1. Introduction
Human papillomavirus (HPV) is a small, non-enveloped DNA virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8 kb in length. It is estimated that 20% of human cancers are caused by infections, and that approximately 12% of the total human cancer burden has a viral association (1). HPV causes 5% of all human cancers and approximately 100% of all cervical cancers (39). HPV is also responsible for a very high percentage of other anogenital cancers, and up to 70% of Head-and-Neck Cancers, which includes those of the oral cavity, oropharynx, sinus, tonsil, and larynx (40). Overall, over 600,000 new cancer cases every year are caused by high-risk HPV types, with types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 being defined by WHO as cancer-causing in the cervix (41). In addition, HPV infection is the most common viral sexually transmitted infection worldwide (42).
From previous research, we know that HPV can enter the cells through clathrin-dependent or non-clathrin-dependent pathways (43); it then travels to the early endosome, and from the early endosome to the late endosome (44). During the acidification process the viral capsid begins a process of disassembly (45) and this allows a small portion of L2 to beome exposed and span the endosomal membrance into the cytosol (46). This exposure of L2 sequences allows the viral protein to recruit various cargo sorting components (47). This in turn promotes endosomal tubulation and trafficking of the L2 DNA complex away from the late endosome (48), whilst the majority of L1 is left behind and is subsequently degraded within the lysozome (49). During this process the L2/DNA complex is transported to the trans-Golgi network (TGN) and stays there until mitosis, when the nuclear envelope breaks down and thereby allows access to nuclear components and subsequent nuclear retention (50). Whilst a great deal is already known about the different cellular partners which facilitate this entry process and trafficking steps within the cell, we were nonetheless interested in investigating other possible L2 interacting partners which may play a role in the infectious entry process. To do this we performed proteomic analysis of L2 interacting proteins. In the study presented here, we have found that COPB1, a component of the COP1 cargo protein complex is an interacting partner of L2 during HPV infectious entry. We have found that HPV requires COPI cargo proteins for a successful infection.
As from previous studies we know that there are two types of COP protein COPI and COPII (12), they are composed of five subunits COPI is composed of (α-COP), (β’-COP), (ε-COP (β-COP), (γ-COP), (δ-COP), (ζ-COP) and COPII is composed of Sar1, Sec23, Sec24, Sec13, and Sec31 (7), each subunit perform a specific function,(53) COPII proteins transport newly synthesized proteins from ER to TGN, (51) While COPI proteins transports recycling proteins from cis Golgi networks to ER (52), basically both COP proteins are required for transportation of large molecules from one enclosed compartment in to another, formation of membrane domains within the cells, large transport carriers, their connection with the cytoskeleton and interaction with the target organelles are the basic functions of COP proteins (8).
2. Materials and Methods
2.1. Antibodies
The following primary antibodies were employed in this study: Mouse anti-HPV-16 L1 (CAMVIR-1; Santa Cruz) with rabbit anti-COPB1 (Abcam), In Western blotting tests, the secondary antibodies horseradish peroxidase-conjugated swine anti-rabbit and rabbit anti-mouse (Dako), as well as the anti-HA mouse (Santa Cruise), HA rabbit, and HA goat (Thermofisher), were used.
2.2. Plasmids
The plasmids expressing FLAG-HA-tagged HPV-16 L2 have been previously described (26), as have the expression vectors for the HPV-16 L1 and HPV-16 L2 full-length GST fusion proteins (23). DNA sequencing was used to authenticate the plasmids produced by the GENEART site-directed mutagenesis system (Invitrogen) for the production of shortened versions of GST-tagged HPV-16 L2, which were made using custom-designed oligonucleotides (Eurofins MWG). HPV-16 L1 and L2 were expressed in a codon-optimized bicistronic fashion using plasmid p16shell L2-3×FLAG-HA, in which L2 is tagged with FLAG and hemagglutinin (HA) tags (24). The firefly luciferase gene-carrying pGL3 Luci construct was obtained from Promega. Genscript was used to create the murine Papilloma virus L2 protein construct. The plasmids expressing HPV-2, HPV-5, BPV-1, SfPV-1, Mus musculus papillomavirus type 1 (MmuPV-1), and MCV capsid proteins were the gift of Dr Chris B. Buck; HPV-16 and HPV-31 capsid protein expression plasmids were the gift of Dr Michelle A. Ozbun; HPV-18 capsid protein expression plasmid was the gift of Dr Samuel K. Campos, the GST BPV-1 L2 construct was the gift of Dr Patricio I. Meneses, and the GST HPV-11 L2 construct was the gift of Dr Bob Garcea, all of which were sequenced for verification.
2.3. Cell culture and transfections.
The human embryonic kidney cells (HEK293 and HEK293TT-21), and human skin keratinocytes (HaCaT) were cultured in Dulbecco’s modified Eagle medium, supplemented with 10% foetal bovine serum, 100 ug/ml penicillin-streptomycin, and 300 mg/ml glutamine.
HEK293 and HEK293TT cells were transfected using the calcium phosphate precipitation technique (22). For siRNA experiments, HaCaT cells were transfected using Lipofectamine RNAiMAX (Invitrogen) with an ON-TARGETplus SMARTpool of siRNAs against COPB1 (Dharmacon): (5′-CCAAGAUUGCAUUGCGCUA), (5′-CAUAUAAGAAUUCGUGCAA), (5′-AUUAUUAAGGAGAGCGACA), and (5′-CCUCAUGACUUCGCAAAUA). In parallel, a scrambled siSTABLE nontargeting siRNA (Dharmacon) was employed as a control.
2.4. Cell viability assay
A Trypan Blue exclusion assay was used to count the number of live cells. After being seeded in a 6-well plate, the HaCaT cells were treated with either a control siRNA (siCTRL) or siRNA that selectively targeted COPB1 for 48 hours. The cells were trypsinized, collected, and stained with 0.2% trypan blue after 48 hours. Using a typical hemocytometer, cells were counted directly under a microscope to measure the quantity of viable cells. A one-tailed t test was used to assess statistical significance after normalising the number of viable cells in siCOPB1-treated samples to the number of viable cells obtained for siCTRL-treated samples in each experiment. Western blotting of the cellular lysates was used to verify siCOPB1's effectiveness.
2.5. Infectivity assays.
In order to assess the infectivity in HaCaT cells, they were treated for 48 hours with either a control siRNA (siCTRL) or a siRNA specifically targeting COPB1. HPV-16 PsVs were added to these cells at concentrations of 300, 150, or 50 vge/cell, depending on the circumstances in each instance. Using a luciferase assay equipment (Promega), firefly luciferase activity was measured 48 hours later as an indicator of infection. To ensure that the luciferase assays used equivalent protein inputs, the total cell protein was measured. A one-tailed t test was used to assess the statistical significance of the decrease in luciferase activity upon si-COPB1-treated cells, relative to the luciferase readings for siCTRL-treated cells in each experiment. Additionally, the cellular lysates were utilised to validate COPB1's knockdown effectiveness by western blot.
2.6. Synthetic HPV particle production and labeling.
HEK293TT cells were used to create HPV-2,5,16,18,31, PMU, BPV, and PsVs with a luciferase reporter plasmid (pGL3 Luci), as previously reported (28). Using solutions of bovine serum albumin (BSA) at various concentrations as standards, the purity and capsid protein content of the PsV preparations were assessed using SDS-PAGE and Coomassie brilliant blue staining. Using a standard curve of the published plasmid DNA, the encapsidated DNA was subjected to real-time PCR analysis, allowing the copy number of each PsVs preparation to be determined. The HPV-16 L1-only VLPs were produced by employing a PsV-like procedure.
2.7. Fusion protein purification and binding assays.
As previously reported (27), GST-tagged fusion proteins or GST alone were produced, purified, and immobilized on glutathione agarose (Sigma). After thorough washing, the GST bead-bound proteins were identified by western blotting with selected primary and secondary antibodies and by Ponceau staining. HaCat cell lysates were treated with GST-tagged fusion proteins for one hour at 4°C.
2.8. Mass spectrometry analysis.
FLAG-HA-tagged HPV-16 L2 expression plasmid or, as a control, empty plasmid were transfected into HEK293 cells. After 48 hours, cell extracts were produced using mass spectrometry lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 50 mM NaF, 1 mM EDTA, 0.5% NP-40), and they were then incubated for one hour at 4°C on a rotating wheel with EZview Red Anti-HA affinity gel beads (Sigma). The beads were washed using phosphate-buffered saline (PBS) after first being washed with wash buffer (25 mM HEPES [pH 7.0], 0.1% NP-40, and 150 mM NaCl), and then proteome analysis was performed as previously described (23).
In short, 50 ng of sequencing-grade trypsin in 20 mM triethanolamine bicarbonate (pH 8.5) was used for 12 hours at room temperature to elute proteins from the affinity beads. Following digestion, the beads' supernatant was extracted, and they were then quickly incubated with another aliquot of 20 mM triethanolamine bicarbonate (pH 8.5). This aliquot was then extracted and combined with the first supernatant. Formic acid was added to 0.1% to halt the reactions. The resultant mixture was then desalted using C18 STAGE (Stop and Go Extraction) tips and lyophilized until it was completely dry. Nanobore columns were constructed using in-house constructed columns packed with 15 cm of 3-μm Ascentis RPA particles (Sigma) using a high-pressure column loader. After being desalted, the samples were reconstituted in 0.1% formic acid and put onto the column. A discontinuous gradient of 0% to 80% acetonitrile in 0.1% formic acid was used to create the column 60 minutes and poured straight into the Amazon ETD ion trap mass spectrometer's opening (Bruker). During the liquid chromatography (LC) separation, eight data-dependent tandem mass spectrometry (MS/MS) scans were conducted in succession, after one complete scan (375 to 1,700 m/z). Using the Global Proteome Machine interfaced with X!Tandem, raw data files from the Amazon ETD instrument were converted to mgf files by a data analysis software package (Bruker). Then, the files were searched against the Ensembl human protein database and the NCBInr Viral database. The matches were filtered at a 1% false discovery rate.
3. Results
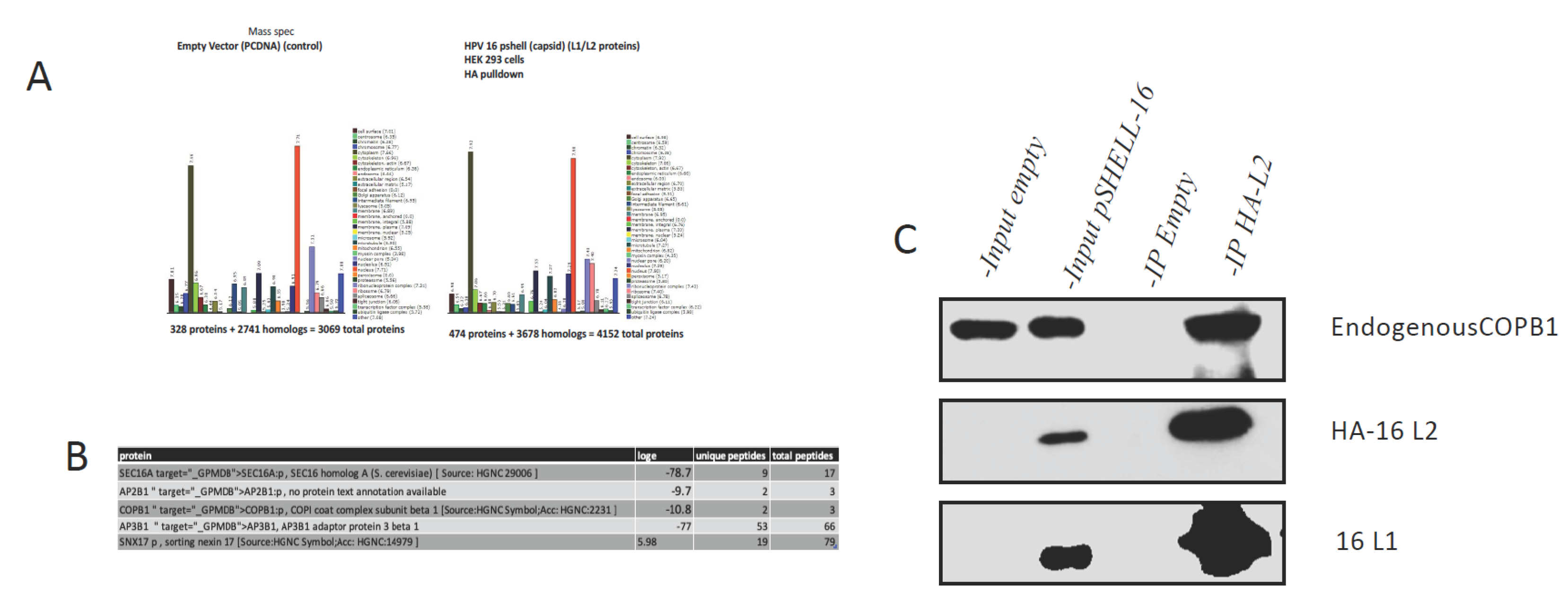
3.1. Mass spectrometry assay identifies COPB1 cargo protein as an HPV-16 interacting protein
To begin to investigate which cellular proteins might be involved in HPV virus entry, we performed mass spectrometry assays. W
e transfected HEK293 cells with HPV capsid proteins, p16 shell L1/L2 proteins where L2 is HA and FLAG tagged, or with empty vector pCDNA (as a control). After overnight incubation, cells were lysed, the HPV p16 shell L1/L2 proteins where L2 protein is HA and FLAG tagged was immunoprecipitated with anti-HA-conjugated agarose beads and the co-precipitated proteins were analyzed by mass spectrometry. As can be seen in
Figure 1, from this analysis we found several interesting cargo proteins that coprecipitated in the samples containing
HPV p16 shell L1/L2 proteins where L2 protein is HA and FLAG tagged, but were absent from the empty vector samples. These proteins are found in the cellular compartments where HPV is trafficked
(endosome, TGN, and Nucleus), and include: Adaptor protein 2 (AP2) in the cell membrane, Adaptor protein 1 (AP1) in endosomes, Adaptor protein 3 (AP3) in the TGN, COPB1 protein between the TGN and ER, and SEC16A at the ER exit site. We were particularly interested in L1 and L2’s interaction with COPB1, as this has not previously been described. To validate this interaction, we performed western blots on the cell extracts used in the immunoprecipitation:
Figure 1C shows a representative western blot, performed to confirm the binding of COPB1 with HPV-16 pseudovirion (L1/L2).
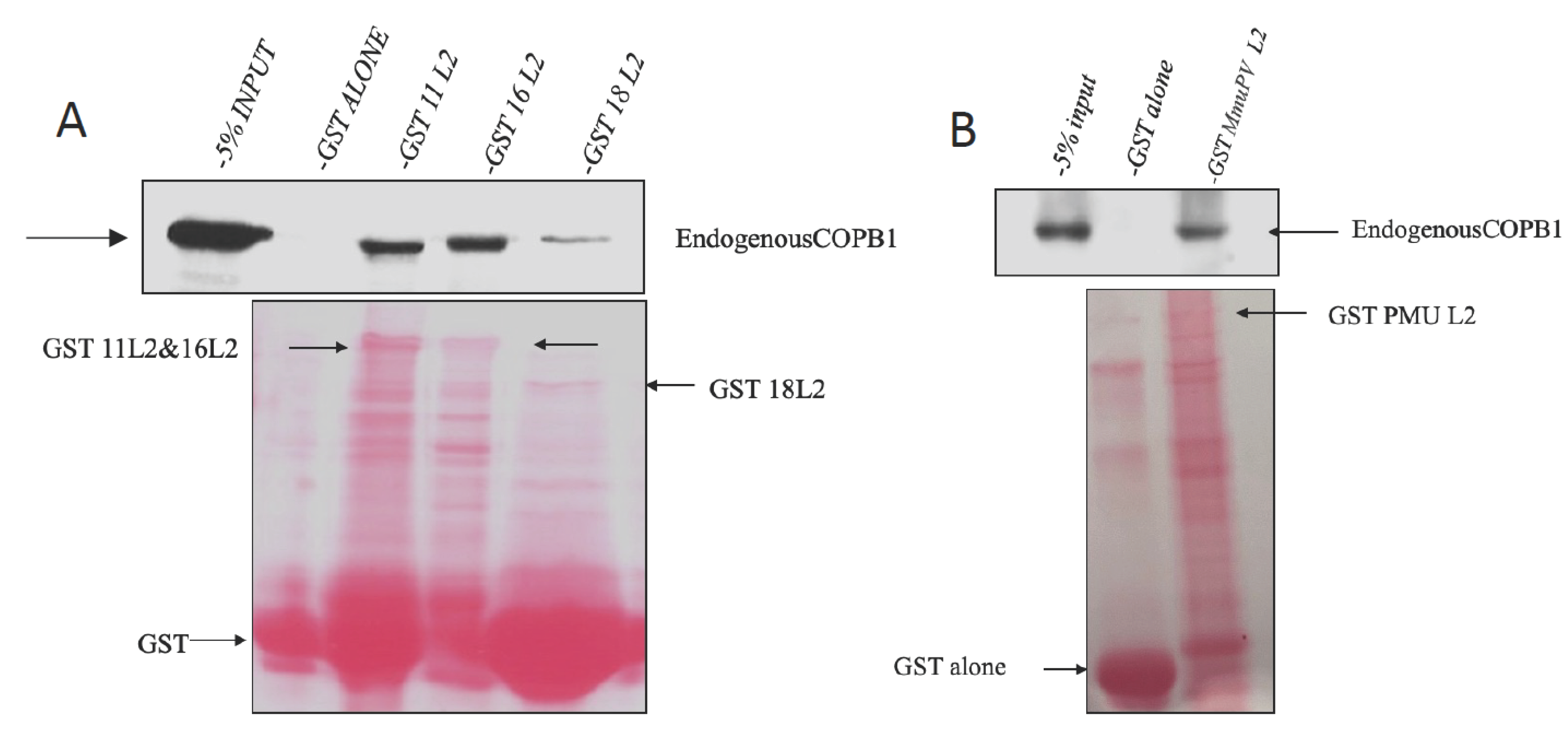
3.2. GST-L2 fusion protein binds COPB1 in vitro
Having found a number of new candidate interacting partners of HPV-16 L2, we first wanted to determine whether COPB1 proteins could associate with HPV-16 L2 in simple protein-protein interaction assays
in vitro. We also wanted to ascertain whether these interactions were specific for HPV-16 L2 or whether they were conserved among other HPV types. In order to do this, HACAT cell lysates were incubated with GST alone, or GST-11 L2 (a low-risk virus), GST-16 L2 or GST-18 L2 (high-risk viruses) and GST
MmuPV-L2 (murine papillomavirus) fusion proteins immobilised on Glutathione-agarose overnight at 4°C. After extensive washing, bound proteins were eluted and analysed by SDS-PAGE and western blot, probed with antibodies against COPB1 endogenous proteins. As can be seen in
Figure 2, HPV-16 L2 interacts strongly with COPB1. Interestingly, similar results are also obtained with HPV-11 and HPV-18 L2 and
MmuPV L2 proteins, indicating that these interactions are conserved across multiple Papillomavirus types.
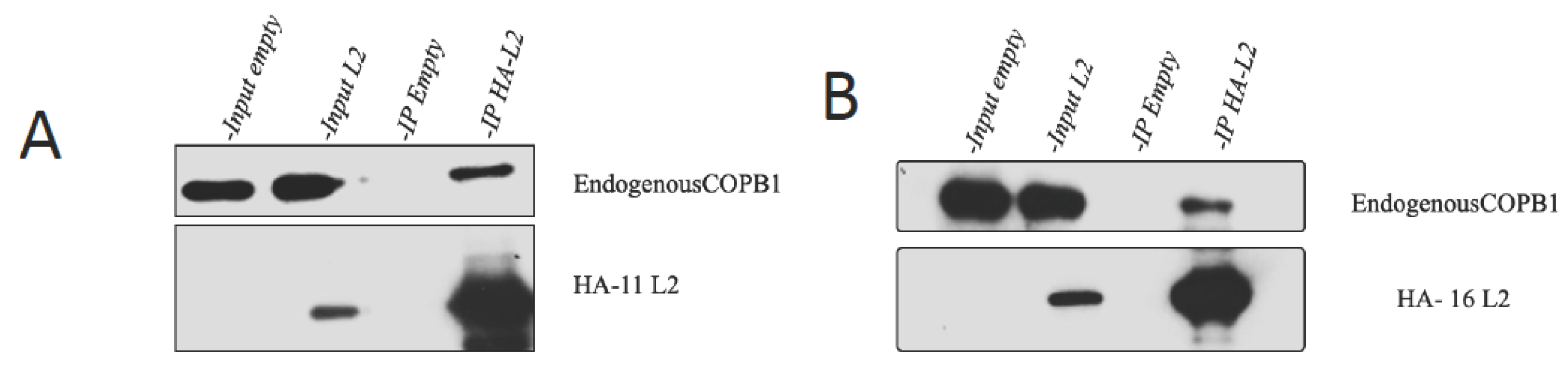
3.3. HPV-16 AND HPV-11 L2 interact with COPB1 in vivo.
From the GST pulldown, we have shown that there is a strong binding between HPV-16 L2 and COPB1. Therefore, we further proceeded to confirm the interaction between HPV-16 and HPV-11 L2 and COPB1 by performing a co-immunoprecipitation. HA- and FLAG-tagged HPV-16 and HPV-11 L2 proteins were overexpressed overnight in HEK293 cells. Cell lysates were immunoprecipitated with anti-HA agarose beads; the bound proteins were analyzed by SDS-PAGE and western blot, probed for endogenous COPB1. The results in Figure 3 show that there is a strong interaction between HPV-16 L2 and COPB1, compared with control (empty vector).
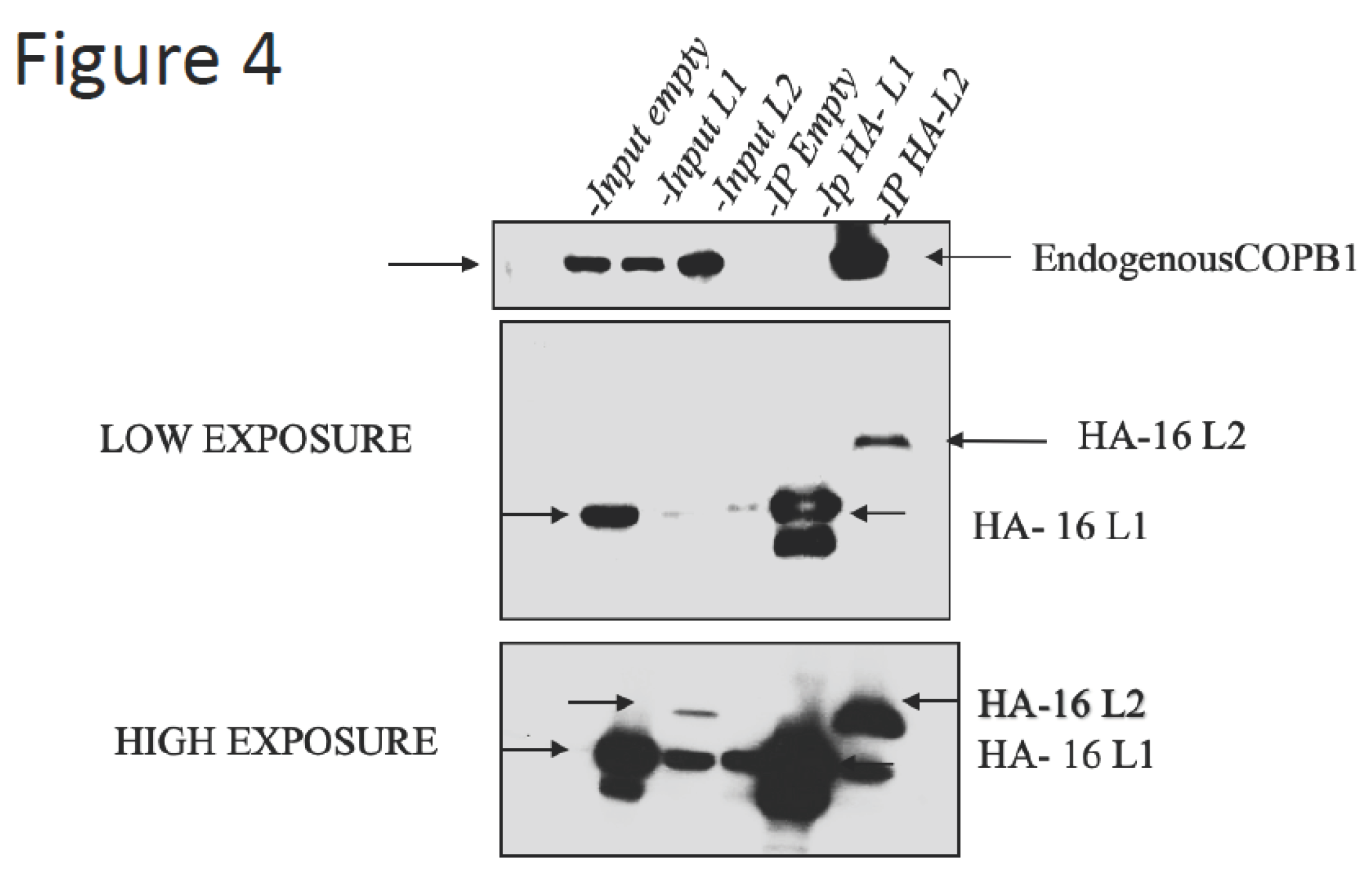
3.4. HPV-16 L2, but not L1, binds to COPB1.
We were further interested to know whether the COPB1 binding to L2 is specific, or whether it can also bind to L1, and furthermore whether the L2 interaction with COPB1 could be affected by the presence of L1. To examine this, we repeated the co-immunoprecipitation experiment: HA- and FLAG-tagged HPV-16 L2 and L1 proteins were overexpressed overnight in HEK293 cells. Cell lysates were immunoprecipitated with anti-HA agarose beads, then the bound proteins were analyzed by SDS-PAGE and western blot, probed for endogenous COPB1. The results in
Figure 4 confirm that there is a strong interaction between HPV 16 L2 and COPB1 whilst L1 fails to interact with COPB1 and has no influence over the L2-COPB1 association.
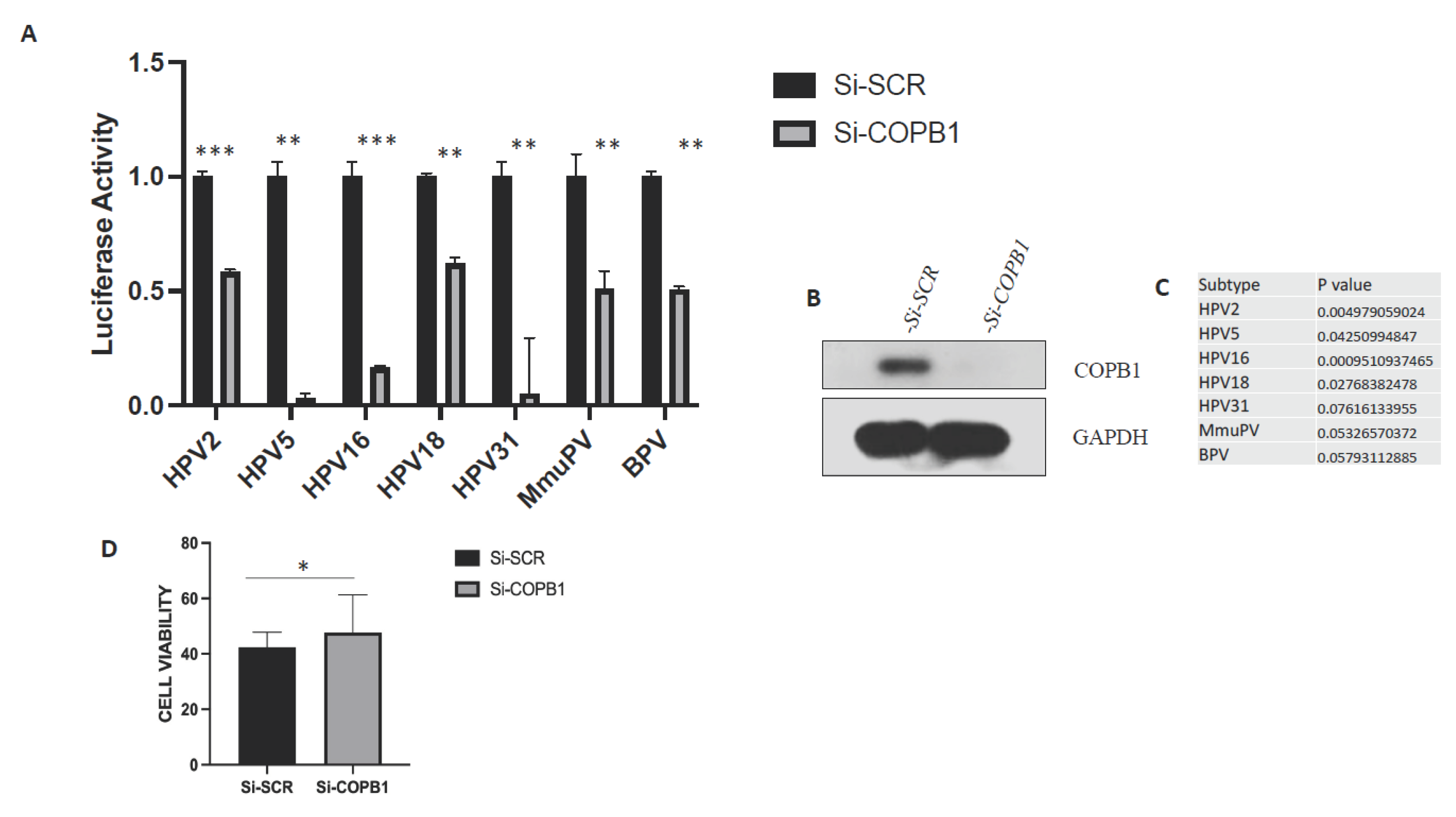
3.5. HPV infection requires COPB1.
Since L2 is essential for HPV infection, we were interested to know how much the COPB1 interaction might contribute to a successful infection. To examine this, we transfected HACAT cells with siRNAs to knockdown COPB1 expression. After 48h, the cells were infected with 150 VGE of PsVs from HPV-2, -5, -16, -18, -31 as well as PsVs from bovine and murine papillomaviruses (BPV-1 and MmuPV, respectively). each having a Luciferase-expressing plasmid as a reporter genome; after a further 48h the cells were harvested, and Luciferase activity was measured by luminometer. The protein concentrations were also measured, and the luciferase activities normalized by protein concentration. The COPB1 knockdown levels were also confirmed by western blot. As shown by the data in Figure 5, knockdown of COPB1 results in a marked decrease in infectivity of all HPV types (more than 80% with HPV-16), compared with control, although it is interesting to note that there is wide variation among the different types as to the degree of loss of infectivity, with HPV5, 16 and 31 being particularly susceptible. Interestingly BPV and MmuPV infectivity is also reduced, indicating that the requirement for COPB1 is conserved across many different Papillomavirus types and we have also checked the cell viability upon COPB1 knockdown compare to control A Trypan Blue exclusion assay was used to count the number of live cells. After being seeded in a 6-well plate, the HaCaT cells were treated with either a control siRNA (siCTRL) or siRNA that selectively targeted COPB1 for 48 hours. The cells were trypsinized, collected, and stained with 0.2% trypan blue after 48 hours. Using a typical hemocytometer, cells were counted directly under a microscope to measure the quantity of viable cells. A one-tailed t test was used to assess statistical significance after normalising the number of viable cells in siCOPB1-treated samples to the number of viable cells obtained for siCTRL-treated samples in each experiment. Western blotting of the cellular lysates was used to verify siCOPB1's effectiveness.
4. Discussion
From previous studies we know that HPV entry is a multistep process, and that HPV uses the cellular machinery for entry and trafficking to the cell nucleus (54). Using a proteomic screen we have found several interesting cargo proteins as potential interacting partners of HPV-16 L2. One of these cargo proteins was COPB1, which is a COPI protein subunit that is responsible for cargo binding and has the function of transporting cargo from TGN to ER (5). After confirming the binding between COPBI and HPV-11, -16, and -18 L2 proteins, through GST pulldown and co-immunoprecipitation assays, we investigated whether COPI has any role in the infectious entry process of HPV. Upon knockdown of COPB1 we found that there is more than 80% reduction in HPV-16 PsVs infectivity. We also investigated the effect of COPB1 knockdown on the infectivity of both high-risk and low-risk HPV types, and found a similar requirement for COPB1 for infectivity of diverse HPV types. Finally we also analysed the effects on infectivity of other papillomaviruses - Bovine papillomavirus (BPV) and Murine papillomavirus (MmuPV) - and found that COPB1 knockdown reduces BPV and MmuPV infectivity, Interestingly, we also confirmed that MmuPV L2 can bind COPB1 in GST pulldown assays. As we can see in figure 5 that some of HPVs are more sensitive upon COPB1 knockdown and some are less sensitive, which could be due to binding strength, as from GST pulldown in figure 2, we can see that COPB1 interacts strong with HPV-16 L2 as compared to HPV-18 L2 and the same goes with infectivity (HPV-16 is more sensitive upon COPB1 knockdown as compared to HPV-18). Thus, from this study, we have understood that COPB1 is a common cargo from TGN towards cell Nucleus for many HPV types, as it is localized in specific parts of the cell that are traversed by most viruses en route to the cell nucleus through this path. This suggests that COPB1 could be a good target for blocking virus entry into the cell nucleus. It would also be important to check the infectivity of newly synthesized viruses from nucleus upon COPII protein knockdown and to check their interaction with virus cargo proteins.
References
- Schelhaas, M.; Shah, B.; Holzer, M.; Blattmann, P.; Kühling, L.; Day, P.M.; Schiller, J.T.; Helenius, A. Entry of Human Papillomavirus Type 16 by Actin-Dependent, Clathrin- and Lipid Raft-Independent Endocytosis. PLOS Pathog. 2012, 8, e1002657. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV entry into cells. Mutat. Res. Mol. Mech. Mutagen. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Day, P.M.; Thompson, C.D.; Schowalter, R.M.; Lowy, D.R.; Schiller, J.T. Identification of a Role for the trans -Golgi Network in Human Papillomavirus 16 Pseudovirus Infection. J. Virol. 2013, 87, 3862–3870. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Massimi, P.; Pim, D.; Marušič, M.B.; Myers, M.P.; Garcea, R.L.; Banks, L. Phosphorylation of Human Papillomavirus Type 16 L2 Contributes to Efficient Virus Infectious Entry. J. Virol. 2019, 93, e00128-19. [Google Scholar] [CrossRef] [PubMed]
- Duden, R. ER-to-Golgi transport: COP I and COP II function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Duden, R. ER-to-Golgi transport: COP I and COP II function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Miller, E.A. COP-coated vesicles. Current Biology 2016, 26, R54–R57. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Lippincott-Schwartz, J. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 2003, 4, 409–414. [Google Scholar] [CrossRef]
- Basukala, O.; Trejo-Cerro, O.; Myers, M.P.; Pim, D.; Massimi, P.; Thomas, M.; Guarnaccia, C.; Owen, D.; Banks, L. HPV-16 E7 Interacts with the Endocytic Machinery via the AP2 Adaptor μ2 Subunit. mBio 2022, 13, e0230222. [Google Scholar] [CrossRef]
- Shin, J.; Nile, A.; Oh, J.-W. Role of adaptin protein complexes in intracellular trafficking and their impact on diseases. Bioengineered 2021, 12, 8259–8278. [Google Scholar] [CrossRef]
- Park, S.Y.; Guo, X. Adaptor protein complexes and intracellular transport. Biosci. Rep. 2014, 34, 381–390. [Google Scholar] [CrossRef]
- Duden, R. ER-to-Golgi transport: COP I and COP II function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef]
- Day, P.M.; Thompson, C.D.; Schowalter, R.M.; Lowy, D.R.; Schiller, J.T. Identification of a Role for the trans -Golgi Network in Human Papillomavirus 16 Pseudovirus Infection. J. Virol. 2013, 87, 3862–3870. [Google Scholar] [CrossRef]
- Valdano, M.B.; Massimi, P.; Broniarczyk, J.; Pim, D.; Myers, M.; Gardiol, D.; Banks, L. Human Papillomavirus Infection Requires the CCT Chaperonin Complex. J. Virol. 2021, 95, e01943-20. [Google Scholar] [CrossRef]
- Pim, D.; Broniarczyk, J.; Siddiqa, A.; Massimi, P.; Banks, L. Human Papillomavirus 16 L2 Recruits both Retromer and Retriever Complexes during Retrograde Trafficking of the Viral Genome to the Cell Nucleus. J. Virol. 2021, 95, e02068-20. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.A.; Oorschot, V.; Hesser, B.A.; Austin, C.D.; Scheller, R.H.; Klumperman, J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004, 164, 1065–1076. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Keiffer, T.R.; Bienkowska-Haba, M.; Luszczek, W.; Guion, L.G.M.; Müller, M.; Sapp, M. Topography of the Human Papillomavirus Minor Capsid Protein L2 during Vesicular Trafficking of Infectious Entry. J. Virol. 2015, 89, 10442–10452. [Google Scholar] [CrossRef] [PubMed]
- Tsakogiannis, D.; Nikolaidis, M.; Zagouri, F.; Zografos, E.; Kottaridi, C.; Kyriakopoulou, Z.; Tzioga, L.; Markoulatos, P.; Amoutzias, G.D.; Bletsa, G. Mutation Profile of HPV16 L1 and L2 Genes in Different Geographic Areas. Viruses 2022, 15, 141. [Google Scholar] [CrossRef]
- Schoppe, J.; Schubert, E.; Apelbaum, A.; Yavavli, E.; Birkholz, O.; Stephanowitz, H.; Han, Y.; Perz, A.; Hofnagel, O.; Liu, F.; et al. Flexible open conformation of the AP-3 complex explains its role in cargo recruitment at the Golgi. J. Biol. Chem. 2021, 297, 101334. [Google Scholar] [CrossRef]
- McMahon, H.T.; Mills, I.G. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr. Opin. Cell Biol. 2004, 16, 379–391. [Google Scholar] [CrossRef]
- Beck, R.; Ravet, M.; Wieland, F.; Cassel, D. The COPI system: Molecular mechanisms and function. FEBS Lett. 2009, 583, 2701–2709. [Google Scholar] [CrossRef]
- Drake, M.T.; Zhu, Y.; Kornfeld, S. The Assembly of AP-3 Adaptor Complex-containing Clathrin-coated Vesicles on Synthetic Liposomes. Mol. Biol. Cell 2000, 11, 3723–3736. [Google Scholar] [CrossRef]
- Darsow, T.; Burd, C.G.; Emr, S.D. Acidic Di-leucine Motif Essential for AP-3–dependent Sorting and Restriction of the Functional Specificity of the Vam3p Vacuolar t-SNARE. J. Cell Biol. 1998, 142, 913–922. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Massimi, P.; Bergant, M.; Banks, L. Human Papillomavirus Infectious Entry and Trafficking Is a Rapid Process. J. Virol. 2015, 89, 8727–8732. [Google Scholar] [CrossRef]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005, 119, 445–462. [Google Scholar]
- Graham, F.L.; Van Der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Marušič, M.B.; Ozbun, M.A.; Campos, S.K.; Myers, M.P.; Banks, L. Human Papillomavirus L2 Facilitates Viral Escape from Late Endosomes via Sorting Nexin 17. Traffic 2011, 13, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kazakov, T.; Popa, A.; DiMaio, D. Vesicular Trafficking of Incoming Human Papillomavirus 16 to the Golgi Apparatus and Endoplasmic Reticulum Requires γ-Secretase Activity. mBio 2014, 5, e01777–14. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.; Massimi, P.; Pim, D.; Broniarczyk, J.; Banks, L. Human Papillomavirus 16 Infection Induces VAP-Dependent Endosomal Tubulation. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Marušič, M.B.; Mencin, N.; Ličen, M.; Banks, L.; Grm, H.S. Modification of Human Papillomavirus Minor Capsid Protein L2 by Sumoylation. J. Virol. 2010, 84, 11585–11589. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Massimi, P.; Jenkins, J.; Banks, L. HPV-18 E6 mediated inhibition of p53 DNA binding activity is independent of E6 induced degradation. Oncogene 1995, 10, 261–8. [Google Scholar]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005, 119, 445–462. [Google Scholar]
- Valdano, M.B.; Massimi, P.; Broniarczyk, J.; Pim, D.; Myers, M.; Gardiol, D.; Banks, L. Human Papillomavirus Infection Requires the CCT Chaperonin Complex. J. Virol. 2021, 95, e01943-20. [Google Scholar] [CrossRef]
- Bergant, M.; Banks, L. SNX17 Facilitates Infection with Diverse Papillomavirus Types. J. Virol. 2013, 87, 1270–1273. [Google Scholar] [CrossRef]
- Matsuoka, K.; Morimitsu, Y.; Uchida, K.; Schekman, R. Coat Assembly Directs v-SNARE Concentration into Synthetic COPII Vesicles. Mol. Cell 1998, 2, 703–708. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, P.; Crite, M.; Lindsay, C.V.; DiMaio, D. Retromer stabilizes transient membrane insertion of L2 capsid protein during retrograde entry of human papillomavirus. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Duden, R. ER-to-Golgi transport: COP I and COP II function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Miller, E.A. COP-coated vesicles. Curr. Biol. 2016, 26, R54–R57. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D'Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Skoulakis, A.; Fountas, S.; Mantzana-Peteinelli, M.; Pantelidi, K.; Petinaki, E. Prevalence of human papillomavirus and subtype distribution in male partners of women with cervical intraepithelial neoplasia (CIN): a systematic review. BMC Infect. Dis. 2019, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.A.; Boulet, G.A.; Renoux, V.M.; O Delvenne, P.; Bogers, J.-P.J. Mechanisms of cell entry by human papillomaviruses: an overview. Virol. J. 2010, 7, 11–11. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.; Broniarczyk, J.; Banks, L. Papillomaviruses and Endocytic Trafficking. Int. J. Mol. Sci. 2018, 19, 2619. [Google Scholar] [CrossRef] [PubMed]
- Morante, A.V.; Baboolal, D.D.; Simon, X.; Pan, E.C.-Y.; Meneses, P.I. Human Papillomavirus Minor Capsid Protein L2 Mediates Intracellular Trafficking into and Passage beyond the Endoplasmic Reticulum. Microbiol. Spectr. 2022, 10, e0150522. [Google Scholar] [CrossRef]
- Calton, C.M.; Bronnimann, M.P.; Manson, A.R.; Li, S.; Chapman, J.A.; Suarez-Berumen, M.; Williamson, T.R.; Molugu, S.K.; Bernal, R.A.; Campos, S.K. Translocation of the papillomavirus L2/vDNA complex across the limiting membrane requires the onset of mitosis. PLOS Pathog. 2017, 13, e1006200. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Ring, N.; Massimi, P.; Giacca, M.; Banks, L. HPV-16 virions can remain infectious for 2 weeks on senescent cells but require cell cycle re-activation to allow virus entry. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Siddiqa, A.; Broniarczyk, J.; Banks, L. Papillomaviruses and Endocytic Trafficking. Int. J. Mol. Sci. 2018, 19, 2619. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Guion, L.G.M.; Keiffer, T.R.; Sapp, M. Human Papillomavirus Major Capsid Protein L1 Remains Associated with the Incoming Viral Genome throughout the Entry Process. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Weisberg, A.S.; Thompson, C.D.; Hughes, M.M.; Pang, Y.Y.; Lowy, D.R.; Schiller, J.T. Human Papillomavirus 16 Capsids Mediate Nuclear Entry during Infection. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Peotter, J.; Kasberg, W.; Pustova, I.; Audhya, A. COPII-mediated trafficking at the ER/ERGIC interface. Traffic 2019, 20, 491–503. [Google Scholar] [CrossRef]
- Sohn, K.; Orci, L.; Ravazzola, M.; Amherdt, M.; Bremser, M.; Lottspeich, F.; Fiedler, K.; Helms, J.B.; Wieland, F.T. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J. Cell Biol. 1996, 135, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Szul, T.; Sztul, E. COPII and COPI Traffic at the ER-Golgi Interface. Physiology 2011, 26, 348–364. [Google Scholar] [CrossRef] [PubMed]
- A Ozbun, M.; Campos, S.K. The long and winding road: human papillomavirus entry and subcellular trafficking. Curr. Opin. Virol. 2021, 50, 76–86. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).