Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Lactate and Cancer
- 1)
- Malignant cells’ increased glucose uptake and metabolism.
- 2)
- Glycolytic metabolism which occurs even in the presence of oxygen (Warburg effect).
- 3)
- Decreased activity of the pyruvate dehydrogenase (PDH) complex.
- 4)
- Increased activity of pyruvate dehydrogenase kinases (PDK)
- 5)
- Increased expression and activity of the lactate extrusion proteins MCT1 and MCT4.
- 6)
- Increased expression and activity of the glycolytic enzymes.
- 1)
- 2)
- 3)
- 4)
- 5)
- 6)
- 7)
- Lactate activates HIF-1α in non-glycolytic cells (but not in glycolytic cells) which in turn further stimulates glycolysis and angiogenesis [50].
- 8)
- 9)
- Lactate has a positive correlation with radioresistance [53].
- 10)
- It increases hyaluronan production involved in migration and growth [54].
- 11)
- Lactate modulates the tumor microenvironment [55].
- 12)
- 13)
- Lactylation has also been found to be an important mechanism of post-translational modification of proteins associated with poor prognosis in cancer progression [59].
- 14)
- Lactate is an important source of energy for oxidative lactophagic cells [60].
- 15)
- The pyruvate to lactate conversion by lactate dehydrogenase regenerates nicotinamide adenine dinucleotide (NAD+) in the cytoplasm [61] and NAD+ is an important metabolite for redox processes.
1.2. Dichloroacetate (DCA)
1.2.1. Therapeutic History of Dichloroacetate
1.2.2. DCA Enters the Oncology Terrain
- 1)
- To summarize and review published data on DCA use in cancer.
- 2)
- To establish an impartial view of benefits/harm that DCA may cause.
- 3)
- To determine the role that DCA should or should not have in cancer treatment.
- 4)
- To evaluate the opportunity and convenience of further research.
- 5)
- To find out if DCA deserves to be rescued from the hands of unqualified people and introduced as a complementary treatment for cancer.
1.2.3. Chemistry and Pharmacology of DCA
- 1)
- doubts about the possibility of translating findings in other animals to humans.
- 2)
- the importance of the inter-species differences of the clearance mechanism.
1.2.4. Mechanism of Action
- a)
- an inhibitory system represented by the four isoforms of PDK and
- b)
- a "disinhibitory" system represented by the two PDH phosphatases (see below)
2.
2.1. PDH Complex
2.2. PDK Family of Enzymes
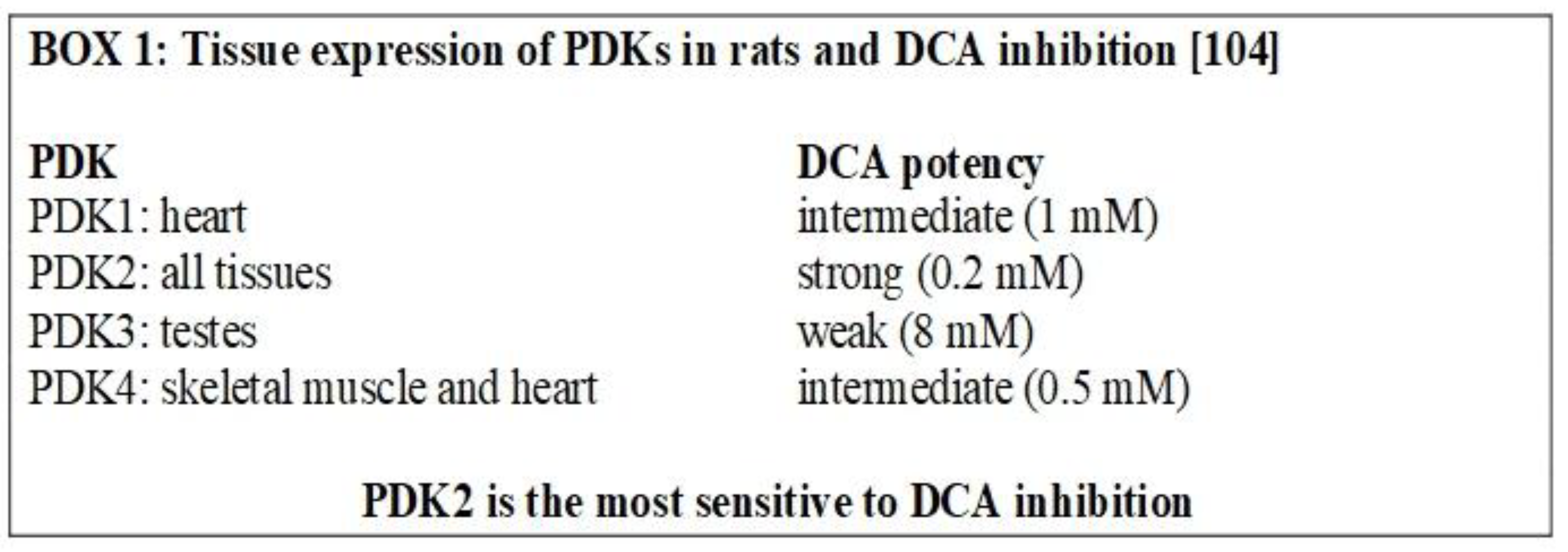
2.3. Inhibition of PDKs

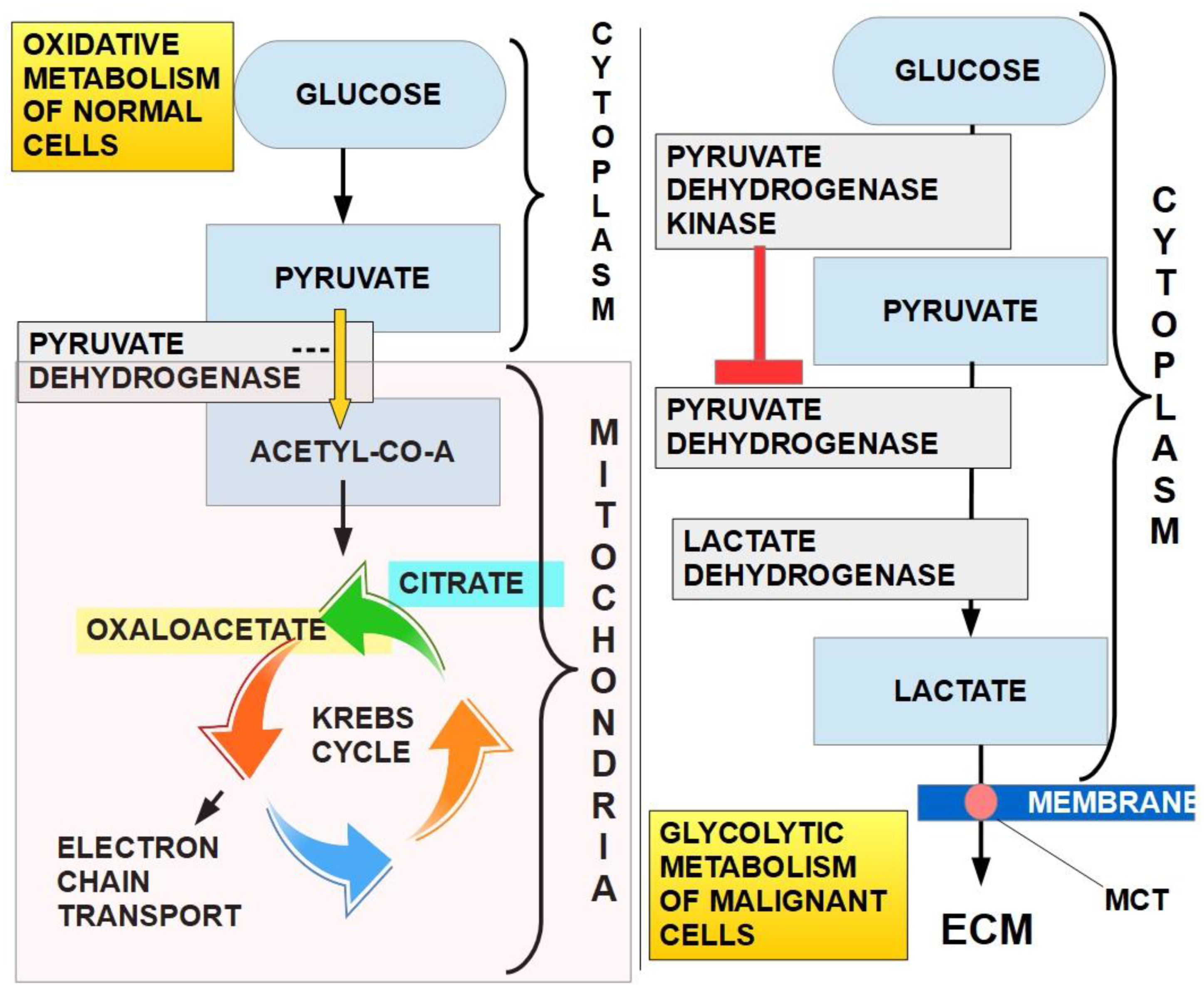
2.4. Pyruvate Dehydrogenase Phosphatases (PDP)
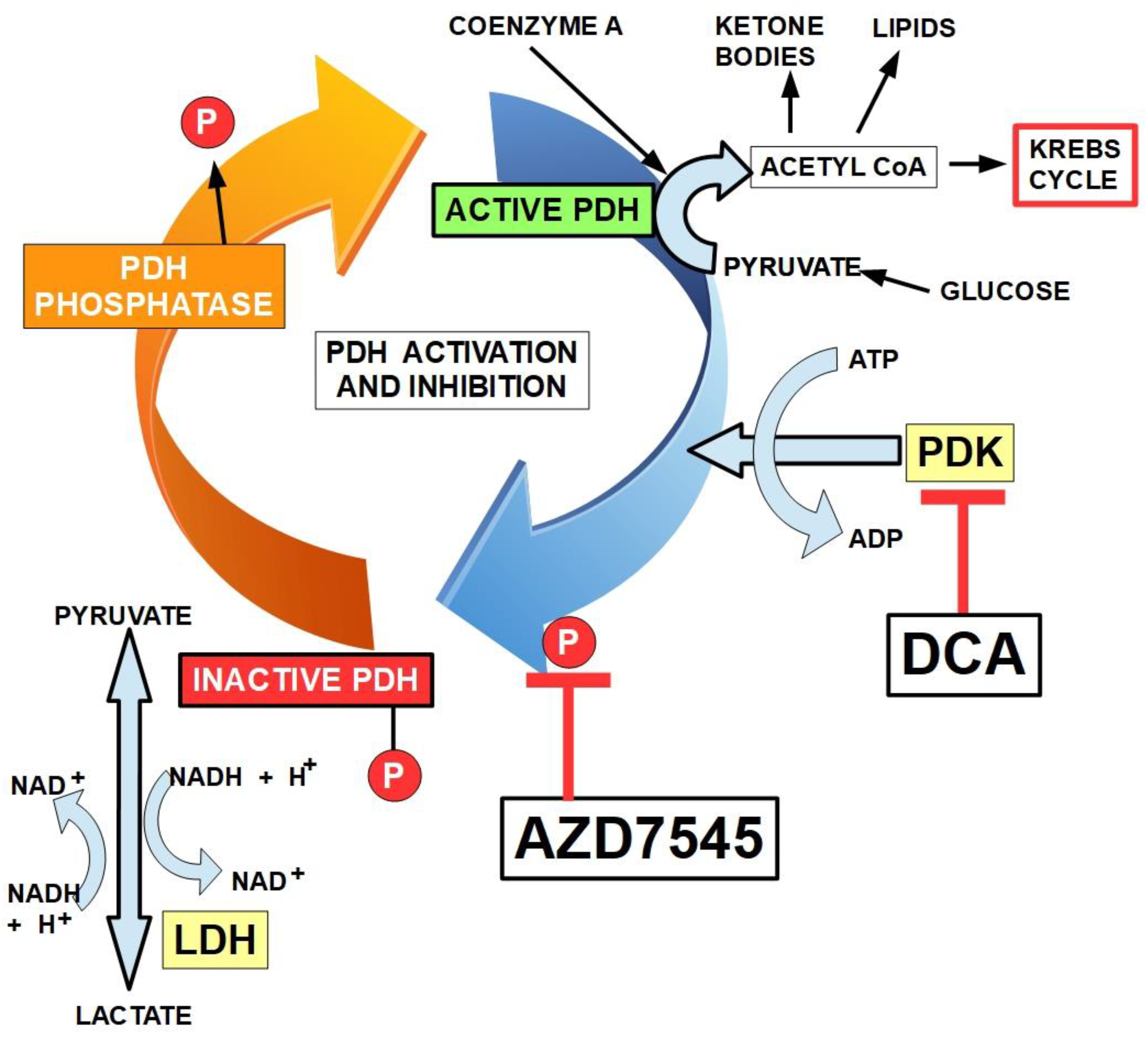
2.5. Mechanism of Action of DCA
Lactylation and DCA
- 1)
- 2)
- can external lactate that enters the cell by MCT1 activity be integrated into lactylation, or is it required that it be converted into pyruvate? If external lactate (from the lactate shuttle) participates significantly in lactylation, DCA would be ineffective in preventing it. Thus far, there is only evidence of lactylation that originates from lactate metabolically produced by the cell [149]. However, for now there is no evidence that excludes lactate imported into cells from participating in lactylation.
3. Experimental Evidence on DCA Activity in Cancer
3.1. Breast Cancer
3.2. Prostate Cancer
- 1)
- in prostate cancer cells Krebs cycle oxaloacetic acid is not regenerated but produced from imported aspartate [175].
- 2)
- prostate cancer is initially not a glycolytic type of tumor, rather it adopts a lipogenic phenotype until some critical time later, during its progression when it becomes glycolytic. This last peculiarity in metabolism contrasts with breast cancer where glycolysis is the predominant metabolic feature from very early stages. This also explains the reasons why fluorodeoxyglucose positron emission scans are of little help at initial stages of prostate cancer [176] and become useful in the advanced stages [177].
3.3. Colon Cancer
3.4. Melanoma
3.5. Glioblastoma
3.6. Hematopoietic Tumors
3.6.1. Myeloma
3.6.2. Lymphoma
3.6.3. Leukemia
3.7. Ovarian, Cervical and Uterine Cancer
3.8. Lung Cancer
3.8.1. Non Small Cell Lung Cancer (NSCLC)
3.8.2. Small Cell Lung Cancer (SCLC)
3.9. Head and Neck Squamous Cell Carcinoma (HNSCC)
3.10. Renal Tumors
3.11. Pancreatic Cancer
3.12. Hepatocarcinoma
3.13. Other Tumors
4. Resistance to DCA
5. DCA and Some Interesting Associations
5.1. DCA and Metformin
5.2. DCA and COX2 Inhibitors
5.3. DCA and Lipoic Acid
5.4. DCA and 2D-Deoxy Glucose (2DG)
5.5. DCA and Bicarbonate
5.6. DCA and Sulindac
5.7. Mitaplatin
5.8. Thiamin
5.9. DCA and Betulinic Acid
5.10. DCA and Rapamycin
5.11. DCA and Vemurafenib
5.12. DCA and Ivermectin
5.13. DCA and TRAIL Liposomes
5.14. DCA and 5-Fluorouracil (5-FU)
5.15. DCA and Chemotherapeutic Drugs in General
5.16. DCA and Salinomycin
5.17. DCA and Propranolol
5.18. DCA and All-Transretinoic Acid (ATRA)
5.19. DCA and Radiotherapy
5.20. DCA and Omeprazol
5.21. DCA and 2-Methoxiestradiol
5.22. DCA and Sirtinol
5.23. DCA and EGFR Tyrosine Kinase Inhibitors
6. DCA and T Cells
7. Side Effects, Toxicity and Doses
8. DCA Dosage
9. DCA Concentrations in Humans and Animals: A Key Issue
10. DCA Derivatives
11. Clinical Cases
11.1. Clinical Trials
Clinical Trials with Poor Results
12. Negative Results with DCA
13. Discussion
- a)
- cells that survive can switch to another type of metabolism such as mitochondrial oxidative metabolism;
- b)
- there may be a persistence of oxidative cells that are not affected by DCA;
- c)
- DCA is cytostatic rather than cytotoxic, and both require very high concentrations;
- d)
- the tumor is predominantly oxidative.
- 1)
- DCA responders: the glycolytic phenotype is due to up-regulation of PDK.
- 2)
- DCA non responders: the glycolytic phenotype is not dependent on PDK up-regulation.
14. Future Perspectives
15. General Conclusions
16. Main Conclusion
17. Declarations
Author Contributions
Funding
Conflicts of Interest
References
- Warburg, O.; Wind, F.; Negelein, E. THE METABOLISM OF TUMORS IN THE BODY. The Journal of General Physiology 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell metabolism 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nature reviews Cancer 2013, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Gramm, C.F.; Scorrano, L.; Zhang, C.Y.; Krauss, S.; Ranger, A.M.; Datta, S.R.; Greenberg, M.E.; Licklider, L.J.; Lowell, B.B.; Gygi, S.P. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003, 424, 952. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A.; Serizawa, S.; Tachibana, K.; Sakurada, K.; Samejima, H.; Kuchino, Y.; Kitanaka, C. Critical role for mitochondrial oxidative phosphorylation in the activation of tumor suppressors Bax and Bak. Journal of the National Cancer Institute 2006, 98, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Moraes, C.T. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-xL in osteosarcoma cells. Journal of Biological Chemistry 2000, 275, 7087–7094. [Google Scholar] [CrossRef]
- Park, S.Y.; Chang, I.; Kim, J.Y.; Kang, S.W.; Park, S.H.; Singh, K.; Lee, M.S. Resistance of mitochondrial DNA-depleted cells against cell death: role of mitochondrial superoxide dismutase. Journal of Biological Chemistry. 2004, 279, 7512–7520. [Google Scholar] [CrossRef]
- Harris, M.H.; Vander Heiden, M.G.; Kron, S.J.; Thompson, C.B. Role of oxidative phosphorylation in Bax toxicity. Molecular and cellular biology 2000, 20, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Lin, Z.; Ertel, A.; Flomenberg, N.; Witkiewicz, A.K.; Birbe, R.C.; Howell, A.; Pavlides, S.; Gandara, R.; Pestell, R.G.; Sotgia, F.; Philp, N.J.; Lisanti, M.P. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011, 10, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.I. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation. Oncology letters 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. The Journal of physiology 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Reviews Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; T Supuran, C.; O Alfarouk, K. The Warburg effect and the Hallmarks of Cancer. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2017, 17, 164–170. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Verduzco, D.; Rauch, C.; Muddathir, A.K.; Adil, H.B.; Elhassan, G.O.; Ibrahim, M.E.; Orozco, J.D.; Cardone, R.A.; Reshkin, S.J.; Harguindey, S. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience 2014, 1, 777. [Google Scholar] [CrossRef]
- Harguindey, S.; Reshkin, S.J. The new pH-centric anticancer paradigm in Oncology and Medicine"; SCB, 2017. In Seminars in cancer biology 2017 Apr (Vol. 43, pp. 1–4). [CrossRef]
- Walenta, S.; Mueller-Klieser, W.F. Lactate: mirror and motor of tumor malignancy. InSeminars in radiation oncology 2004 Jul 1 (Vol. 14, No. 3, 267-274). WB Saunders. [CrossRef]
- Newell, K.; Franchi, A.; Pouyssegur, J.; Tannock, I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proceedings of the National Academy of Sciences 1993, 90, 1127–1131. [Google Scholar] [CrossRef]
- Yamagata, M.; Hasuda, K.; Stamato, T.; Tannock, I.F. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. British journal of cancer 1998, 77, 1726. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Meredith, D.; Fox, J.E.; Manoharan, C.; Davies, A.J.; Halestrap, A.P. Basigin (CD147) Is the Target for Organomercurial Inhibition of Monocarboxylate Transporter Isoforms 1 and 4 THE ANCILLARY PROTEIN FOR THE INSENSITIVE MCT2 IS EMBIGIN (gp70). Journal of Biological Chemistry 2005, 280, 27213–27221. [Google Scholar] [CrossRef] [PubMed]
- Polański, R.; Hodgkinson, C.L.; Fusi, A.; Nonaka, D.; Priest, L.; Kelly, P.; Trapani, F.; Bishop, P.W.; White, A.; Critchlow, S.E.; Smith, P.D. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clinical cancer research 2013, 20, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.; Binet, I.; Steger, U.; Bundick, R.; Ferguson, D.; Murray, C.; Donald, D.; Wood, K. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation 2007, 84, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Ovens, M.J.; Davies, A.J.; Wilson, M.C.; Murray, C.M.; Halestrap, A.P. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochemical Journal 2010, 425, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.M.; Hutchinson, R.; Bantick, J.R.; Belfield, G.P.; Benjamin, A.D.; Brazma, D.; Bundick, R.V.; Cook, I.D.; Craggs, R.I.; Edwards, S.; Evans, L.R. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nature chemical biology 2005, 1, 371. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Otsuka, Y.; Itagaki, S.; Hirano, T.; Iseki, K. Inhibitory effects of statins on human monocarboxylate transporter 4. International journal of pharmaceutics 2006, 317, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Antunes, B.; Batista, A.; Pinto-Ribeiro, F.; Baltazar, F.; Afonso, J. In vivo anticancer activity of AZD3965: a systematic review. Molecules 2021, 27, 181. [Google Scholar] [CrossRef] [PubMed]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. International journal of oncology 2011, 39, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Leukel, P.; Doerfelt, A.; Beier, C.P.; Dettmer, K.; Oefner, P.J.; Kastenberger, M.; Kreutz, M.; Nickl-Jockschat, T.; Bogdahn, U.; Bosserhoff, A.K. Lactate promotes glioma migration by TGF-β2–dependent regulation of matrix metalloproteinase-2. Neuro-oncology 2009, 11, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Oommen, S.; Gohil, K.; Brooks, G.A. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. The FASEB Journal 2007, 21, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor–macrophage interplay to promote breast cancer metastasis. Proceedings of the National Academy of Sciences 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? . The Journal of pathology 2013, 230, 350–355. [Google Scholar] [CrossRef]
- Zong, J.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Tumor-derived factors modulating dendritic cell function. Cancer Immunology, Immunotherapy. 2016, 65, 821–33. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Puig-Kröger, A.; Muniz-Pello, O.; Selgas, R.; Criado, G.; Bajo, M.A.; Sánchez-Tomero, J.A.; Alvarez, V.; del Peso, G.; Sánchez-Mateos, P.; Holmes, C.; Faict, D. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. Journal of Leucocyte Biology 2003, 73, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; Renner, K.; Timischl, B.; Mackensen, A.; Kunz-Schughart, L.; Andreesen, R.; Krause, S.W.; Kreutz, M:. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Constant, J.S.; Feng, J.J.; Zabel, D.D.; Yuan, H.; Suh, D.Y.; Scheuenstuhl, H.; Hunt, T.K.; Hussain, M.Z. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen 2000, 8, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Milovanova, T.N.; Bhopale, V.M.; Sorokina, E.M.; Moore, J.S.; Hunt, T.K.; Hauer-Jensen, M.; Velazquez, O.C.; Thom, S.R. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol Cell Biol 2008, 28, 6248–6261. [Google Scholar] [CrossRef]
- Hunt, T.K.; Aslam, R.S.; Beckert, S.; et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal, 1115. [Google Scholar] [CrossRef]
- Beckert, S.; Farrahi, F.; Aslam, R.S.; et al. Lactate stimulates endothelial cell migration. Wound Repair Regen. [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer research 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Walenta, S.; Salameh, A.; Lyng, H.; Evensen, J.F.; Mitze, M.; Rofstad, E.K.; Mueller-Klieser, W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. The American journal of pathology 1997, 150, 409. [Google Scholar] [PubMed]
- Schwickert, G.; Walenta, S.; Sundfør, K.; Rofstad, E.K.; Mueller-Klieser, W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer research 1995, 55, 4757–4759. [Google Scholar] [PubMed]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.G.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell cycle 2010, 9, 3506–3514. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Prisco, M.; Ertel, A.; Tsirigos, A.; Lin, Z.; Pavlides, S.; Wang, C.; Flomenberg, N.; Knudsen, E.S.; Howell, A.; Pestell, R.G. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell cycle 2011, 10, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Shegay, P.V.; Zabolotneva, A.A.; Shatova, O.P.; Shestopalov, A.V.; Kaprin, A.D. Evolutionary view on lactate-dependent mechanisms of maintaining cancer cell stemness and reprimitivization. Cancers 2022, 14, 4552. [Google Scholar] [CrossRef] [PubMed]
- De Saedeleer, C.J.; Copetti, T.; Porporato, P.E.; Verrax, J.; Feron, O.; Sonveaux, P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PloS one 2012, 7, e46571. [Google Scholar] [CrossRef] [PubMed]
- Samuvel, D.J.; Sundararaj, K.P.; Nareika, A.; Lopes-Virella, M.F.; Huang, Y. Lactate boosts TLR4 signaling and NF-κB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. The Journal of Immunology 2009, 182, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Shime, H.; Yabu, M.; Akazawa, T.; Kodama, K.; Matsumoto, M.; Seya, T.; Inoue, N. Tumor-Secreted Lactic Acid Promotes IL-23/IL-17 Proinflammatory Pathway. J. Immunol 2008, 180, 7175–7183. [Google Scholar] [CrossRef] [PubMed]
- Sattler, U.G.; Meyer, S.S.; Quennet, V.; Hoerner, C.; Knoerzer, H.; Fabian, C.; Yaromina, A.; Zips, D.; Walenta, S.; Baumann, M.; Mueller-Klieser, W. Glycolytic metabolism and tumour response to fractionated irradiation. Radiotherapy and oncology 2010, 94, 102–109. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronidases in cancer biology. InHyaluronan in Cancer Biology 2009 (pp. 207-220). Academic Press. Elsevier. Robert Stein Editor. First Edition Amsterdam, The Netherlands.
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Frontiers in oncology 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Julian, C.G.; Matarazzo, C.; Martinez, J.; Brooks, G.A. Is lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Frontiers in oncology 2020, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.T.; Wellen, K.E. Histone lactylation links metabolism and gene regulation. Nature 2019, 574, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Wang, H.; Li, Q.; Liu, S.; Qu, K.; Liu, C.; Zhang, J. Lactylation: a Passing Fad or the Future of Posttranslational Modification. Inflammation 2022, 45, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int J Mol Sci 2022, 23, 11943. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The tortuous path of lactate shuttle discovery: From cinders and boards to the lab and ICU. Journal of sport and health science 2020, 9, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Hopp, A.K.; Grüter, P.; Hottiger, M.O. Regulation of glucose metabolism by NAD+ and ADP-ribosylation. Cells 2019, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- Kocianova, E.; Piatrikova, V.; Golias, T. Revisiting the Warburg Effect with Focus on Lactate. Cancers 2022, 14, 6028. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wan, J.; Cai, K.; Song, H.; Wang, Y.; Sun, W.; Hu, J. Understanding the contribution of lactate metabolism in cancer progress: a perspective from isomers. Cancers 2022, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Harman, E.M.; Curry, S.H.; Baumgartner, T.G.; Misbin, R.I. Treatment of lactic acidosis with dichloroacetate. New England Journal of Medicine 1983, 309, 390–396. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Lorenz, A.C.; Thomas, R.G.; Harman, E.M. Dichloroacetate in the treatment of lactic acidosis. Annals of internal medicine 1988, 108, 58–63. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Kerr, D.S.; Barnes, C.; Bunch, S.T.; Carney, P.R.; Fennell, E.M.; Felitsyn, N.M.; Gilmore, R.L.; Greer, M.; Henderson, G.N.; Hutson, A.D. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics 2006, 117, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Irsigler, K.; Brändle, J.; Kaspar, L.; Kritz, H.; Lageder, H.; Regal, H. Treament of biguanide-induced lactic acidosis with dichloroacetate. 3 case histories. Arzneimittel-Forschung 1979, 29, 555–559. [Google Scholar] [PubMed]
- Stacpoole, P.W.; Wright, E.C.; Baumgartner, T.G.; Bersin, R.M.; Buchalter, S.; Curry, S.H.; Duncan, C.A.; Harman, E.M.; Henderson, G.N.; Jenkinson, S.; Lachin, J.M. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. New England Journal of Medicine 1992, 327, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Agbenyega, T.; Angus, B.J.; Bedu-Addo, G.; Ofori-Amanfo, G.; Henderson, G.; Szwandt, I.S.; O'BRIEN, R. , Stacpoole, P.W. Pharmacokinetics and pharmacodynamics of dichloroacetate in children with lactic acidosis due to severe malaria. QJM: An International Journal of Medicine 1995, 88, 341–349. [Google Scholar] [PubMed]
- Stacpoole, P.W.; Barnes, C.L.; Hurbanis, M.D.; Cannon, S.L.; Kerr, D.S. Treatment of congenital lactic acidosis with dichloroacetate. Archives of disease in childhood 1997, 77, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; Harry, G. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Plas, D.R.; Thompson, C.B. Cell metabolism in the regulation of programmed cell death. Trends in Endocrinology & Metabolism 2002, 13, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Agbenyega, T.; Planche, T.; Bedu-Addo, G.; Ansong, D.; Owusu-Ofori, A.; Bhattaram, V.A.; Nagaraja, N.V.; Shroads, A.L.; Henderson, G.N.; Hutson, A.D.; Derendorf, H. Population kinetics, efficacy, and safety of dichloroacetate for lactic acidosis due to severe malaria in children. The Journal of Clinical Pharmacology 2003, 43, 386–396. [Google Scholar] [CrossRef] [PubMed]
- 2005. Dichloroacetic Acid in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality. Downloaded December 26, 2023 from https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/dichloroaceticacid0505.pdf?sfvrsn=2246389b_4.
- Stacpoole, P.W. Clinical Physiology and Pharmacology of GSTZ1/MAAI. Biochemical Pharmacology 2023, 115818. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.H.; Lorenz, A.; Chu, P.I.; Limacher, M.; Stacpoole, P.W. Disposition and pharmacodynamics of dichloroacetate (DCA) and oxalate following oral DCA doses. Biopharm Drug Dispos 1991, 12, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Pharmacokinetics of Sodium Dichloroacetate. PhD dissertation. Gainesville, FL:University of Florida, 1987. Downloaded from https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Chu+P-I.+Pharmacokinetics+of+Sodium+Dichloroacetate.+PhD+dissertation.+Gainesville%2C+FL%3AUniversity+of+Florida%2C+1987.&btnG= Accessed February 2023.
- Evans, O.B. Dichloroacetate tissue concentration and its relationship to hypolactatemia and pyruvate dehydrogenase activity by dichloroacetate. Biochemical Pharmacology 1982, 31, 3124–3126. [Google Scholar] [CrossRef] [PubMed]
- Shroads, A.L.; Guo, X.; Dixit, V.; Liu, H.P.; James, M.O.; Stacpoole, P.W. Age-dependent kinetics and metabolism of dichloroacetate: possible relevance to toxicity. Journal of Pharmacology and Experimental Therapeutics 2008, 324, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.C.; Smeltz, M.G.; Hu, Z.; Rowland-Faux, L.; Zhong, G.; Lorenzo, R.J.; Cisneros, K.V.; Stacpoole, P.W.; James, M.O. Regulation of dichloroacetate biotransformation in rat liver and extrahepatic tissues by GSTZ1 expression and chloride concentration. Biochem Pharmacol 2018, 152, 236–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- James, M.O.; Yan, Z.; Cornett, R.; Jayanti, V.M.; Henderson, G.N.; Davydova, N.; Katovich, M.J.; Pollock, B.; Stacpoole, P.W. Pharmacokinetics and metabolism of [14C] dichloroacetate in male sprague-dawley rats: Identification of glycine conjugates, including hippurate, as urinary metabolites of dichloroacetate. Drug Metabolism and Disposition 1998, 26, 1134–1143. [Google Scholar] [PubMed]
- Wells, P.G.; Moore, G.W.; Rabin, D.; Wilkinson, G.R.; Oates, J.A.; Stacpoole, P.W. Metabolic effects and pharmacokinetics of intravenously administered dichloroacetate in humans. Diabetologia 1980, 19, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W. The pharmacology of dichloroacetate. Metabolism-Clinical and Experimental 1989, 38, 1124–1144. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Moore, G.W.; Kornhauser, D.M. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N Engl J Med 1978, 298, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Leon, A.; Schultz, I.R.; Xu, G.; Bull, R.J. Pharmacokinetics and metabolism of dichloroacetate in the F344 rat after prior administration in drinking water. Toxicology and applied pharmacology 1997, 146, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lukas, G.; Vyas, K.H.; Brindle, S.D.; Le Sher, A.R.; Wagner, W.E. Biological disposition of sodium dichloroacetate in animals and humans after intravenous administration. Journal of pharmaceutical sciences 1980, 69, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Maisenbacher, H.W.; Shroads, A.L.; Zhong, G.; Daigle, A.D.; Abdelmalak, M.M.; Samper, I.S.; Mincey, B.D.; James, M.O.; Stacpoole, P.W. Pharmacokinetics of oral dichloroacetate in dogs. Journal of biochemical and molecular toxicology 2013, 27, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Lane, J.R.; Turkel, C.C.; Capparelli, E.V.; Dziewanowska, Z.; Fox, A.W. Dichloroacetate: population pharmacokinetics with a pharmacodynamic sequential link model. The Journal of Clinical Pharmacology 2001, 41, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Henderson, G.N.; Yan, Z.; James, M.O. Clinical pharmacology and toxicology of dichloroacetate. Environmental health perspectives 1998, 106, 989. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Webster, L.; Mackey, J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. British journal of cancer 2008, 99, 989. [Google Scholar] [CrossRef]
- Stockwin, L.H.; Yu, S.X.; Borgel, S.; Hancock, C.; Wolfe, T.L.; Phillips, L.R.; Hollingshead, M.G.; Newton, D.L. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. International journal of cancer 2010, 127, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Constantin-Teodosiu, D. Regulation of muscle pyruvate dehydrogenase complex in insulin resistance: effects of exercise and dichloroacetate. Diabetes & metabolism journal 2013, 37, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M.; McCormack, J.G.; Rutter, G.A.; Burnett, P.; Edgell, N.J.; Moule, S.K.; Diggle, T.A. The hormonal regulation of pyruvate dehydrogenase complex. Advances in enzyme regulation 1996, 36, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Schoenmann, N.; Tannenbaum, N.; Hodgeman, R.M.; Raju, R.P. Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1667. [Google Scholar] [CrossRef]
- Popov, K.M.; Zhao, Y.; Shimomura, Y.; Kuntz, M.J.; Harris, R.A. Branched-chain alpha-ketoacid dehydrogenase kinase. Molecular cloning, expression, and sequence similarity with histidine protein kinases. Journal of Biological Chemistry 1992, 267, 13127–13130. [Google Scholar] [CrossRef] [PubMed]
- Gudi, R.; Melissa, M.B.; Kedishvili, N.Y.; Zhao, Y.; Popov, K.M. Diversity of the pyruvate dehydrogenase kinase gene family in humans. Journal of Biological Chemistry 1995, 270, 28989–28994. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.; Scherer, S.W.; Xi, T.; Majer, M.; Nickle, D.C.; Rommens, J.M.; Popov, K.M.; Harris, R.A.; Riebow, N.L.; Xia, J.; Tsui, L.C. Cloning and characterization of PDK4 on 7q21. 3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. Journal of Biological Chemistry 1996, 271, 22376–22382. [Google Scholar] [CrossRef]
- Simon, M.C. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab 2006, 3, 150–151. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell metabolism 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, T.; Saramäki, A.; Malinen, M.; Rieck, M.; Väisänen, S.; Huotari, A.; Herzig, K.H.; Müller, R.; Carlberg, C. Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor β/δ. Journal of molecular biology 2007, 372, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Peters, J.M.; Harris, R.A. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor α. Biochemical and biophysical research communications 2001, 287, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Contractor, T.; Harris, C.R. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer research 2011. [Google Scholar] [CrossRef]
- Yeaman, S.J.; Hutcheson, E.T.; Roche, T.E.; Pettit, F.H.; Brown, J.R.; Reed, L.J.; Watson, D.C.; Dixon, G.H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry 1978, 17, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Bowker-Kinley, M.M.; Davis, I.W.; Pengfei, W.U.; Harris, A.R.; Popov, MK. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochemical Journal 1998, 329, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.; Ramos, H.; Soares, J.; Saraiva, L. p53 and glucose metabolism: an orchestra to be directed in cancer therapy. Pharmacological research 2018, 131, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Li, J.; Chuang, J.L.; Chuang, D.T. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure 2007, 15, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Morley, A. An evolutionary perspective on the Crabtree effect. Frontiers in molecular biosciences 2014, 1, 17. [Google Scholar] [CrossRef]
- Saunier, E.; Benelli, C.; Bortoli, S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. International journal of cancer 2016, 138, 809–817. [Google Scholar] [CrossRef]
- Holness, M.J.; and Sugden, M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans 2003, 31, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, S.; Cooper, R.H.; Randle, P.J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochemical Journal 1974, 141, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Kankotia, S.; Stacpoole, P.W. Dichloroacetate and cancer: new home for an orphan drug? . Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2014, 1846, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, S.; Randle, P.J. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication). Biochem J 1973, 134, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Lorini, M.; Ciman, M. Hypoglycaemic action of diisopropylammonium salts in experimental diabetes. Biochemical pharmacology 1962, 11, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. Dichloroacetate induced intracellular acidification in glioblastoma: in vivo detection using AACID-CEST MRI at 9.4 Tesla. Journal of neuro-oncology 2018, 136, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Anemone, A.; Consolino, L.; Conti, L.; Reineri, F.; Cavallo, F.; Aime, S.; Longo, D.L. In vivo evaluation of tumour acidosis for assessing the early metabolic response and onset of resistance to dichloroacetate by using magnetic resonance pH imaging. International journal of oncology 2017, 51, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Albatany, M.; Li, A.; Meakin, S.; Bartha, R. In vivo detection of acute intracellular acidification in glioblastoma multiforme following a single dose of cariporide. International journal of clinical oncology 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Xiong, H.; Zhou, P.; Ni, Z.; Yang, T.; Zhang, Y.; Zeng, Y.; He, J.; Yang, F.; Zhang, N. Inhibition of COX2 enhances the chemosensitivity of dichloroacetate in cervical cancer cells. Oncotarget 2017, 8, 51748. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Treading slowly through hypoxic waters: dichloroacetate to the rescue! J Physiol 2018, 596, 2957–2958. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Rajendran, G.; Harris, R.A.; Taylor JA 3rd. Metabolic Flexibility in Cancer: Targeting the Pyruvate Dehydrogenase Kinase:Pyruvate Dehydrogenase Axis. Mol Cancer Ther 2019, 18, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lee, W.D.; Leitner, B.P.; Zhu, W.; Fosam, A.; Li, Z.; Gaspar, R.C.; Halberstam, A.A.; Robles, B.; Rabinowitz, J.D.; Perry, R.J. Dichloroacetate as a novel pharmaceutical treatment for cancer-related fatigue in melanoma. American Journal of Physiology-Endocrinology and Metabolism 2023, 325, E363–E375. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, E.A.; Mahmoud, H.M.; Azouz, A.A. Management of ulcerative colitis by dichloroacetate: Impact on NFATC1/NLRP3/IL1B signaling based on bioinformatics analysis combined with in vivo experimental verification. Inflammopharmacology 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. JNCI: Journal of the National Cancer Institute 2017, 109, djx071. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and cancer: an overview towards clinical applications. Oxidative medicine and cellular longevity 2019, 2019, 8201079. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Felts, J.M. Diisopropylammonium dichloroacetate (DIPA) and sodium dichloroacetate (DCA): Effect on glucose and fat metabolism in normal and diabetic tissue. Metabolism 1970, 19, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Harwood, H.J.; Varnado, C.E. Regulation of rat liver hydroxymethylglutaryl coenzyme A reductase by a new class of noncompetitive inhibitors. Effects of dichloroacetate and related carboxylic acids on enzyme activity. The Journal of clinical investigation 1983, 72, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.W.; Swift, L.L.; Rabinowitz, D.; Crofford, O.B.; Oates, J.A.; Stacpoole, P.W. Reduction of serum cholesterol in two patients with homozygous familial hypercholesterolemia by dichloroacetate. Atherosclerosis 1979, 33, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Stakišaitis, D.; Kapočius, L.; Kilimaitė, E.; Gečys, D.; Šlekienė, L.; Balnytė, I.; Palubinskienė, J.; Lesauskaitė, V. Preclinical Study in Mouse Thymus and Thymocytes: Effects of Treatment with a Combination of Sodium Dichloroacetate and Sodium Valproate on Infectious Inflammation Pathways. Pharmaceutics 2023, 15, 2715. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; Ding, J. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Qu, J.; Li, P.; Sun, Z. Histone lactylation regulates cancer progression by reshaping the tumor microenvironment. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Huang, D.; Jiang, Y.; Hou, J.; Tian, M.; Li, J.; Sun, L.; Zhang, Y.; Zhang, T.; Li, Z.; Li, Z. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Frontiers in oncology 2021, 11, 647559. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, Z.; Li, Z.; Jing, D.S.; Fan, G.X.; Liu, M.Q.; Zhuo, Q.F.; Ji, S.R.; Yu, X.J.; Xu, X.W.; Qin, Y. Lactate-induced protein lactylation: A bridge between epigenetics and metabolic reprogramming in cancer. Cell proliferation 2023, e13478. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Lv, Y.; Dai, X. Lactate, histone lactylation and cancer hallmarks. Expert Reviews in Molecular Medicine 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Q.; Zheng, J.; Yang, Y.; Zhang, X.; Ma, A.; Qin, Y.; Qin, Z.; Zheng, X. The function and mechanism of lactate and lactylation in tumor metabolism and microenvironment. Genes & Diseases 2023, 10, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zou, X.; Yang, S.; Zhang, A.; Li, N.; Ma, Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Frontiers in Immunology 2023, 14, 1149989. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Li, Y.; Li, S.; Liu, S.; Cheng, Y. Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis and stemness of esophageal cancer cells. Molecular and Cellular Biochemistry 2024, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zheng, Z.; Bian, C.; Chang, S.; Bao, J.; Yu, H.; Xin, Y.; Jiang, X. Functions and mechanisms of lactylation in carcinogenesis and immunosuppression. Frontiers in Immunology 2023, 14, 1253064. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ying, T.; Yuan, J.; Wang, Y.; Su, X.; Chen, S.; Zhao, Y.; Zhao, Y.; Sheng, J.; Teng, L.; Luo, C. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocrine-Related Cancer 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhao, X.; Liu, X. Hypoxia induced β-catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Experimental Cell Research 2023, 422, 113439. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell metabolism 2023, 35, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, W.; Li, N.; Ma, Y.; Yao, M.; Wang, G.; He, S.; Li, W.; Tan, J.; Lu, Q.; Hou, S. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biology 2023, 24, 87. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Wang, X.; Mang, G.; Chen, J.; Yan, X.; Tong, Z.; Yang, Q.; Wang, M.; Chen, L.; Sun, P. Histone lactylation boosts reparative gene activation post–myocardial infarction. Circulation Research 2022, 131, 893–908. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ji, Z.; Gong, Y.; Fan, L.; Xu, P.; Chen, X.; Miao, J.; Zhang, K.; Zhang, W.; Ma, P.; Zhao, H. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell reports 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Huang, H.; Li, M.; Chen, Y. Proteomic analysis identifies PFKP lactylation in SW480 colon cancer cells. Iscience 2024, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, T.; Meng, D.; Lv, W.; Ye, J.; Cheng, L.; Hu, J. BZW2 Modulates Lung Adenocarcinoma Progression through Glycolysis-Mediated IDH3G Lactylation Modification. Journal of Proteome Research 2023, 22, 3854–3865. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Mao, L.; Wang, J.; Zhang, X.; Wu, M.; Wen, Q.; Yu, S.C. Beyond metabolic waste: lysine lactylation and its potential roles in cancer progression and cell fate determination. Cellular Oncology 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Varner, E.L.; Trefely, S.; Bartee, D.; von Krusenstiern, E.; Izzo, L.; Bekeova, C.; O'Connor, R.S.; Seifert, E.L.; Wellen, K.E.; Meier, J.L.; Snyder, N.W. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biology 2020, 10, 200187. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Yruela, C.; Bæk, M.; Monda, F.; Olsen, C.A. Chiral posttranslational modification to lysine ε-amino groups. Accounts of Chemical Research 2022, 55, 1456–1466. [Google Scholar] [CrossRef]
- Patel, R.; Kumar, A.; Lokhande, K.B.; Swamy, K.V.; Sharma, N.K. Molecular Docking and Simulation Studies Predict Lactyl-CoA as the Substrate for P300 Directed Lactylation. This manuscript has been released as a Pre-Print at “bioRxiv” downloaded from https://chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c74e8d4c8919380fad3a55/original/molecular-docking-and-simulation-studies-predict-lactyl-co-a-as-the-substrate-for-p300-directed-lactylation.pdf. Accessed 02/21/2024.
- Wu, H.; Huang, H.; Zhao, Y. Interplay between metabolic reprogramming and post-translational modifications: from glycolysis to lactylation. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. Glucose metabolism and glucose transporters in breast cancer. Frontiers in cell and developmental biology 2021, 9, 728759. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, X.; Wang, J.; Liu, B. Glycolysis-related lncRNA TMEM105 upregulates LDHA to facilitate breast cancer liver metastasis via sponging miR-1208. Cell Death & Disease 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Schreier, A.; Zappasodi, R.; Serganova, I.; Brown, K.A.; Demaria, S.; Andreopoulou, E. Facts and Perspectives: Implications of tumor glycolysis on immunotherapy response in triple negative breast cancer. Frontiers in Oncology 2023, 12, 1061789. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.; Ulloa, V.; Rodríguez, F.; Reinicke, K.; Yañez, A.J.; García, M.D.; Medina, R.A.; Carrasco, M.; Barberis, S.; Castro, T.; Martínez, F. Differential subcellular distribution of glucose transporters GLUT1–6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. Journal of cellular physiology 2006, 207, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ciavardelli, D.; Rossi, C.; Barcaroli, D.; Volpe, S.; Consalvo, A.; Zucchelli, M.; De Cola, A.; Scavo, E.; Carollo, R.; D'agostino, D.; Forlì, F. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell death & disease 2014, 5, e1336. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.M.; Shin, Y.; Lee, E.J.; Lee, S.; Jeong, S.H.; Kong, H.K.; Park, E.Y.; Kim, H.K.; Han, J.; Chang, M.; Park, J.H. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PloS one 2015, 10, e0132285. [Google Scholar] [CrossRef] [PubMed]
- Littleflower, A.B.; Parambil, S.T.; Antony, G.R.; Subhadradevi, L. The determinants of metabolic discrepancies in aerobic glycolysis: Providing potential targets for breast cancer treatment. Biochimie 2024, 220(107) 121. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.L.; Eubank, W.B.; Mankoff, D.A. FDG PET, PET/CT, and breast cancer imaging. Radiographics 2007, 207, S215–S229. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Fadia, M.; Dahlstrom, J.E.; Parish, C.R.; Board, P.G.; Blackburn, A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast cancer research and treatment 2010, 120, 253–260. [Google Scholar] [CrossRef]
- Gang, B.P.; Dilda, P.J.; Hogg, P.J.; Blackburn, A.C. Dichloroacetate reverses the Warburg effect, inhibiting growth and sensitizing breast cancer cells towards apoptosis. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia (PA): AACR; C.ancer Res 2012;72(8 Suppl):Abstract nr 3228.
- Harting, T.P.; Stubbendorff, M.; Hammer, S.C.; Schadzek, P.; Ngezahayo, A.; Escobar, H.M.; Nolte, I. Dichloroacetate affects proliferation but not apoptosis in canine mammary cell lines. PloS one 2017, 12, e0178744. [Google Scholar] [CrossRef] [PubMed]
- De Preter, G. , Neveu, M.-A., Danhier, P., Brisson, L., Payen, V.L., Porporato, P.E., … Gallez, B. (2016). Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. ( 7(3), 2910–2920. [CrossRef] [PubMed]
- Blackburn, A.C.; Rooke, M.; Sun, R.C.; Fadia, M.; Dahlstrom, J.E.; Board, P.G. Reversal of the glycolytic phenotype with dichloroacetate in a mouse mammary adenocarcinoma model. Pathology 2010, 42, S61–S62. [Google Scholar] [CrossRef]
- Wang, M.; Liao, C.; Hu, Y.; Pan, Q.; Jiang, J. Sensitization of breast cancer cells to paclitaxel by dichloroacetate through inhibiting autophagy. Biochemical and biophysical research communications 2017, 489, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H. , Seo, S.-K., Park, Y., Kim, E.-K., Seong, M.-K., Kim, H.-A., … Park, I.-C. (). Dichloroacetate potentiates tamoxifen-induced cell death in breast cancer cells via downregulation of the epidermal growth factor receptor. Oncotarget 2016, 7, 59809–59819. [Google Scholar] [CrossRef]
- Haugrud, A.B.; Zhuang, Y.; Coppock, J.D.; Miskimins, W.K. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast cancer research and treatment 2014, 147, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Board, P.G.; Blackburn, A.C. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Molecular cancer 2011, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Lam, Y.M.; Leung, Y.C.; Hu, X.; Chen, X.; Cheung, E.; Tam, K.Y. Combined use of arginase and dichloroacetate exhibits anti-proliferative effects in triple negative breast cancer cells. J Pharm Pharmacol 2019, 71, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Martin, N.K. Bicarbonate and dichloroacetate: evaluating pH altering therapies in a mouse model for metastatic breast cancer. BMC cancer 2011, 11, 235. [Google Scholar] [CrossRef]
- Hong, S.E.; Shin, K.S.; Lee, Y.H.; Seo, S.K.; Yun, S.M.; Choe, T.B.; Kim, H.A.; Kim, E.K.; Noh, W.C.; Kim, J.I.; Hwang, C.S. Inhibition of S6K1 enhances dichloroacetate-induced cell death. Journal of Cancer Research and Clinical Oncology 2015, 141. [Google Scholar] [CrossRef] [PubMed]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677. [Google Scholar] [CrossRef]
- Lefort, N.; Brown, A.; Lloyd, V.; Ouellette, R.; Touaibia, M.; Culf, A.S.; Cuperlovic-Culf, M. 1H NMR metabolomics analysis of the effect of dichloroacetate and allopurinol on breast cancers. Journal of pharmaceutical and biomedical analysis 2014, 93, 77–85. [Google Scholar] [CrossRef] [PubMed]
- 172 de Mey, S.; Dufait, I.; Jiang, H.; Corbet, C.; Wang, H.; Van De Gucht, M.; Kerkhove, L.; Law, K.L.; Vandenplas, H.; Gevaert, T.; Feron, O. Dichloroacetate radiosensitizes hypoxic breast cancer cells. International Journal of Molecular Sciences 2020, 21, 9367. [Google Scholar] [CrossRef]
- Gholami, L.; Attari, F.; Talkhabi, M.; Saadatpour, F. MDA-MB-231. Nova Biologica Reperta 2023 10(1):1-10Doi: 10.29252/nbr.10.1.1 Downloaded from https://scholar.google.com/scholar?cluster=12666777980647358402&hl=en&as_sdt=0,5&as_ylo=2023. Accessed January 2024. 2023. [Google Scholar]
- Cutruzzolà, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose metabolism in the progression of prostate cancer. Frontiers in physiology 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Molecular cancer 2006, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Salminen, E.; Hogg, A.; Binns, D.; Frydenberg, M.; Hicks, R. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncologica 2002, 41, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Akhurst, T.; Osman, I.; Nunez, R.; Macapinlac, H.; Siedlecki, K.; Verbel, D.; Schwartz, L.; Larson, S.M.; Scher, H.I. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 2002, 59, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yacoub, S.; Shiverick, K.T.; Namiki, K.; Sakai, Y.; Porvasnik, S.; Urbanek, C.; Rosser, C.J. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. The Prostate 2008, 68, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.H.; Withers, H.G.; Garbens, A.; Love, H.D.; Magnoni, L.; Hayward, S.W.; Moyes, C.D. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochimica et Biophysica Acta (BBA)-Bioenergetics 2009, 1787, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Harting, T.; Stubbendorff, M.; Willenbrock, S.; Wagner, S.; Schadzek, P.; Ngezahayo, A.; Escobar, H.M.; Nolte, I. The effect of dichloroacetate in canine prostate adenocarcinomas and transitional cell carcinomas in vitro. International journal of oncology 2016, 49, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liang, H.; Guan, G. Dichloroacetate enhances the cytotoxic effect of Cisplatin via decreasing the level of FOXM1 in prostate cancer. Int J Clin Med 2016, 9, 11044–11050. [Google Scholar]
- Olszewski, U.; Poulsen, T.T.; Ulsperger, E.; Poulsen, H.S.; Geissler, K.; Hamilton, G. In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds. Clinical pharmacology: advances and applications. [CrossRef]
- Chaudhary, A.K.; Bhat, T.A.; Kumar, S.; Kumar, A.; Kumar, R.; Underwood, W.; Koochekpour, S.; Shourideh, M.; Yadav, N.; Dhar, S.; Chandra, D. Mitochondrial dysfunction-mediated apoptosis resistance associates with defective heat shock protein response in African–American men with prostate cancer. British journal of cancer 2016, 114, 1090. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.W.; Kasai, M.; Yamamoto, S.; Fukuhara, H.; Karashima, T.; Kurabayashi, A.; Furihata, M.; Hanazaki, K.; Inoue, K.; Ogura, S.I. Metabolic shift towards oxidative phosphorylation reduces cell-density-induced cancer-stem-cell-like characteristics in prostate cancer in vitro. Biology Open 2023, 12, bio059615–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, L.; Oyang, L.; Liang, J.; Tan, S.; Wu, N.; Yi, P.; Pan, Q.; Rao, S.; Han, Y.; Tang, Y. The POU2F1-ALDOA axis promotes the proliferation and chemoresistance of colon cancer cells by enhancing glycolysis and the pentose phosphate pathway activity. Oncogene 2022, 41, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.S.; Wang, J.Y.; Chung, F.Y.; Lee, S.C.; Huang, M.Y.; Kuo, C.W.; Yang, M.J.; Lin, S.R. Significance of the glycolytic pathway and glycolysis related-genes in tumorigenesis of human colorectal cancers. Oncology reports 2008, 19, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Madhok, B.M.; Yeluri, S.; Perry, S.L.; Hughes, T.A.; Jayne, D.G. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. British journal of cancer 2010, 102, 1746. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Hill, D.K.; Andrejeva, G.; Boult, J.K.; Troy, H.; Fong, A.L.; Orton, M.R.; Panek, R.; Parkes, H.G.; Jafar, M.; Koh, D.M. Dichloroacetate induces autophagy in colorectal cancer cells and tumours. British journal of cancer 2014, 111, 375. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.M.; Ho, N.; Morrison, J.; Farias, N.R.; Mosser, D.D.; Coomber, B.L. Dichloroacetate affects proliferation but not survival of human colorectal cancer cells. Apoptosis 2015, 20, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, X.; Pan, S.; Wang, L.; Dou, W.; Liu, J.; Shi, X. Dichloroacetate attenuates the stemness of colorectal cancer cells via trigerring ferroptosis through sequestering iron in lysosomes. Environmental toxicology 2021, 36, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Xie, G.; He, J.; Li, J.; Pan, F.; Liang, H. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. BioMed Research International. 2011. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, L.; Li, L.; Li, L.; Zhu, L.; Wang, Y.; Huang, X.; Hou, Y.; Zhu, D.; Zou, H.; Gu, Y. Dichloroacetate restores colorectal cancer chemosensitivity through the p53/miR-149-3p/PDK2-mediated glucose metabolic pathway. Oncogene 2020, 39, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, D.; Zhu, L.; Hou, Y.; Hou, L.; Huang, X.; Li, L.; Wang, Y.; Li, L.; Zou, H.; Wu, T. Dichloroacetate overcomes oxaliplatin chemoresistance in colorectal cancer through the miR-543/PTEN/Akt/mTOR pathway. Journal of Cancer 2019, 10, 6037. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Andrews, D.; Blackburn, A.C. Long-term stabilization of stage 4 colon cancer using sodium dichloroacetate therapy. World journal of clinical cases 2016, 4, 336. [Google Scholar] [CrossRef]
- Hall, A.; Meyle, K.D.; Lange, M.K.; Klima, M.; Sanderhoff, M.; Dahl, C.; Abildgaard, C.; Thorup, K.; Moghimi, S.M.; Jensen, P.B.; Bartek, J. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the V600EBRAF oncogene. Oncotarget 2013, 4, 584. [Google Scholar] [CrossRef]
- Falkenius, J.; Lundeberg, J.; Johansson, H.; Tuominen, R.; Frostvik-Stolt, M.; Hansson, J.; Brage, S.E. High expression of glycolytic and pigment proteins is associated with worse clinical outcome in stage III melanoma. Melanoma research 2013, 23, 452–460. [Google Scholar] [CrossRef]
- Koch, A.; Ebert, E.V.; Seitz, T.; Dietrich, P.; Berneburg, M.; Bosserhoff, A.; Hellerbrand, C. Characterization of glycolysis-related gene expression in malignant melanoma. Pathology-Research and Practice 2020, 216, 152752. [Google Scholar] [CrossRef] [PubMed]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Sierra-Rivera, C.A.; Zapata-Benavides, P.; Hernandez, D.F.; Chávez-Reyes, A.; Rivera-Morales, L.G.; Tamez-Guerra, R.; Rodríguez-Padilla, C. In vitro and in vivo antitumoral activitiy of sodium dichloroacetate (DCA-Na) against murine melanoma. African Journal of Microbiology Research 2012, 6, 4782–4796. [Google Scholar] [CrossRef]
- Chaube, B.; Malvi, P.; Singh, S.V.; Mohammad, N.; Meena, A.S.; Bhat, M.K. Targeting metabolic flexibility by simultaneously inhibiting respiratory complex I and lactate generation retards melanoma progression. Oncotarget 2015, 6, 37281. [Google Scholar] [CrossRef]
- Pópulo, H.; Caldas, R.; Lopes, J.M.; Pardal, J.; Máximo, V.; Soares, P. Overexpression of pyruvate dehydrogenase kinase supports dichloroacetate as a candidate for cutaneous melanoma therapy. Expert opinion on therapeutic targets 2015, 19, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, C.; Dahl, C.; Basse, A.L.; Ma, T.; Guldberg, P. Bioenergetic modulation with dichloroacetate reduces the growth of melanoma cells and potentiates their response to BRAF V600E inhibition. Journal of translational medicine 2014, 12, 247. [Google Scholar] [CrossRef]
- Zheng, M.F.; Shen, S.Y. DCA increases the antitumor effects of capecitabine in a mouse B16 melanoma allograft and a human non-small cell lung cancer A549 xenograft. Cancer chemotherapy and pharmacology 2013, 72, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Andrews, D.; Shainhouse, J.; Blackburn, A.C. Long-term stabilization of metastatic melanoma with sodium dichloroacetate. World journal of clinical oncology 2017, 8, 371. [Google Scholar] [CrossRef]
- Reuss, A.M.; Groos, D.; Buchfelder, M.; Savaskan, N. The acidic brain—glycolytic switch in the microenvironment of malignant glioma. International journal of molecular sciences 2021, 22, 5518. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Shingu, T.; Feng, L.; Chen, Z.; Ogasawara, M.; Keating, M.J.; Kondo, S.; Huang, P. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. Journal of Biological Chemistry 2011, 286, 32843–32853. [Google Scholar] [CrossRef]
- Sanzey, M.; Abdul Rahim, S.A.; Oudin, A.; Dirkse, A.; Kaoma, T.; Vallar, L.; Herold-Mende, C.; Bjerkvig, R.; Golebiewska, A.; Niclou, S.P. Comprehensive analysis of glycolytic enzymes as therapeutic targets in the treatment of glioblastoma. PloS one 2015, 10, e0123544. [Google Scholar] [CrossRef] [PubMed]
- Stanke, K.M.; Wilson, C.; Kidambi, S. High expression of glycolytic genes in clinical glioblastoma patients correlates with lower survival. Frontiers in Molecular Biosciences 2021, 8, 752404. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Wilson, E.B.; Short, S.; Melcher, A.A.; Biggs, M.; Diakos, C.I.; Howell, V.M. Glycolysis and fatty acid oxidation inhibition improves survival in glioblastoma. Frontiers in Oncology 2021, 11, 633210. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.L.; Mackey, J.R.; Fulton, D.; Abdulkarim, B. Metabolic modulation of glioblastoma with dichloroacetate. Science translational medicine 2010, 2, 31ra34. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhao, X.; Ren, W.; Wang, X.; Yu, K.F.; Li, D.; Zhang, X.; Zhang, Q. Antitumor activity of dichloroacetate on C6 glioma cell: in vitro and in vivo evaluation. OncoTargets and therapy. [CrossRef]
- Kumar, K. , Wigfield, S., Gee, H.E., Devlin, C.M., Singleton, D., Li, J.-L., … Ivan, M. (2013). Dichloroacetate Reverses the Hypoxic Adaptation to Bevacizumab and Enhances its Antitumor Effects in Mouse Xenografts. ( 91(6), 749–758. [CrossRef] [PubMed]
- Morfouace, M.; Lalier, L.; Oliver, L.; Cheray, M.; Pecqueur, C.; Cartron, P.F.; Vallette, F.M. Control of glioma cell death and differentiation by PKM2–Oct4 interaction. Cell death & disease 2014, 5, e1036. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Boichuk, I.V.; Solyanik, G.I. Hypoxia enhances antitumor activity of dichloroacetate. Experimental oncology. 2: 4). [PubMed]
- Vella, S.; Conti, M.; Tasso, R.; Cancedda, R.; Pagano, A. Dichloroacetate inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. International journal of cancer 2012, 130, 1484–1493. [Google Scholar] [CrossRef]
- Sradhanjali, S.; Tripathy, D.; Rath, S.; Mittal, R.; Reddy, M.M. Overexpression of pyruvate dehydrogenase kinase 1 in retinoblastoma: A potential therapeutic opportunity for targeting vitreous seeds and hypoxic regions. PloS one 2017, 12, e0177744. [Google Scholar] [CrossRef]
- Park, J.M.; Recht, L.D.; Josan, S.; Merchant, M.; Jang, T.; Yen, Y.F.; Hurd, R.E.; Spielman, D.M.; Mayer, D. Metabolic response of glioma to dichloroacetate measured in vivo by hyperpolarized 13C magnetic resonance spectroscopic imaging. Neuro-oncology 2013, 15, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Fedorchuk, A.G.; Pyaskovskaya, O.N.; Gorbik, G.V.; Prokhorova, I.V.; Kolesnik, D.L.; Solyanik, G.I. Effectiveness of sodium dichloroacetate against glioma C6 depends on administration schedule and dosage. Exp Oncol. [PubMed]
- Wicks, R.T.; Azadi, J.; Mangraviti, A.; Zhang, I.; Hwang, L.; Joshi, A.; Bow, H.; Hutt-Cabezas, M.; Martin, K.L.; Rudek, M.A.; Zhao, M. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro-oncology 2014, 17, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Korsakova, L.; Krasko, J.A.; Stankevicius, E. Metabolic-targeted combination therapy with dichloroacetate and metformin suppresses glioblastoma cell line growth in vitro and in vivo. in vivo 2021, 35, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hau, E.; Joshi, S.; Dilda, P.J.; McDonald, K.L. Sensitization of glioblastoma cells to irradiation by modulating the glucose metabolism. Molecular cancer therapeutics 2015, 14, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.M. , Shen, H., McKelvey, K.J., Gee, H.E., & Hau, E. (2021). Targeting glucose metabolism of cancer cells with dichloroacetate to radiosensitize high-grade gliomas. International Journal of Molecular Sciences. [CrossRef]
- Jiang, W.; Finniss, S.; Cazacu, S.; Xiang, C.; Brodie, Z.; Mikkelsen, T.; Poisson, L.; Shackelford, D.B.; Brodie, C. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget 2016, 7, 56456. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, I.V.; Pyaskovskaya, O.N.; Kolesnik, D.L.; Solyanik, G.I. Influence of metformin, sodium dichloroacetate and their combination on the hematological and biochemical blood parameters of rats with gliomas C6. Experimental oncology. 2018. Т. 40, № 3. — С. 205-210. [PubMed]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Yurchenko, O.V.; Solyanik, G.I. Metformin enhances antitumor action of sodium dichloroacetate against glioma C6. Experimental Oncology 2019, 41, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yu, M.; Tsoli, M.; Chang, C.; Joshi, S.; Liu, J.; Ryall, S.; Chornenkyy, Y.; Siddaway, R.; Hawkins, C.; Ziegler, D.S. Targeting reduced mitochondrial DNA quantity as a therapeutic approach in pediatric high-grade gliomas. Neuro-oncology 2020, 22, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zhang, L.; Zhao, C.; Wang, D. Phosphorylated form of pyruvate dehydrogenase α1 mediates tumor necrosis factor α-induced glioma cell migration. Oncology Letters 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Duraj, T.; García-Romero, N.; Carrión-Navarro, J.; Madurga, R.; Ortiz de Mendivil, A.; Prat-Acin, R.; Garcia-Cañamaque, L.; Ayuso-Sacido, A. Beyond the Warburg effect: Oxidative and glycolytic phenotypes coexist within the metabolic heterogeneity of glioblastoma. Cells 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Griguer, C.E.; Oliva, C.R.; Gillespie, G.Y. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. Journal of neuro-oncology. [CrossRef]
- Shibao, S.; Minami, N.; Koike, N.; Fukui, N.; Yoshida, K.; Saya, H.; Sampetrean, O. Metabolic heterogeneity and plasticity of glioma stem cells in a mouse glioblastoma model. Neuro-oncology 2018, 20, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Boag, J.M.; Beesley, A.H.; Firth, M.J.; Freitas, J.R.; Ford, J.; Hoffmann, K.; Cummings, A.J.; de Klerk, N.H.; Kees, U.R. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia 2006, 20, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Hulleman, E.; Kazemier, K.M.; Holleman, A.; VanderWeele, D.J.; Rudin, C.M.; Broekhuis, M.J.; Evans, W.E.; Pieters, R.; Den Boer, M.L. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood 2009, 113, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Buentke, E.; Nordström, A.; Lin, H.; Björklund, A.C.; Laane, E.; Harada, M.; Lu, L.; Tegnebratt, T.; Stone-Elander, S.; Heyman, M.; Söderhäll, S.; Porwit, A.; Ostenson, C.G.; Shoshan, M.; Tamm, K.P.; Grandér, D. Glucocorticoid-induced cell death is mediated through reduced glucose metabolism in lymphoid leukemia cells. Blood Cancer, J 2011, 1, e31. [Google Scholar] [CrossRef] [PubMed]
- Samuels, A.L.; Heng, J.Y.; Beesley, A.H.; Kees, U.R. Bioenergetic modulation overcomes glucocorticoid resistance in T-lineage acute lymphoblastic leukaemia. Br J Haematol 2014, 165, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; McBrayer, S.K.; Rosen, S.T. Targeting the Warburg effect in hematological malignancies: from PET to therapy. Curr Opin Oncol 2009, 21, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Howman, R.A.; Neeson, P.J.; Berridge, M.V.; Ritchie, D.S. The level of glycolytic metabolism in acute myeloid leukemia blasts at diagnosis is prognostic for clinical outcome. Journal of leukocyte biology 2011, 89, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Xia, J.; Xu, H.; Frech, I.; Tricot, G.; Zhan, F. NEK2 promotes aerobic glycolysis in multiple myeloma through regulating splicing of pyruvate kinase. Journal of hematology & oncology 2017, 10, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Paschou, S.A.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Metabolic disorders in multiple myeloma. International Journal of Molecular Sciences 2021, 22, 11430. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.Y.; McGee, S.L.; Connor, T.; Mottram, B.; Wilkinson, A.; Whitehead, J.P.; Vuckovic, S.; Catley, L. Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. British journal of cancer 2013, 108, 1624. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Sasano, T.; Arima, Y.; Kushima, S.; Tsujita, K.; Matsuoka, M.; Hata, H. A novel PDK1 inhibitor, JX06, inhibits glycolysis and induces apoptosis in multiple myeloma cells. Biochemical and Biophysical Research Communications 2022, 587, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S. , Kawano, Y., Yuki, H., Okuno, Y., Nosaka, K., Mitsuya, H., & Hata, H. (2013). PDK1 inhibition is a novel therapeutic target in multiple myeloma. ( 108(1), 170–178. [CrossRef] [PubMed]
- Tian, D.D.; Bennett, S.K.; Coupland, L.A.; Forwood, K.; Lwin, Y.; Pooryousef, N.; Tea, I.; Truong, T.T.; Neeman, T.; Crispin, P.; D’Rozario, J. GSTZ1 genotypes correlate with dichloroacetate pharmacokinetics and chronic side effects in multiple myeloma patients in a pilot phase 2 clinical trial. Pharmacology research & perspectives 2019, 7, e00526. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Rimondi, E.; Melloni, E.; Gilli, P.; Bertolasi, V.; Casciano, F.; Rigolin, G.M.; Zauli, G.; Secchiero, P. Metformin combined with sodium dichloroacetate promotes B leukemic cell death by suppressing anti-apoptotic protein Mcl-1. Oncotarget 2016, 7, 18965. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kant, S.; Singh, S.M. Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicology and applied pharmacology 2013, 273, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Flavin, D.F. Non-Hodgkin's lymphoma reversal with dichloroacetate. Journal of oncology. 2010; Article ID 414726. [CrossRef]
- Strum, S.B.; Adalsteinsson, Ö.; Black, R.R.; Segal, D.; Peress, N.L.; Waldenfels, J. Case report: sodium dichloroacetate (DCA) inhibition of the “Warburg Effect” in a human cancer patient: complete response in non-Hodgkin’s lymphoma after disease progression with rituximab-CHOP. Journal of bioenergetics and biomembranes 2013, 45, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Abramek, J.; Bogucki, J.; Ziaja-Sołtys, M.; Stępniewski, A.; Bogucka-Kocka, A. Effect of sodium dichloroacetate on apoptotic gene expression in human leukemia cell lines. Pharmacological Reports 2019, 71, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, C.; Melloni, E.; Casciano, F.; Rigolin, G.M.; Rimondi, E.; Celeghini, C.; Brunelli, L.; Cuneo, A.; Secchiero, P.; Zauli, G. Sodium dichloroacetate exhibits anti-leukemic activity in B-chronic lymphocytic leukemia (B-CLL) and synergizes with the p53 activator Nutlin-3. Oncotarget 2014, 5, 4347. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, C.; Brunelli, L.; Melloni, E.; Pastorelli, R.; Casciano, F.; Rimondi, E.; Rigolin, G.M.; Cuneo, A.; Secchiero, P.; Zauli, G. The anti-leukemic activity of sodium dichloroacetate in p53mutated/null cells is mediated by a p53-independent ILF3/p21 pathway. Oncotarget 2015, 6, 2385. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Sadowska, M.; Carter-Cooper, B.; Bhatnagar, V.; van der Merwe, I.; Levis, M.J.; Sausville, E.A.; Lapidus, R.G. Perturbation of cellular oxidative state induced by dichloroacetate and arsenic trioxide for treatment of acute myeloid leukemia. Leukemia research 2015, 39, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Queckborner, S.; Turnbull, A.; Michie, C.O.; Williams, A.R.; Rye, T.; Gourley, C.; Langdon, S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC cancer 2018, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, L.; Chai, W.; Zhao, T.; Jin, X.; Guo, X.; Han, L.; Yuan, C. Dichloroacetic acid upregulates apoptosis of ovarian cancer cells by regulating mitochondrial function. OncoTargets and therapy. 1729. [Google Scholar] [CrossRef]
- Saed, G.M.; Fletcher, N.M.; Jiang, Z.L.; Abu-Soud, H.M.; Diamond, M.P. Dichloroacetate induces apoptosis of epithelial ovarian cancer cells through a mechanism involving modulation of oxidative stress. Reproductive sciences 2011, 18, 1253–1261. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Vančo, J.; Dvořák, Z. Half-sandwich Ru (II) and Os (II) bathophenanthroline complexes containing a releasable dichloroacetato ligand. Molecules 2018, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Priego-Hernández, V.D.; Arizmendi-Izazaga, A.; Soto-Flores, D.G.; Santiago-Ramón, N.; Feria-Valadez, M.D.; Navarro-Tito, N.; Jiménez-Wences, H.; Martínez-Carrillo, D.N.; Salmerón-Bárcenas, E.G.; Leyva-Vázquez, M.A.; Illades-Aguiar, B. Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines. Pathogens 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, B.S.; Yu, D.H.; Lu, Q.; Ma, J.; Qi, H.; Fang, C.; Chen, H.Z. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. International journal of oncology 2011, 38, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhang, Y.Z.; Wang, Y.S.; Ma, X.X. Identification of novel cell glycolysis related gene signature predicting survival in patients with endometrial cancer. Cancer cell international 2019, 19, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Huggins, G.S.; Debidda, M.; Munshi, N.C.; De Vivo, I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecologic oncology 2008, 109, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.H.; Zhou, C.; Bae-Jump, V. Dichloroacetate inhibits cell proliferation and induces apoptosis in human endometrial cancer cell lines. Cancer Research. 2016 Jul 15;76(14_Supplement):2993. [CrossRef]
- Li, X.B.; Gu, J.D.; Zhou, Q.H. Review of aerobic glycolysis and its key enzymes–new targets for lung cancer therapy. Thoracic cancer 2015, 6, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Leko, P.; Stacher-Priehse, E.; Brcic, L.; El-Heliebi, A.; Hofmann, L.; Quehenberger, F.; Hrzenjak, A.; Popper, H.H.; Olschewski, H.; Leithner, K. Distribution and prognostic significance of gluconeogenesis and glycolysis in lung cancer. Molecular oncology 2020, 14, 2853–2867. [Google Scholar] [CrossRef]
- Xu, J.Q.; Fu, Y.L.; Zhang, J.; Zhang, K.Y.; Ma, J.; Tang, J.Y.; Zhang, Z.W.; Zhou, Z.Y. Targeting glycolysis in non-small cell lung cancer: Promises and challenges. Frontiers in Pharmacology 2022, 13, 1037341. [Google Scholar] [CrossRef] [PubMed]
- Oylumlu, E.; yin Ng, Y. EVALUATION OF ANTIPROLIFERATIVE EFFECTS OF SODIUM DICHLOROACETATE ON LUNG ADENOCARCINOMA. Turkish Journal of Biochemistry/Turk Biyokimya Dergisi. 2019 Dec 3;44. Downloaded from https://openurl.ebsco.com/EPDB%3Agcd%3A11%3A2866491/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A140410362&crl=c Accessed 20 december 2023.
- Allen, K.T.; Chin-Sinex, H.; DeLuca, T.; Pomerening, J.R.; Sherer, J.; Watkins III JB, Foley, J. ; Jesseph, J.M.; Mendonca, M.S. Dichloroacetate alters Warburg metabolism, inhibits cell growth, and increases the X-ray sensitivity of human A549 and H1299 NSC lung cancer cells. Free Radical Biology and Medicine 2015, 89, 263–273. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, D.; Hou, B.; Liu, Q.X.; Chen, Q.; Deng, X.F.; Yu, Z.B.; Dai, J.G.; Zheng, H. Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer management and research. 1231. [Google Scholar] [CrossRef]
- Sun, H. , Zhu, A., Zhou, X., & Wang, F. (2017). Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget, 2017; 8, 52642–52650. [Google Scholar] [CrossRef]
- Yang, Z.; Tam, K.Y. Anti-cancer synergy of dichloroacetate and EGFR tyrosine kinase inhibitors in NSCLC cell lines. European journal of pharmacology 2016, 789, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Al-Azawi, A.; Sulaiman, S.; Arafat, K.; Yasin, J.; Nemmar, A.; Attoub, S. Impact of sodium dichloroacetate alone and in combination therapies on lung tumor growth and metastasis. International journal of molecular sciences 2021, 22, 12553. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, Y.; Wu, P.; Ran, M.; Xu, N.; Shan, W.; Sha, O.; Tam, K.Y. Dichloroacetophenone biphenylsulfone ethers as anticancer pyruvate dehydrogenase kinase inhibitors in non-small cell lung cancer models. Chemico-Biological Interactions 2023, 378, 110467. [Google Scholar] [CrossRef] [PubMed]
- Fiebiger, W.; Olszewski, U.; Ulsperger, E.; Geissler, K.; Hamilton, G. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clinical and Translational Oncology 2011, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Boychuk, I.V.; Dasyukevich, O.I.; Melnikov, O.R.; Tarasov, A.S.; Solyanik, G.I. Effect of dichloroacetate on Lewis lung carcinoma growth and metastasis. Experimental oncology. 1: 2). [PubMed]
- Feng, M. , Wang, J., & Zhou, J. (2023). Unraveling the therapeutic mechanisms of dichloroacetic acid in lung cancer through integrated multi-omics approaches: metabolomics and transcriptomics. Frontiers in Genetics. [CrossRef]
- Penticuff, J.C. , Woolbright, B.L., Sielecki, T.M., Weir, S.J., and Taylor, J.A. MIF family proteins in genitourinary cancer: Tumorigenic roles and therapeutic potential. Nat. Rev. Urol. 2019, 16, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.C.; de Araújo, J.M.; Fernandes, T.A.; Cobucci, R.N.; Lanza, D.C.; Andrade, V.S.; Fernandes, J.V. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer. Pathology & Oncology Research. [CrossRef]
- Yasasever, V. , Camlica, H., Duranyildiz, D., Oguz, H., Tas, F., & Dalay, N. (2007). Macrophage migration inhibitory factor in cancer. Cancer investigation, 25(8), 715-719. [CrossRef] [PubMed]
- Zheng, X. , Wei, Y., Huang, T., Wei, X., Sun, S., Wang, T., & Zhao, Z. (2023). Role of macrophage migration inhibitory factor for stem cells. Chinese Journal of Tissue Engineering Research, 27(15), 2395. [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat Rev Dis Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Morita, M.; Tanuma, N. A metabolic vulnerability of small-cell lung cancer. Oncotarget 2018, 9, 32278–32279. [Google Scholar] [CrossRef]
- Gao, C.; Shen, Y.; Jin, F.; Miao, Y.; Qiu, X. Cancer stem cells in small cell lung cancer cell line H446: higher dependency on oxidative phosphorylation and mitochondrial substrate-level phosphorylation than non-stem cancer cells. PLoS One 2016, 11, e0154576. [Google Scholar] [CrossRef]
- Grass, G.D.; Scott, K.E.; Fernandez, M.R.; Stewart, P.A.; Kang, Y.P.; Yang, C.; DeNicola, G.M.; Bannister, T.D.; Cubitt, C.L.; Haura, E.B.; Cleveland, J.L. Targeting Lactate Transport in Small-Cell Lung Cancer. International Journal of Radiation Oncology, Biology, Physics 2018, 102, e182. [Google Scholar] [CrossRef]
- Choi, J.E.; Sebastian, C.; Ferrer, C.M.; Lewis, C.A.; Sade-Feldman, M.; LaSalle, T.; Gonye, A.; Lopez, B.G.; Abdelmoula, W.M.; Regan, M.S.; Cetinbas, M. A unique subset of glycolytic tumour-propagating cells drives squamous cell carcinoma. Nature metabolism 2021, 3, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Somers, K.D.; Merrick, M.A.; Lopez, M.E.; Incognito, L.S.; Schechter, G.L.; Casey, G. Frequent p53 mutations in head and neck cancer. Cancer Research 1992, 52, 5997–6000. [Google Scholar] [PubMed]
- Lui, V.W.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; Freilino, M. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer discovery 2013, 3, 761–769. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.M.; Rothenberg, S.M.; Faquin, W.C.; Chan, A.W.; Clark, J.R.; Ellisen, L.W.; Wirth, L.J. Mutation frequency in 15 common cancer genes in high-risk head and neck squamous cell carcinoma. Head & neck 2014, 36, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D.; Gooding, W.E. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clinical Cancer Research 2006, 12, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.A.; Boyle, J.O.; Koch, W.M.; Goodman, S.N.; Hruban, R.H.; Eby, Y.J.; Couch, M.J.; Forastiere, A.A.; Sidransky, D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. New England Journal of Medicine 1995, 332, 712–717. [Google Scholar] [CrossRef]
- Landor, S.K.; Mutvei, A.P.; Mamaeva, V.; Jin, S.; Busk, M.; Borra, R.; Grönroos, T.J.; Kronqvist, P.; Lendahl, U.; Sahlgren, C.M. Hypo-and hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proceedings of the National Academy of Sciences 2011, 108, 18814–18819. [Google Scholar] [CrossRef]
- Slaninova, V.; Krafcikova, M.; Perez-Gomez, R.; Steffal, P.; Trantirek, L.; Bray, S.J.; Krejci, A. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open biology 2016, 6, 150155. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Kuang, S. Notch signaling as a novel regulator of metabolism. Trends in Endocrinology & Metabolism 2015, 26, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Najy, A.J.; Huang, W.; Sethi, S.; Snyder, M.; Sakr, W.; Dyson, G.; Hüttemann, M.; Lee, I.; Ali-Fehmi, R.; Franceschi, S. HPV-associated differential regulation of tumor metabolism in oropharyngeal head and neck cancer. Oncotarget 2017, 8, 51530. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Agriesti, F.; Scrima, R.; Laurenzana, I.; Perrone, D.; Tataranni, T.; Mazzoccoli, C.; Muzio, L.L.; Capitanio, N.; Piccoli, C. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: a metabolic perspective of treatment. Oncotarget 2015, 6, 1217. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, H.F.; Guo, Y.Z.; Wang, P.Y.; Liu, Z.S.; Gao, H.D.; Xie, W.L. Combination of Taxol® and dichloroacetate results in synergistically inhibitory effects on Taxol-resistant oral cancer cells under hypoxia. Molecular medicine reports 2015, 11, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Park, J.Y.; Kim, E.H.; Jang, H.J.; Kwon, M. Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer. Cancer Letters 2016, 371, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Mazurczak, M.; Dib, E.G.; Bleeker, J.S.; Geeraerts, L.H.; Tinguely, M.; Lohr, M.M.; McGraw, S.C.; Jensen, A.W.; Ellison, C.A.; Black, L.J. Phase II study of dichloroacetate, an inhibitor of pyruvate dehydrogenase, in combination with chemoradiotherapy for unresected, locally advanced head and neck squamous cell carcinoma. Investigational New Drugs 2022, 40, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Inanc, S.; Keles, D.; Eskiizmir, G.; Basbinar, Y.A.; OKTAY, G. Metformin And Dichloroacetate Combination Exert A Synergistic Effect On Cell Viability Of Oral Squamous Cell Carcinoma. ENT Updates 2019, 9, 68–73. [Google Scholar] [CrossRef]
- Morais, M.; Dias, F.; Teixeira, A.L.; Medeiros, R. MicroRNAs and altered metabolism of clear cell renal cell carcinoma: Potential role as aerobic glycolysis biomarkers. Biochimica et Biophysica Acta (BBA)-General Subjects 2017, 1861, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Liu, M.; Xu, Y.; Wu, G. Glycolysis-related genes serve as potential prognostic biomarkers in clear cell renal cell carcinoma. Oxidative medicine and cellular longevity. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Shuch, B.; Linehan, W.M.; Srinivasan, R. Aerobic glycolysis: a novel target in kidney cancer. Expert review of anticancer therapy 2013, 13, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Balan, M.; Sabarwal, A.; Choueiri, T.K.; Pal, S. Metabolic reprogramming in renal cancer: Events of a metabolic disease. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2021, 1876, 188559. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Poma, J.; Trilla-Fuertes, L.; López-Vacas, R.; López-Camacho, E.; García-Fernández, E.; Pertejo, A.; Lumbreras-Herrera, M.I.; Zapater-Moros, A.; Díaz-Almirón, M.; Dittmann, A.; Fresno Vara, J.Á. Proteomics Characterization of Clear Cell Renal Cell Carcinoma. Journal of Clinical Medicine 2023, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Xavier, C.E.; Emaldi, M.; Mingo, J.; Øyjord, T.; Mælandsmo, G.M.; Fodstad, Ø.; Errarte, P.; Larrinaga, G.; Llarena, R.; López, J.I.; Pulido, R. The expression pattern of pyruvate dehydrogenase kinases predicts prognosis and correlates with immune exhaustion in clear cell renal cell carcinoma. Scientific Reports 2023, 13, 7339. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, A.; Dromparis, P.; Saleme, B.; Gurtu, V.; Watson, K.; Paulin, R.; Zervopoulos, S.; Stenson, T.; Sutendra, G.; Pink, D.B.; Carmine-Simmen, K. Metabolic modulation of clear-cell renal cell carcinoma with dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase. European urology 2016, 69, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Q.; Tang, H.Y.; Wang, C.D.; Sang, B.T.; Liu, X.; Yi, F.P.; Liu, G.L.; Wu, X.M. Influence of Dichloroacetate on Wilms’ Tumor in vitro. Annals of Clinical & Laboratory Science 2022, 52, 101–108. [Google Scholar]
- Kalay, S.; Dogan, A.; Turkan, A.; Demiroglu-Zergeroglu, A. Dicholoroacetate exerts anti-cancer activity on human renal cell carcinoma cells. Turkish Journal of Biochemistry 2017, 42, 577–585. [Google Scholar] [CrossRef]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose metabolism in pancreatic cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; Wright, E.M. Functional expression of sodium-glucose transporters in cancer. Proceedings of the National Academy of Sciences 2015, 112, E4111–E4119. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, N.V. , Yabuuchi, S., Pai, S.G., De Oliveira, E., Kamphorst, J.J., Rabinowitz, J.D.,... & Dang, C.V. Treatment of pancreatic cancer patient–derived xenograft panel with metabolic inhibitors reveals efficacy of phenformin. Clinical Cancer Research, 2017; 23, 5639–5647. [Google Scholar] [CrossRef]
- Tavares-Valente, D.; Cannone, S.; Greco, M.R.; Carvalho, T.M.; Baltazar, F.; Queirós, O.; Agrimi, G.; Reshkin, S.J.; Cardone, R.A. Extracellular Matrix Collagen I Differentially Regulates the Metabolic Plasticity of Pancreatic Ductal Adenocarcinoma Parenchymal Cell and Cancer Stem Cell. Cancers 2023, 15, 3868. [Google Scholar] [CrossRef]
- Shimoni-Sebag, A.; Abramovich, I.; Agranovich, B.; Sirovsky, Y.; Stossel, C.; Atias, D.; Raitses-Gurevich, M.; Glick-Gorman, Y.; Margalit, O.; Regev, D.; Tal, R. The pentose-phosphate pathway induces pancreatic cancer radioresistance, a preclinical study with clinical validation. Cancer Research. 2023 Apr 4;83(7_Supplement):2411. [CrossRef]
- Feuerecker, B. , Biechl, P., Veltkamp, C., Saur, D., & Eisenreich, W. Metabolic response of pancreatic carcinoma cells under treatment with dichloroacetate. Metabolites, 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, T. , Agriesti, F., Pacelli, C., Ruggieri, V., Laurenzana, I., Mazzoccoli, C.,... & Piccoli, C. (). Dichloroacetate affects mitochondrial function and stemness-associated properties in pancreatic cancer cell lines. Cells 2019, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xiong, X.; Huang, G.; Liu, J.; Sheng, S.; Wang, H.; Qin, W. Dichloroacetate enhances adriamycin-induced hepatoma cell toxicity in vitro and in vivo by increasing reactive oxygen species levels. PLoS One 2014, 9, e92962. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Xie, P.; Dai, X.F.; Chen, L.X.; Sun, L.B.; Li, T.; He, W.H.; Xu, Z.Z.; Huang, G.; He, F.T.; Lian, J.Q. Dichloroacetate enhances the antitumor effect of pirarubicin via regulating the ROS-JNK signaling pathway in liver cancer cells. Cancer Drug Resistance 2020, 3, 947. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Lee, M.; Park, M.; Kim, S.Y.; Shim, M.S.; Lee, C.Y.; Choi, D.H.; Cho, Y. Metformin and dichloroacetate suppress proliferation of liver cancer cells by inhibiting mTOR complex 1. International Journal of Molecular Sciences 2021, 22, 10027. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Akazawa, T.; Aoki, M.; Kuze, B.; Mizuta, K.; Ito, Y.; Inoue, N. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. International journal of cancer 2013, 133, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Rooke, M.; Coupland, L.A.; Truong, T.; Blackburn, A.C. Dichloroacetate is an effective treatment for sarcoma models in vitro and in vivo. Cancer & Metabolism. [CrossRef]
- Ishiguro, T.; Ishiguro, M.; Ishiguro, R.; Iwai, S. Cotreatment with dichloroacetate and omeprazole exhibits a synergistic antiproliferative effect on malignant tumors. Oncology letters 2012, 3, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Dromparis, P.; Kinnaird, A.; Stenson, T.H.; Haromy, A.; Parker, J.M.; McMurtry, M.S.; Michelakis, E.D. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 2013, 32, 1638. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.M.; Baghdadi, H.; Ahmed, N.S.; Almaramhy, H.H.; Mahmoud, A.A.; El-Sawy, S.A.; Ayat, M.; Elshazley, M.; Abdel-Aziz, W.; Abdel-Latif, H.M.; Ibrahim, W. Dichloroacetate is an antimetabolite that antagonizes acetate and deprives cancer cells from its benefits: A novel evidence-based medical hypothesis. Medical Hypotheses 2019, 122, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Khyzhnyak, S.V.; Sorokina, L.V.; Stepanova, L.I.; Kaplia, A.A. Functional and dynamic state of inner mitochondrial membrane of sarcoma 37 in mice under administration of sodium dichloroacetate. The Ukrainian biochemical journal. 2014, 106–118. [Google Scholar] [CrossRef]
- Choi, Y.W.; Lim, I.K. Sensitization of metformin-cytotoxicity by dichloroacetate via reprogramming glucose metabolism in cancer cells. Cancer letters 2014, 346, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Hur, H.; Ham, I.H.; Yun, J.; Lee, J.Y.; Shim, W.; Kim, Y.B.; Lee, G.; Han, S.U.; Cho, Y.K. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Experimental cell research 2014, 321, 219–230. [Google Scholar] [CrossRef]
- Badr, M.M.; Qinna, N.A.; Qadan, F.; Matalka, K.Z. Dichloroacetate modulates cytokines toward T helper 1 function via induction of the interleukin-12–interferon-γ pathway. OncoTargets and Therapy, 2014, 7, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yuan, P.; Yu, W.; Lin, J.; Xu, A.; Xu, X.; Lou, J.; Yu, T.; Qian, C.; Liu, B.; Song, J. Mitochondria-targeting polymer micelle of dichloroacetate induced pyroptosis to enhance osteosarcoma immunotherapy. ACS nano 2022, 16, 10327–10340. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K. , Yan, S., Lam, J.S.M., Feng, Y., Khan, M., Chen, C.,... & Ho, J.C.M. (2022). Disturbance of the Warburg effect by dichloroacetate and niclosamide suppresses the growth of different sub-types of malignant pleural mesothelioma in vitro and in vivo. Frontiers in Pharmacology. [CrossRef]
- Qin, H.; Zheng, G.; Li, Q.; Shen, L. Metabolic reprogramming induced by DCA enhances cisplatin sensitivity through increasing mitochondrial oxidative stress in cholangiocarcinoma. Frontiers in Pharmacology. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Bott, A.J.; Maimouni, S.; Zong, W.X. The pleiotropic effects of glutamine metabolism in cancer. Cancers 2019, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Klose, K.; Packeiser, E.M.; Müller, P.; Granados-Soler, J.L.; Schille, J.T.; Goericke-Pesch, S.; Kietzmann, M.; Escobar, H.M.; Nolte, I. Metformin and sodium dichloroacetate effects on proliferation, apoptosis, and metabolic activity tested alone and in combination in a canine prostate and a bladder cancer cell line. PLoS One 2021, 16, e0257403. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.; Sivaprakasam, S.; Bhutia, Y.D.; Boettger, T.; Singh, N.; Ganapathy, V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J 2015, 469, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Gopal, E.; Miyauchi, S.; Prasad, P.D. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005, 33, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardo, J. and Rhoden, K.J. Atlas of genetcs and cytogenetcs in oncology and haematology. 2009. Downloaded from https://atlasgeneticsoncology.org/gene/44089/slc5a8 Accessed 01/24/2024.
- Whitman, S.P.; Hackanson, B.; Liyanarachchi, S.; Liu, S.; Rush, L.J.; Maharry, K.; Margeson, D.; Davuluri, R.; Wen, J.; Witte, T.; Yu, L. DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood, The Journal of the American Society of Hematology 2008, 112, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Myeroff, L.; Smiraglia, D.; Romero, M.F.; Pretlow, T.P.; Kasturi, L.; Lutterbaugh, J.; Rerko, R.M.; Casey, G.; Issa, J.P.; Willis, J. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proceedings of the National Academy of Sciences 2003, 100, 8412–8417. [Google Scholar] [CrossRef] [PubMed]
- Porra, V.; Ferraro-Peyret, C.; Durand, C.; Selmi-Ruby, S.; Giroud, H.; Berger-Dutrieux, N.; Decaussin, M.; Peix, J.L.; Bournaud, C.; Orgiazzi, J.; Borson-Chazot, F.; Dante, R.; Rousset, B. Silencing of the tumor suppressor gene SLC5A8 is associated with BRAF mutations in classical papillary thyroid carcinomas. J Clin Endocrinol Metab 2005, 90, 3028–3035. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Maunakea, A.; Jun, P.; Bollen, A.W.; Hodgson, J.G.; Goldenberg, D.D.; Weiss, W.A.; Costello, J.F. Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res 2005, 65, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Helm, J.F.; Zheng, W.; Ly, Q.P.; Hodul, P.J.; Centeno, B.A.; Malafa, M.P. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas 2008, 36, e32–e39. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Toyota, M.; Akino, K.; Suzuki, H.; Kusano, M.; Satoh, A.; Mita, H.; Sasaki, Y.; Nojima, M.; Yanagihara, K.; Hinoda, Y.; Tokino, T.; Imai, K. Aberrant methylation and histone deacetylation associated with silencing of SLC5A8 in gastric cancer. Tumour Biol. [CrossRef]
- Li, B.; Li, X.; Ni, Z.; Zhang, Y.; Zeng, Y.; Yan, X.; Huang, Y.; He, J.; Lyu, X.; Wu, Y.; Wang, Y. Dichloroacetate and metformin synergistically suppress the growth of ovarian cancer cells. Oncotarget 2016, 7, 59458. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.P.; Poff, A.M.; Koutnik, A.P.; D’Agostino, D.P. Complex I inhibition augments dichloroacetate cytotoxicity through enhancing oxidative stress in VM-M3 glioblastoma cells. PloS one 2017, 12, e0180061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. Journal of neurochemistry 2002, 80, 780–787. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. Journal of Biological Chemistry 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; Chandel, N.S. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife, 2014; 3. [Google Scholar] [CrossRef]
- Algire, C.; Moiseeva, O.; Deschênes-Simard, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Viollet, B.; Ferbeyre, G.; Pollak, M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prevention Research 2012, 5, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; O'Neill, L.A. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. Journal of Biological Chemistry 2015, 290, 20348–20359. [Google Scholar] [CrossRef]
- De Haes, W.; Frooninckx, L.; Van Assche, R.; Smolders, A.; Depuydt, G.; Billen, J.; Braeckman, B.P.; Schoofs, L.; Temmerman, L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proceedings of the National Academy of Sciences 2014, 111, E2501–E2509. [Google Scholar] [CrossRef]
- Bridges, H.R.; Jones, A.J.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochemical Journal 2014, 462, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; De, B.B.; Gunsalus, I.C.; Hornberger CS, Jr. Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. [CrossRef]
- Korotchkina, L.G.; Sidhu, S.; Patel, M.S. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res, 1083. [Google Scholar] [CrossRef]
- Konrad, T.; Vicini, P.; Kusterer, K.; Höflich, A.; Assadkhani, A.; Böhles, H.J.; Sewell, A.; Tritschler, H.J.; Cobelli, C.; Usadel, K.H. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes care 1999, 22, 280–287. [Google Scholar] [CrossRef] [PubMed]
- 349. Mahban Rahimifard, Mona Navaei-Nigjeh, Maryam Baeeri, et al. Multiple protective mechanisms of alpha-lipoic acid in oxidation, apoptosis and inflammation against hydrogen peroxide induced toxicity in human lymphocytes. Molecular and Cellular Biochemistry. [CrossRef]
- Schwartz, L.; Abolhassani, M.; Guais, A.; Sanders, E.; Steyaert, J.M.; Campion, F.; Israël, M. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: preliminary results. Oncology reports 2010, 23, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Nickel, A.; Daniel, H. α-lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2−. -generation. Apoptosis 2005, 10, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Moungjaroen, J.; Nimmannit, U.; Callery, P.S.; Wang, L.; Azad, N.; Lipipun, V.; Chanvorachote, P.; Rojanasakul, Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. Journal of Pharmacology and Experimental Therapeutics 2006, 319, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Simbula, G.; Columbano, A.; Ledda-Columbano, G.M.; Sanna, L.; Deidda, M.; Diana, A.; Pibiri, M. Increased ROS generation and p53 activation in α-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis 2007, 12, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Pirsig, S.; Seidl, C.; Aichler, M.; Feuchtinger, A.; Bruchelt, G.; Senekowitsch-Schmidtke, R. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer biology & therapy 2012, 13, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Pyaskovskaya, O.N.; Kolesnik, D.L.; Fedorchuk, A.G.; Prokhorova, I.V.; Solyanik, G.I. 2-Deoxy-D-glucose enhances dichloroacetate antitumor action against Lewis lung carcinoma. Exp Oncol 2016, 38, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhong, X.; Yuan, Y. Does baking soda function as a magic bullet for patients with cancer? A mini review. Integrative cancer therapies. 1534. [Google Scholar] [CrossRef]
- Ayyanathan, K. , Kesaraju, S., Dawson-Scully, K., & Weissbach, H. (2012). Combination of Sulindac and Dichloroacetate Kills Cancer Cells via Oxidative Damage. ( 7(7), e39949. [CrossRef]
- Dhar, S.; Lippard, S.J. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proceedings of the National Academy of Sciences 2009, 106, 22199–22204. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Marrache, S.; Harn, D.A.; Dhar, S. Mito-DCA: a mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS chemical biology 2014, 9, 1178–1187. [Google Scholar] [CrossRef]
- Hanberry, B.S.; Berger, R.; Zastre, J.A. High-dose vitamin B1 reduces proliferation in cancer cell lines analogous to dichloroacetate. Cancer chemotherapy and pharmacology 2014, 73, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Ghosh, M.; Dutta, S.K. A potent tumoricidal co-drug ‘Bet-CA’-an ester derivative of betulinic acid and dichloroacetate selectively and synergistically kills cancer cells. Scientific reports 2015, 5, 7762. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. , Liang, K., Hou, C. et al. Dichloroacetic acid and rapamycin synergistically inhibit tumor progression. J. Zhejiang Univ. Sci. B. [CrossRef]
- Bonner, M.Y.; Karlsson, I.; Rodolfo, M.; Arnold, R.S.; Vergani, E.; Arbiser, J.L. Honokiol bis-dichloroacetate (Honokiol DCA) demonstrates activity in vemurafenib-resistant melanoma in vivo. Oncotarget 2016, 7, 12857. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T. , Ishiguro, R.H., Ishiguro, M., Toki, A., & Terunuma, H. (2022). Synergistic anti-tumor effect of dichloroacetate and ivermectin. Cureus. [CrossRef]
- Marco-Brualla, J.; de Miguel, D.; Martínez-Lostao, L.; Anel, A. DR5 Up-Regulation Induced by Dichloroacetate Sensitizes Tumor Cells to Lipid Nanoparticles Decorated with TRAIL. Journal of Clinical Medicine 2023, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Mironiuk-Puchalska, E.; Karatsai, O.; Żuchowska, A.; Wróblewski, W.; Borys, F.; Lehka, L.; Rędowicz, M.J.; Koszytkowska-Stawińska, M. Development of 5-fluorouracil-dichloroacetate mutual prodrugs as anticancer agents. Bioorganic Chemistry 2023, 140, 106784. [Google Scholar] [CrossRef] [PubMed]
- Korga, A.; Ostrowska, M.; Iwan, M.; Herbet, M.; Dudka, J. Inhibition of glycolysis disrupts cellular antioxidant defense and sensitizes HepG2 cells to doxorubicin treatment. FEBS Open Bio 2019, 9, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Choudhary, D.; Mikhalyuk, A.; Trammel, C.; Shanmugam, S.; Abbott, E.; Pilbeam, C.C.; Taylor III, JA. The role of pyruvate dehydrogenase kinase-4 (PDK4) in bladder cancer and chemoresistance. Molecular Cancer Therapeutics 2018, 17, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Fekir, K.; Dubois-Pot-Schneider, H.; Desert, R.; Daniel, Y.; Glaise, D.; Rauch, C.; Morel, F.; Fromenty, B.; Musso, O.; Cabillic, F.; Corlu, A. Retrodifferentiation of human tumor hepatocytes to stem cells leads to metabolic reprogramming and chemoresistance. Cancer research 2019, 79, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Skeberdytė, A.; Sarapinienė, I.; Aleksander-Krasko, J.; Stankevičius, V.; Sužiedėlis, K.; Jarmalaitė, S. Dichloroacetate and salinomycin exert a synergistic cytotoxic effect in colorectal cancer cell lines. Scientific reports 2018, 8, 17744. [Google Scholar] [CrossRef]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol reduces cancer risk: a population-based cohort study. Medicine. [CrossRef]
- Wang, F.; Liu, H.; Wang, F.; Xu, R.; Wang, P.; Tang, F.; Zhang, X.; Zhu, Z.; Lv, H.; Han, T. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Molecular medicine reports 2018, 17, 5213–5221. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer by the beta-blocker propranolol. Anti-cancer drugs 2009, 20, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Ma, Q.; Wang, L.; Hu, H.; Li, J.; Zhang, D.; Zhang, M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncology reports 2009, 22, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Propranolol as an anti-cancer agent. ecancermedicalscience. [CrossRef]
- Lucido, C.T.; Miskimins, W.K.; Vermeer, P.D. Propranolol promotes glucose dependence and synergizes with dichloroacetate for anti-cancer activity in HNSCC. Cancers 2018, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Guha, I.; Chatterjee, A.; Banerji, A. Anti-cancer potential of all-trans retinoic acid (ATRA): A Review. InProceedings of the Zoological Society 2013 Jun (Vol. 66, pp. 1–7). Springer-Verlag. [CrossRef]
- Hoffman, E.; Mielicki, W. All-trans retinoic acid (ATRA) in prevention and cancer therapy. Advances in Hygiene and Experimental Medicine. 2010 Jun 9;64. Downloaded from http://www.phmd.pl/fulltxt.php?ICID=911863. Accesed February 2023.
- Abildgaard, C.; Dahl, C.; Abdul-Al, A.; Christensen, A.; Guldberg, P. Inhibition of retinoic acid receptor β signaling confers glycolytic dependence and sensitization to dichloroacetate in melanoma cells. Oncotarget 2017, 8, 84210. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, Q.; Jiang, F.; Yu, D.; Mao, Q.; Xia, W.; Shi, R.; Wang, J.; Xu, L. Diisopropylamine dichloroacetate enhances radiosensitization in esophageal squamous cell carcinoma by increasing mitochondria-derived reactive oxygen species levels. Oncotarget 2016, 7, 68170. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, J.; Ge, S.; Su, Y.; Fan, X. Novel insight into metabolic reprogrammming in cancer radioresistance: A promising therapeutic target in radiotherapy. International Journal of Biological Sciences 2023, 19, 811. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.F.; Nagamine, M.K.; Nowosh, V.; Machado, F.T.; Massoco, C.O.; Souza-Pinto, N.C.; Dagli, M.L. Antineoplastic effects of sodium dichloroacetate and omeprazole, alone or in combination, on canine oral mucosal melanoma cells. Frontiers in Veterinary Science. [CrossRef]
- Romero, Y.; Castillejos-López, M.; Romero-García, S.; Aguayo, A.S.; Herrera, I.; Garcia-Martin, M.O.; Torres-Espíndola, L.M.; Negrete-García, M.C.; Olvera, A.C.; Huerta-Cruz, J.C.; Velázquez-Cruz, R. Antitumor therapy under hypoxic microenvironment by the combination of 2-methoxyestradiol and sodium dichloroacetate on human non-small-cell lung cancer. Oxidative Medicine and Cellular Longevity. 2020. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, X.; Wang, K.; Liu, J.; Huang, G. Dichloroacetic acid (DCA) synergizes with the SIRT2 inhibitor Sirtinol and AGK2 to enhance anti-tumor efficacy in non-small cell lung cancer. Cancer Biology & Therapy 2018, 19, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Dyrstad, S.E.; Lotsberg, M.L.; Tan, T.Z.; Pettersen, I.K.; Hjellbrekke, S.; Tusubira, D.; Engelsen, A.S.; Daubon, T.; Mourier, A.; Thiery, J.P.; Dahl, O. Blocking aerobic glycolysis by targeting pyruvate dehydrogenase kinase in combination with EGFR TKI and ionizing radiation increases therapeutic effect in non-small cell lung cancer cells. Cancers 2021, 13, 941. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.L.; Hu, X.; Tam, K.Y. Inhibition of pyruvate dehydrogenase kinase 1 enhances the anti-cancer effect of EGFR tyrosine kinase inhibitors in non-small cell lung cancer. European Journal of Pharmacology 2018, 838, 41–52. [Google Scholar] [CrossRef]
- Frisch, A.; Wang, Y.; Wang, Y.; Lontos, K.; Rivadeneira, D.; Delgoffe, G. 100 Redirecting glucose flux during in vitro expansion improves the in vivo performance of adoptive T cell therapies for cancer. Journal for ImmunoTherapy of Cancer. A: 1;9(Suppl 2). [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Karioti, A.; Antoniadi, G.; Antoniadis, N.; Liakopoulos, V.; Stefanidis, I. Dichloroacetate at therapeutic concentration alters glucose metabolism and induces regulatory T-cell differentiation in alloreactive human lymphocytes. Journal of basic and clinical physiology and pharmacology 2013, 24, 271–276. [Google Scholar] [CrossRef]
- Cluxton, D.; Petrasca, A.; Moran, B.; Fletcher, J.M. Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Frontiers in immunology 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, N.A.; Lopez, V.L.; Bautista, A.D.; Mizisin, L.M.; Torres, B.R.; Shroads, A.L.; Mizisin, A.P.; Stacpoole, P.W. Peripheral neuropathy in rats exposed to dichloroacetate. J Neuropathol Exp Neurol 2009, 68, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Engelstad, K. ; Wei Y et al (2006) Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 66:324–330. [CrossRef]
- Brandsma, D.; Dorlo, T.P.; Haanen, J.H.; Beijnen, J.H.; Boogerd, W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. Journal of neurology 2010, 257, 2099–2100. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.M. Trial of dichloroacetate in MELAS: toxicity overshadows the assessment of potential benefit. Neurology 2006, 66, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Felitsyn, N.; Stacpoole, P.W.; Notterpek, L. Dichloroacetate causes reversible demyelination in vitro: potential mechanism for its neuropathic effect. Journal of neurochemistry 2007, 100, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Deck, M.; Van Hameren, G.; Campbell, G.; Bernard-Marissal, N.; Devaux, J.; Berthelot, J.; Lattard, A.; Médard, J.J.; Gautier, B.; Guelfi, S.; Abbou, S. Physiology of PNS axons relies on glycolytic metabolism in myelinating Schwann cells. Plos one 2022, 17, e0272097. [Google Scholar] [CrossRef] [PubMed]
- Babetto, E.; Wong, K.M.; Beirowski, B. A glycolytic shift in Schwann cells supports injured axons. Nature neuroscience 2020, 23, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Bouçanova, F.; Chrast, R. Metabolic interaction between Schwann cells and axons under physiological and disease conditions. Frontiers in cellular neuroscience 2020, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; Diaz, F. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W.; Martyniuk, C.J.; James, M.O.; Calcutt, N.A. Dichloroacetate-induced peripheral neuropathy. International Review of neurobiology 2019, 145, 211–238. [Google Scholar] [CrossRef] [PubMed]
- Shroads, A.L.; Langaee, T.; Coats, B.S.; Kurtz, T.L.; Bullock, J.R.; Weithorn, D.; Gong, Y.; Wagner, D.A.; Ostrov, D.A.; Johnson, J.A.; Stacpoole, P.W. Human polymorphisms in the glutathione transferase zeta 1/maleylacetoacetate isomerase gene influence the toxicokinetics of dichloroacetate. The Journal of Clinical Pharmacology 2012, 52, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Washington, J.T.; Quintyne, N.J. Dichloroacetate induces different rates of cell death in cancer and noncancer cell lines in vitro. Tumori Journal 2012, 98, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.J.; Sanchez, I.M.; Nelson, M.A.; Larson, J.L.; Lansing, A.J. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology 1990, 63, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.L.; Bull, R.J. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicology and applied pharmacology 1992, 115, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Schwab, S.; Efferth, T. Fatal liver and bone marrow toxicity by combination treatment of dichloroacetate and artesunate in a glioblastoma multiforme patient: case report and review of the literature. Frontiers in Oncology 2016, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.M.; Bull, R.J. Early induction of reparative hyperplasia in the liver of B6C3F1 mice treated with dichloroacetate and trichloroacetate. Toxicology 1990, 64, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, X. , Dixit, V., Liu, H., Shroads, A.L., Henderson, G.N., James, M.O. and Stacpoole, P.W., 2006. Inhibition and recovery of rat hepatic glutathione S-transferase zeta and alteration of tyrosine metabolism following dichloroacetate exposure and withdrawal. Drug metabolism and disposition. [CrossRef]
- Hassoun, E.A.; Dey, S. Dichloroacetate-and trichloroacetate-induced phagocytic activation and production of oxidative stress in the hepatic tissues of mice after acute exposure. Journal of biochemical and molecular toxicology 2008, 22, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, E. , Cearfoss, J., Mamada, S., Al-Hassan, N., Brown, M., Heimberger, K. and Liu, M.C., 2014. The effects of mixtures of dichloroacetate and trichloroacetate on induction of oxidative stress in livers of mice after subchronic exposure. Journal of Toxicology and Environmental Health, Part, A.; 77. [CrossRef]
- James, M.O.; Stacpoole, P.W. Pharmacogenetic considerations with dichloroacetate dosing. Pharmacogenomics 2016, 17, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Ragazzi, E.; Montopoli, M. Mitochondrial involvement in cisplatin resistance. International journal of molecular sciences 2019, 20, 3384. [Google Scholar] [CrossRef]
- Bruzzese, F.; Rocco, M.; Castelli, S.; Di Gennaro, E.; Desideri, A.; Budillon, A. Synergistic antitumor effect between vorinostat and topotecan in small cell lung cancer cells is mediated by generation of reactive oxygen species and DNA damage-induced apoptosis. Molecular cancer therapeutics 2009, 8, 3075–3087. [Google Scholar] [CrossRef]
- Su, L. , Zhang, H., Yan, C., Chen, A., Meng, G., Wei, J., … Ding, Y. Superior anti-tumor efficacy of diisopropylamine dichloroacetate compared with dichloroacetate in a subcutaneous transplantation breast tumor model. Oncotarget, 2016; 7, 65721–65731. [Google Scholar] [CrossRef]
- She, W. , Liu, T., Li, H., Wang, Z., Guo, Z., Liu, Y., & Liu, Y. (2023). Reprogramming Energy Metabolism with Synthesized PDK Inhibitors Based on Dichloroacetate Derivatives and Targeted Delivery Systems for Enhanced Cancer Therapy. Journal of Medicinal Chemistry 2023, 66, 21–14683. [Google Scholar] [CrossRef] [PubMed]
- Trapella, C.; Voltan, R.; Melloni, E.; Tisato, V.; Celeghini, C.; Bianco, S.; Fantinati, A.; Salvadori, S.; Guerrini, R.; Secchiero, P.; Zauli, G. Design, synthesis, and biological characterization of novel mitochondria targeted dichloroacetate-loaded compounds with antileukemic activity. Journal of Medicinal Chemistry 2016, 59, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fereidoonnezhad, M.; Tabaei, S.M.; Sakhteman, A.; Seradj, H.; Faghih, Z.; Faghih, Z.; Mojaddami, A.; Sadeghian, B.; Rezaei, Z. Design, synthesis, molecular docking, biological evaluations and QSAR studies of novel dichloroacetate analogues as anticancer agent. Journal of Molecular Structure 2020, 1221, 128689. [Google Scholar] [CrossRef]
- Yang, Y.; Shang, P.; Cheng, C.; Wang, D.; Yang, P.; Zhang, F.; Li, T.; Lu, A.; Zhao, Y. Novel N-phenyl dichloroacetamide derivatives as anticancer reagents: design, synthesis and biological evaluation. European journal of medicinal chemistry 2010, 45, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Zajac, J.; Kostrhunova, H.; Novohradsky, V.; Vrana, O.; Raveendran, R.; Gibson, D.; Kasparkova, J.; Brabec, V. Potentiation of mitochondrial dysfunction in tumor cells by conjugates of metabolic modulator dichloroacetate with a Pt (IV) derivative of oxaliplatin. Journal of Inorganic Biochemistry 2016, 156, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wu, X.Y.; Luo, O.Y.; Su, L.; Ding, Y.T.; Jiang, Y.; Yu, D.C. Diisopropylamine dichloroacetate alleviates liver fibrosis through inhibiting activation and proliferation of hepatic stellate cells. Int. J. Clin. Exp. Med 2019, 12, 3440–3448. [Google Scholar]
- Yamane, K.; Indalao, I.L.; Chida, J.; Yamamoto, Y.; Hanawa, M.; Kido, H. Diisopropylamine dichloroacetate, a novel pyruvate dehydrogenase kinase 4 inhibitor, as a potential therapeutic agent for metabolic disorders and multiorgan failure in severe influenza. PloS one 2014, 9, e98032. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, H.; Yan, C.; Chen, A.; Meng, G.; Wei, J.; Yu, D.; Ding, Y. Superior anti-tumor efficacy of diisopropylamine dichloroacetate compared with dichloroacetate in a subcutaneous transplantation breast tumor model. Oncotarget 2016, 7, 65721. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Marier, D.; Marsden, E.; Andrews, D.; Eliaz, I. A novel form of dichloroacetate therapy for patients with advanced cancer: a report of 3 cases. Altern Ther Health Med. 2014 Oct 1;20(Suppl 2):21-8. PMID 25362214.
- Khan, A.; Ghen, M. A 15 Year Evolution of Dichloroacetate-Based Metabolic Cancer Therapy: A Review with Case Reports. Medical Research Archives. [CrossRef]
- Ishiguro, T.; Ishiguro, R.; Ishiguro, M.; Iwai, S. Co-treatment of dichloroacetate, omeprazole and tamoxifen exhibited synergistically antiproliferative effect on malignant tumors: in vivo experiments and a case report. Hepato-gastroenterology 2012, 59, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Lemmo, W.; Tan, G. Prolonged Survival After Dichloroacetate Treatment of Non-Small-Cell Lung Carcinoma-Related Leptomeningeal Carcinomatosis. Journal of Medical Cases 2016, 7, 136–142. [Google Scholar] [CrossRef]
- Dunbar, E.M.; Coats, B.S.; Shroads, A.L.; et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 2014, 32, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Christofk, H.R.; Hosmer, W.; et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Research and Clinical Oncology. 2014, 140, 443–52. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Mazurczak, M.; Dib, E.G.; Bleeker, J.S.; Geeraerts, L.H.; Tinguely, M.; Lohr, M.M.; McGraw, S.C.; Jensen, A.W.; Ellison, C.A.; Black, L.J.; Puumala, S.E.; Reed, V.J.; Miskimins, W.K.; Lee, J.H.; Spanos, W.C. Phase II study of dichloroacetate, an inhibitor of pyruvate dehydrogenase, in combination with chemoradiotherapy for unresected, locally advanced head and neck squamous cell carcinoma. Invest New Drugs 2022, 40, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Heshe, D.; Hoogestraat, S.; Brauckmann, C.; Karst, U.; Boos, J.; Lanvers-Kaminsky, C. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs. Cancer chemotherapy and pharmacology 2011, 67, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Lacombe, K.; Adamcic, U.; Minhas, K.; Coomber, B.L. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer letters 2010, 297, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Jajeh, J.; Guinan, P.; Rubenstein, M. In vitro effects of dichloroacetate and CO2 on hypoxic HeLa cells. Anticancer research 2009, 29, 4579–4588. [Google Scholar] [PubMed]
- Zwicker, F.; Kirsner, A.; Peschke, P.; Roeder, F.; Debus, J.; Huber, P.E.; Weber, K.J. Dichloroacetate induces tumor-specific radiosensitivity in vitro but attenuates radiation-induced tumor growth delay in vivo. Strahlentherapie und Onkologie 2013, 189, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Seidl, C.; Pirsig, S.; Bruchelt, G.; Senekowitsch-Schmidtke, R. DCA promotes progression of neuroblastoma tumors in nude mice. American journal of cancer research 2015, 5, 812. [Google Scholar] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nature reviews cancer 2020, 20, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Sounidaki, M.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. In human alloreactive CD4+ T-cells, dichloroacetate inhibits aerobic glycolysis, induces apoptosis and favors differentiation towards the regulatory T-cell subset instead of effector T-cell subsets. Molecular medicine reports 2016, 13, 3370–3376. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, H.; Khakpoor-Kooshe, M.; Jafarzadeh, L.; Masoumi, E.; Fallah-Mehrjardi, K.; Tavassolifar, M.J.; Pawelek, J.M.; Mirzaei, H.R.; Hadjati, J. Restricting Tumor Lactate Metabolism using Dichloroacetate Improves T Cell Functions. BMC Cancer, 06 Jan 2022, 22(1):39. [CrossRef]
- Schoonjans, C.A.; Joudiou, N.; Brusa, D.; Corbet, C.; Feron, O.; Gallez, B. Acidosis-induced metabolic reprogramming in tumor cells enhances the anti-proliferative activity of the PDK inhibitor dichloroacetate. Cancer letters 2020, 470, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, R. , Iannuzzi, C., Manco, G., & Brunetti-Pierri, N. Differential inhibition of PDKs by phenylbutyrate and enhancement of pyruvate dehydrogenase complex activity by combination with dichloroacetate. Journal of inherited metabolic disease 2015, 38, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Škorja Milić, N. , Dolinar, K., Miš, K., Matkovič, U., Bizjak, M., Pavlin, M.,... & Pirkmajer, S. (2021). Suppression of pyruvate dehydrogenase kinase by dichloroacetate in cancer and skeletal muscle cells is isoform specific and partially independent of HIF-1α. International journal of molecular sciences. [CrossRef]
- Tiersma, J.F. , Evers, B., Bakker, B.M., Jalving, M., & de Jong, S. (2022). Pyruvate dehydrogenase kinase inhibition by dichloroacetate in melanoma cells unveils metabolic vulnerabilities. ( 23(7), 3745. [CrossRef] [PubMed]
- Gang, B.P. , Rooke, M., Sun, R.C., Banskota, S., Mishra, S., Dahlstrom, J.E., & Blackburn, A.C. (2023). Pyruvate dehydrogenase kinase expression profile is a biomarker for cancer sensitivity to dichloroacetate-mediated growth inhibition. bioRxiv. [CrossRef]
- Fiorillo, M.; Lamb, R.; Tanowitz, H.B.; Mutti, L.; Krstic-Demonacos, M.; Cappello, A.R.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Repurposing atovaquone: Targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget 2016, 7, 34084. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hardy, M.; Topchyan, P.; Zander, R.; Volberding, P.; Cui, W.; Kalyanaraman, B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Scientific reports 2020, 10, 17872. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Segaran, A.; Lord, S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. InSeminars in Cancer Biology, 86. [CrossRef]
- Gurtu, V.; Webster, L.; Kinnaird, A.; Boukouris, A.; White, C.; Nagendran, J.; Freed, D.H.; Wilkins, M.R.; Michelakis, E. Targeting Pyruvate Dehydrogenase Kinase With Dichloroacetate in Pulmonary Arterial Hypertension: A Variable Clinical Response Driven by Genetic Polymorphisms in Sirtuin-3 and Uncoupling Protein-2. Circulation. 2017 Nov 14;136(suppl_1):A17443-.
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W. , & Dang, C.V. (). Multifaceted roles of glycolytic enzymes. Trends in biochemical sciences 2005, 30, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Parczyk, J. , Ruhnau, J., Pelz, C., Schilling, M., Wu, H., Piaskowski, N.N.,... & Klein, A. (2021). Dichloroacetate and PX-478 exhibit strong synergistic effects in a various number of cancer cell lines. BMC cancer. [CrossRef]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S.; Kong, R. In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Scientific reports 2016, 6, 28479. [Google Scholar] [CrossRef]
- Qiu, X.; Jiang, Z.; Luo, Y.; Tian, D.; Song, T.; Li, Q. PPP3CB Inhibits Cell Proliferation and the Warburg Effect in Bladder Cancer by Blocking PDHK1. Frontiers in Bioscience-Landmark 2024, 29, 48. [Google Scholar] [CrossRef] [PubMed]
- Zajac, J.; Kostrhunova, H.; Novohradsky, V.; Vrana, O.; Raveendran, R.; Gibson, D.; Kasparkova, J.; Brabec, V. Potentiation of mitochondrial dysfunction in tumor cells by conjugates of metabolic modulator dichloroacetate with a Pt (IV) derivative of oxaliplatin. Journal of Inorganic Biochemistry 2016, 156, 89–97. [Google Scholar] [CrossRef]
- Sun, L. , Jiang, Y., Yan, X., Dai, X., Huang, C., Chen, L.,... & Lian, J. (2021). Dichloroacetate enhances the anti-tumor effect of sorafenib via modulating the ROS-JNK-Mcl-1 pathway in liver cancer cells. Experimental Cell Research, 1275. [Google Scholar] [CrossRef]
- Babu, E.; Ramachandran, S.; CoothanKandaswamy, V.; Elangovan, S.; Prasad, P.D.; Ganapathy, V.; Thangaraju, M. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 2011, 30, 4026. [Google Scholar] [CrossRef] [PubMed]
- Kailavasan, M.; Rehman, I.; Reynolds, S.; Bucur, A.; Tozer, G.; Paley, M. NMR-based evaluation of the metabolic profile and response to dichloroacetate of human prostate cancer cells. NMR in biomedicine 2014, 27, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Harris, R.A. Mechanism responsible for the hypoglycemic actions of dichloroacetate and 2-chloropropionate. Archives of Biochemistry and Biophysics 1979, 198, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Kawata, Y.; Moritoh, Y.; Watanabe, M. Dichloroacetate, a pyruvate dehydrogenase kinase inhibitor, ameliorates type 2 diabetes via reduced gluconeogenesis. Heliyon 2022, 8, e08889. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, M.S.; Bonnet, S.; Wu, X.; Dyck, J.R.; Haromy, A.; Hashimoto, K.; Michelakis, E.D. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 2004, 95, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E.; Nance, S. Selective reduction of fatty acid oxidation in colonocytes: correlation with ulcerative colitis. Lipids 1990, 25, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Hayek, A.; Woodside, W.F. Short-term influences of dichloroacetate on genetically hyperlipemic rats. Metabolism 1980, 29, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Dhanesha, N.; Doddapattar, P.; Rodriguez, O.; Sonkar, V.K.; Dayal, S.; Chauhan, A.K. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv 2018, 2, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Oh, C.J.; Park, S.; Lee, J.M.; Go, Y.; Park, B.Y.; Kang, H.J.; Kim, D.W.; Kim, J.E.; Yoo, E.K.; Kim, H.E.; Kim, M.J.; Jeon, Y.H.; Kim, Y.H.; Lee, C.H.; Jeon, J.H.; Lee, I.K. Therapeutic effect of dichloroacetate against atherosclerosis via hepatic FGF21 induction mediated by acute AMPK activation. Exp Mol Med 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Horne, A.W.; Ahmad, S.F.; Carter, R.; Simitsidellis, I.; Greaves, E.; Hogg, C.; Morton, N.M.; Saunders, P.T.K. Repurposing dichloroacetate for the treatment of women with endometriosis. Proc Natl Acad Sci U S A 2019, 116, 25389–25391. [Google Scholar] [CrossRef]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Bolatto, C.; Trías, E.; Gandelman, M.; Radi, R.; Barbeito, L.; Cassina, P. Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis. PloS one 2012, 7, e34776. [Google Scholar] [CrossRef] [PubMed]
- Shangraw, R.E.; Fisher, D.M. Pharmacokinetics and pharmacodynamics of dichloroacetate in patients with cirrhosis. Clinical Pharmacology & Therapeutics 1999, 66, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Shangraw, R.E.; Jahoor, F. Mechanism of dichloroacetate-induced hypolactatemia in humans with or without cirrhosis. Metabolism 2004, 53, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.C.; Solayman, M.H.; Lorenzo, R.J.; Langaee, T.; Stacpoole, P.W.; James, M.O. GSTZ1 expression and chloride concentrations modulate sensitivity of cancer cells to dichloroacetate. Biochimica et Biophysica Acta (BBA)-General Subjects 2016, 1860, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Terado, T.; Tambe, Y.; Mukaisho, K.I.; Kageyama, S.; Kawauchi, A.; Inoue, H. Cryptotanshinone, a novel PDK 4 inhibitor, suppresses bladder cancer cell invasiveness via the mTOR/β-catenin/N-cadherin axis. International journal of oncology 2021, 59, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Tambe, Y.; Terado, T.; Kim, C.J.; Mukaisho, K.; Yoshida, S.; Sugihara, H.; Tanaka, H.; Chida, J.; Kido, H.; Yamaji, K.; et al. Antitumor activity of potent pyruvate dehydrogenase kinase 4 inhibitors from plants in pancreatic cancer. Mol Carcinog. 58:1726–1737. 2019. [CrossRef]
- 468 Sestito, S.; Bacci, A.; Chiarugi, S.; Runfola, M.; Gado, F.; Margheritis, E.; Gul, S.; Riveiro, M.E.; Vazquez, R.; Huguet, S.; Manera, C. Development of potent dual PDK1/AurA kinase inhibitors for cancer therapy: Lead-optimization, structural insights, and ADME-Tox profile. European Journal of Medicinal Chemistry 2021, 226, 113895. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Yadav, V.K.; Kuo, K.T.; Pikatan, N.W.; Lin, C.S.; Chien, M.H.; Lee, W.H.; Hsiao, M.; Chiu, S.C.; Yeh, C.T.; Tsai, J.T. PDK1 inhibitor BX795 improves cisplatin and radio-efficacy in oral squamous cell carcinoma by downregulating the PDK1/CD47/Akt-mediated glycolysis signaling pathway. International journal of molecular sciences 2021, 22, 11492. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; De Franco, M.; Carbone, D.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Moro, S.; Gandin, V. 1, 2, 4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: Synthesis and pharmacological evaluation. European Journal of Medicinal Chemistry 2023, 249, 115134. [Google Scholar] [CrossRef]
- Mitchel, J.; Bajaj, P.; Patil, K.; Gunnarson, A.; Pourchet, E.; Kim, Y.N.; Skolnick, J.; Pai, S.B. Computational identification of stearic acid as a potential PDK1 inhibitor and in vitro validation of stearic acid as colon cancer therapeutic in combination with 5-fluorouracil. Cancer Informatics. 2021 Dec;20:11769351211065979. [CrossRef]
- Chen, Q.; Han, H.; Lin, F.; Yang, L.; Feng, L.; Lai, X.; Wen, Z.; Yang, M.; Wang, C.; Ma, Y.; Yin, T. Novel shikonin derivatives suppress cell proliferation, migration and induce apoptosis in human triple-negative breast cancer cells via regulating PDK1/PDHC axis. Life Sciences 2022, 310, 121077. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody–drug conjugates (ADCs) for cancer therapy. Cancer Cell International 2022, 22, 1–2. [Google Scholar] [CrossRef]
- Wei, G.; Sun, J.; Luan, W.; Hou, Z.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis-M2-TAMs polarization for combined cancer therapy. Journal of medicinal chemistry 2019, 62, 8760–8772. [Google Scholar] [CrossRef] [PubMed]
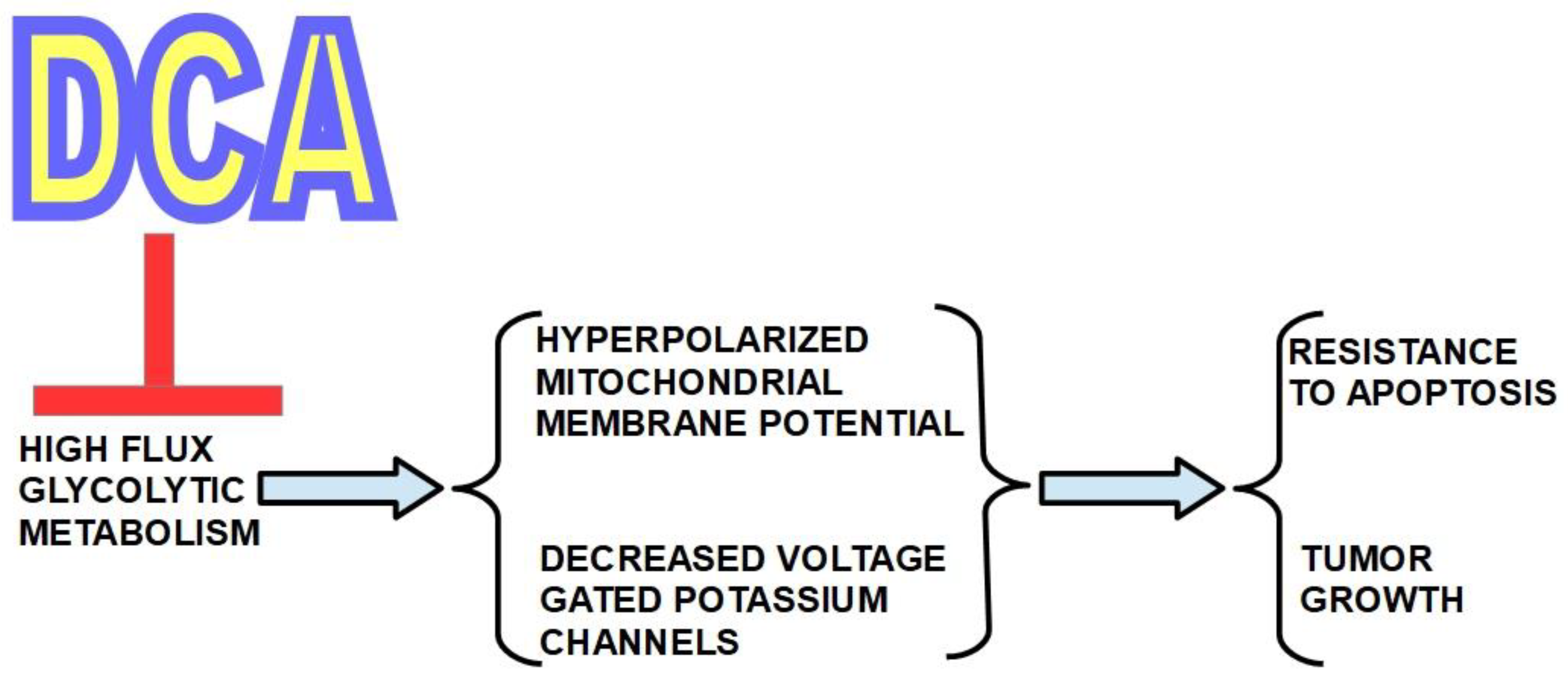

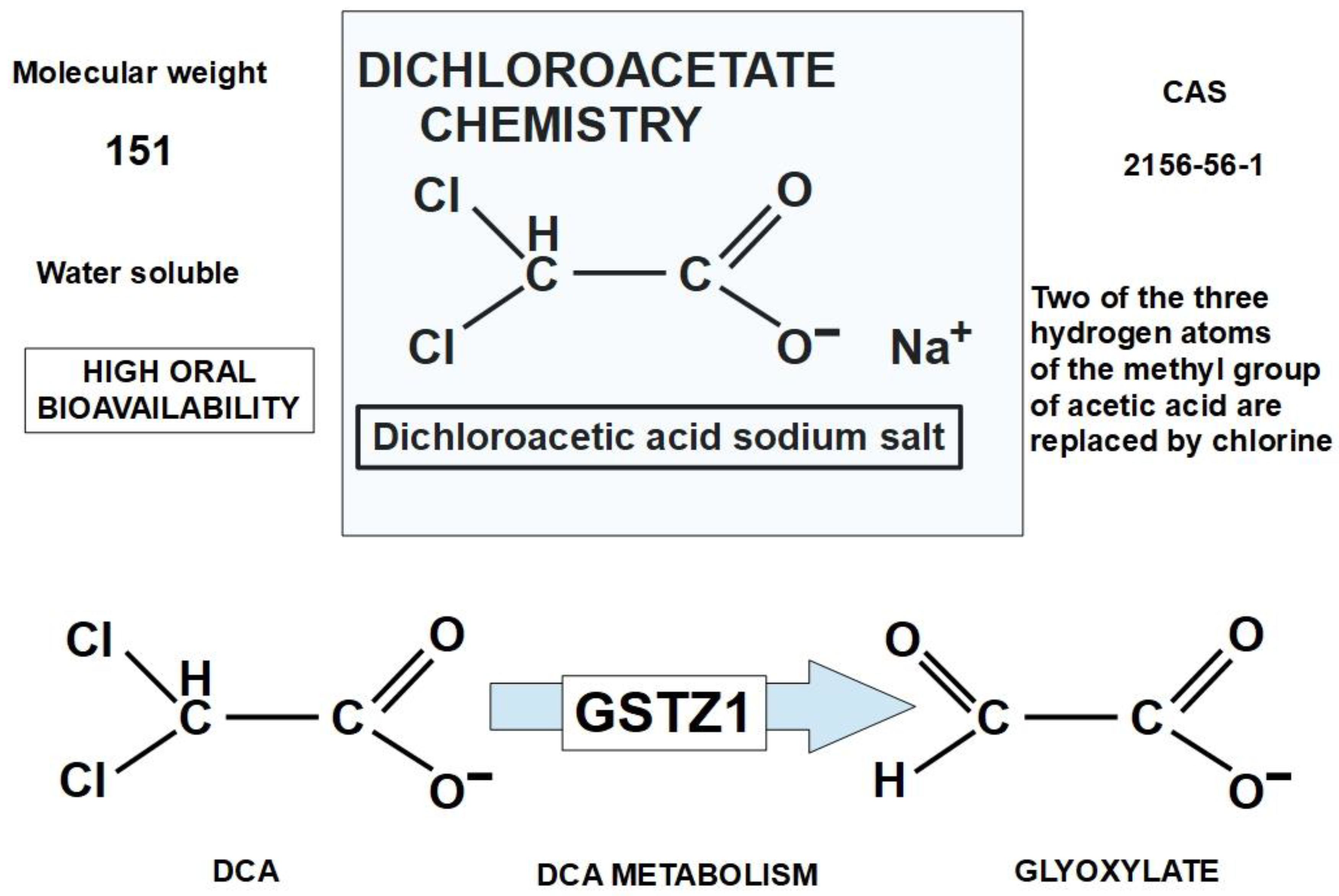
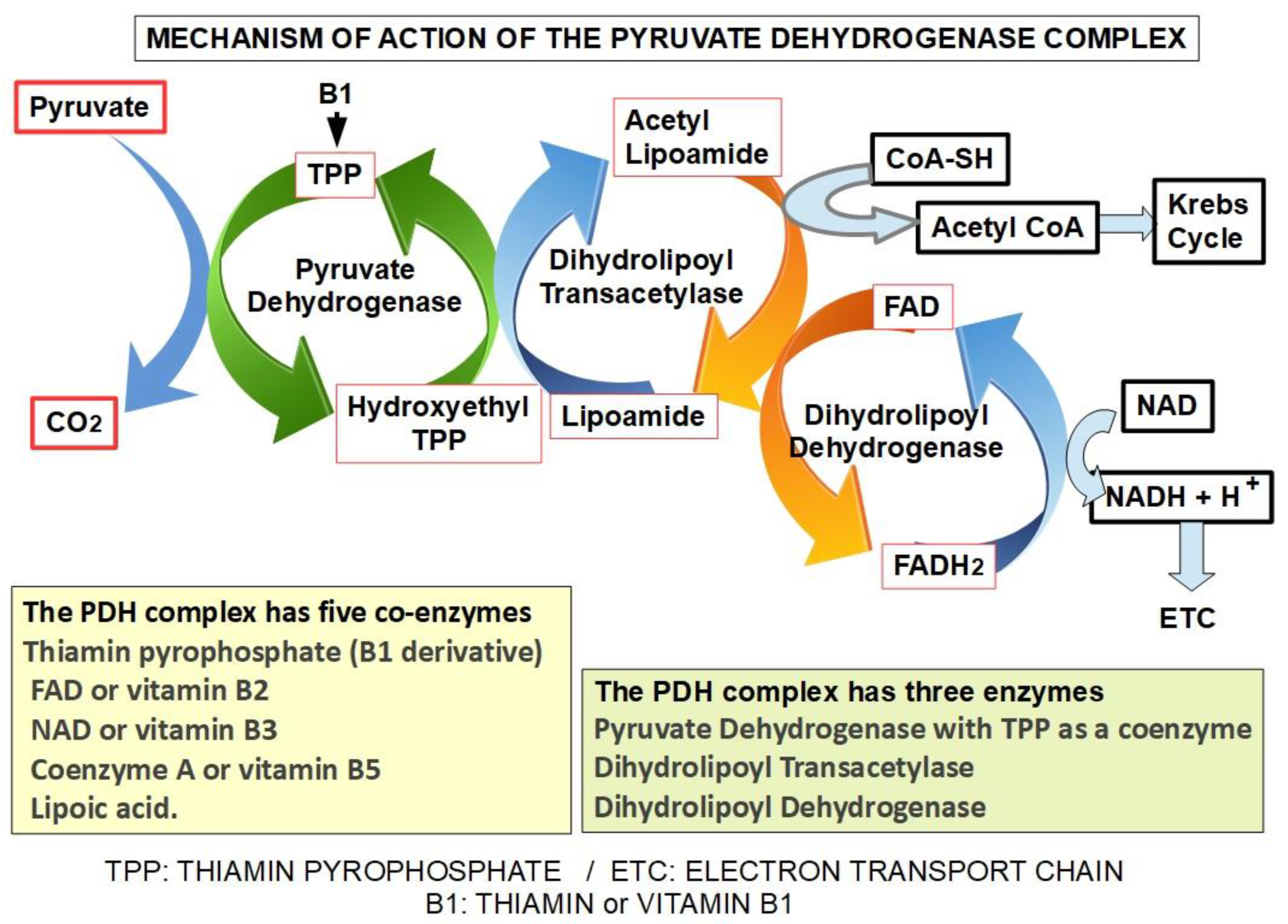
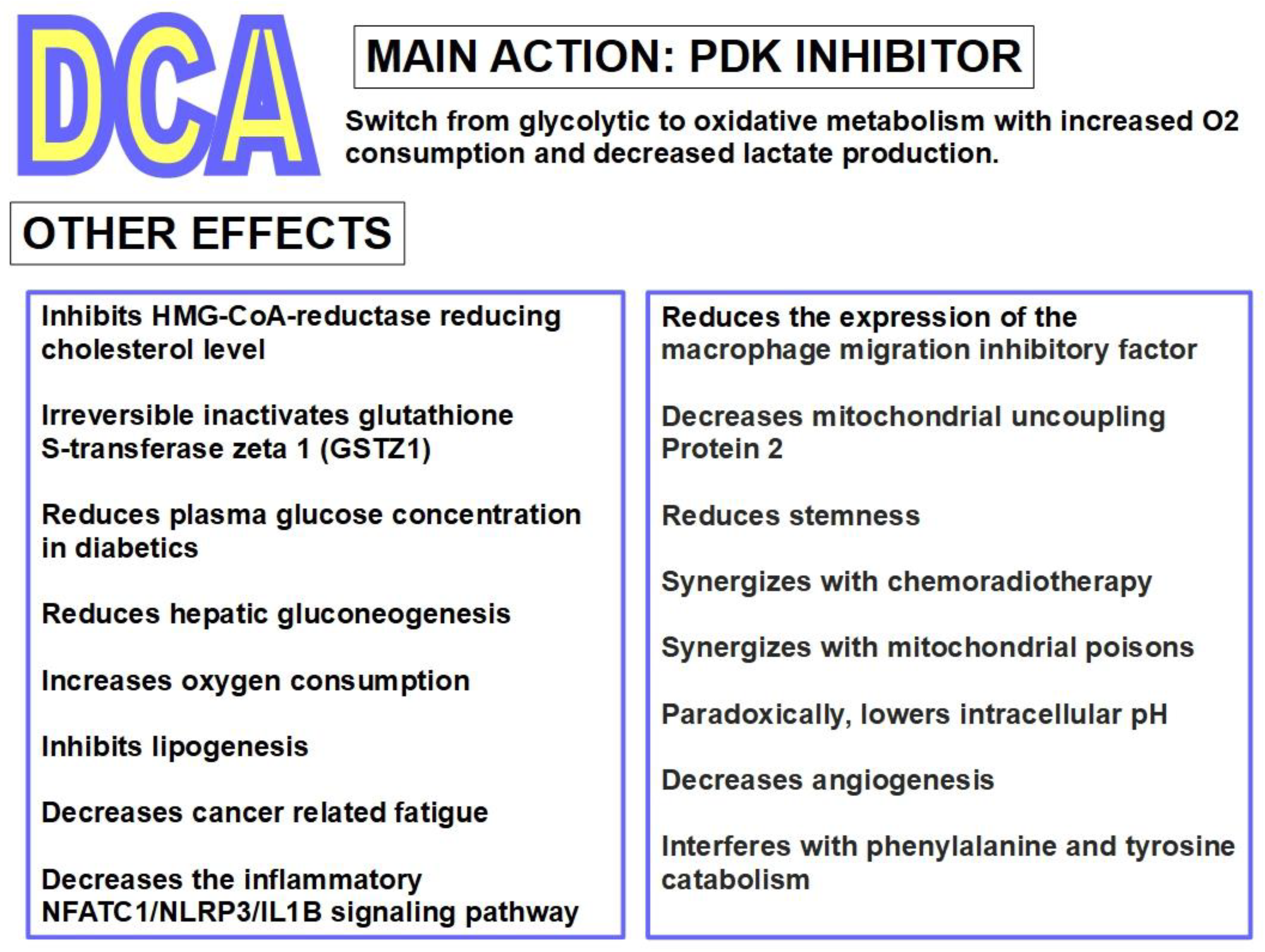
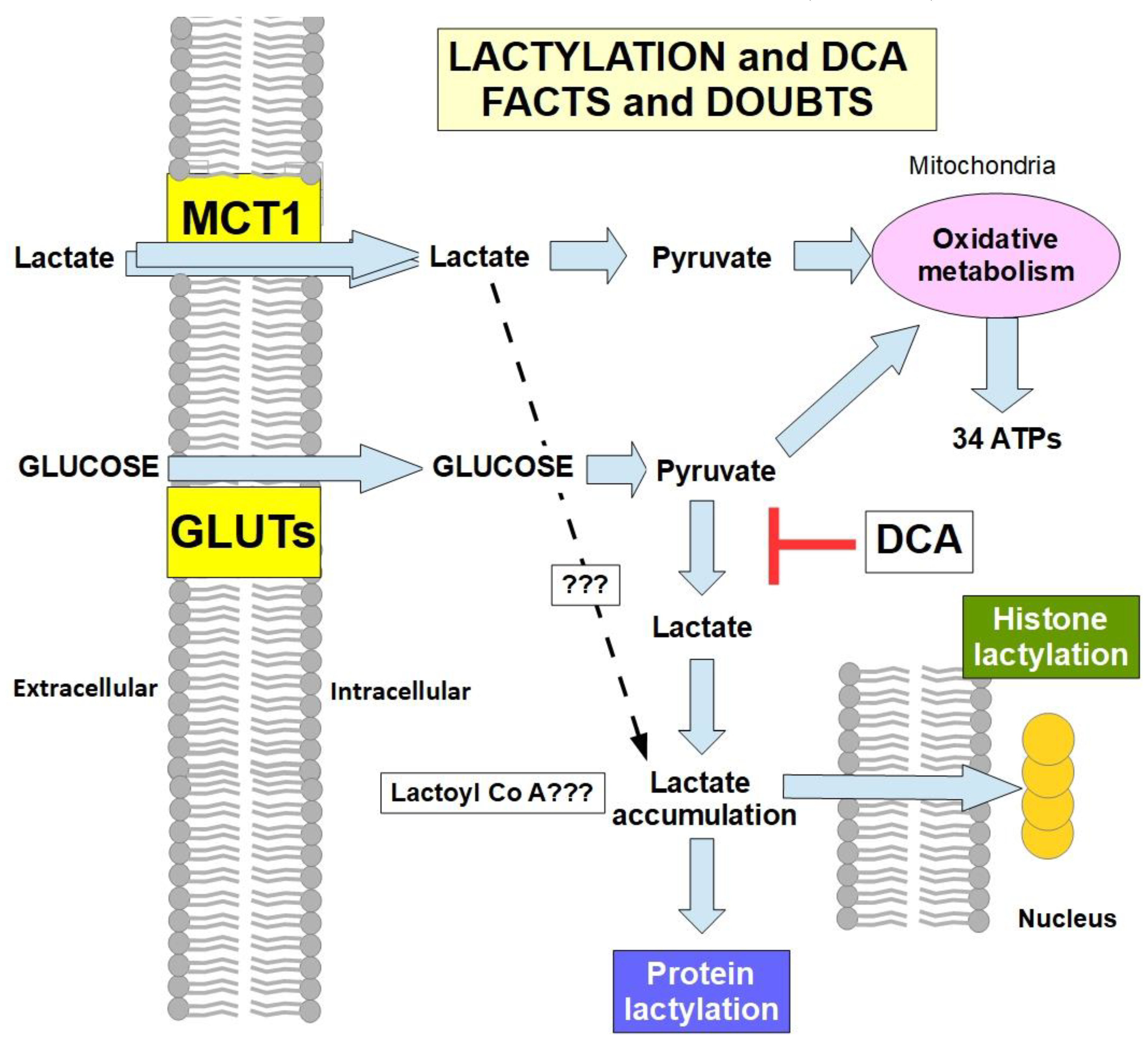
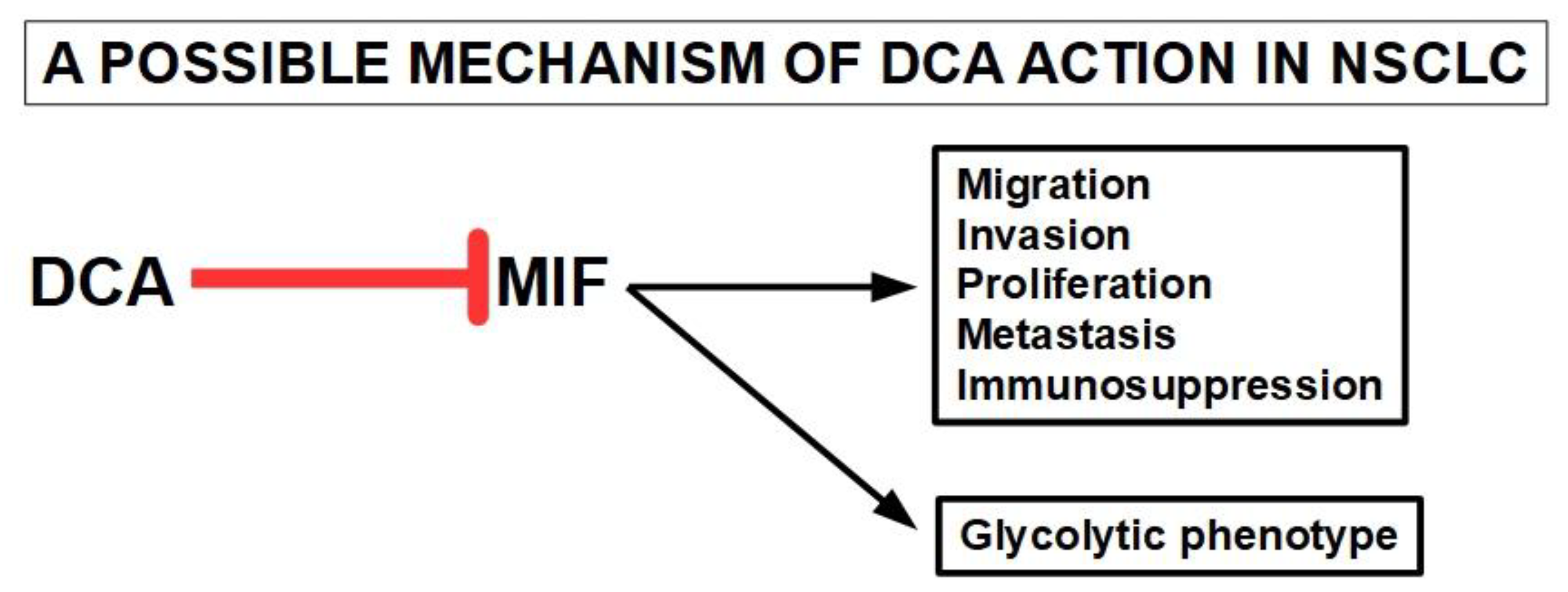
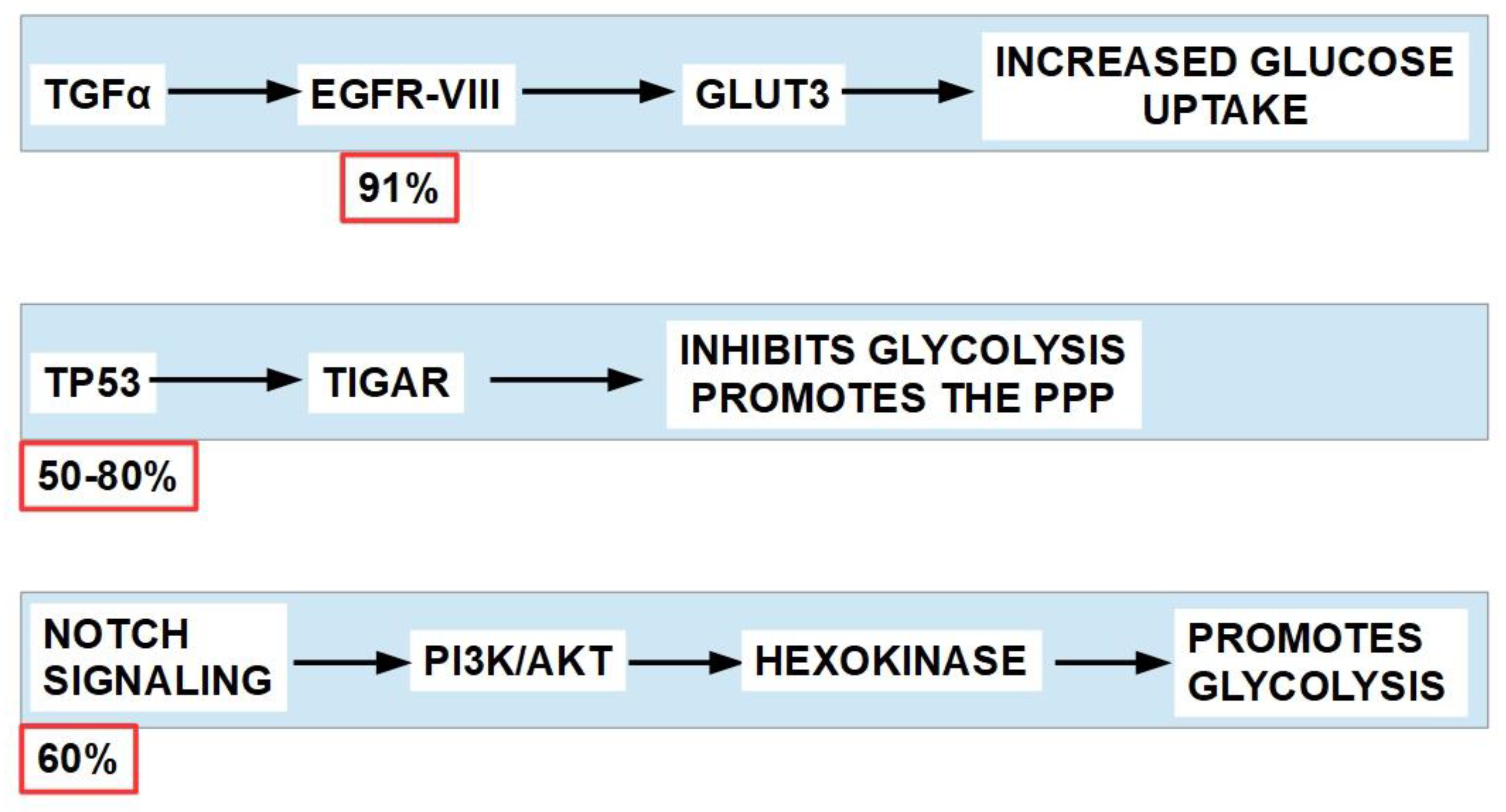
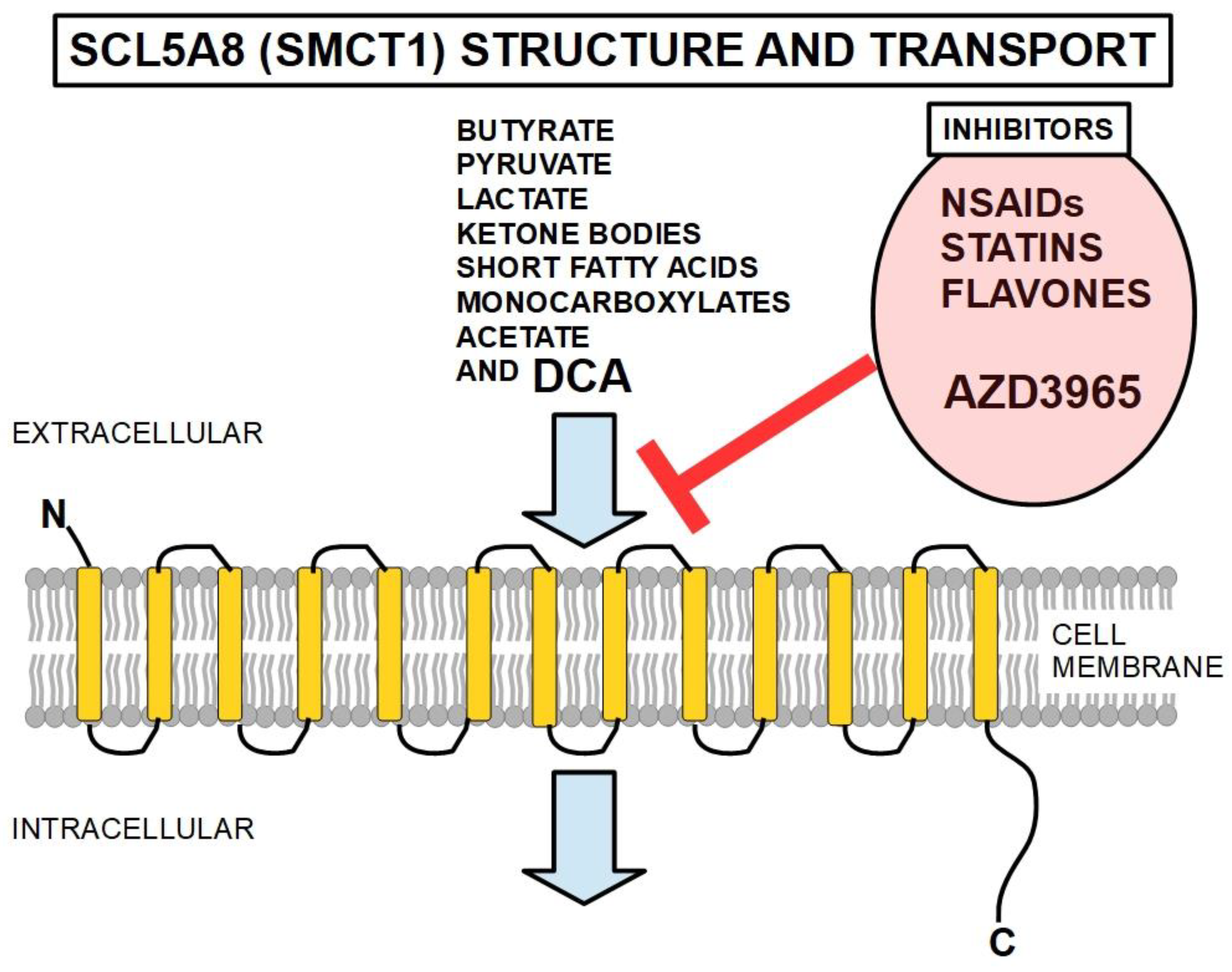
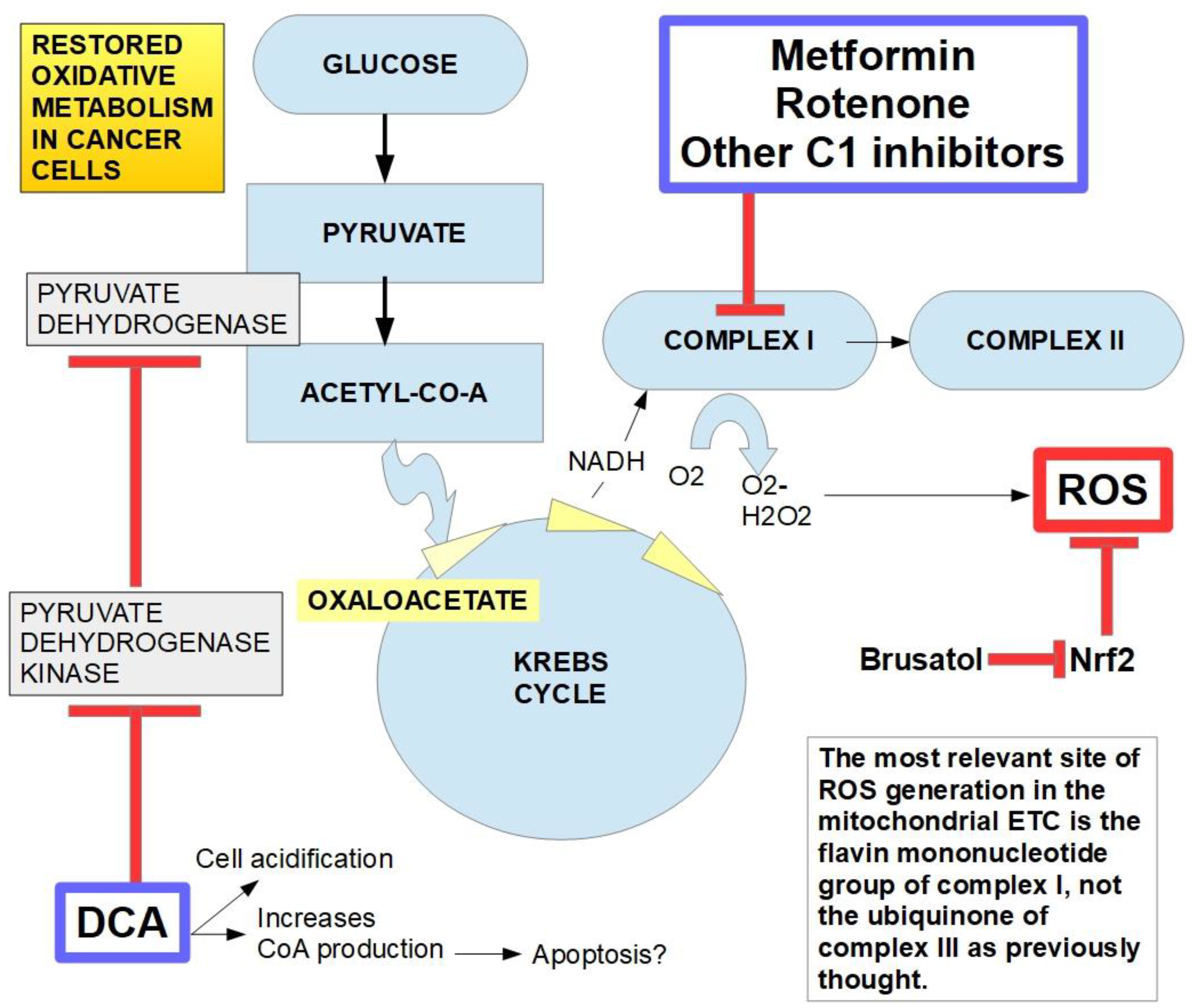

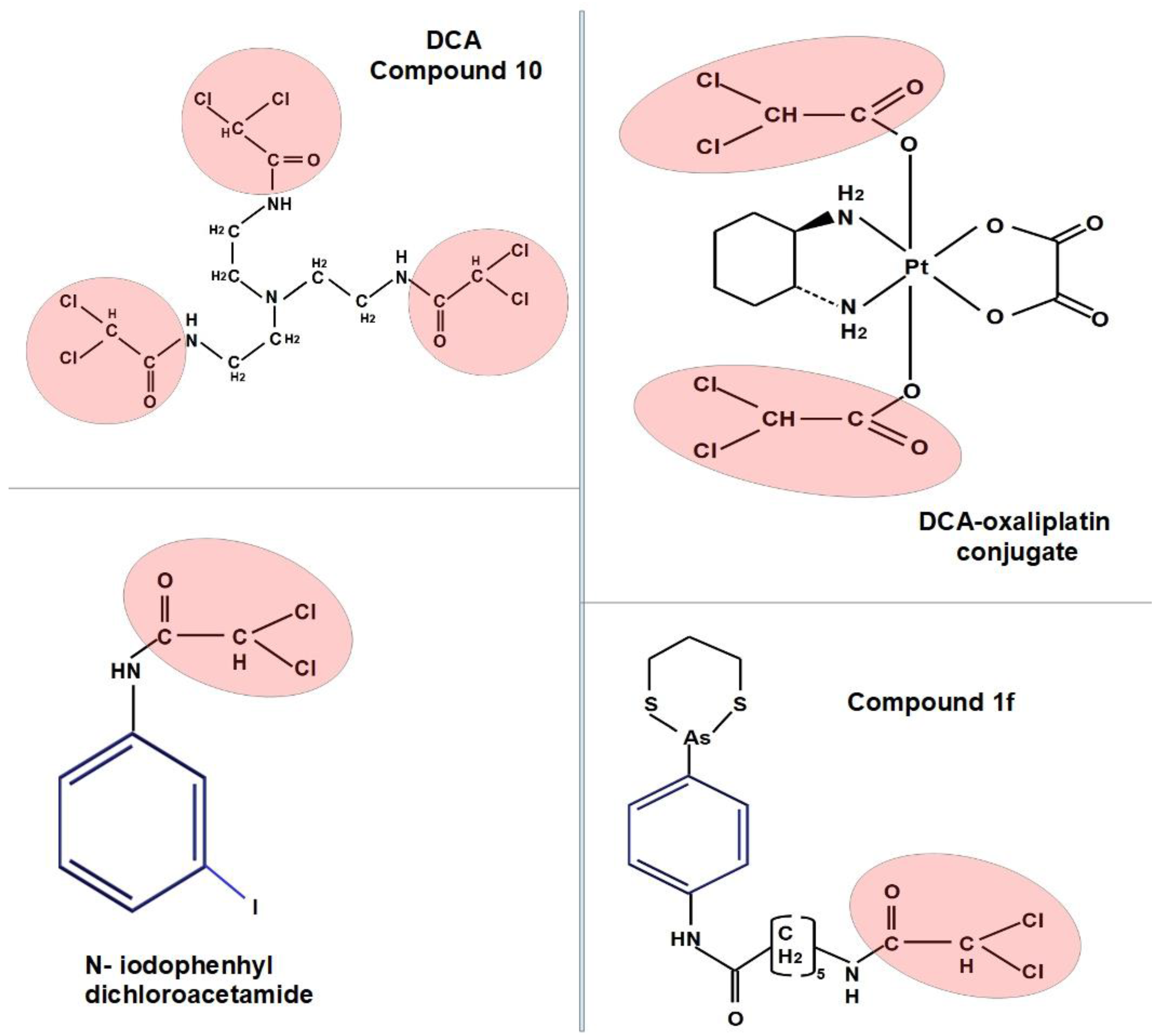
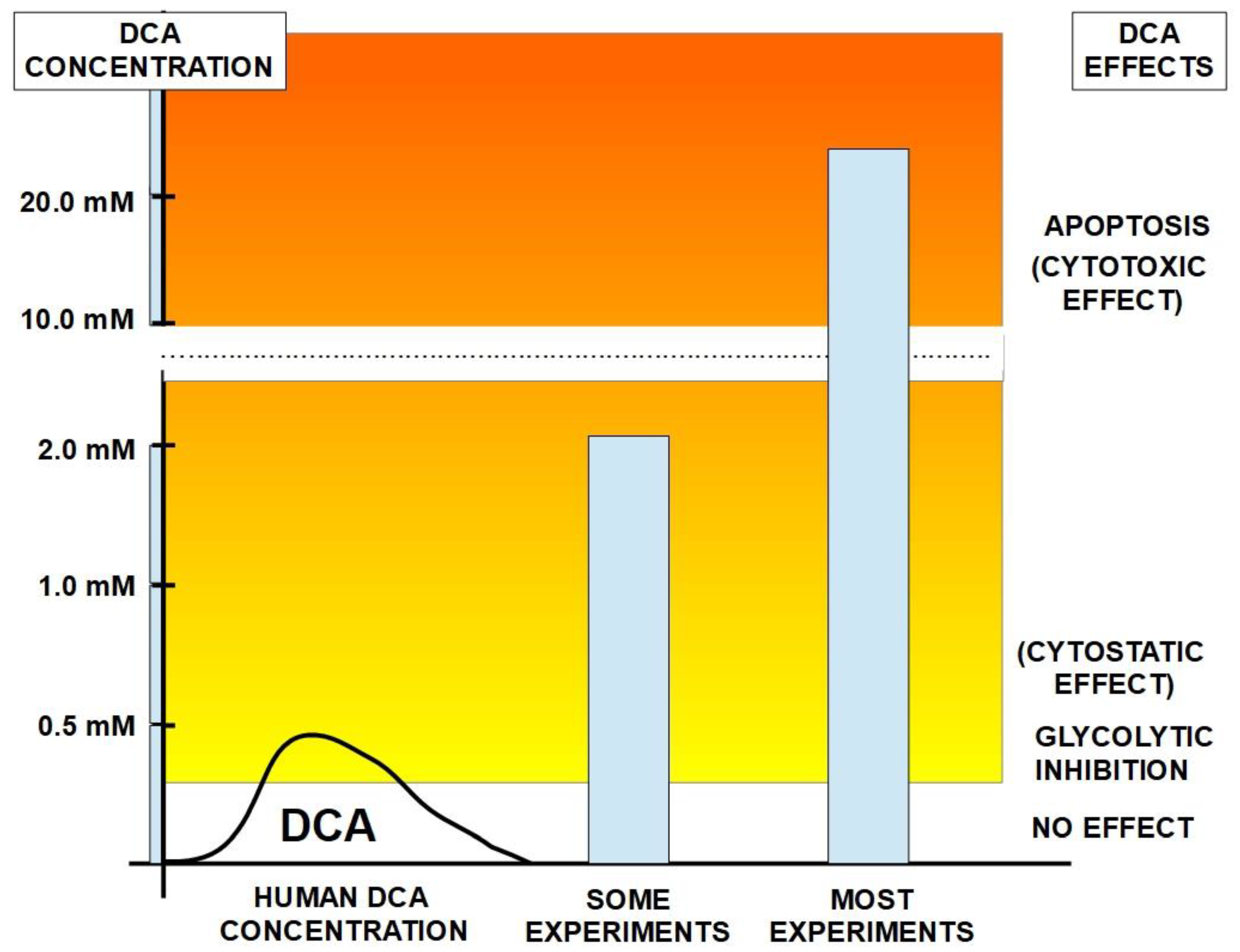
| Reference | Findings |
|---|---|
| Michelakis ED et al, 2010 [209] | The authors tested whether DCA can reverse cancer-specific metabolic and mitochondrial remodeling in glioblastoma. Freshly isolated glioblastomas from 49 patients showed mitochondrial hyperpolarization, which was rapidly reversed by DCA. A separate experiment with five patients had glioblastoma treated with oral DCA for up to 15 months. Three patients showed evidence of tumor regression. DCA depolarized mitochondria, increased mitochondrial reactive oxygen species, and induced apoptosis in GBM cells, as well as in putative GBM stem cells. DCA therapy also inhibited the hypoxia-inducible factor–1α, promoted p53 activation, and suppressed angiogenesis both in vivo and in vitro. |
| Duan, Y.; et al. 2013 [210] | DCA inhibited cell proliferation, induced apoptosis, and arrested C6 glioma cells in S phase. In vivo antitumor testing indicated that DCA markedly inhibited the growth of glioma tumors in brain tumor-bearing rats and tumor-bearing nude mice. DCA significantly induced the ROS production and decreased the mitochondrial membrane potential in tumor tissues. Antiangiogenic effects were also found. |
| Kumar K et al. 2013 [211] | DCA synergistically enhanced the results of bevacizumab antiangiogenic treatment in a xenotransplant mouse model of glioblastoma. |
| Morfouace, M. et al. 2014 [212] | Oct4 is a major regulator of cell pluripotency. DCA increased the amount of Oct4-pyruvate kinase 2 complexes which inhibited Oct4- dependent gene expression, inducing differentiation of glioma stem cells. |
| Kolesnik DL et al. 2014 [213] | Hypoxia enhanced the cytotoxic effects of DCA in glioblastoma cells inducing necrosis of tumor cells. |
| Vella et al. 2012 [214] | DCA had anticancer effects on NB (neuroblastoma) tumor cells which was selectively directed to very malignant NB cells. More differentiated/less malignant NB cells were refractory to DCA treatment. |
| Sradhanjali S et al. 2017 [215] | DCA reduced retinoblastoma cell line and retinoblastoma explant growth and potentiated carboplatin inhibition of retinoblastoma cell growth. |
| Park, J.M.; et al. 2013 [216] | C6 glioma was transplanted into rat brains. Magnetic Resonance imaging in vivo showed that DCA modified PDH flux much more in gliomas compared to normal brains. |
| Fedorchuk, A.G.; et al. 2016 [217] | Experiments with rats with transplanted C6 glioma cells showed that different administration schedules of DCA could cause ambiguous effects: inhibition of tumor growth or stimulation. Prolonged daily administration showed the best antitumor effects. Under hypoxic conditions, anticancer efficacy of DCA was significantly increased. |
| Wicks, R.T.; et al. 2014 [218] | Local delivery (wafers) of DCA to experimental brain tumors in rats caused a significantly increased survival compared with controls and with oral administration of the drug. |
| Korsakova, et al. 2021 [219] | The authors found synergistic apoptotic effects of metformin and DCA in glioblastomas cells in vitro and in vivo with dose dependent cytotoxicity. Cytotoxic activity required DCA concentrations above 10 mM and metformin 5 mM. There were no effects with concentration of 5 mM DCA and 2.5 mM metformin. |
| Shen, H.; et al. 2015 [220] | DCA increased radiosensitivity in orthotopic glioblastoma-bearing mice and of high-grade gliomas. Also Reviewed in Cook, 2021 [221] |
| Jiang, W.; et al. 2016 [222] | DCA increased cytotoxic activity of phenformin on glioblastoma stem cells. |
| Prokorhova, I.V.; et al. 2018 [223] | Co-administration of metformin with DCA improved anemia and thrombocytopenia, produced by glioma C6 growth. |
| Kolesnik, D.L.; et al. 2019 [224] | It was found that metformin increased the cytotoxic activity of DCA against C6 glioma cells, in vitro and in vivo. |
| Shen, H.; et al. 2021 [225] | DCA associated with metformin and radiotherapy increased apoptosis in pediatric glioma cells in vitro and in vivo. A triple combination of DCA, metformin and radiation therapy had more potent effects. |
| Yang, Z.; et al. 2021 [226] | Phosphorylated PDH-A1 mediated tumor necrosis factor-α (TNF-α) -induced cell migration in glioma cells. DCA decreased phosphorylated PDH-A1 levels and reduced glioma cell migration and invasion. |
| References | Organ/Cell | Findings |
|---|---|---|
| Ohashi T et al. 2013 [314] | Macrophages | DCA targets macrophages to suppress activation of the IL-23/IL-17 pathway and prevents the expression of arginase 1 (ARG1) induced by lactic acid. Lactic acid-pretreated macrophages inhibited CD8+T-cell proliferation, but CD8+T-cell proliferation was restored when macrophages were pretreated with lactic acid and DCA. Although DCA treatment alone did not suppress tumor growth, it increased antitumor immunotherapeutic activity. |
| Rooke M et al. 2014 [315] | Sarcoma models in vitro and in vivo. | Three types of cells (mouse fibrosarcoma S180, mouse osteosarcoma K7M2 and human fibrosarcoma HT1080-luc2) were tested with DCA with and without doxorubicin. DCA alone significantly decreased viability at a concentration of 5 mM. At 0.5 mM viability only decreased 15%. Importantly, there were no signs of apoptosis, therefore DCA inhibited proliferation but did not induce apoptosis. DCA had additive effects with low concentrations of doxorubicin. |
| Ishiguro T et al. 2012 [316] | Fibrosarcoma and colon cancer cells, normal human fibroblasts. | The combination of DCA and omeprazol exhibited more potent antitumor activity than DCA alone in tumor cells and did not affect the proliferation of normal cells. Caspase-dependent apoptosis through superoxide production was the suggested the mechanism of action. |
| Sutendra, G.; et al. 2013 [317] | NSCLC and 2335 mammary cells | In a rat xenotransplanted model, DCA reduced NSCLC and breast cancer tumor vascularity in vivo. DCA also inhibited HIF-1 α in tumor cell lines. |
| El Sayed SM et al. 2019 [318] | Cancer cells | The authors propose that DCA is an antagonist to acetate competing for enzymes, thus its therapeutic action would be caused by acetate deprivation. |
| Khyzhnyak, S.V. et al. 2014 [319] | Mouse sarcoma | Examination of sarcoma mitochondria from mice under DCA treatment showed they had a decrease in lactic acid levels and an increase in PDH activity with a decrease in the electron system transport activity and an increase in ROS. |
| Choi YW et al. 2014 [320] | HeLa cells | DCA and metformin acted synergistically enhancing cytotoxicity on cancer cells. |
| Xuan Y et al. 2014 [321] | Gastric cancer cells | DCA decreased resistance to 5-fluorouracil in highly hypoxic gastric cancer cells. |
| Badr MM et al. 2014 [322] | Fibrosarcoma | DCA showed immunomodulatory effects through the interleukin 12-interferon γ pathway. |
| Jin, J. et al. 2022 [323] | Osteosarcoma cell lines | DCA mitochondrial targeting micelles induced pyroptosis increasing the effects of immunotherapy. |
| Lam, S.K.; et al, 2022 [324] | Mesothelioma | The combination of DCA with niclosamide blocked growth and proliferation of different mesothelioma cells in vitro and in vivo. |
| Qin H et al. 2023 [325] | Cholangiocarcinoma | DCA increased sensitivity to cisplatin, changing glycolytic metabolism to an oxidative one and increasing ROS. Chloroquine further increased this sensitivity. |
| Dose in Humans | Concentration Range | Peak Concentration | |
|---|---|---|---|
| IV 10 mg/kg | 19.9 µg/ml | 24.7 µg/ml | 0.16 mM |
| IV 20 mg/kg | 57.3 µg/ml | 74.9 µg/ml | 0.49 mM |
| Oral 25 mg/kg | 41 µg/ml (0.27mM)(slow metabolizer) | ||
| Oral 25 mg/kg | 30 µg/ml (0.20 mM)(quick metabolizer) | ||
| Other sources | |||
| IV 25 mg/kg | 130 µg/ml (0.86 mM) | ||
| After 5 infusions | 163 µg/ml (1.08 mM) | ||
| DOSE IN RATS | |||
| 100 mg/Kg | 120 µg/ml | 164 µg/ml | 1.08 mM |
| DOSE IN DOGS | |||
| 100 mg/Kg | 447 µg/ml | 508 µg/ml | 3.36 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





