Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microalgae Strains, Culture Conditions, and Growth
2.2. Cellular Density Determination
2.3. Toxicity Tests
3. Results and Discussion
3.1. Relationship between Numbers of Cells per mL and Optical Density
3.2. Toxicity of K2Cr2O7 in Green Microalgae
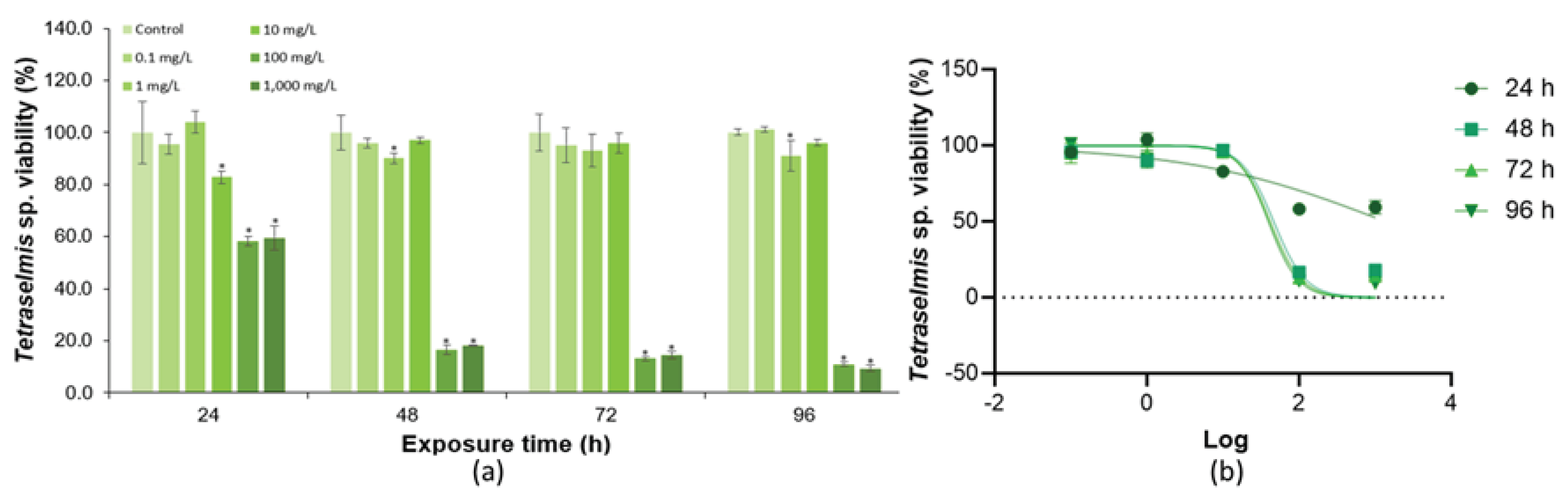
3.3. Toxicity of KCl to Green Microalgae
3.4. Toxicity of SDS to Green Microalgae
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I. Microalgae Taxonomy and Breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI, 2013; pp. 44–53.
- Thoré, E.S.J.; Muylaert, K.; Bertram, M.G.; Brodin, T. Microalgae. Current Biology 2023, 33, R91–R95. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Saxena, R.C. An Introduction to Microalgae: Diversity and Significance. Handbook of Marine Microalgae 2015, 11–24. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Q.; Zhang, Z.; Hu, B.; Chen, J.; Chen, J.; Qian, H. Pollutant Toxicology with Respect to Microalgae and Cyanobacteria. Journal of Environmental Sciences 2021, 99, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Tan, J. Sen; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl Microbiol Biotechnol 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol Adv 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol Adv 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Adams, M.S.; Stauber, J.L.; Jolley, D.F.; Warne, M.S.J. Development and Application of a Multispecies Toxicity Test with Tropical Freshwater Microalgae. Environmental Pollution 2019, 250, 97–106. [Google Scholar] [CrossRef]
- Silva, A.; Figueiredo, S.A.; Sales, M.G.; Delerue-Matos, C. Ecotoxicity Tests Using the Green Algae Chlorella Vulgaris—A Useful Tool in Hazardous Effluents Management. J Hazard Mater 2009, 167, 179–185. [Google Scholar] [CrossRef]
- Boros, B.-V.; Ostafe, V. Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials 2020, 10, 610. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, Fate and Transformation of Emerging Contaminants in Water: An Overarching Review of the Field. Environmental Pollution 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science (1979) 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R.; Sethunathan, N. The Impacts of Environmental Pollutants on Microalgae and Cyanobacteria. Crit Rev Environ Sci Technol 2010, 40, 699–821. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Apte, S.C.; Lim, R.P. Effect of Initial Cell Density on the Bioavailability and Toxicity of Copper in Microalgal Bioassays. Environ Toxicol Chem 2002, 21, 742. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Shen, H. Basic Culturing Techniques. In Handbook of Microalgal Culture; Blackwell Publishing Ltd: Oxford, UK, 2003; pp. 40–56. [Google Scholar]
- Harris, E.H.; Stern, D.B.; Witman, G.B. Chlamydomonas in the Laboratory. In The Chlamydomonas Sourcebook; Harris, E.H., Stern, D.B., Witman, G.B., Eds.; Academic Press: London, 2009; pp. 241–302. ISBN 978-0-12-370873-1. [Google Scholar]
- Prieto, B.; Silva, B.; Lantes, O. Biofilm Quantification on Stone Surfaces: Comparison of Various Methods. Science of The Total Environment 2004, 333, 1–7. [Google Scholar] [CrossRef]
- Prado, R.; García, R.; Rioboo, C.; Herrero, C.; Cid, Á. Suitability of Cytotoxicity Endpoints and Test Microalgal Species to Disclose the Toxic Effect of Common Aquatic Pollutants. Ecotoxicol Environ Saf 2015, 114, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadeh, M.H.; La, H.-J.; Seo, S.-H.; Asgharnejad, H.; Oh, H.-M. Evaluation of Various Techniques for Microalgal Biomass Quantification. J Biotechnol 2015, 216, 90–97. [Google Scholar] [CrossRef]
- Stauber, J.L.; Franklin, N.M.; Adams, M.S. Applications of Flow Cytometry to Ecotoxicity Testing Using Microalgae. Trends Biotechnol 2002, 20, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow Cytometry for the Development of Biotechnological Processes with Microalgae. Biotechnol Adv 2013, 31, 2–16. [Google Scholar] [CrossRef]
- Zhou, F.; Li, C.; Zhu, H.; Li, Y. Determination of Trace Ions of Cobalt and Copper by UV–Vis Spectrometry in Purification Process of Zinc Hydrometallurgy. Optik (Stuttg) 2019, 184, 227–233. [Google Scholar] [CrossRef]
- Parmar, A.; Sharma, S. Derivative UV-Vis Absorption Spectra as an Invigorated Spectrophotometric Method for Spectral Resolution and Quantitative Analysis: Theoretical Aspects and Analytical Applications: A Review. TrAC Trends in Analytical Chemistry 2016, 77, 44–53. [Google Scholar] [CrossRef]
- Dankowska, A.; Domagała, A.; Kowalewski, W. Quantification of Coffea Arabica and Coffea Canephora Var. Robusta Concentration in Blends by Means of Synchronous Fluorescence and UV-Vis Spectroscopies. Talanta 2017, 172, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.L.; Hansen, B.W. Evaluation of the Robustness of Optical Density as a Tool for Estimation of Biomass in Microalgal Cultivation: The Effects of Growth Conditions and Physiological State. Aquac Res 2019, 50, 2698–2706. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Mirzabeygi, V.; Davallo, M. Use of Continuous Wavelet Transform Approach for Simultaneous Quantitative Determination of Multicomponent Mixture by UV–Vis Spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc 2018, 201, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lúcia, H.R.R.; Alexandre, A.; Maria, T.R.-R.; Nelson, F.F. Algal Density Assessed by Spectrophotometry: A Calibration Curve for the Unicellular Algae Pseudokirchneriella Subcapitata. Journal of Environmental Chemistry and Ecotoxicology 2011, 3, 225–228. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and Application of Microalgae. J Ind Microbiol Biotechnol 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Chu, S.P. The Influence of the Mineral Composition of the Medium on the Growth of Planktonic Algae: Part I. Methods and Culture Media. J Ecol 1942, 30, 284. [Google Scholar] [CrossRef]
- ABNT ABNT NBR 12648:2018 - Ecotoxicologia Aquática - Toxicidade Crônica - Método de Ensaio Com Algas (Chlorophyceae) 2018.
- Tompkins, J.; DeVille, M.; Day, J.; Turner, M. Culture Collection of Algae and Protozoa: Catalogue of Strains; Ambleside, UK, 1995. [Google Scholar]
- ABNT ABNT NBR 16181:2021 - Ecotoxicologia Aquática - Toxicidade Crônica - Método de Ensaio Com Microalgas Marinhas 2021.
- Zhang, T.; Tai, F.; Hu, L.; Chen, S. Method for Extracting Pigment Characteristic Spectra from the Phytoplankton Absorption Spectrum. Opt Express 2023, 31, 22233. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P. Photosynthesis in Plants and Algae. Anticancer Res 2022, 42, 5035–5041. [Google Scholar] [CrossRef]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Ali, M.S. Spectral Characterization of Bioactive Compounds from Microalgae: N. Oculata and C. Vulgaris. Biocatal Agric Biotechnol 2019, 19, 101094. [Google Scholar] [CrossRef]
- da Silva, J.C.; Lombardi, A.T. Chlorophylls in Microalgae: Occurrence, Distribution, and Biosynthesis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer International Publishing: Cham, 2020; pp. 1–18. ISBN 978-3-030-50971-2. [Google Scholar]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth Kinetic Models for Microalgae Cultivation: A Review. Algal Res 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Arora, M.; Anil, A.C.; Leliaert, F.; Delany, J.; Mesbahi, E. Tetraselmis Indica (Chlorodendrophyceae, Chlorophyta), a New Species Isolated from Salt Pans in Goa, India. Eur J Phycol 2013, 48, 61–78. [Google Scholar] [CrossRef]
- Lortou, U.; Gkelis, S. Polyphasic Taxonomy of Green Algae Strains Isolated from Mediterranean Freshwaters. Biol Res-Thessaloniki 2019, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Trenkenshu, R.P. Calculation of the Specific Growth Rate of Microalgae. Marine Biological Journal 2019, 4, 100–108. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of Photoperiods on the Growth Rate and Biomass Productivity of Green Microalgae. Bioprocess Biosyst Eng 2014, 37, 735. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Lim, D.K.Y.; Schenk, P.M. Effects of Long Chain Fatty Acid Synthesis and Associated Gene Expression in Microalga Tetraselmis Sp. Marine Drugs 2014, Vol. 12, Pages 3381-3398 2014, 12, 3381–3398.
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Gómez, R.V.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A Simple Spectrophotometric Method for Biomass Measurement of Important Microalgae Species in Aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Murado, M.A.; Prieto, M.A. NOEC and LOEC as Merely Concessive Expedients: Two Unambiguous Alternatives and Some Criteria to Maximize the Efficiency of Dose–Response Experimental Designs. Science of The Total Environment 2013, 461–462, 576–586. [Google Scholar] [CrossRef]
- Bagchi, D. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent Chromium Reduction Ability and Bioremediation Potential of Aspergillus Flavus CR500 Isolated from Electroplating Wastewater. Chemosphere 2019, 237, 124567. [Google Scholar] [CrossRef]
- Shi, L.; Xue, J.; Liu, B.; Dong, P.; Wen, Z.; Shen, Z.; Chen, Y. Hydrogen Ions and Organic Acids Secreted by Ectomycorrhizal Fungi, Pisolithus Sp1, Are Involved in the Efficient Removal of Hexavalent Chromium from Waste Water. Ecotoxicol Environ Saf 2018, 161, 430–436. [Google Scholar] [CrossRef]
- Park, G.-S.; Lee, S.-H.; Lee, S.-M. Phytoplankton as Standard Test Species for Marine Ecotoxicological Evaluation. Journal of Environmental Science International 2005, 14, 1129–1139. [Google Scholar] [CrossRef]
- Bucková, M.; Hegrová, J.; Jandová, V.; Svoboda, J.; Huzlík, J.; Ličbinský, R. Study of Bioaccumulation of Cr, Ni and Zn by the Green Alga Desmodesmus Subspicatus and Assessment of the Toxic Effect of Selected Elements and Their Mixtures on Algae Cells. J Appl Phycol 2023, 35, 2241–2256. [Google Scholar] [CrossRef]
- Berden-Zrimec, M.; Drinovec, L.; Zrimec, A.; Tišler, T. Delayed Fluorescence in Algal Growth Inhibition Tests. Cent Eur J Biol 2007, 2, 169–181. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological Functions of Mineral Macronutrients. Curr Opin Plant Biol 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.L.; Kumar, H.D.; Rai, L.C.; Singh, J.B. Potassium Salts Inhibit Growth of the Cyanobacteria Microcystis Spp. in Pond Water and Defined Media: Implications for Control of Microcystin-Producing Aquatic Blooms. Appl Environ Microbiol 1997, 63, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.T.; Zehnder, A.J.B.; Escher, B.I. Membrane Toxicity of Linear Alcohol Ethoxylates. Environ Toxicol Chem 1999, 18, 2767–2774. [Google Scholar] [CrossRef]
- Reunova, Y.A.; Ayzdaycher, N.A. Effects of Detergent on Chlorophyll a Content and Quantity Dynamics of Microalga Chroomonas Salina (Wils.) Butch. (Cryptophyta). Int J Algae 2003, 5, 90–97. [Google Scholar] [CrossRef]
- Markina, Zh. V. Effects of Sodium Dodecyl Sulfate on the Growth Dynamics and Physiological State of the Microalga Dunaliella Salina (Chlorophyta). Russ J Mar Biol 2010, 36, 191–194. [Google Scholar] [CrossRef]
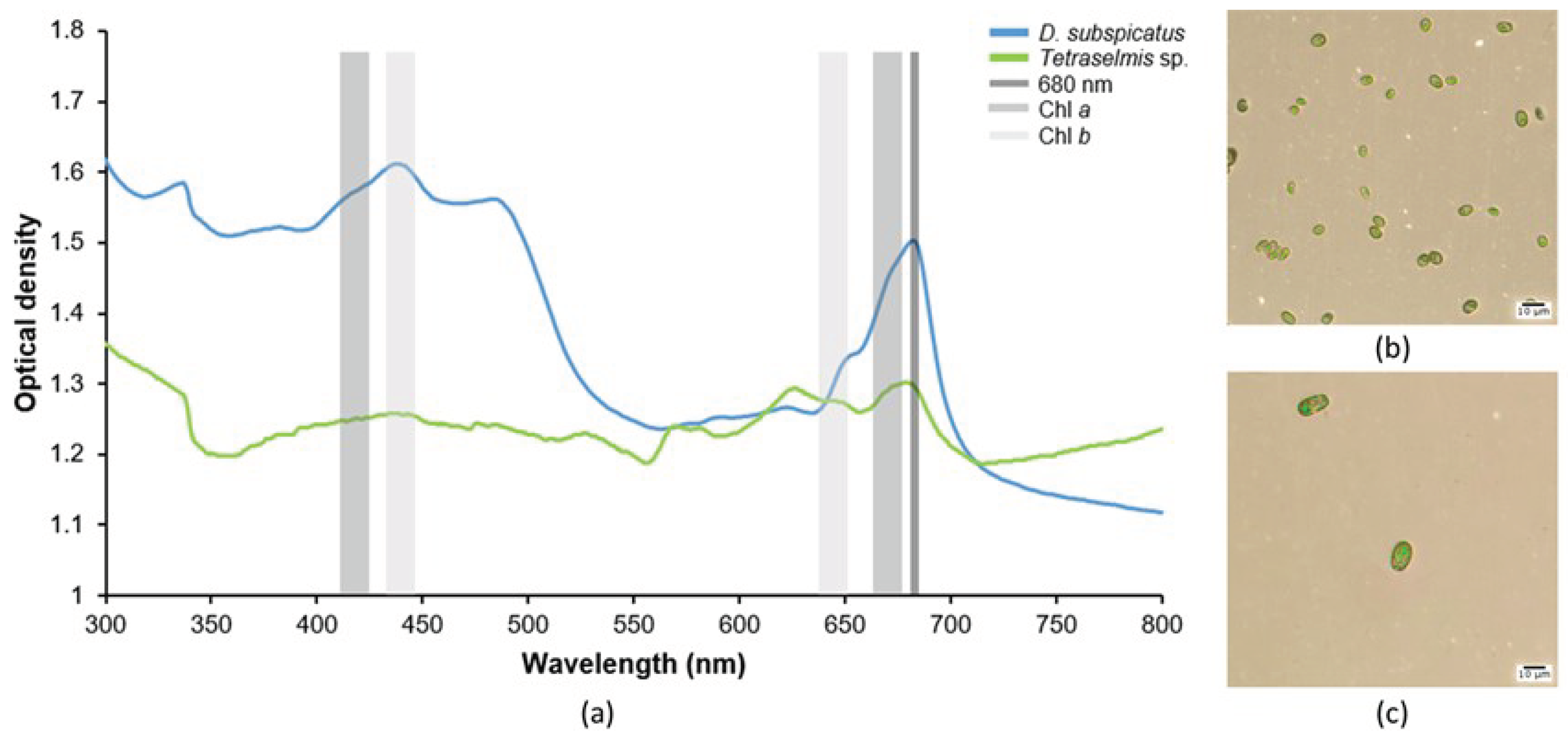
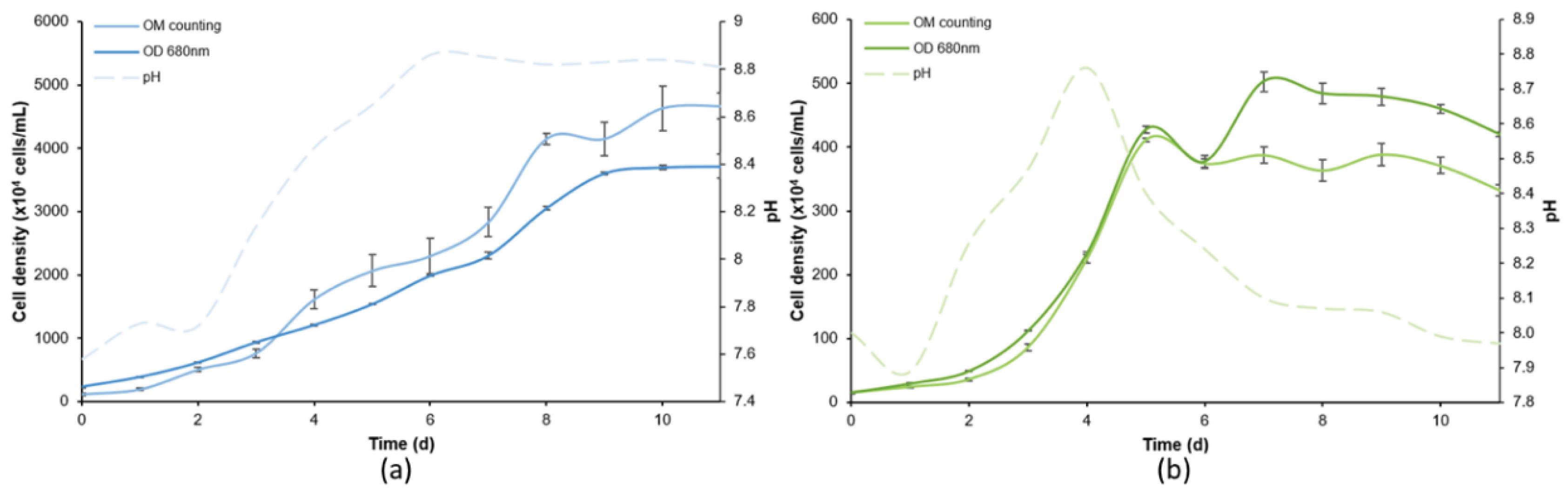
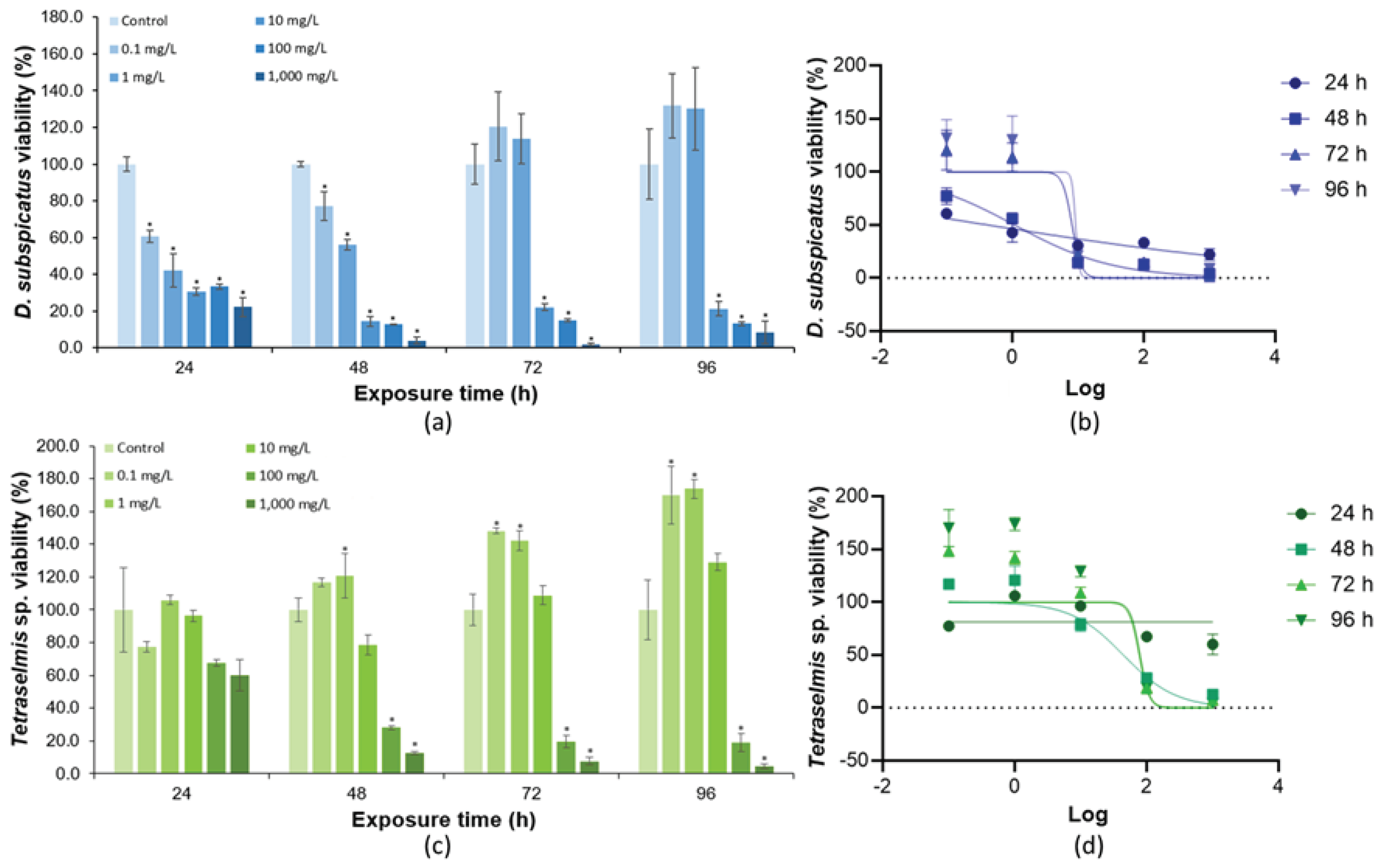
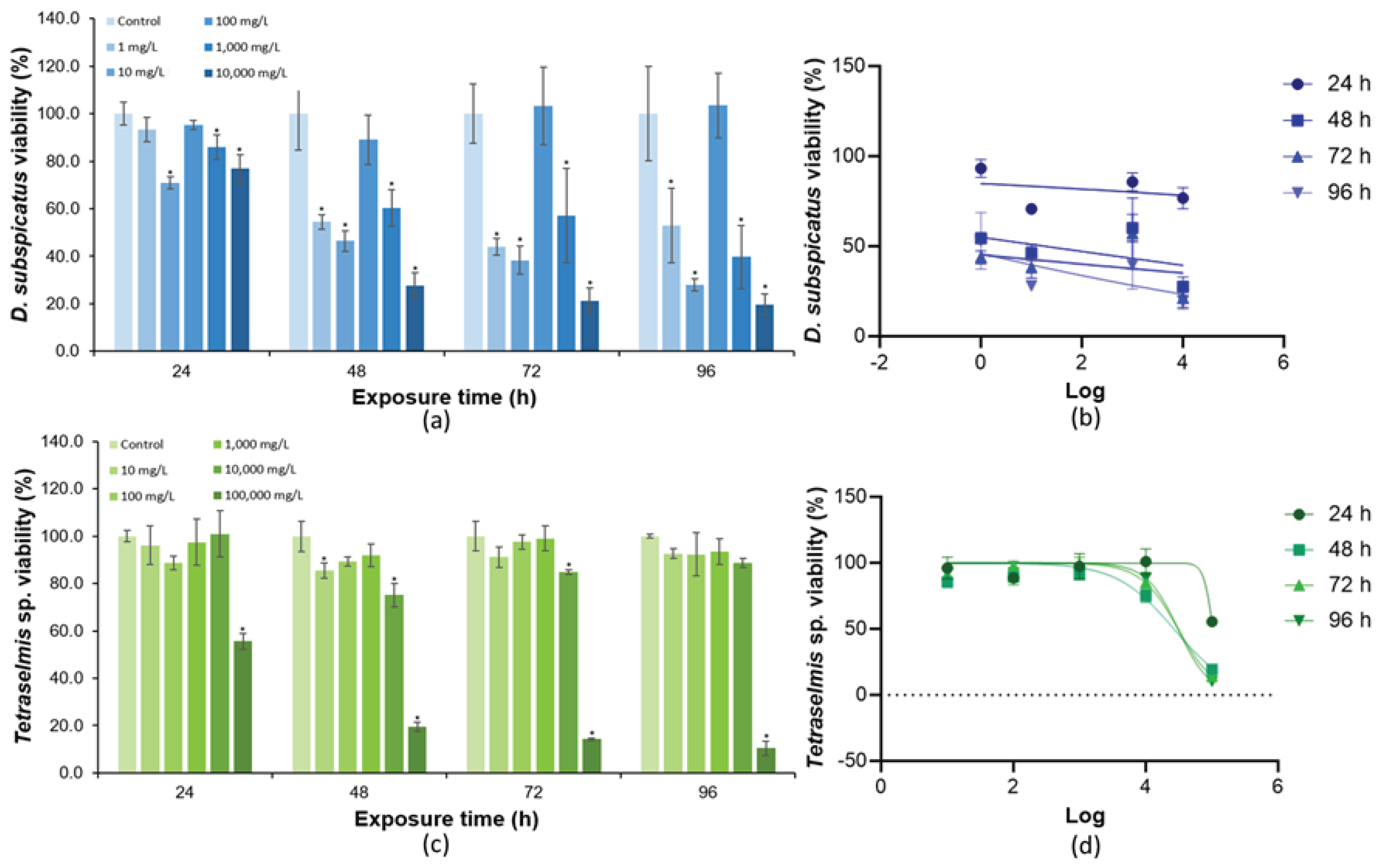
| Specie | Culture | a | b | R2 | F |
|---|---|---|---|---|---|
| D. subspicatus | 1 | 1.573 x 10-01 | 1.252 x 10-07 | 0.996 | 766.7*** |
| 2 | 3.640 x 10-03 | 1.362 x 10-07 | 0.999 | 3280*** | |
| Pooled | 7.354 x 10-02 | 1.313 x 10-07 | 0.980 | 399.1*** | |
| Tetraselmis sp. | 3 | 3.592 x 10-02 | 2.918 x 10-07 | 0.997 | 1113*** |
| 4 | -3.144 x 10-04 | 3.359 x 10-07 | 0.998 | 1345*** | |
| Pooled | 1.771 x 10-02 | 3.151 x 10-07 | 0.992 | 1038*** |
| Microalgae | Cell density technique | μ (d-1) | Doubling time (h) |
|---|---|---|---|
| D. subspicatus | OM | 1.00 | 16.56 |
| OD | 0.71 | 23.52 | |
| Tetraselmis sp. | OM | 3.13 | 5.28 |
| OD | 2.35 | 6.96 |
|
Toxicity Data |
D. subspicatus | Tetraselmis sp. | ||||
|---|---|---|---|---|---|---|
| K2Cr2O7 | KCl | SDS | K2Cr2O7 | KCl | SDS | |
| Exposure time | 72 h | 48 h | 72 h | 48 h | 72 h | 72 h |
| EC50 (mg/L) | 8.1 | 19.57 | - | 44.5 | 30,908 | 41.6 |
| R2 | 0.9120 | 0.2277 | - | 0.8967 | 0.9774 | 0.9564 |
| NOEC (mg/L) | 1 | <1 | 100 | 0.1 | 1,000 | 10 |
| LOEC (mg/L) | 10 | 1 | 1,000 | 1 | 10,000 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





