.; Ramawat, K.G., Eds.; Springer Nature: Switzerland, pp. 403-425, 2020.
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, Compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [CrossRef]
- Romero, H.; Pott, D.M.; Vallarino, J.G.; Osorio, S. (2021). Metabolomics based evaluation of crop quality changes as a consequence of climate change. Metabolites 2021, 11, 461. [CrossRef]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815-819. [CrossRef]
| Constituents | RICal | RILit | % |
| Ethyl butanoate | 799 | 802a | 2.1 |
| (3Z)-Hexenol | 848 | 850a | 0.6 |
| n-Hexanol | 860 | 863a | 1.1 |
| 2-Heptanol | 894 | 891b | 1.2 |
| α-Pinene | 932 | 932a | 17.4 |
| Hexanoic acid (Caproic acid) | 970 | 967a | 0.1 |
| β-Pinene | 976 | 974a | 6.8 |
| Myrcene | 989 | 988a | 0.4 |
| Hexyl acetate | 1011 | 1007a | 0.8 |
| Limonene | 1027 | 1024a | 1.7 |
| (E)-β-Ocimene | 1045 | 1044a | 0.8 |
| p-Mentha-2,4(8)-diene | 1087 | 1085a | 1.4 |
| Terpinen-4-ol | 1176 | 1174a | 0.3 |
| α-Terpineol | 1189 | 1186a | 9.6 |
| n-Hexyl 2-methyl butanoate | 1236 | 1233a | 1.4 |
| δ-Elemene | 1336 | 1335 | 4.1 |
| Hexyl hexanoate | 1385 | 1382a | 3.5 |
| β-Elemene | 1392 | 1389a | 1.8 |
| (E)-Caryophyllene | 1419 | 1417a | 0.4 |
| β-Chamigrene | 1475 | 1476a | 1.2 |
| γ-Muurolene | 1481 | 1478a | 2.6 |
| Germacrene D | 1486 | 1484a | 0.5 |
| β-Selinene | 1492 | 1489a | 1.3 |
| (Z)-α-Bisabolene | 1502 | 1506a | 0.5 |
| δ-Cadinene | 1523 | 1522a | 0.7 |
| Citronellyl butanoate | 1532 | 1530a | 15.6 |
| Caryophyllene oxide | 1583 | 1582a | 0.4 |
| Junenol | 1618 | 1618a | 1.9 |
| 1-epi-Cubenol | 1628 | 1627a | 0.3 |
| γ-Eudesmol | 1631 | 1630a | 0.8 |
| epi-α-Muurolol | 1642 | 1640a | 3.2 |
| Cubenol | 1646 | 1645a | 0.8 |
| Pogostol | 1654 | 1651a | 13.5 |
| Monoterpene hydrocarbons Oxygenated monoterpenes Sesquiterpene hydrocarbons Oxygenated sesquiterpenes Fatty acid derivatives |
28.5 | ||
| 25.5 | |||
| 13.1 | |||
| 20.9 | |||
| 10.8 | |||
| Total (%) | 98.8 | ||
| Constituents | RICal | RILit | % |
| n-Octane | 797 | 800a | 0.1 |
| Myrcene | 989 | 988a | 1.6 |
| Limonene | 1027 | 1024a | 0.1 |
| (Z)-β-Ocimene | 1034 | 1032a | 4.6 |
| (E)-β-Ocimene | 1045 | 1044a | 11.1 |
| Linalool | 1098 | 1095a | 0.3 |
| allo-Ocimene | 1127 | 1128a | 0.1 |
| δ-Terpineol | 1161 | 1162a | 0.3 |
| α-Terpineol | 1189 | 1186a | 0.1 |
| δ-Elemene | 1337 | 1335a | 0.4 |
| β-Elemene | 1391 | 1389a | 1.5 |
| (E)-Caryophyllene | 1419 | 1417a | 1.5 |
| γ-Elemene | 1433 | 1434a | 1.5 |
| α-Humulene | 1453 | 1452a | 0.1 |
| β-Chamigrene | 1475 | 1476a | 0.6 |
| γ-Muurolene | 1480 | 1478a | 0.9 |
| β-Selinene | 1485 | 1489a | 0.2 |
| δ-Selinene | 1490 | 1492a | 0.2 |
| Curzerene | 1497 | 1499a | 30.5 |
| cis-α-Bisabolene | 1509 | 1506a | 0.1 |
| δ-Cadinene | 1523 | 1522a | 0.1 |
| γ-Cuprenene | 1534 | 1532a | 0.1 |
| α-Cadinene | 1537 | 1537a | 1.1 |
| Selina-4(15),7(11)-diene | 1541 | 1540a | 0.2 |
| Germacrene B | 1556 | 1557a | 2.3 |
| Spathulenol | 1576 | 1577a | 0.4 |
| Cubeban-11-ol | 1593 | 1595a | 0.2 |
| cis-β-Elemenone | 1589 | 1589a | 0.3 |
| trans-β-Elemenone | 1603 | 1601a | 4.1 |
| 1,10-di-epi-Cubenol | 1617 | 1618a | 0.4 |
| 10-epi-γ-Eudesmol | 1625 | 1622a | 0.1 |
| γ-Eudesmol | 1631 | 1630a | 0.5 |
| epi-α-Muurolol | 1641 | 1640a | 0.2 |
| Pogostol | 1654 | 1651a | 1.2 |
| Atractylone | 1659 | 1657a | 13.1 |
| Selin-11-en-4-α-ol | 1662 | 1658a | 0.2 |
| Germacrone | 1692 | 1693a | 15.4 |
| Zizanal | 1694 | 1697a | 2.1 |
| Maiurone | 1709 | 1709a | 0.3 |
| γ-Eudesmol acetate | 1780 | 1783a | 1.3 |
| Monoterpene hydrocarbons Oxygenated monoterpenes Sesquiterpene hydrocarbons Oxygenated sesquiterpenes Fatty acid derivatives |
17.5 | ||
| 0.7 | |||
| 41.3 | |||
| 39.8 | |||
| 0.1 | |||
| Total (%) | 99.4 | ||
| Constituents | RICal | RILit | % |
| 2,4-Dimethyl-3-pentanone | 795 | 788a | 0.1 |
| (3Z)-Hexenal | 798 | 797a | 0.1 |
| Furfural | 827 | 828a | 0.3 |
| (2E)-Hexenal | 847 | 846a | 0.1 |
| (3Z)-Hexenol | 851 | 850a | 0.1 |
| α-Thujene | 925 | 924a | 0.3 |
| α-Pinene | 934 | 932a | 55.8 |
| α-Fenchene | 946 | 945a | 0.1 |
| Camphene | 947 | 946a | 0.2 |
| β-Pinene | 976 | 974a | 2.6 |
| Myrcene | 989 | 988a | 0.1 |
| α-Phellandrene | 1005 | 1002a | 0.1 |
| α-Terpinene | 1016 | 1014a | 0.1 |
| p-Cymene | 1023 | 1020a | 0.1 |
| Limonene | 1027 | 1024a | 3.7 |
| 1,8-Cineole | 1030 | 1026a | 0.1 |
| (Z)-β-Ocimene | 1035 | 1032a | 0.2 |
| (E)-β-Ocimene | 1046 | 1044a | 13.1 |
| γ-Terpinene | 1057 | 1054a | 0.3 |
| Terpinolene | 1087 | 1086a | 2.9 |
| endo-Fenchol | 1112 | 1114a | 0.3 |
| α-Campholenal | 1125 | 1122a | 0.1 |
| trans-Pinocarveol | 1138 | 1135a | 0.1 |
| cis-β-Terpineol | 1143 | 1140a | 0.1 |
| Camphene hydrate | 1147 | 1145a | 0.1 |
| Borneol | 1164 | 1165a | 0.3 |
| Terpinen-4-ol | 1176 | 1174a | 0.3 |
| α-Terpineol | 1190 | 1186a | 10.0 |
| γ-Terpineol | 1196 | 1199a | 0.1 |
| (E)-Caryophyllene | 1419 | 1417a | 4.2 |
| γ-Elemene | 1433 | 1434a | 0.1 |
| α-Humulene | 1453 | 1452a | 0.2 |
| Bicyclogermacrene | 1496 | 1497a | 0.1 |
| δ-Amorphene | 1508 | 1511a | 0.1 |
| δ-Cadinene | 1523 | 1522a | 0.1 |
| Germacrene B | 1556 | 1559a | 0.4 |
| Monoterpene hydrocarbons | 79.6 | ||
| Oxygenated monoterpenes | 11.5 | ||
| Sesquiterpene hydrocarbons | 5.2 | ||
| Fatty acid derivatives | 0.7 | ||
| Total (%) | 97.0 | ||
| Constituents | RICal | RILit | % |
| Hexanal | 800 | 801a | 15.4 |
| (2E)-Hexenal | 847 | 846a | 21.7 |
| n-Hexanol | 862 | 863a | 2.2 |
| (3Z)-Hexenyl acetate | 1006 | 1004a | 0.5 |
| 2-Ethylhexanol | 1026 | 1030a | 1.8 |
| (Z)-β-Ocimene | 1036 | 1032a | 2.6 |
| (E)-β-Ocimene | 1046 | 1044a | 1.3 |
| α-Copaene | 1376 | 1374a | 0.6 |
| (E)-Caryophyllene | 1420 | 1417a | 4.1 |
| β-Selinene | 1487 | 1489a | 1.6 |
| α-Selinene | 1496 | 1498a | 1.1 |
| Caryophyllene oxide | 1583 | 1582a | 9.2 |
| Ledol | 1604 | 1602a | 0.9 |
| Humulene epoxide II | 1609 | 1608a | 0.6 |
| Muurola-4,10(14)-dien-1-β-ol | 1629 | 1630a | 4.8 |
| Caryophylla-4(12),8(13)-dien-5-α-ol | 1636 | 1639a | 3.3 |
| Caryophylla-4(12),8(13)-dien-5-β-ol | 1637 | 1639a | 10.5 |
| α-Muurolol | 1641 | 1644a | 1.4 |
| β-Eudesmol | 1645 | 1649a | 2.4 |
| Pogostol | 1648 | 1651a | 8.3 |
| 14-hydroxy-9-epi-(E)-Caryophyllene | 1664 | 1668a | 2.4 |
| Monoterpene hydrocarbons | 3.9 | ||
| Sesquiterpene hydrocarbons | 7.4 | ||
| Oxygenated sesquiterpenes | 43.8 | ||
| Fatty acid derivatives | 41.6 | ||
| Total (%) | 96.7 | ||
| Constituents | RICal | RILit | % |
| Ethyl butanoate | 799 | 802a | 12.1 |
| Butyl acetate | 806 | 807a | 0.2 |
| (2E)-Hexenal | 846 | 846a | 2.6 |
| n-Hexanol | 860 | 863a | 0.8 |
| Isopentyl acetate | 870 | 869a | 0.1 |
| Methyl hexanoate | 920 | 921a | 0.6 |
| α-Pinene | 932 | 932a | 9.2 |
| Camphene | 947 | 946a | 0.1 |
| Benzaldehyde | 955 | 952a | 1.1 |
| Hexanoic acid | 970 | 967a | 0.1 |
| β-Pinene | 976 | 974a | 0.4 |
| 6-methyl-5-Hepten-2-one | 984 | 981a | 0.4 |
| Myrcene | 989 | 988a | 0.9 |
| Butyl butanoate | 994 | 993a | 0.2 |
| Ethyl hexanoate | 998 | 997a | 5.9 |
| (3Z)-Hexenyl acetate | 1005 | 1004a | 2.3 |
| Hexyl acetate | 1011 | 1007a | 0.6 |
| p-Cymene | 1023 | 1020a | 0.3 |
| Limonene | 1027 | 1024a | 25.2 |
| 1,8-Cineole | 1030 | 1026a | 0.2 |
| γ-Terpinene | 1057 | 1054a | 0.3 |
| Methyl octanoate | 1123 | 1123a | 0.1 |
| (3Z)-Hexenyl butanoate | 1185 | 1184a | 1.2 |
| Hexyl butanoate | 1191 | 1191a | 1.4 |
| Ethyl octanoate | 1196 | 1196a | 0.1 |
| α-Copaene | 1376 | 1374 | 0.9 |
| (3Z)-Hexenyl hexanoate | 1380 | 1378a | 0.7 |
| Geranyl acetate | 1383 | 1379a | 0.2 |
| Hexyl hexanoate | 1385 | 1382a | 0.4 |
| iso-Italicene | 1403 | 1401a | 0.1 |
| Acora-3,7(14)-diene | 1408 | 1407a | 0.2 |
| α-Cedrene | 1412 | 1410a | 0.6 |
| (E)-Caryophyllene | 1420 | 1417a | 1.1 |
| β-Santalene | 1460 | 1457a | 0.2 |
| α-Acoradiene | 1464 | 1464a | 0.4 |
| 10-epi-β-Acoradiene | 1479 | 1474a | 0.4 |
| Ar-Curcumene | 1482 | 1479a | 0.9 |
| α-Zingiberene | 1495 | 1493a | 0.1 |
| (Z)-α-Bisabolene | 1502 | 1503a | 0.2 |
| β-Bisabolene | 1508 | 1506a | 2.7 |
| α-Bulnesene | 1512 | 1509a | 0.7 |
| β-Curcumene | 1515 | 1514a | 1.8 |
| δ-Cadinene | 1524 | 1522a | 0.9 |
| (E)-γ-Bisabolene | 1532 | 1529a | 0.9 |
| (E)-Nerolidol | 1563 | 1561a | 0.4 |
| Caryophyllene oxide | 1583 | 1582a | 0.2 |
| Cedrol | 1601 | 1600a | 0.1 |
| 10-epi-γ-Eudesmol | 1625 | 1622a | 0.7 |
| α-Acorenol | 1630 | 1632a | 1.1 |
| Gossonorol | 1637 | 1636a | 0.2 |
| epi-α-Cadinol | 1641 | 1638a | 0.6 |
| Hinesol | 1644 | 1640a | 0.3 |
| α-Muurolol | 1646 | 1644a | 0.6 |
| α-Cadinol | 1654 | 1652a | 0.5 |
| 14-hydroxy-(Z)-Caryophyllene | 1664 | 1666a | 0.7 |
| epi-β-Bisabolol | 1670 | 1670a | 9.8 |
| epi-α-Bisabolol | 1683 | 1683a | 0.8 |
| α-Bisabolol | 1685 | 1685a | 1.8 |
| (2E,6Z)-Farnesal | 1714 | 1713a | 0.3 |
| (2Z,6E)-Farnesol | 1721 | 1722a | 0.4 |
| (2E-6E)-Farnesal | 1741 | 1740a | 0.4 |
| Monoterpene hydrocarbons | 36.4 | ||
| Oxygenated monoterpenes | 0.4 | ||
| Sesquiterpene hydrocarbons | 12.1 | ||
| Oxygenated sesquiterpenes | 18.9 | ||
| Benzenoids/Phenylpropanoids | 1.1 | ||
| Fatty acid derivatives | 29.8 | ||
| Total (%) | 98.7 | ||
| Classes of compounds (%) | Esti | Euni | Mdub | Pgua | Pgui |
| Monoterpene hydrocarbons (MH) | 28.5 | 17.5 | 79.6 | 3.9 | 36.4 |
| Oxygenated monoterpenes (OM) | 25.5 | 0.7 | 11.5 | - | 0.4 |
| Sesquiterpene hydrocarbons (SH) | 13.1 | 41.3 | 5.2 | 7.4 | 12.1 |
| Oxygenated sesquiterpenes (OS) | 20.9 | 39.8 | - | 43.8 | 18.9 |
| Fatty acids derivatives (FA) | 10.8 | 0.1 | 0.7 | 41.6 | 29.8 |
| Benzenoids/Phenylpropanoids (B/P) | - | - | - | - | 1.1 |
| Total (%) | 98.8 | 99.4 | 97.0 | 96.7 | 98.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





