Submitted:
11 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. As(V) and As(III) Incorporation by Lactobacilli Strains
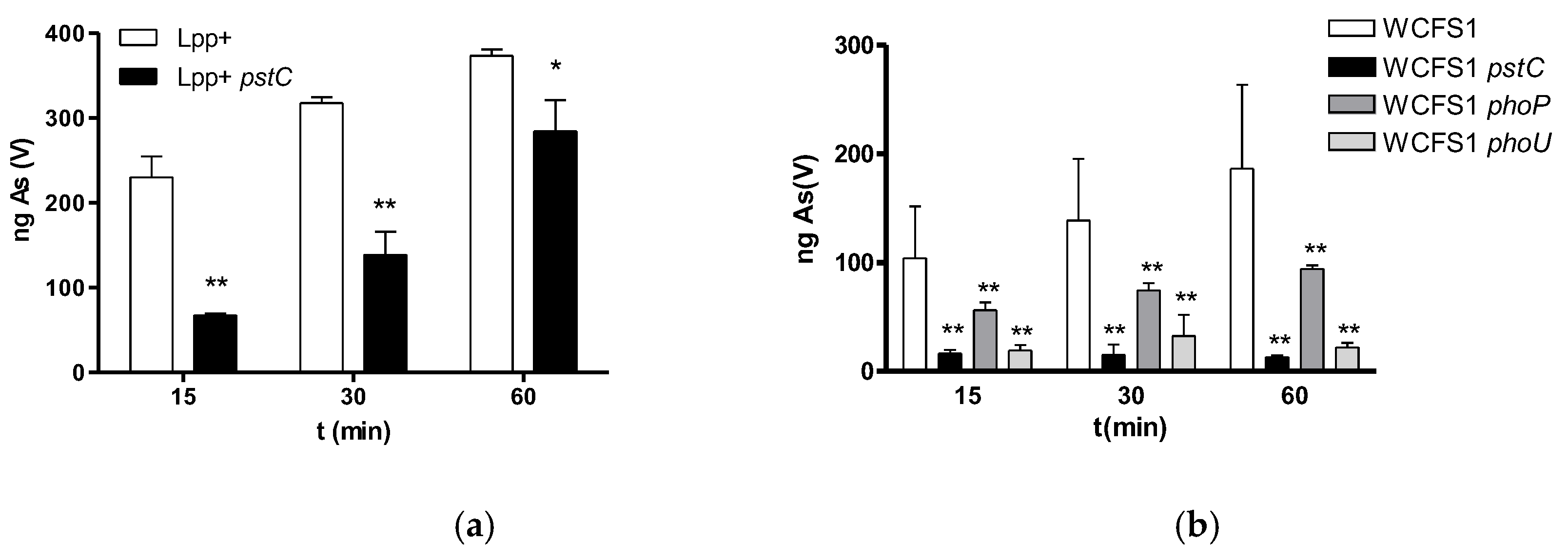
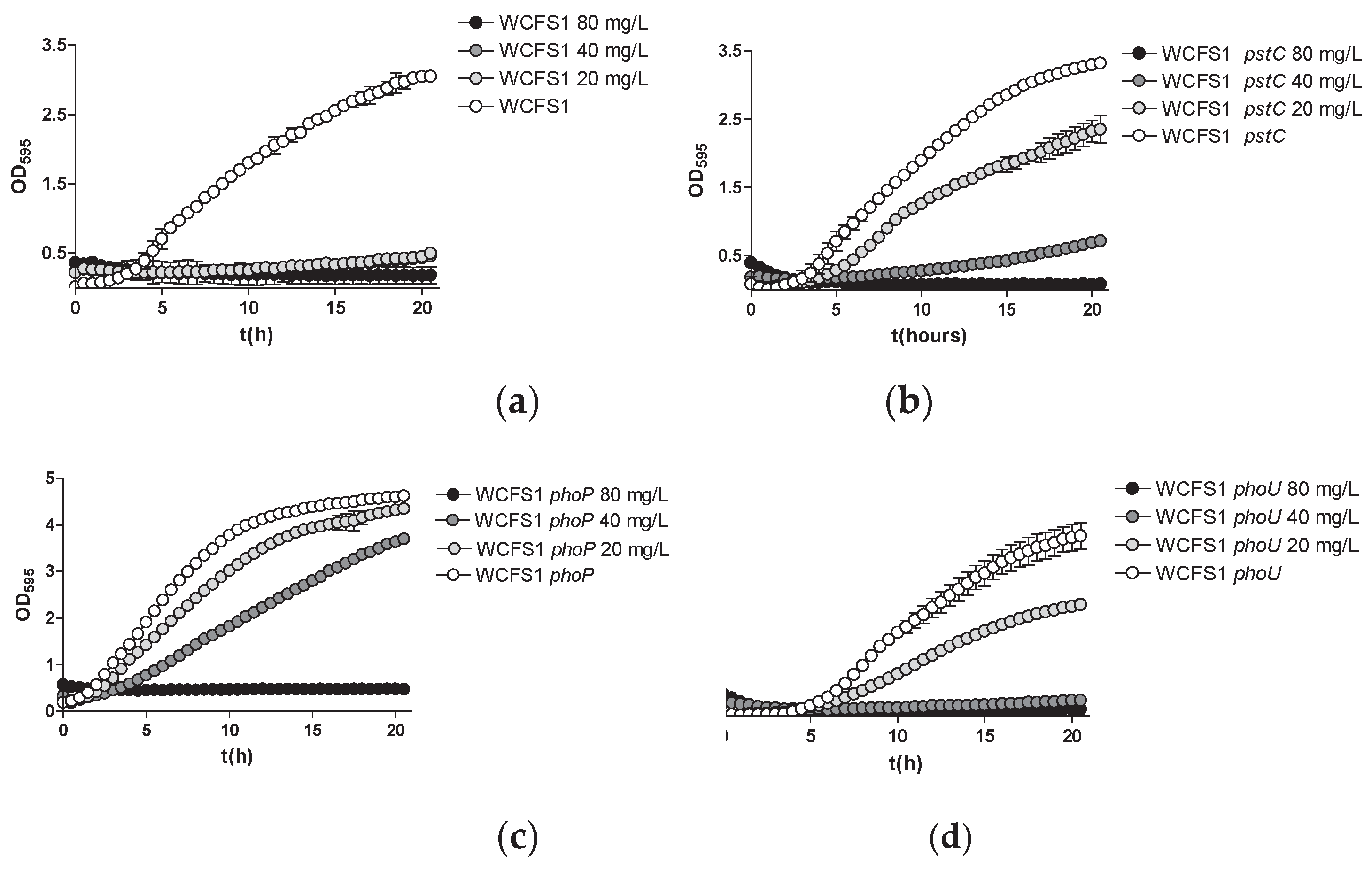
2.2. Mutation of Pst Genes Coding for ABC Phosphate Transporters Impacts As(V) Uptake and Toxicity
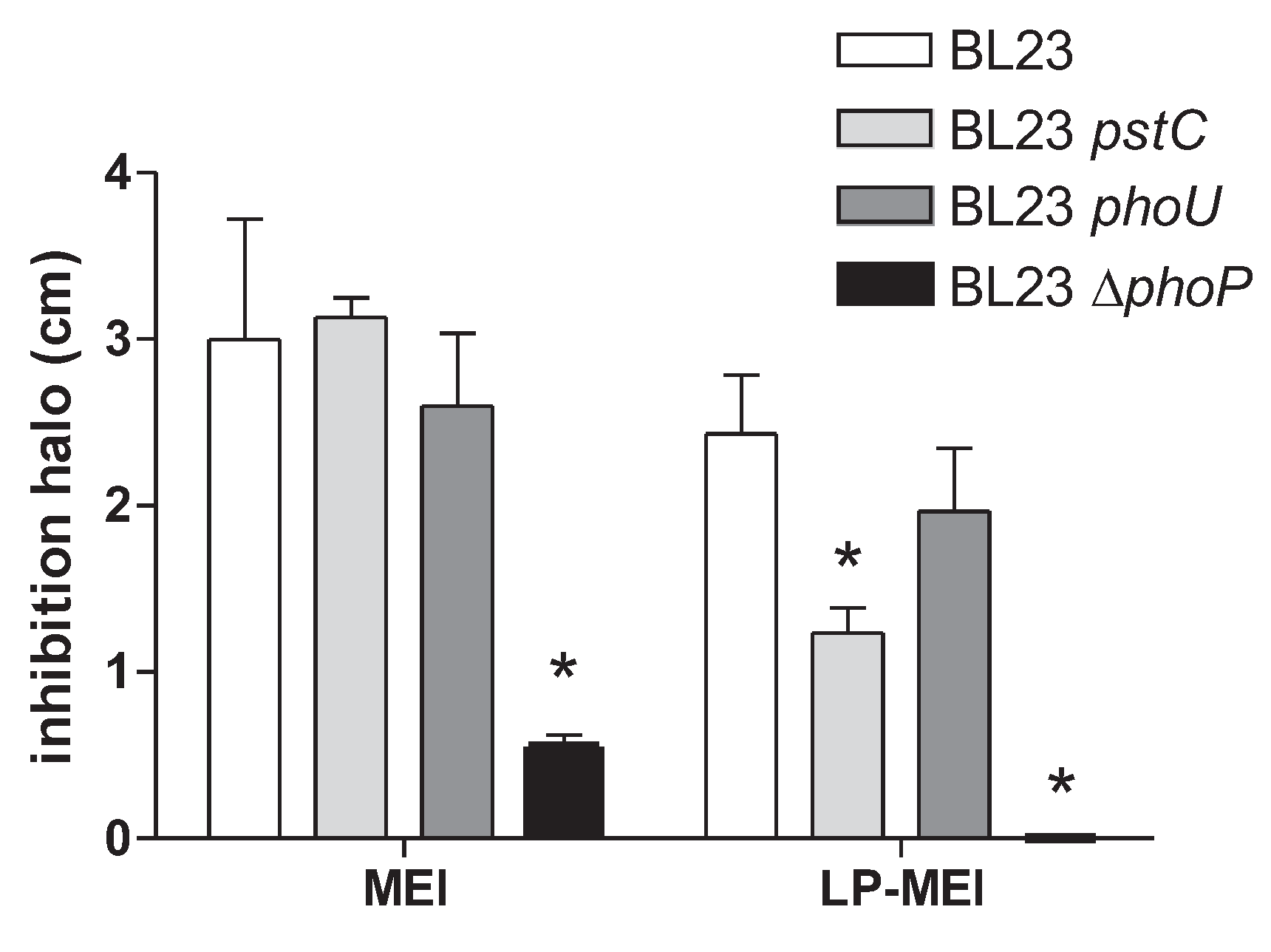
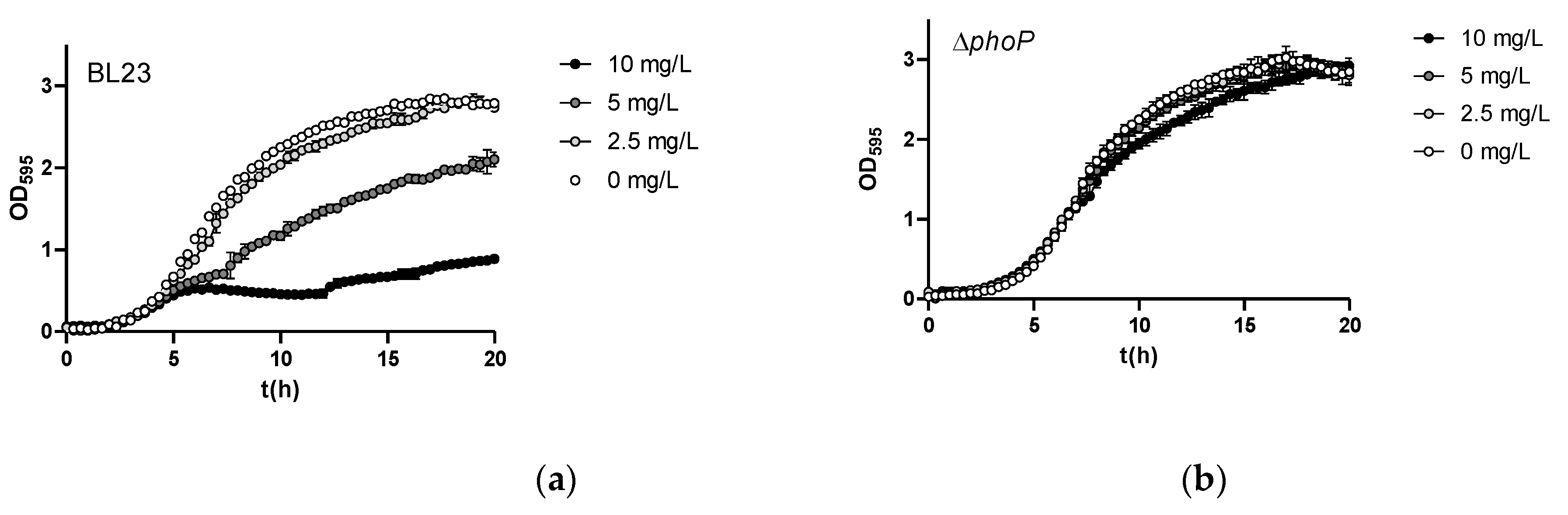
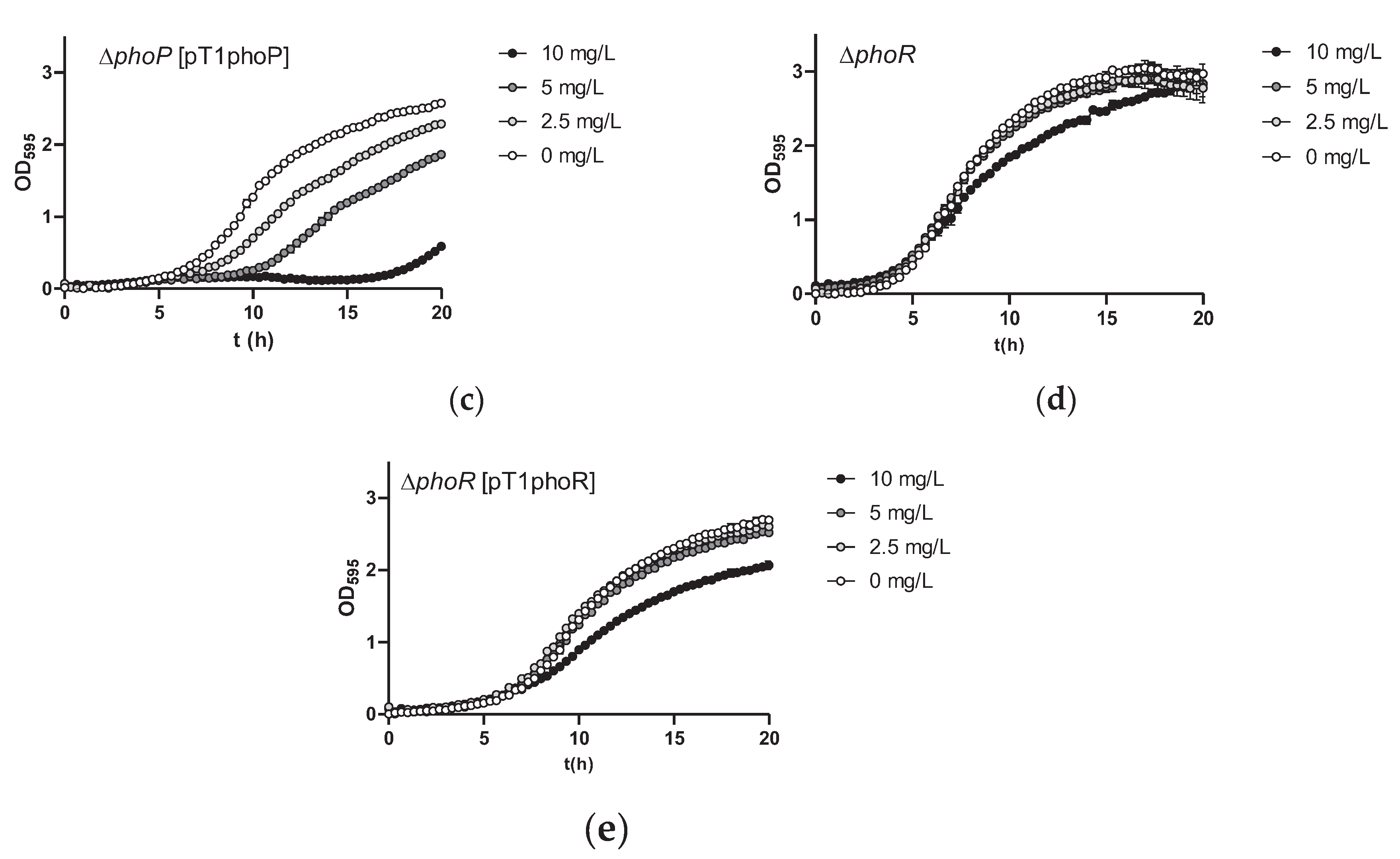
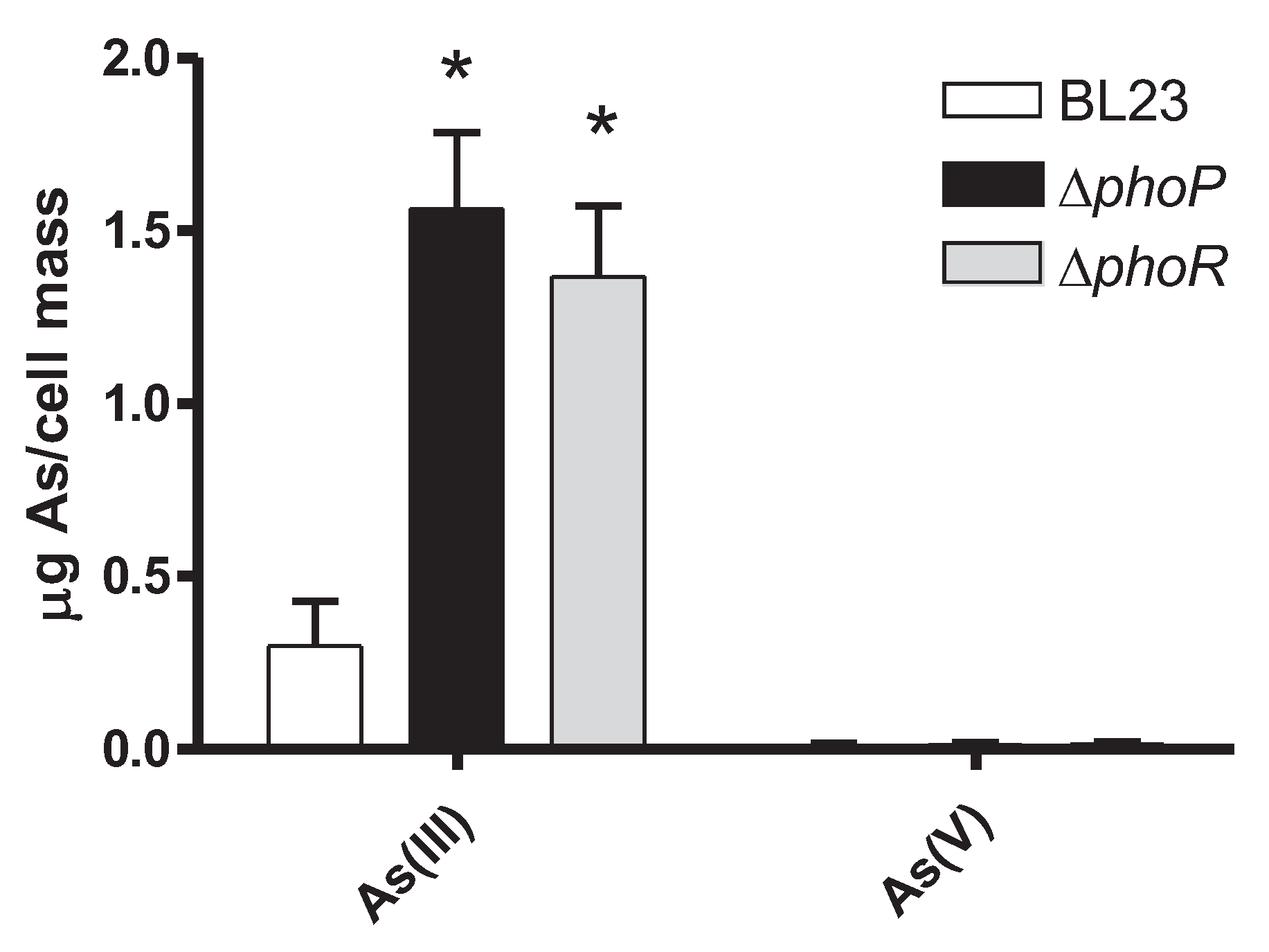
2.3. Mutations in the PhoPR TCS Result in Increased As(III) Resistance in Lc. paracasei
2.4. No Differences in As(III) Oxidation Are Observed in Lc. paracasei phoP or phoR Mutants
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture Conditions
4.2. Construction of Strains Mutated in Pst and Pho Genes
4.3. Strains Complementation
4.4. As Toxicity, Incorporation and Speciation Assays
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO, Arsenic fact sheet. 2012, No. 372, Geneva.
- Mayne, S. T. , The FDA's action plan to reduce dietary exposure to arsenic, lead, cadmium, and mercury for infants and young children. Am J Clin Nutr 2023, 117, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Burton, J. P.; Reid, G. , Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol 2012, 78, 6397–404. [Google Scholar] [CrossRef] [PubMed]
- Chiocchetti, G.M.; Jadán-Piedra, C.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. Use of lactic acid bacteria and yeasts to reduce exposure to chemical food contaminants and toxicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Ren, Q.; Liu, Z.; Zhang, X.; Wu, Z. The Involvement of Lactic Acid Bacteria and Their Exopolysaccharides in the Biosorption and Detoxication of Heavy Metals in the Gut. Biol. Trace Element Res. 2024, 202, 671–684. [Google Scholar] [CrossRef]
- Rodríguez-Viso, P.; Domene, A.; Vélez, D.; Devesa, V.; Zúñiga, M.; Monedero, V. Protective effects of oral administration of lactic acid bacteria strains against methylmercury-induced intestinal toxicity in a murine model. Food Chem. Toxicol. 2024, 114461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gu, S.; Liu, D.; Zhao, L.; Xia, S.; He, X.; Chen, H.; Ge, J. , Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-kappaB signaling cascades. Front Microbiol 2018, 9, 2425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, L.; Shen, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. Int. J. Mol. Sci. 2021, 22, 11045. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.P.; Burton, J.; Gloor, G.B.; Reid, G. Randomized Open-Label Pilot Study of the Influence of Probiotics and the Gut Microbiome on Toxic Metal Levels in Tanzanian Pregnant Women and School Children. mBio 2014, 5, e01580–14. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Protano, C.; Schiavi, E.; Marconi, E.; Capobianco, D.; Massimi, L.; Ristorini, M.; Baldassarre, M.E.; Laforgia, N.; Vitali, M.; et al. A prophylactic multi-strain probiotic treatment to reduce the absorption of toxic elements: In-vitro study and biomonitoring of breast milk and infant stools. Environ. Int. 2019, 130, 104818. [Google Scholar] [CrossRef]
- Jadán-Piedra, C.; Alcántara, C.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. The use of lactic acid bacteria to reduce mercury bioaccessibility. Food Chem. 2017, 228, 158–166. [Google Scholar] [CrossRef]
- Domene, A.; Orozco, H.; Rodríguez-Viso, P.; Monedero, V.; Zúñiga, M.; Vélez, D.; Devesa, V. Lactobacillus strains reduce the toxic effects of a subchronic exposure to arsenite through drinking water. Environ. Res. 2024, 245, 117989. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. K. , Assessment of Lactobacillus rhamnosus mediated protection against arsenic-induced toxicity in zebrafish: a qPCR-based analysis of Firmicutes and Bacteroidetes groups and embryonic development. Arch Microbiol 2023, 205, 316. [Google Scholar] [CrossRef]
- Bora, S.; Lakshman, M.; Madhuri, D.; Kalakumar, B.; Udayakumar, M. Protective Effect of Lactobacillus sporogenes against Arsenic-Induced Hematological Alterations in Male Albino Wistar Rats. Biol. Trace Element Res. 2022, 200, 4744–4749. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Al-Turki, I.A.; Ramadan, M.F. Application of lactic acid bacteria in removing heavy metals and aflatoxin B1 from contaminated water. Water Sci. Technol. 2016, 74, 625–638. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Sorribas, V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate. Toxicol. Appl. Pharmacol. 2008, 232, 125–134. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef]
- Gardner, S. G.; McCleary, W. R. , Control of the phoBR regulon in Escherichia coli. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.G.; Johns, K.D.; Tanner, R.; McCleary, W.R. The PhoU Protein from Escherichia coli Interacts with PhoR, PstB, and Metals To Form a Phosphate-Signaling Complex at the Membrane. J. Bacteriol. 2014, 196, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Revilla-Guarinos, A.; Zúñiga, M. Influence of Two-Component Signal Transduction Systems of Lactobacillus casei BL23 on Tolerance to Stress Conditions. Appl. Environ. Microbiol. 2011, 77, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.J.; Vivó, M.d.L. .; Puig, S.; Zúñiga, M.; Monedero, V.; Devesa, V.; Vélez, D. In vitro evaluation of the efficacy of lactobacilli and yeasts in reducing bioavailability of inorganic arsenic. LWT 2020, 126, 109272. [Google Scholar] [CrossRef]
- Alcántara, C.; Jadán-Piedra, C.; Vélez, D.; Devesa, V.; Zúñiga, M.; Monedero, V. Characterization of the binding capacity of mercurial species in Lactobacillus strains. J. Sci. Food Agric. 2017, 97, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Boekhorst, J.; Van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Cesena, C.; Morelli, L.; Alander, M.; Siljander, T.; Tuomola, E.; Salminen, S.; Mattila-Sandholm, T.; Vilpponen-Salmela, T.; von Wright, A. Lactobacillus crispatus and its Nonaggregating Mutant in Human Colonization Trials. J. Dairy Sci. 2001, 84, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.; del Río. R.; Giner-Lamia, J.; Huerta-Cepas, J. GeCoViz: genomic context visualisation of prokaryotic genes from a functional and evolutionary perspective. Nucleic Acids Res. 2022, 50, W352–W357. [Google Scholar] [CrossRef] [PubMed]
- Mazé, A.; Boël, G.; Zúñiga, M.; Bourand, A.; Loux, V.; Yebra, M.J.; Monedero, V.; Correia, K.; Jacques, N.; Beaufils, S.; et al. Complete Genome Sequence of the Probiotic Lactobacillus casei Strain BL23. J. Bacteriol. 2010, 192, 2647–2648. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Trajtenberg, F. , Two-component sensing and regulation: how do histidine kinases talk with response regulators at the molecular level? Annual Review of Microbiology 2019, 73, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, T.; Finell, M.; Salminen, S. Arsenic removal by native and chemically modified lactic acid bacteria. Int. J. Food Microbiol. 2007, 120, 173–178. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2018, 126, 1149–1160. [Google Scholar] [CrossRef]
- Atalla, A.; Schumann, W. , The pst operon of Bacillus subtilis is specifically induced by alkali stress. Journal of Bacteriology 2003, 185, 5019–5022. [Google Scholar] [CrossRef]
- Morohoshi, T.; Maruo, T.; Shirai, Y.; Kato, J.; Ikeda, T.; Takiguchi, N.; Ohtake, H.; Kuroda, A. , Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl Environ Microbiol 2002, 68, 4107–10. [Google Scholar] [CrossRef]
- Rosen, B.P. Biochemistry of arsenic detoxification. FEBS Lett. 2002, 529, 86–92. [Google Scholar] [CrossRef]
- Isokpehi, R.D.; Udensi, U.K.; Simmons, S.S.; Hollman, A.L.; Cain, A.E.; Olofinsae, S.A.; Hassan, O.A.; Kashim, Z.A.; Enejoh, O.A.; Fasesan, D.E.; et al. Evaluative Profiling of Arsenic Sensing and Regulatory Systems in the Human Microbiome Project Genomes. Microbiol. Insights 2014, 7, MBI–S18076. [Google Scholar] [CrossRef] [PubMed]
- Gustaw, K.; Koper, P.; Polak-Berecka, M.; Rachwał, K.; Skrzypczak, K.; Waśko, A. Genome and Pangenome Analysis of Lactobacillus hilgardii FLUB—A New Strain Isolated from Mead. Int. J. Mol. Sci. 2021, 22, 3780. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, Y.; Wei, S.; Li, Y.; Li, X.; Wang, Q.; Wang, G. Regulation of arsenite oxidation by the phosphate two-component system PhoBR in Halomonas sp. HAL1. Front. Microbiol. 2015, 6, 923. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Shi, M.; Zhang, Y.; Wang, G. Regulation of antimonite oxidation and resistance by the phosphate regulator PhoB in Agrobacterium tumefaciens GW4. Microbiol. Res. 2019, 226, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: a huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef]
- Alcántara, C.; Coll-Marqués, J.M.; Jadán-Piedra, C.; Vélez, D.; Devesa, V.; Zúñiga, M.; Monedero, V. Polyphosphate in Lactobacillus and Its Link to Stress Tolerance and Probiotic Properties. Front. Microbiol. 2018, 9, 1944. [Google Scholar] [CrossRef]
- Leloup, L.; Ehrlich, S.D.; Zagorec, M.; Morel-Deville, F. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 1997, 63, 2117–2123. [Google Scholar] [CrossRef]
- Aukrust, T.; Blom, H. Transformation of Lactobacillus strains used in meat and vegetable fermentations. Food Res. Int. 1992, 25, 253–261. [Google Scholar] [CrossRef]
- Posno, M.; Leer, R.J.; van Luijk, N.; van Giezen, M.J.F.; Heuvelmans, P.T.H.M.; Lokman, B.C.; Pouwels, P.H. Incompatibility of Lactobacillus Vectors with Replicons Derived from Small Cryptic Lactobacillus Plasmids and Segregational Instability of the Introduced Vectors. Appl. Environ. Microbiol. 1991, 57, 1822–1828. [Google Scholar] [CrossRef]
- Schotte, L.; Steidler, L.; Vandekerckhove, J.; Remaut, E. Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzym. Microb. Technol. 2000, 27, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Holo, H.; Nes, I. F. , High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 1989, 55, 3119–23. [Google Scholar] [PubMed]
- Clemente, M.J.; Devesa, V.; Vélez, D. In Vitro Reduction of Arsenic Bioavailability Using Dietary Strategies. J. Agric. Food Chem. 2017, 65, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Ilgen, G.; Fecher, P. Quantitative chemical extraction for arsenic speciation in rice grains. J. Anal. At. Spectrom. 2010, 25, 800–802. [Google Scholar] [CrossRef]
- Domínguez-González, M.R.; Barciela-Alonso, M.C.; Calvo-Millán, V.G.; Herbello-Hermelo, P.; Bermejo-Barrera, P. The bioavailability of arsenic species in rice. Anal. Bioanal. Chem. 2020, 412, 3253–3259. [Google Scholar] [CrossRef]
| Strain designation and collection code | % As(III) retention | % As(V) retention | % DMA retention |
|---|---|---|---|
| BL7 Levilactobacillus brevis DSMZa 1268 | 0.48 ± 0.08 | 0.06 ± 0.01 | 0.09 ± 0.02 |
| BL10 Lactobacillus acidophilus ATCCb 9224 | 3.28 ± 1.40 | 0.80 ± 0.18 | 0.74 ± 0.13 |
| BL17 Lactobacillus acidophilus ATCCb 4356 | 3.99 ± 0.95 | 0.41 ± 0.27 | 0.88 ± 0.27 |
| BL23 Lacticaseibacillus paracasei CECTc 5275 | 0.08 ± 0.07 | 0.09 ± 0.03 | 0.03 ± 0.02 |
| BL36 Levilactobacillus brevis ATCCb 14869 | 3.01 ± 0.19 | 2.92 ± 0.05 | 0.35 ± 0.01 |
| BL73 Lactobacillus acidophilus CNRZd 55 | 2.60 ± 0.32 | 0.77 ± 0.42 | 0.83 ± 0.35 |
| BL75 Lactobacillus acidophilus CNRZd 21 | 3.27 ± 0.06 | 0.49 ± 0.12 | 0.51 ± 0.35 |
| BL166 Lactiplantibacillus plantarum WCFS1e | 1.26 ± 0.12 | 0.01 ± 0.03 | 0.47 ± 0.05 |
| BL221 Lactobacillus crispatus M247f | 1.20 ± 0.10 | 0.36 ± 0.14 | 0.24 ± 0.08 |
| BL278 Lactobacillus crispatus DSMZa 20584 | 1.38 ± 0.04 | 0.27 ±0.01 | 0.37 ± 0.04 |
| BL279 Lactobacillus acidophilus CECTc 4529 | 1.25 ± 0.12 | 0.20 ± 0.09 | 0.41 ± 0.10 |
| BL280 Lactobacillus acidophilus CECTc 4179 | 2.41 ± 0.15 | 0.41 ± 0.13 | 0.76 ± 0.41 |
| Lpp+ Lactiplantibacillus plantarumg | 2.55 ± 0.16 | 5.70 ± 0.16 | 1.12 ± 0.09 |
| Strain | genotype | reference |
|---|---|---|
| Lp. plantarum WCFS1 | wild type | [23] |
| Lp. plantarum DC421 | WCFS1 pstC::pRV300; eryRa | This work |
| Lp. plantarum DC423 | WCFS1 phoP::pRV300; eryR | This work |
| Lp. plantarum DC425 | WCFS1 phoU::pRV300; eryR | This work |
| Lp. plantarum Lpp+ | wild type | [38] |
| Lp. plantarum DC424 | Lpp+ pstC::pRV300; eryR | This work |
| Lc. paracasei BL23 | wild type | [26] |
| Lc. paracasei DC399 | BL23 pstC::pRV300; eryR | This work |
| Lc. paracasei TC04 | BL23 phoP::pRV300; eryR | [20] |
| Lc. paracasei DC398 | BL23 phoU::pRV300; eryR | This work |
| Lc. paracasei DC487 | BL23 phoP | This work |
| Lc. paracasei DC488 | BL23 phoP [pT1phoP]; eryR | This work |
| Lc. paracasei DC489 | BL23 phoR | This work |
| Lc. paracasei DC490 | BL23 phoR [pT1phoR]; eryR | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





