Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
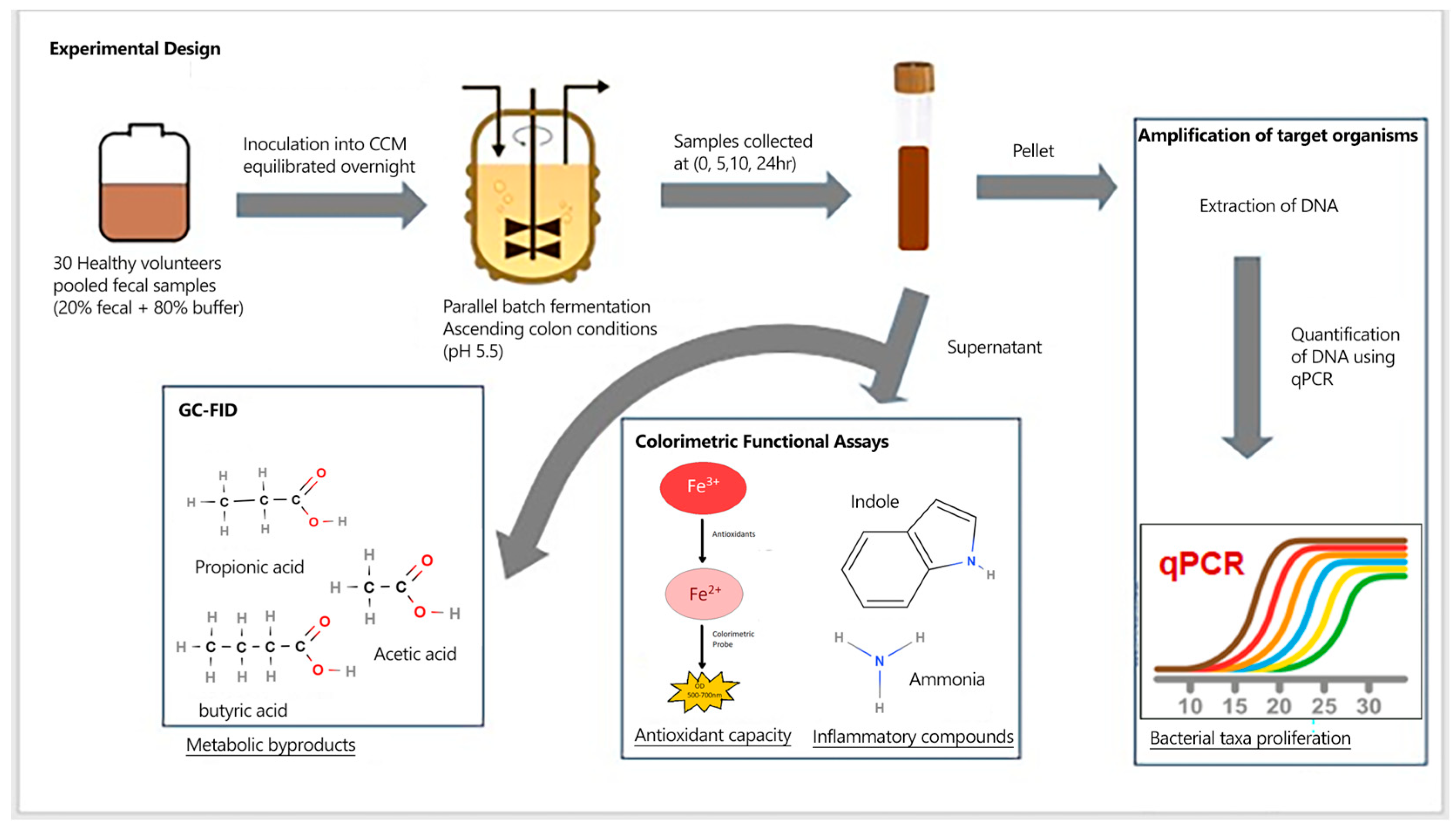
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Blend Formulations
2.3. Study Design and Supplementation Parameters
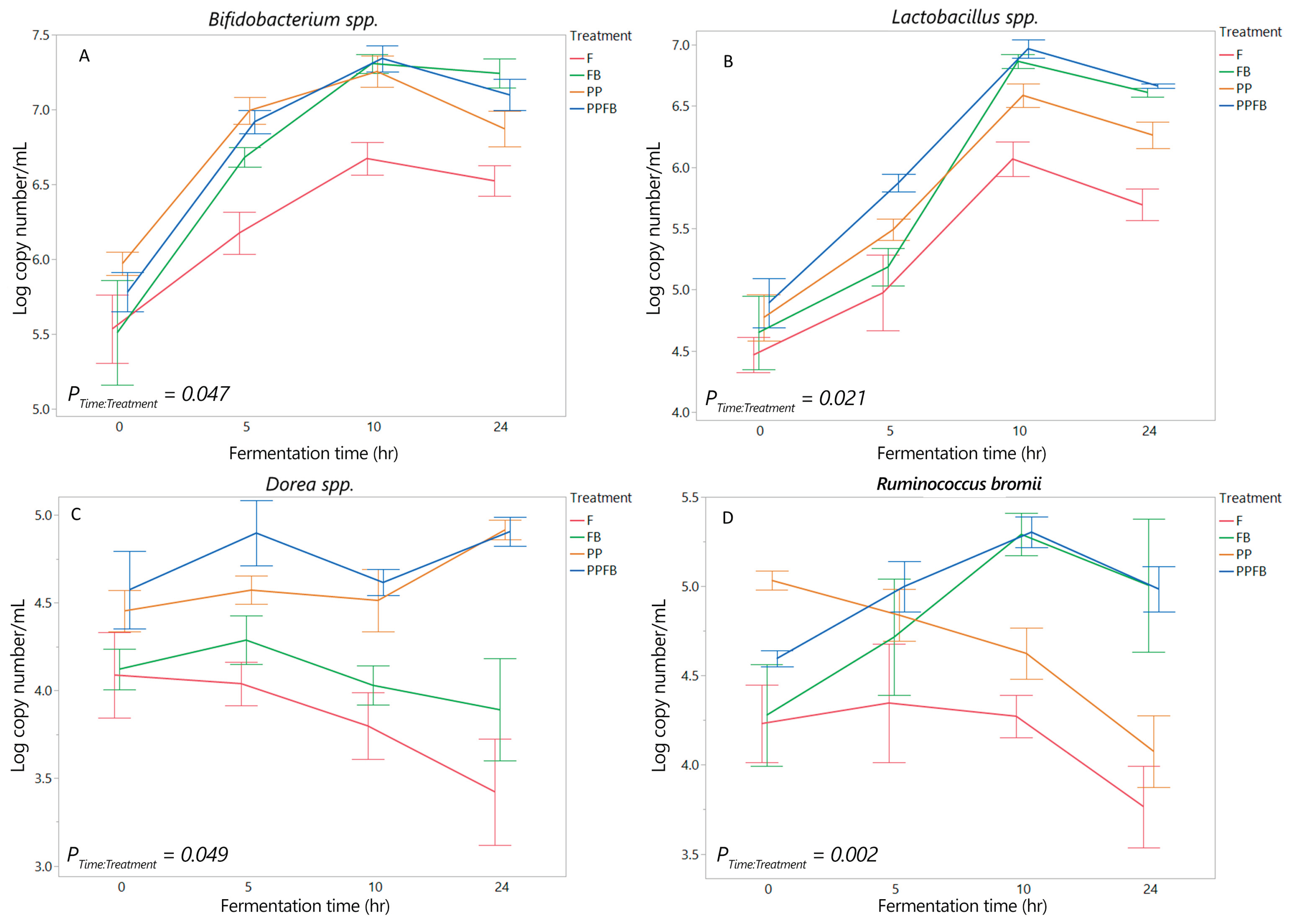
2.4. Targeted Keystone Bacterial Taxa Analysis
2.5. Metabolite Analysis
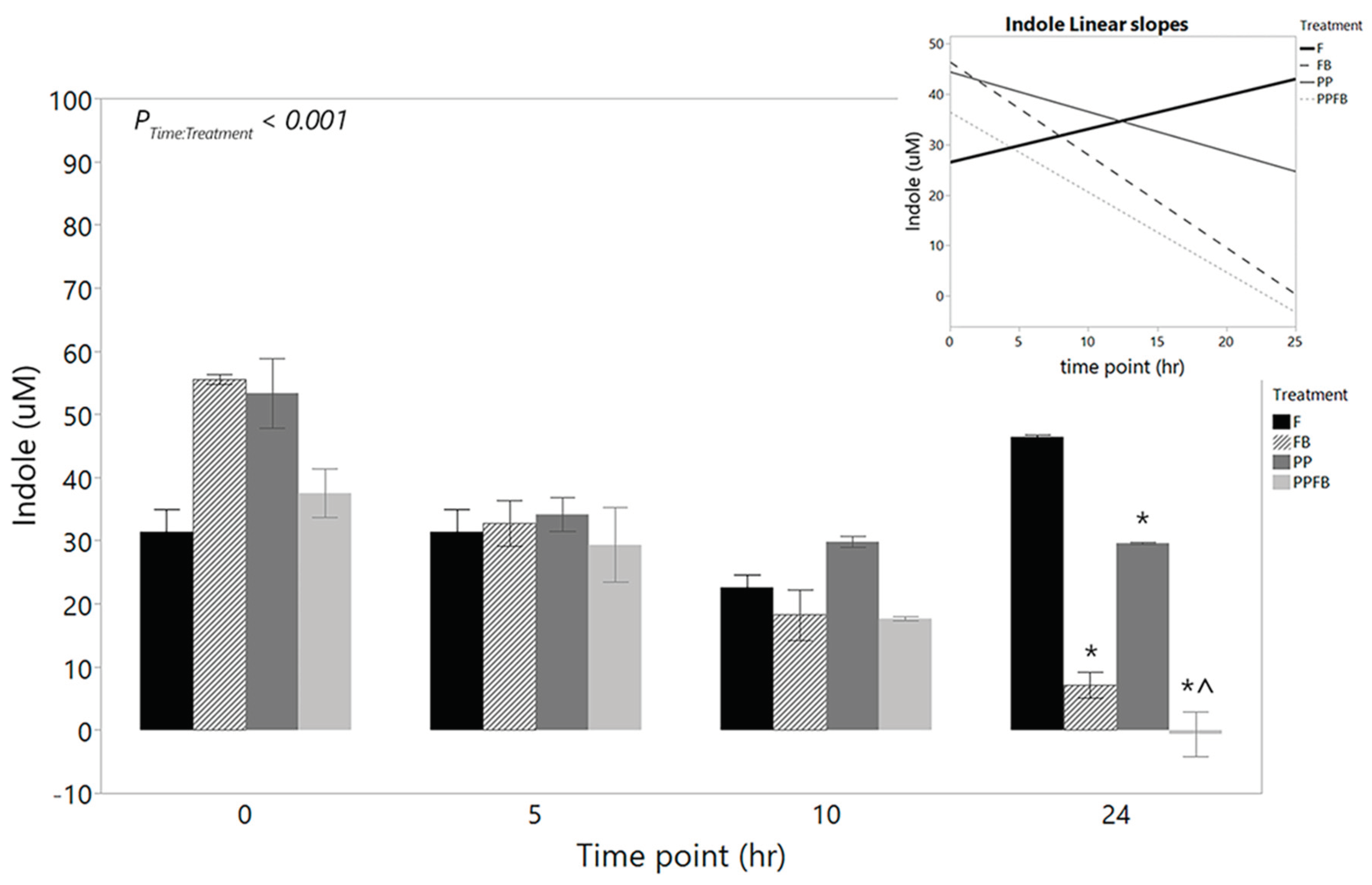
2.6. Influence of Supplementation on Indole
2.7. Influence of supplementation on Ammonia
2.8. Influence of Supplementation on Antioxidant Capacity
2.9. Statistical Analysis
3. Results
3.1. Effects of Polyphenol and Fiber Supplementation on Bacterial Abundance
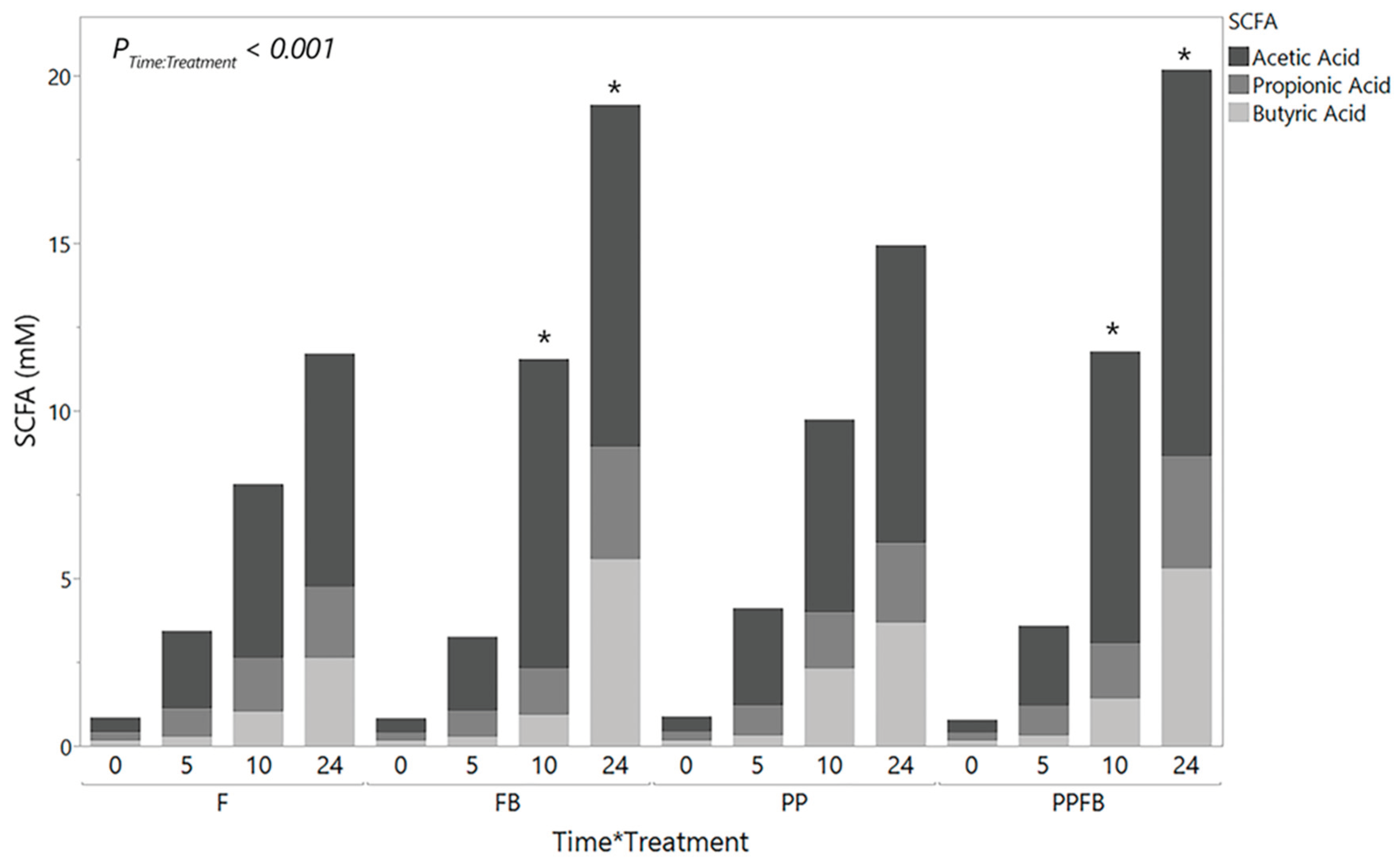
3.2. Effects of Polyphenol and Fiber Supplementation on Metabolite Concentrations
3.2.1. Changes in Total SCFA Concentrations
3.2.2. Changes in Indole Concentrations
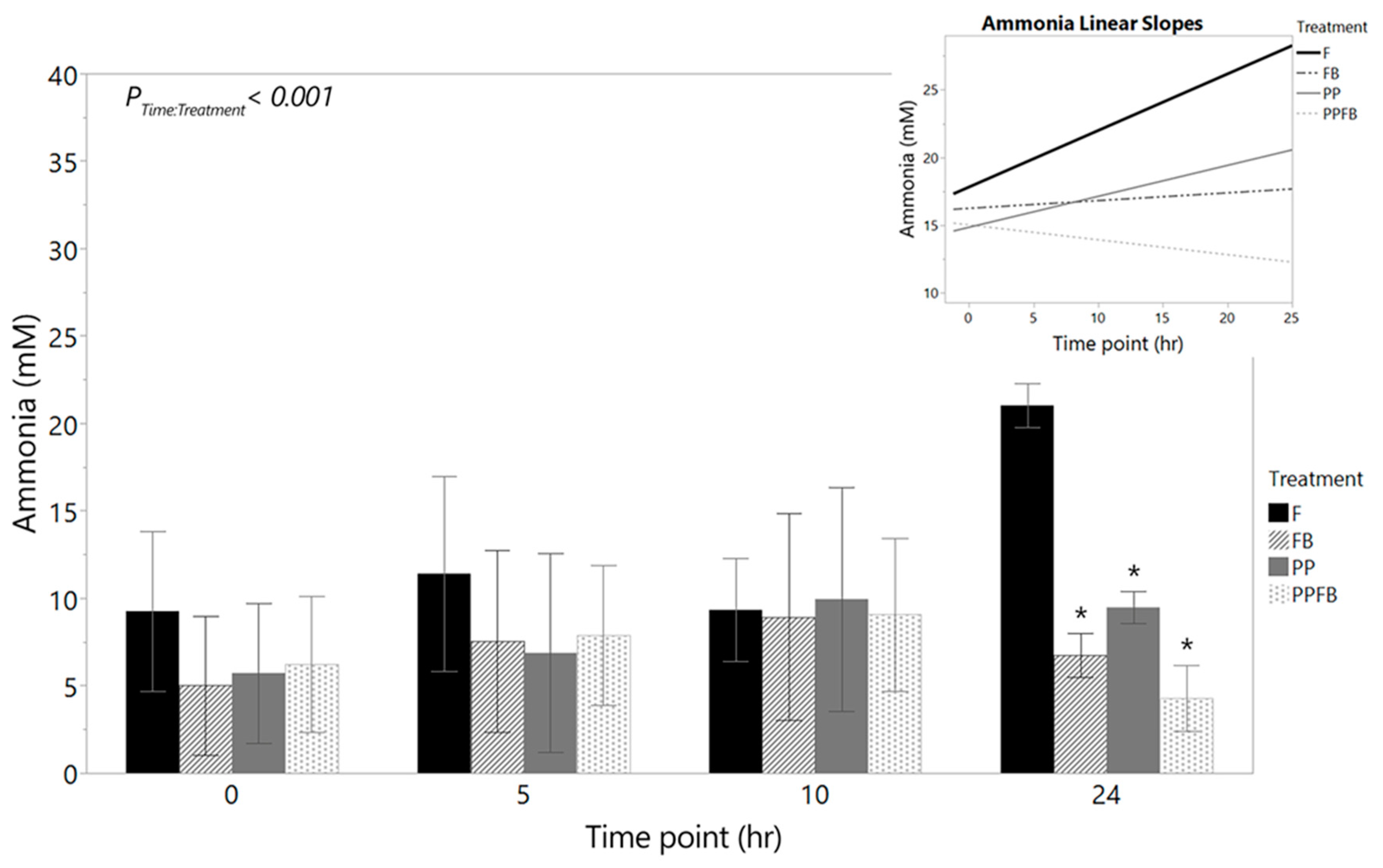
3.2.3. Changes in Ammonia Concentrations
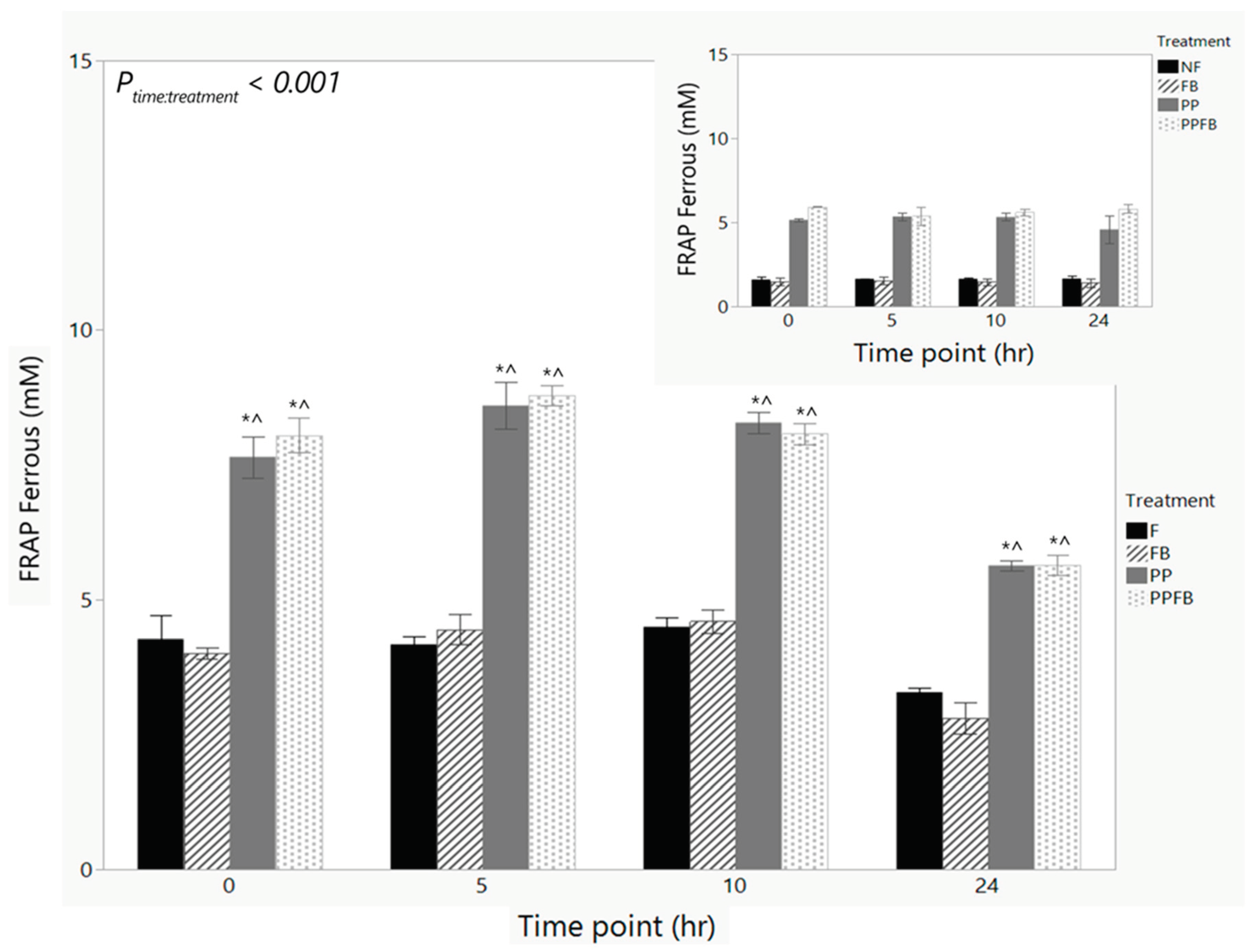
3.2.4. Changes in Antioxidant Capacity
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nova, E.; Gomez-Martinez, S.; Gonzalez-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: metabolic and health benefits. Br J Nutr 2010, 104 Suppl 2, S1–63. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Nieman, D.C.; Neilson, A.P.; Lila, M.A. Enhancing the Cognitive Effects of Flavonoids With Physical Activity: Is There a Case for the Gut Microbiome? Front Neurosci-Switz 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Rohs, E.; Birkett, A. Comparative Effect of 22 Dietary Sources of Fiber on Gut Microbiota of Healthy Humans in vitro. Front Nutr 2021, 8, 700571. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit Rev Biotechnol 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.H.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci Tech 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J Aoac Int 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, and/or Fiber. J Agr Food Chem 2012, 60, 8776–8782. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Wang, Y.H.; Lin, Y.; Lang, Y.X.; Li, E.H.; Zhang, X.Y.; Zhang, Q.; Feng, Y.; Meng, X.J.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Di Pede, G.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. In vitro (poly)phenol catabolism of unformulated- and phytosome-formulated cranberry ( Vaccinium macrocarpon ) extracts. Food Res Int 2021, 141. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Yang, K.D.; Zhu, J.J. Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: promise for improving human health. Adv Nutr 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Pantoja-Feliciano, I.G.; Soares, J.W.; Doherty, L.A.; Karl, J.P.; McClung, H.L.; Armstrong, N.J.; Branck, T.A.; Arcidiacono, S. Acute stressor alters inter-species microbial competition for resistant starch-supplemented medium. Gut Microbes 2019, 10, 439–446. [Google Scholar] [CrossRef]
- Verbeke, K.; Boobis, A.R.; Chiodini, A.; Edwards, C.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; Tuohy, K.; Van Tol, R. Microbial metabolites as potential markers for health benefits of prebiotics. Ann Nutr Metab 2015, 67, 271–272. [Google Scholar]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front Microbiol 2018, 9, 2013. [Google Scholar] [CrossRef]
- McClung, H.L.; Armstrong, N.J.; Hennigar, S.R.; Staab, J.S.; Montain, S.J.; Karl, J.P. Randomized Trial Comparing Consumption of Military Rations to Usual Intake for 21 Consecutive Days: Nutrient Adequacy and Indicators of Health Status. J Acad Nutr Diet 2020, 120, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Armstrong, N.J.; Player, R.A.; Rood, J.C.; Soares, J.W.; McClung, H.L. The Fecal Metabolome Links Diet Composition, Foacidic positive ion conditions, chromatographicallyod Processing, and the Gut Microbiota to Gastrointestinal Health in a Randomized Trial of Adults Consuming a Processed Diet. J Nutr 2022, 152, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- McInnes, P. Manual of Procedures for Human Microbiome Project Core Microbiome Sampling-Protocol A. 2010, 12.0.
- Pantoja-Feliciano, I.G.; Karl, J.P.; Perisin, M.; Doherty, L.A.; McClung, H.L.; Armstrong, N.J.; Renberg, R.; Racicot, K.; Branck, T.; Arcidiacono, S.; et al. In vitro gut microbiome response to carbohydrate supplementation is acutely affected by a sudden change in diet. Bmc Microbiol 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb Ecol 1998, 35, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Ramiro-Garcia, J.; Koenen, M.E.; Venema, K. To pool or not to pool? Impact of the use of individual and pooled fecal samples for in vitro fermentation studies. J Microbiol Methods 2014, 107, 1–7. [Google Scholar] [CrossRef]
- Reichardt, N.; Vollmer, M.; Holtrop, G.; Farquharson, F.M.; Wefers, D.; Bunzel, M.; Duncan, S.H.; Drew, J.E.; Williams, L.M.; Milligan, G.; et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. Isme J 2018, 12, 610–622. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Available online: https://www.thermofisher.com/us/en/home/brands/thermo-scientific/molecular-biology/molecular-biology-learning-center/molecular-biology-resource-library/thermo-scientific-web-tools/qpcr-efficiency-calculator.html (accessed on 15 June, 2022).
- Integrated DNA Technologies. Available online: https://www.idtdna.com/pages/education/decoded/article/calculations-converting-from-nanograms-to-copy-number (accessed on 15, June 2022).
- Patrone, V.; Vajana, E.; Minuti, A.; Callegari, M.L.; Federico, A.; Loguercio, C.; Dallio, M.; Tolone, S.; Docimo, L.; Morelli, L. Postoperative Changes in Fecal Bacterial Communities and Fermentation Products in Obese Patients Undergoing Bilio-Intestinal Bypass. Front Microbiol 2016, 7, 200. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. Plos One 2012, 7, e42529. [Google Scholar] [CrossRef]
- Blackmore, T.M.; Dugdale, A.; Argo, C.M.; Curtis, G.; Pinloche, E.; Harris, P.A.; Worgan, H.J.; Girdwood, S.E.; Dougal, K.; Newbold, C.J.; et al. Strong stability and host specific bacterial community in faeces of ponies. Plos One 2013, 8, e75079. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Frontiers in Microbiology 2018, 9. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol Nutr Food Res 2011, 55, S44–S55. [Google Scholar] [CrossRef] [PubMed]
- Ntemiri, A.; Ghosh, T.S.; Gheller, M.E.; Tran, T.T.T.; Blum, J.E.; Pellanda, P.; Vlckova, K.; Neto, M.C.; Howell, A.; Thalacker-Mercer, A.; et al. Whole Blueberry and Isolated Polyphenol-Rich Fractions Modulate Specific Gut Microbes in an In Vitro Colon Model and in a Pilot Study in Human Consumers. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Solch-Ottaiano, R.J.; Judkins, T.C.; Matott, S.H.; McDermott, C.E.; Nieves Jr, C.; Wang, Y.; Colee, J.; Tagliamonte, M.S.; Dissanayake, U.; Mai, V.L.; et al. High polyphenolic cranberry beverage alters specific fecal microbiota but not gut permeability following aspirin challenge in healthy obese adults: A randomized, double-blind, crossover trial. J Funct Foods 2022, 99. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.L.; Sun, Y.K.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X.X. Fermentation in vitro of EGCG, GCG and EGCG3 '' Me isolated from Oolong tea by human intestinal microbiota. Food Res Int 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Lakshmanan, A.P.; Mingione, A.; Pivari, F.; Dogliotti, E.; Brasacchio, C.; Murugesan, S.; Cusi, D.; Lazzaroni, M.; Soldati, L.; Terranegra, A. Modulation of gut microbiota: The effects of a fruits and vegetables supplement. Front Nutr 2022, 9, 930883. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: a Closer Look at the Lungs. Infect Immun 2021, 89. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Wang, X.D.; Gibson, G.R.; Costabile, A.; Sailer, M.; Theis, S.; Rastall, R.A. Prebiotic Supplementation of In Vitro Fecal Fermentations Inhibits Proteolysis by Gut Bacteria, and Host Diet Shapes Gut Bacterial Metabolism and Response to Intervention. Appl Environ Microb 2019, 85. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; et al. Aronia (Aronia melanocarpa) Polyphenols Modulate the Microbial Community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and Decrease Secretion of Proinflammatory Markers in a Caco-2/endothelial Cell Coculture Model. Mol Nutr Food Res 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Havlik, J.; Marinello, V.; Gardyne, A.; Hou, M.; Mullen, W.; Morrison, D.J.; Preston, T.; Combet, E.; Edwards, C.A. Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet-Microbe-Host Interaction. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. Fems Microbiol Rev 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Gibson, G.R.; Beatty, E.; Cummings, J.H. Estimation of Short-Chain Fatty-Acid Production from Protein by Human Intestinal Bacteria Based on Branched-Chain Fatty-Acid Measurements. Fems Microbiol Ecol 1992, 101, 81–88. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. Fems Microbiol Ecol 1998, 25, 355–368. [Google Scholar] [CrossRef]
- Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of Indole and Indole-Related Compounds by the Intestinal Microbiota and Consequences for the Host: The Good, the Bad, and the Ugly. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Goto, K.; Kanaya, S.; Ishigami, T.; Hara, Y. Effects of tea polyphenols on fecal conditions, part 2. The effects of tea catechins on fecal conditions of elderly residents in a long-term care facility. J Nutr Sci Vitaminol 1999, 45, 135–141. [Google Scholar] [CrossRef]
- Mezes, M.; Erdelyi, M. [Antioxidant effect of the fibre content of foods]. Orv Hetil 2018, 159, 709–712. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallares, N.; Marti-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants-Basel 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burillo, S.; Rufian-Henares, J.A.; Pastoriza, S. Towards an improved global antioxidant response method (GAR plus ): Physiological-resembling in vitro digestion-fermentation method. Food Chemistry 2018, 239, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J Food Sci 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. Approved for public release. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




