1. Introduction
Since their first development in the early 2000s, lanthanide-doped upconversion nanoparticles (UCNPs) have attracted extensive attention in various cutting-edge bioapplications such as deep tissue bioimaging, biosensing and nanomedicine [
1].The ability of UCNPs to convert incident light in the near-infrared (NIR) region into high-energy ultraviolet or visible emission via an anti-Stokes process has been exploited [
2,
3]. This allows not only relatively deep light penetration and low photodamage effects, but also reduced autofluorescence, light scattering, and phototoxicity, enabling simultaneous applications of luminescent nanomaterials in areas such as precise theranostics, in vivo optogenetics and environmental hazard control [
4,
5,
6].
Important prerequisites for the use of UCNPs in biomedical applications are hydrophilicity and dispersibility in biological buffers, together with chemical and colloidal stability and high signal reproducibility [
7]. These properties are exhibited by homogeneous phase-pure particles prepared under specific conditions. Other characteristics of UCNPs influencing their use are biocompatibility and non-toxicity, which are affected by particle size, shape, chemical composition and surface structure [
8]. Dissolution of UCNPs in aqueous media can induce cytotoxicity in biological systems, especially under conditions of high dilution in aqueous media [
9,
10,
11,
12]. A tendency of Ln fluorides to dissolve in phosphate buffers due to the release of Ln3+ and F- ions, which can lead to a gradual loss of luminescence intensity, uneven particle brightness and even localized cytotoxicity has been recently confirmed [
13]. In addition, the release of Y3+ ions can affect geno- and/or cytotoxicity and possible neuronal damage [
14], which is a barrier for medical applications [
11]. The toxicity assessment of UCNPs is therefore a relevant issue since many researchers are using, them for various applications, such as bioimaging or drug delivery.
There are several methods for producing rare earth-doped UCNPs, e.g., high-temperature coprecipitation [
15], thermal decomposition [
16], hydro(solvo)thermal [
17], microwave [
18], or microemulsion synthesis [
19]. Among these procedures, the most popular preparation of monodisperse crystalline UCNPs of various sizes and compositions is the aforementioned “user-friendly” coprecipitation using oleic acid as a stabilizer [
20]. Recently, some studies have appeared dealing with the protection of UCNPs by introducing a surface layer that provides both their colloidal stability and insolubility in aqueous media, as well as suppresses luminescence quenching and minimizes particle toxicity [
12,
21]. The advantage of UCNP surface modification is also the possibility of introducing functional groups allowing the attachment of a large number of biomolecules such as peptides, proteins, antibodies, DNAs, drugs, photosensitizers, etc., which facilitate specific targeting and treatment. These functional groups consist of carboxyl, amino, thiol, maleimide, aldehyde, phosphate, bisphosphonate, sulfonate and even o-nitrobenzyl groups [
22]. General surface engineering strategies of UCNPs include ligand oxidation, replacement or removal of hydrophobic stabilizers, also silanization, layer-by-layer assembly, coating with amphiphilic polymers, etc. [
23]. This allowed the modification of UCNPs with poly(ethylene glycol) (PEG) and its derivatives [
24,
25], amphiphilic chitosan [
26], poly(acrylic acid) [
27], polyethyleneimine [
28], polyvinylpyrrolidone [
29], poly(D,L-lactic-co-glycolic acid) [
30], poly(isobutylene-alt-maleic anhydride) [
31], etc. The surface design of UCNPs can also be optimized by encapsulating them in polymers via microemulsion polymerization of monomers such as ethylene glycol methyl ether acrylate, 2-hydroxyethyl and glycidyl methacrylate, acrylic acid, or by “grafting-from” and “grafting-on” methods [
32,
33]. The quality of the surface composition is a key factor affecting the circulation time of UCNPs in the bloodstream and/or passing through cell membranes, which is crucial for their bioapplications. The recommended size for optimal UCNP penetration is less than 100 nm. However, this size may also pose a risk of toxicity due to their potential to penetrate cellular structures and organs via the circulatory system. In addition, UCNPs can generate reactive oxygen species (ROS) that can induce DNA damage, which can lead to damage not only affecting cell growth through protein oxidation, but also impacting mitochondrial respiration [
34]. Nanoparticles administered intravenously circulate through the bloodstream, especially to the liver and spleen, kidneys, heart, lungs, bone marrow and brain. Retention in the blood and organs strongly depends on the surface properties of the nanoparticle material. Coatings that promote interactions of NPs with cell membranes promote internalization by different cell types, while the use of biologically inert coatings, i.e., PEG, leads to prolonged circulation of these NPs in the bloodstream. Moreover, the rate and mechanism of uptake and clearance is cell/tissue dependent and varies between NPs of different hydrodynamic size, composition, shape, charge and surface functional groups [
35,
36].
In this work, we prepared NaYF4:Yb,Er-based UCNPs and investigated the dependence of their morphology, size and type of coating on their cytotoxicity as determined on rMSCs and C6 cells using the xCELLigence real time proliferation assay. We also investigated their internalization in cells and DNA oxidative damage caused by coated and uncoated, small and large UCNPs. As a proof of concept, UCNPs were also tested in experimental animals and imaged in vivo.
2. Results and Discussion
This work is a continuation of our previous papers, in which we investigated the design and properties of small and large surface-engineered UCNPs with emphasis on their colloidal and chemical stability [
37,
38]. In this report, we compared the two types of UCNPs, small spherical (S-UCNPs) and large hexagonal (L-UCNPs), prepared by high-temperature coprecipitation of the respective lanthanide chlorides depending on the reaction conditions (
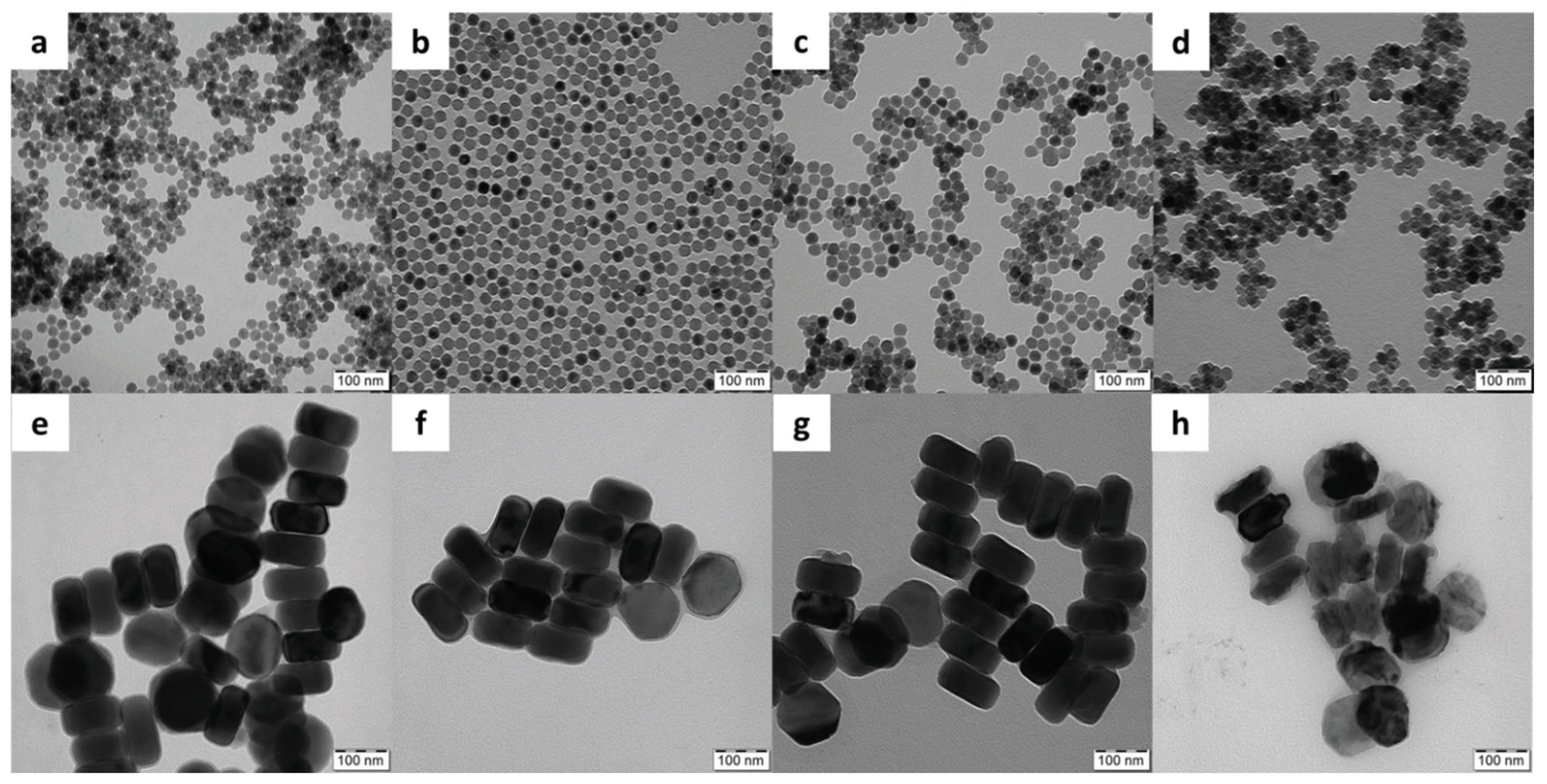
Figure 1), in terms of morphology, cytotoxicity and detection of DNA damage by the comet assay. While the spherical particles were 25 nm in diameter, their hexagonal counterparts were ~120 nm in size; both types had a relatively narrow particle size distribution (
Đ = 1.01), which is essential for biomedical applications as it allows control of particle properties and reproducibility of results. Moreover, three non-toxic biocompatible polymer coatings, namely Ale-PEG, Ale-P(DMA-AEA) and PMVEMA, were selected to ensure the colloidal stability of the particles in media [
37]. The properties of these coatings differed; while nonionic Ale-PEG is known for its antifouling properties [
39], the positively charged Ale-P(DMA-AEA) coating of UCNPs promoted their engulfment by cells [
40]. The latter coating also has the advantage of the presence of reactive amino groups available for prospective attachment of different biomolecules. Both Ale-PEG and Ale-(PDMA-AEA) were terminated with a bisphosphonate group that forms highly stable metal-
bisphosphonate complexes firmly anchoring the polymers to the particle surface. In contrast, negatively charged PMVEMA has multiple carboxyl groups that can coordinate with lanthanide surface ions and/or react with various compounds.
Because all polymers did not exhibit phase contrast in the TEM images, the TEM micrographs of the polymer-coated UCNPs did not differ from those of the starting uncoated particles (
Figure 1). The hydrodynamic diameter (
Dh) of S-UCNPs and S-UCNP@Ale-PEG, S-UCNP@Ale(PDMA-AEA) or S-UCNP@PMVEMA nanoparticles was 101, 68, 102, and 112 nm, respectively. Their ζ-potential, which depends on surface chemistry of the particle, was 30, 8, 27, and -32 mV for neat, Ale-PEG-, Ale(PDMA-AEA)-, and PMVEMA-modified particles, respectively (
Table 1). All studied L-UCNPs had comparable ζ-potential as that of S-UCNPs, but due to their larger size (according to TEM) they had larger
Dh. The resulting
Dh values of L-UCNP, L-UCNP@Ale-PEG, L-UCNP@Ale(PDMA-AEA), and L-UCNP@PMVEMA nanoparticles were 174, 158, 160, and 234 nm, respectively, and the corresponding ζ-potential was 28, 4, 22, and -46 mV, respectively (
Table 1). Monitoring the fluoride ions dissolved in media in which the particles were aged showed that their degradation depended on many parameters, including size, coating type, temperature, and the medium used [
37,
38]. All types of selected coatings were shown to decrease particle degradation in different aqueous media, with the exception of PMVEMA, which slightly increased particles dissolution in water. It was also shown that dissolution of UCNPs was low in water and DMEM, moderate in artificial lysosomal fluid and pronounced in PBS. Increasing temperature directly increased dissolution of particles regardless of their size, coating, and aging medium. Moreover, independently on the type of coating the L-UCNPs dissolved less than the S-UCNPs due to a smaller surface-to-volume ratio. This observation was also confirmed by the ICP-MS, where the amount of Y and Yb was measured in leaches. The results showed that S-UCNPs were more soluble in culture media (Y and Yb within the range of 2-8 µg/ml) compared to the L-UCNPs, where both elements were present at less than 2 µg/ml. Therefore, parameters like particle size, ζ-potential, storage medium can influence particle cytotoxicity due to the interaction of the particles or their degradation products with cells. Our results are in an agreement with other studies in which the stability of NaYF
4:Yb
3+,Er
3+ UCNPs stabilized with phosphonate coatings alendronate and ethylenediamine tetra(methylene phosphonic acid) in PBS or culture medium at room temperature and 37 °C was also investigated. [
41,
42]
Internalization of UCNPs in Cell Cytoplasm
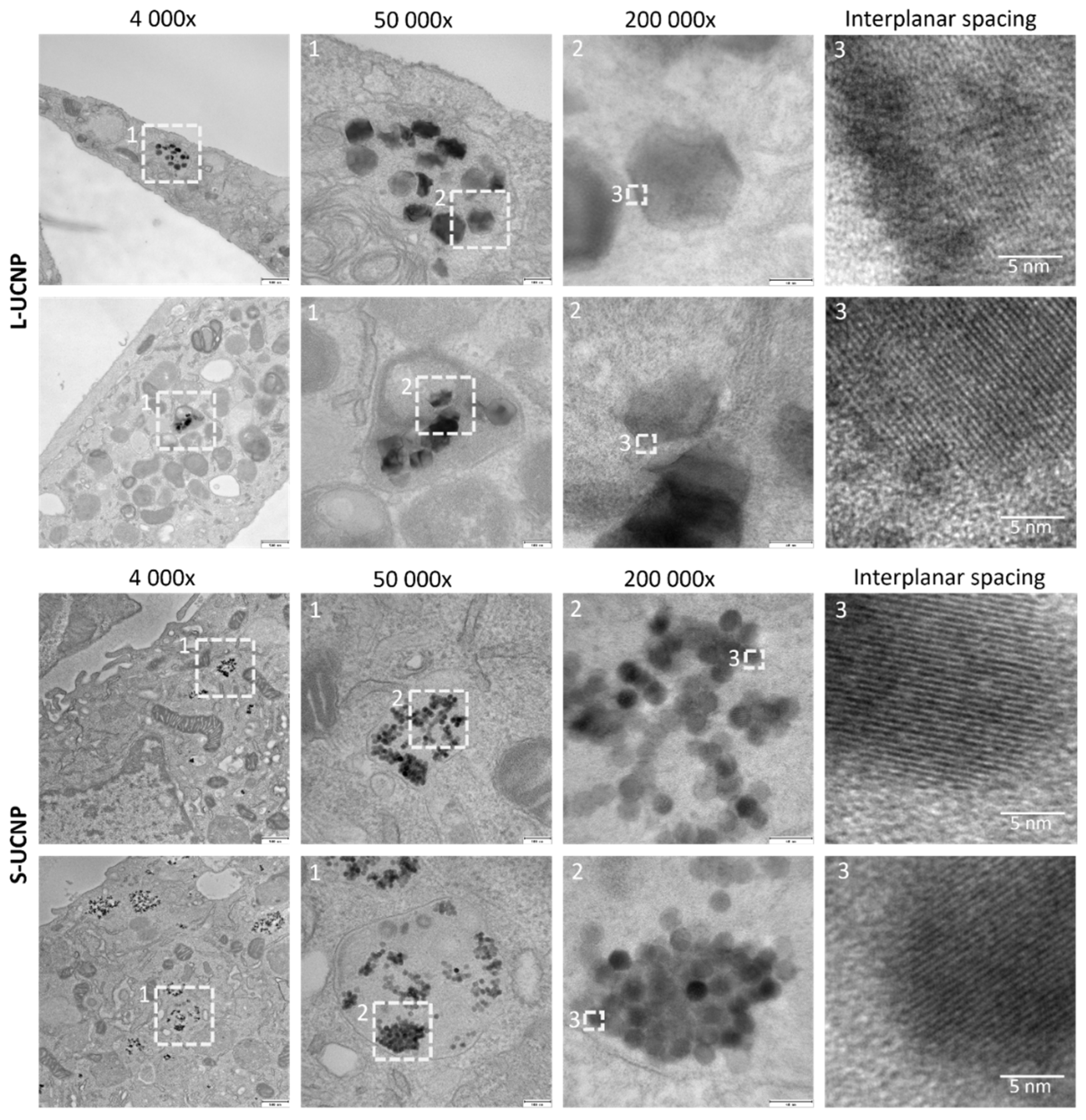
Both types of nanoparticles (L-UCNPs and S-UCNPs) were internalized into membrane delimited endosomal compartments with noticeable aggregation of nanoparticles into clusters within the lumen of endosomes (
Figure 2). Lower magnifications (4,000× and 50,000×) demonstrated position of particle-containing endosomes within the cell, HRTEM images (200 000×) demonstrated crystalline structure of nanoparticles with discernible interplanar spacing (
Figure 2, enlarged on the right). Various densities of L-UCNP nanoparticles (an average diameter of 120 nm) and S-UCNP nanoparticles (an average diameter of 25 nm) were found within the section of 60 nm.
Cytotoxicity by xCELLigence Assay
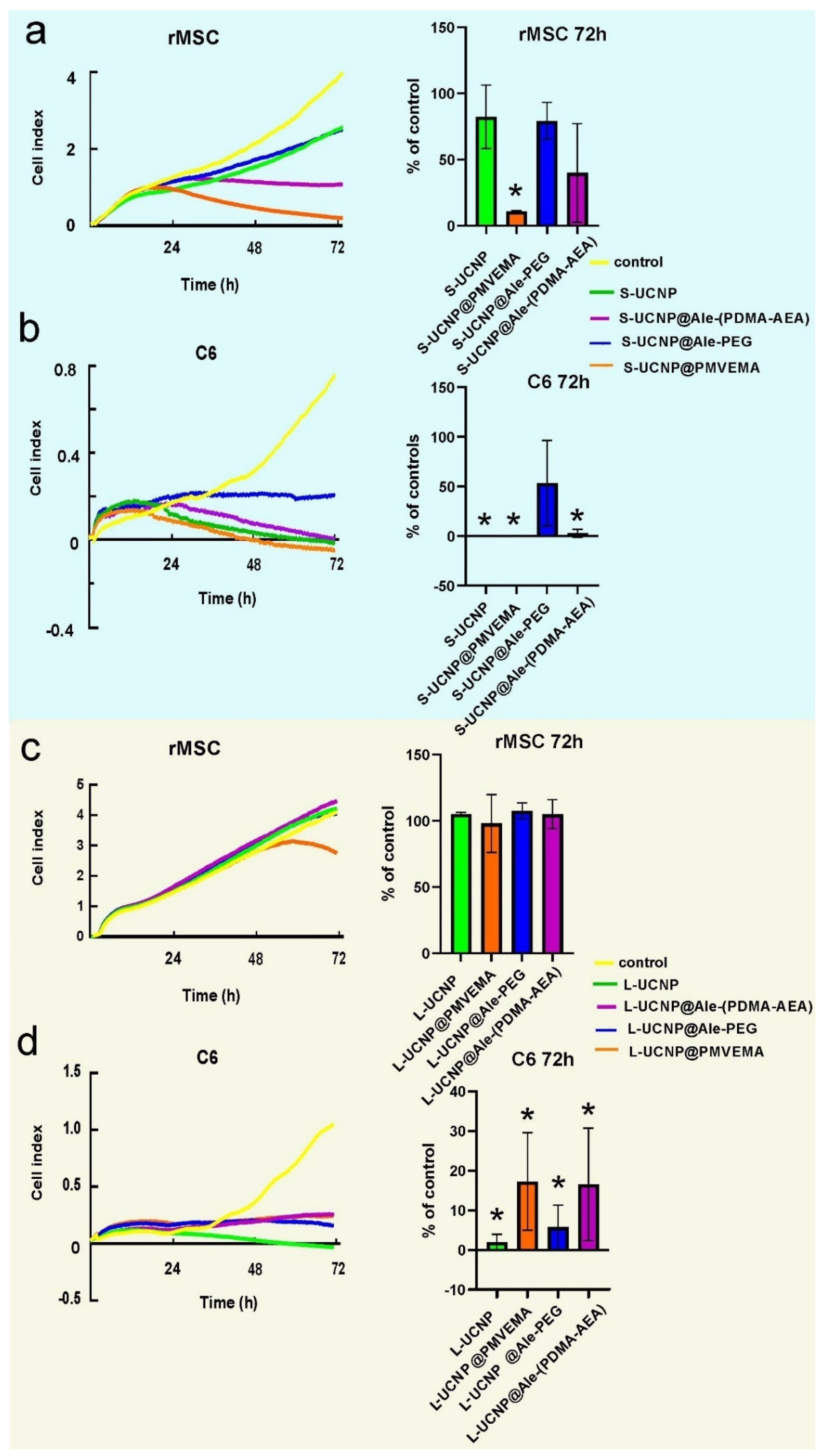
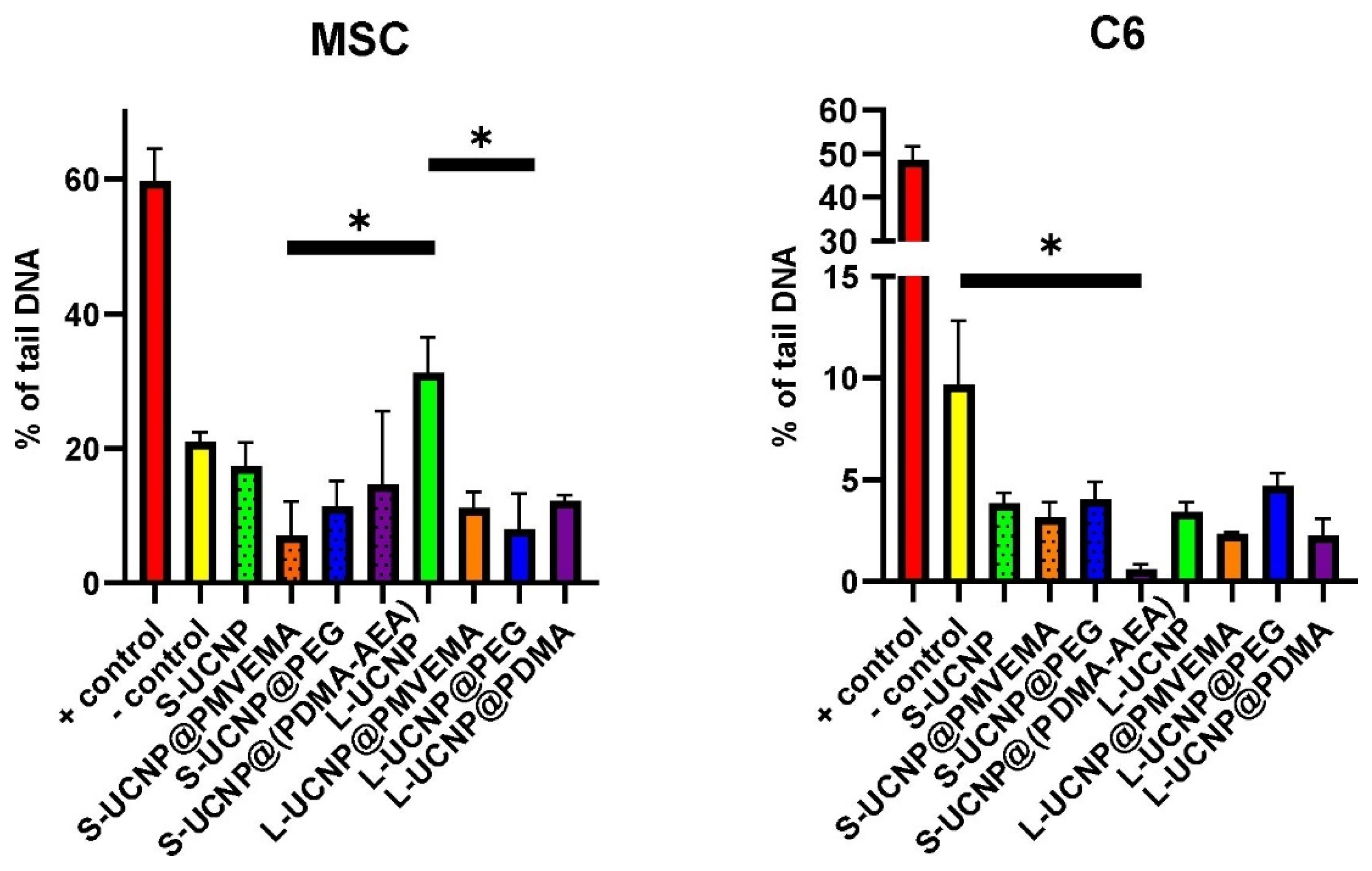
To monitor cell growth dynamics, the viability of C6 and rMSCs incubated with Ale-PEG-, Ale-(PDMA-AEA), and PMVEMA-coated L- and S-UCNPs for 3 days was examined using a real-time proliferation assay. The advantage of this test is the long-term monitoring of cell growth in real time and not only in discrete or terminal values (as MTT assay). The test is performed in wells coated with gold electrodes, where cells growth is expressed as a cell index correlating with an increase in electrical impedance. Cell proliferation curves were determined after incubation with both particle types and their leaches (
Figure 3 and
Figure 4). Particles were used at a concentration of 20 µg/ml, at which the viability of C6 and rMSCs in previous experiments using Alamar blue or MTT assay reached >80 % [
37,
38]. The growth of healthy non-tumor rMSCs incubated with any type of UCNPs for 1 day was similar to the control. After 2 days of incubation of rMSCs with S-UCNP@PMVEMA, there was a dramatic decrease in cell proliferation, which continued for the next 24 h (
p = 0.031). In the presence of S-UCNP@Ale-(PDMA-AEA) particles, cell growth decreased slightly; then remained unchanged over 72 h (
Figure 3a). Cell viability in the presence of S-UCNP@Ale-PEG and neat S-UCNP remained ~80 %. We speculate that uncoated S-UCNPs partially aggregated and thus were not uniformly dispersed in the well. Therefore, the cytotoxicity was lower than that of the S-UCNP@Ale-(PDMA-AEA) particles. In contrast, all types of L-UCNPs had no effect on cell growth, except L-UCNP@PMVEMA, where rMSC proliferation decreased after 48 h of incubation (
Figure 3c). In general, S-UCNPs impaired cancer C6 cell viability more significantly than rMSCs. While control cells proliferated rapidly for all 3 days, C6 cells incubated with S-UCNPs for 1 day stopped growing. The viability of C6 cells in the presence of S-UCNP@Ale-PEG particles remained constant but decreased after incubation with neat S-UCNPs for 72 h (
p = 0.0142) or their analogues coated with Ale-(PDMA-AEA) (
p = 0.0162) or PMVEMA (
p = 0.0142;
Figure 3 b). Similar results were achieved with L-UCNPs, especially neat ones, which stopped the proliferation of C6 cells that detached from the bottom of the well (
Figure 3 d). Prolonged exposure to all polymer-coated L-UCNPs (72 h) at a concentration of 20 µg/ml reduced cell growth (
p <0.0001) as did neat L-UCNPs (
p <0.0001). Our results are in an agreement with MTS assay studies that reported a 67 % decrease in viability of bone marrow-derived stem cells after 48 h of exposure to UCNPs [
43] while shorter incubation times did not affect cell viability. Also, UCNPs coated with polyethylenimine (25 µg/ml) exposed to MSCs for 2 days slightly decreased cell viability to 85 % [
44]. Different coatings of UCNPs were also tested on HaCaT keratinocytes by WST-8 assay and similarly, prolonged exposure to UCNPs for 48 hours resulted in a viability drop when compared to 24h [
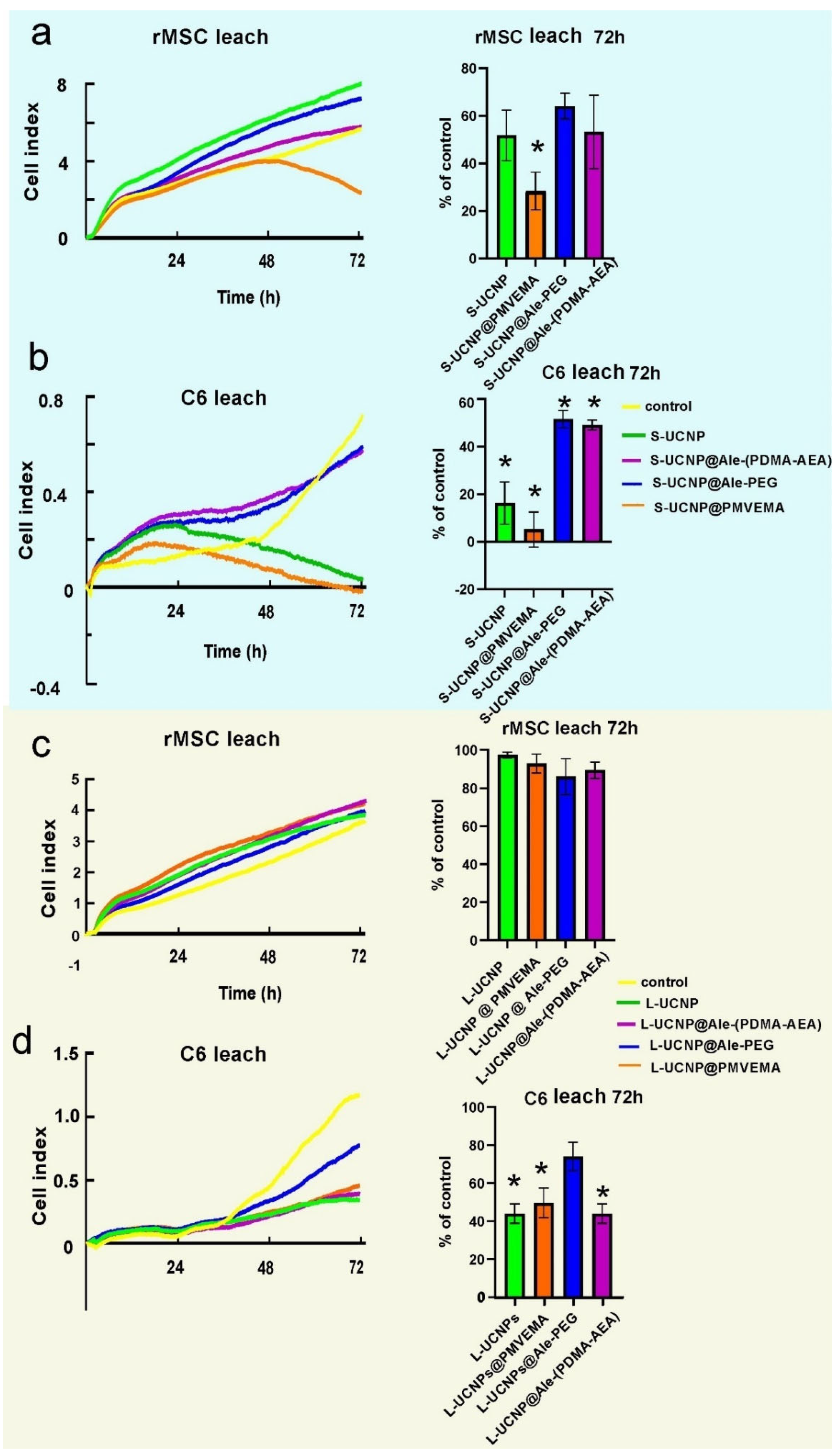
21]. In contrast to nanoparticles, rMSCs incubated with particle leaches from neat S-UCNPs, S-UCNP@PMVEMA, S-UCNP@Ale-(PDMA-AEA), and S-UCNP@Ale-PEG proliferated even faster than in the control group (
Figure 4 a), indicating stimulation of cell growth. In the case of C6 cells, their growth was faster after incubation with leaches for 24 h; in later time periods (72 h), leaches from neat S-UCNPs (
p = 0.0015), S-UCNP-Ale-PEG (
p = 0.0119), S-UCNPs@Ale-(PDMA-AEA) (
p = 0.0098) and S-UCNP@PMVEMA (
p = 0.0009) were toxic (
Figure 4 b). On the other hand, the leaches from L-UCNPs did not affect rMSC proliferation (
Figure 4 c); however, C6 growth was slowed down after the incubation with L-UCNP leaches for 72 h (
p = 0.0086), L-UCNP@PMVEMA leaches (
p = 0.0129) and L-UCNP@Ale-(PDMA-AEA) leaches (
p = 0.0086); except for L-UCNP@Ale-PEG leaches, where the cell proliferation decreased only marginally (
Figure 4 d).
Thus, it can be concluded that the real time proliferation assay in cells incubated with UCNPs gave similar results to MTT or alamar blue done in previous experiments [
37,
38], while the leaches, especially from L-UCNPs, were less harmful. In general, L-UCNPs were less toxic than S-UCNPs due to the lower surface-to-volume ratio. L-UCNP nanoparticles almost did not affect the viability of rMSCs, while the viability of C6 significantly decreased to 30% or less. The viability of rMSCs decreased in the order S-UCNP@Ale-(PDMA-AEA) > S-UCNP@Ale-PEG > S-UCNPs > S-UCNP@PMVEMA. The trend was also similar for C6 cells, which readily internalized particles depending on the incubation time. In contrast, rMSCs engulfed particles much less after three days of incubation than after one day [
37]. The best biocompatibility was achieved with Ale-(PDMA-AEA) and Ale-PEG coatings, which can be attributed to their hydrophilicity. There are alternative approaches to assess NP cytotoxicity: for example, Das et al. [
45] conducted a study on the toxic effects of three types of functionalized UCNPs: oleate ligand-UCNPs, PEG-UCNPs, and bilayered PEG-oleate-UCNPs. They used calcein and propidium iodide viability assay and concluded that bilayer UCNPs exhibit significant toxicity due to functionalization. In another study, Malvindi et al. [
46] evaluated the cytotoxicity of silica-coated iron oxide NPs using the WST-8 ([2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] method and the lactate dehydrogenase release assay (LDH assay) to analyze cell viability and cell membrane integrity. The NPs showed good internalization in HeLa cells with no observed toxicity. Meindl et al. [
47], on the other hand, assessed the cytotoxicity of UCNPs by measuring intracellular calcium, providing an example of an alternative approach to assess toxicity..
In Vivo Study
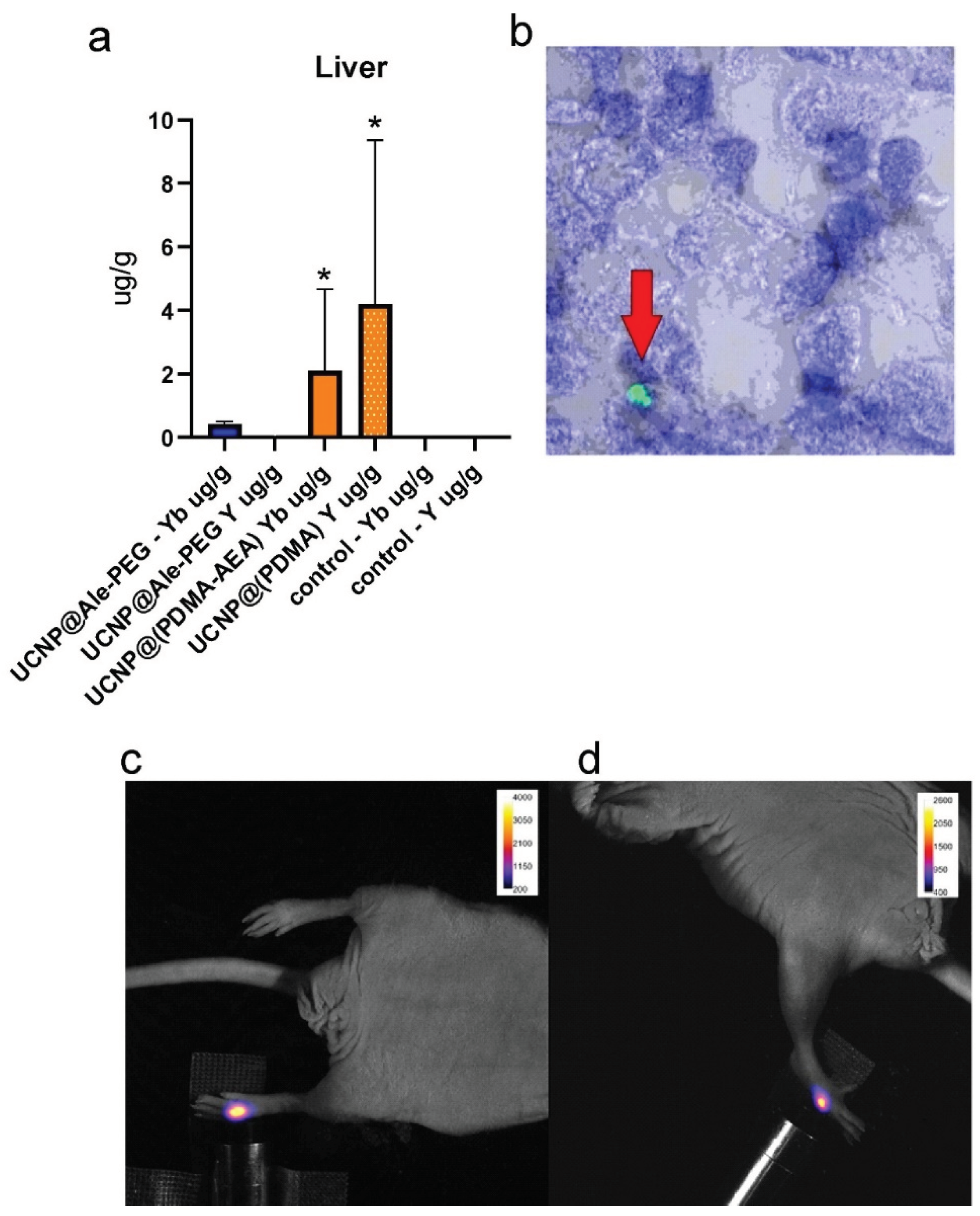
Nanoparticles were investigated in vivo after subcutaneous application in NuNu mice (males, 6-7 weeks old). Upconversion enabled detection of a signal at 535 nm after excitation in near infrared area (980 nm;
Figure 6). After systemic (retroorbital) application, in vivo imaging failed to detect the particles. While near infrared excitation light easily penetrates the tissue and may excite, weaker fluorescent light at 535 nm is absorbed. Therefore, the possibility to obtain the fluorescence signal at this wavelength from organs located deep in the body (e.g., the liver) is very limited. The nanoparticles were detected
postmortem on excised organs. Fluorescence microscopy proved the presence of L-UCNP@Ale-(PDMA-AEA) in the liver only (
Figure 6). L-UCNP@Ale-PEG particles were not detected in any organ. These results were supported by ICP-MS, which confirmed the presence of Yb and Y atoms from the nanoparticles only in the liver. The amount of Yb and Y detected from Ale-PEG-coated UCNPs was significantly lower than that from Ale-(PDMA-AEA)-coated nanoparticles (
p <0.0001), where a statistically significant difference was observed compared to the control group. A statistically significant difference between UCNP@Ale-PEG and the control group was not observed; the particles were almost completely eliminated from the body after more than 96 h (
Figure 6 a). No Yb and Y was detected by ICP-MS in the kidney. These experiments confirmed fast elimination of the coated UCNPs from the organism and also suggested the hepatobiliary excretion route. Contrary to this, Zhou et al. [
49] observed a tendency for NaYF
4:Yb,Er@SiO
2 to accumulate in the liver after entering the bloodstream. Large amount of NaYF
4:Yb,Er@SiO
2 was observed in the liver tissue on days 1 and 7 after intravenous administration of these nanoparticles to mice at a dose of 20 mg/kg, suggesting internalization into hepatocytes. However, histology did not reveal any pathological changes in the liver tissue. While fluorescence particle detection in vivo is very difficult, it does not compromise future use of UCNPs in photodynamic therapy in combination with a suitable photosensitizer. If the particles are carefully navigated, they can be excited by NIR light deep in the tissue. Generated fluorescence light can irradiate an immediate vicinity only, so it may excite a suitable photosensitizer locally with no possible harm to other tissues or organs.
4. Materials and Methods
Small (S) spherical and large (L) hexagonal UCNPs were prepared by high-temperature coprecipitation of lanthanide chlorides and their surface was modified with poly(ethylene glycol) (PEG) and poly(N,N-dimethylacrylamide-co-2-aminoethylacrylamide) [P(DMA-AEA)], both terminated with an alendronate (Ale) anchoring group, and poly(methyl vinyl ether-co-maleic acid) (PMVEMA) according to our previous work [
37,
38]. For cell culturing phosphate-buffered saline (PBS), Dulbecco’s modified Eagle medium (DMEM), supplemented with fetal bovine serum (FBS) (both from Merck; Darmstadt, Germany), a combination of primocin and penicillin - streptomycin (Gibco; Life Technologies, Grand Island, NY, USA) and trypsin (Sigma-Aldrich, St. Luis, MO, USA) was used. For preparation of samples for electron microscopy, we utilized PHEM buffer (pH 7.4) OsO4 solution (Thermo Fisher, Waltham, MA, USA), K3(Fe(CN6)) (VWR International, Radnor, PA, USA), low melting agarose (LMP) (Sigma-Aldrich, St. Luis, MO, USA) , uranyl acetate (Sigma-Aldrich, St. Luis, MO, USA) and Epon HARD complete resin (Electron Microscopis Sciences; Hatfield, USA); for fluorescent microscopy 4′,6-diamidino-2-phenylindole dihydrochloride ( DAPI; Invitrogen; Carlsbad, USA) was obtained.
All other chemicals were purchased from Sigma-Aldrich , or LachNer (Neratovice, Czech Republic).
Distilled and demineralized water (conductivity ˂ 0.1 µS/cm; Millipore; Bedford, MA, USA) was used to prepare all solutions.
Preparation of Leachates
For toxicity analysis of the leachates, these were prepared from dispersions of particles in the culture medium. The nanoparticles were mixed with the medium to a concentration of 20 µl/ml, the mixture was placed in an incubator at 37 °C for three days, the dispersion was centrifuged (160 rcf) for 5 min and the supernatant was analyzed using a real time proliferation assay.
C6 Cell Line
To initiate cell culture, C6 cells were thawed and rinsed in cold PBS. After washing, they were seeded in DMEM, supplemented with FBS and a combination of primocin and penicillin - streptomycin.
Mesenchymal Stem Cells
Rat mesenchymal stem cells (rMSCs) were derived by aspirating bone marrow from the rat’s bone. The harvested bone marrow was subsequently washed twice with PBS. The rinsed bone marrow was then cultured in cell culture flasks containing DMEM. The culture medium was enriched with FBS, along with the addition of primocin and penicillin-streptomycin. The culture flasks were placed in an incubator at 37 °C and 5 % CO2 atmosphere and the culture medium were changed twice a week. Upon reaching approximately 70 % confluence, the cells were passaged using trypsin at 37 °C for 4 min. Trypsin treatment was terminated by FBS. The cells were subsequently washed with PBS and either utilized for the experiment or reseeded for further culture. Both cell cultures were maintained at 37 °C in 5 % CO2 atmosphere. The culture medium was refreshed twice a week to maintain cell viability and growth.
Electron Microscopy
The internalization of the nanoparticles into the C6 and MSCs was investigated by transmission electron microscopy (TEM). The samples were initially centrifuged at 300 g for 5 min at room temperature (RT). They were then fixed with a solution containing 2.5 % glutaraldehyde, 2 % formaldehyde and 0.1 M PHEM buffer (pH 7.4) for 10 min at RT and incubated on ice for 1 h. After fixation, the samples underwent three 5-min ice-cold washes with 0.1 M PHEM buffer (pH 7.4).
Following the washing, the samples were contrasted in 1 % OsO4 solution and 1.5 % K3(Fe(CN6)) in Milli-Q water on ice for 1 h. The samples were washed three times for 5 min each with PHEM buffer (pH 7.4) while kept on ice. Subsequently, the samples were centrifuged at 400 g for 2-5 min at 38 °C, embedded in 2 % LMP at 38 °C, and polymerized for 20 min on ice. Afterward, cubes were cut from the samples, quickly washed with cold Milli-Q water and stained in a 1 % uranyl acetate aqueous solution at RT for 30 min. Dehydration involved washing with a series of cold ethanol solutions (30-50-70-80 %) for 5 min each on ice, followed by RT-warmed 90-100 % ethanol for 5 min at RT. The samples were then transferred into anhydrous acetone at RT, infiltrated and embedded in epoxy resin kit EMBED 812 (EMS#14120) and polymerized at 40 °C for 1 h and at 60 °C for 72 h in an oven. Ultrathin sections were cut on a LEICA UC7 ultramicrotome, collected on formvar carbon coated copper slot grids and post-contrasted with 4 % uranyl acetate and lead citrate. Images were acquired on a JEM 2100 Plus transmission electron microscope (JEOL; Akishima, Japan) operated at 200 kV using TVIPS XF 416 camera.
Real Time Proliferation Assay
Briefly, the culture medium (50 µl) was pipetted into a special E-plate with gold electrodes at the bottom of wells and the background impedance was determined. The cell attachment and proliferation were measured as a change of impedance between electrodes and expressed as a unitless cell index. The appropriate number of cells (5,000 rMSCs or 2,500 C6 cells per well) was added in culture medium (100 µl) and the cells were allowed to attach to the bottom of well for 2 h. This was followed by the addition of nanoparticles or their leaches in medium (50 µl); the final particle concentration was kept at 20 µg/ml per well. The plates were then inserted into the xCELLigence RTCA DP real-time cell analyzer (ACEA Biosciences, now Agilent Technologies; Santa Clara, CA, USA) and the changes in impedance were recorded every 20 min for 3 days. All experiments were done in triplicates and repeated three times.
Pilot In Vivo Imaging
As a proof of principle, the large hexagonal nanoparticles (120 nm) were applied to mice and their fluorescence was detected. For in vivo study, the most successful candidates from in vitro investigation were selected, where large nanoparticles with Ale-PEG and Ale-(PDMA-AEA) coating were less toxic.
CD-1® nude mice (Crl:CD1-FOXn1nu), 6 weeks old, were used throughout the experiments. First experiment involved application of nanoparticle dispersions (10 µL of UCNP@Ale-PEG or UCNP@Ale-(PDMA-AEA), concentration 4 mg/mL) subcutaneously to a mouse and scanning using an optical imager Bruker Xtreme (Bruker, Billerica, MA, USA). Excitation of the injection site was performed by a laser diode (980 nm, 100 mW) and a signal at 535 nm was detected (exposition 5 s, field of view 72×72 mm, no binning).
To evaluate biodistribution of the nanoparticles, 100 µL of UCNP@Ale-PEG or UCNP@Ale-(PDMA-AEA) dispersion (concentration 50 µg/mL of mouse blood) was applied retroorbitally to mice (each nanoparticle type to 5 mice). Animals were scanned at similar experimental conditions (an optical imager Bruker Xtreme, excitation using a laser diode at 980 nm, 100 mW, emission at 535 nm, exposition 1 s, field of view 190×190 mm, no binning). Due to a small area irradiated by the diode, it was not possible to excite nanoparticles in the whole animal body. Therefore, only selected organs (the liver, spleen, kidney) were excited in several subsequent measurements. A group of two animals served as a control.
After 96 h, mice were deeply anesthetized with chloralhydrate in a concentration of 400 mg/kg intraperitoneally and then transcardially perfused first with phosphate buffer (PB) and then with 4 % paraformaldehyde in PB. Paraformaldehyde-fixed tissue samples (kidney and liver) were cryopreserved in sucrose solution with gradually increasing concentration (10, 20 and 30 % sucrose in deionized water). The tissue was then embedded in OCT mounting media (VWR; Radnor, USA). Sections were cut on a Cryostar NX70 cryostat (Thermo Fisher Scientific;Waltham, MA, USA) to 30 µm sections (liver and kidney) and stained with DAPI for 10 min. Finally, content of Yb and Y was quantified by inductively coupled plasma mass spectrometry (ICP-MS).
The animal experiments were performed in accordance with national and international guidelines for laboratory animal care and were approved by the Laboratory Animal Care and Use Committee of the First Faculty of Medicine, Charles University, and the Ministry of Education, Youth and Sports of the Czech Republic (MSMT 46304/2020-3).
Laser Scanning Confocal Microscopy
To visualize UCNPs, Carl Zeiss LSM 880 NLO microscope (Oberkochen, Germany), equipped with 40× NA1.1 water immersion objective and a 32 GaAsP array spectral detector covering emission from 410 to 694 nm and operated at single photon counting mode for maximum SNR, was used. Lambda mode at full spectral resolution was used to spectrally prove UCNPs emission (two characteristic distinct and narrow peaks at 544 nm and 660 nm.) and a channel mode was used to combine DAPI (405 nm excitation, 410-500 nm emission, UCNPs (974 nm excitation, 535-565 nm and 650-668 nm emission bands, and transmitted light signals. 974 nm excitation by a TiSa laser (80 MHz, 350 fs laser pulse width at sample plane) provided the highest emission intensity for upconversion and was used for imaging. The 974 nm laser power was kept low at < 50 µW at sample plane. To capture the slow emission of UCNPs (excited state lifetime on the order of hundreds o f microseconds), the scanning speed was the slowest possible, 132 µs per pixel and the pinhole was opened to 300 um (around 4 Airy units). Pixel size was 132 nm, image size 512×512 pixels, bidirectional scan.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
A NexION 350D ICP-MS instrument (PerkinElmer; Shelton, USA) equipped with Universal Cell Technology™ for spectral interference elimination was used for ICP-MS measurement. The sample introduction system included an internal peristaltic pump with Tygon® tubing (0.38 mm internal diameter), a polytetrafluorethylene concentric nebulizer, and a glass cyclonic spray chamber with a volume of 100 mL. For measurement of 89Y and 174Yb isotopes, the samples were diluted with 2 % nitric acid and spiked with the internal standard (IS) solution (103Rh).
Calibration Y and Yb solutions and IS solution were prepared from solutions of concentration 1.000±0.002 g/L (Merck, Darmstad, Deutschland).
Statistical Methods
The data are presented as the mean ± standard deviation (SD). Statistical analyses were conducted using the GraphPad Software (GraphPad Prism, version 9), employing a one-way ANOVA test followed by Tukey post hoc test. A significance threshold of p <0.05 was applied to determine statistical significance.