Submitted:
23 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Inclusion Criteria
2.3. Information Sources and Search Strategies
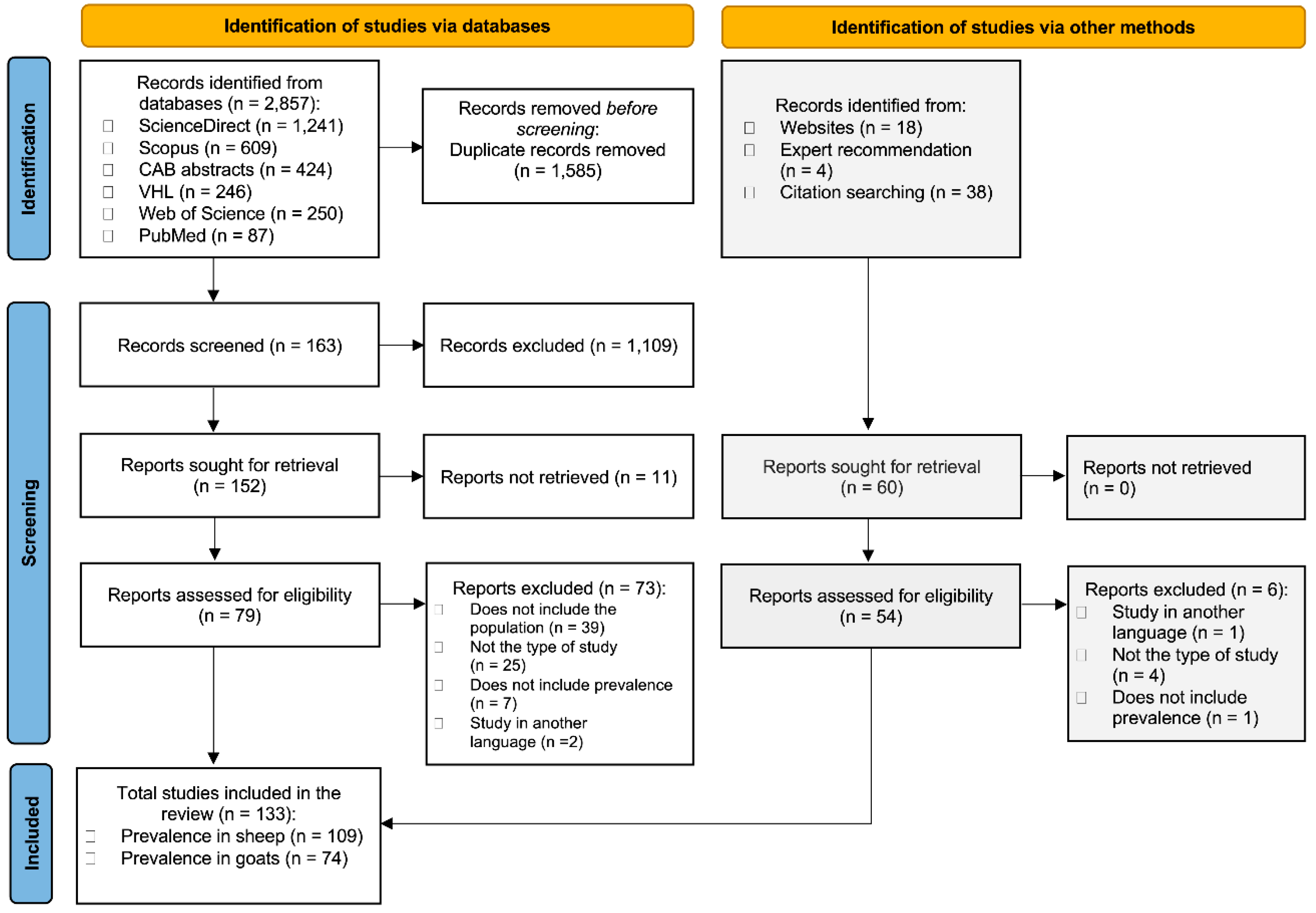
2.4. Study Selection Process
2.5. Information Collection Process and Extracted Data
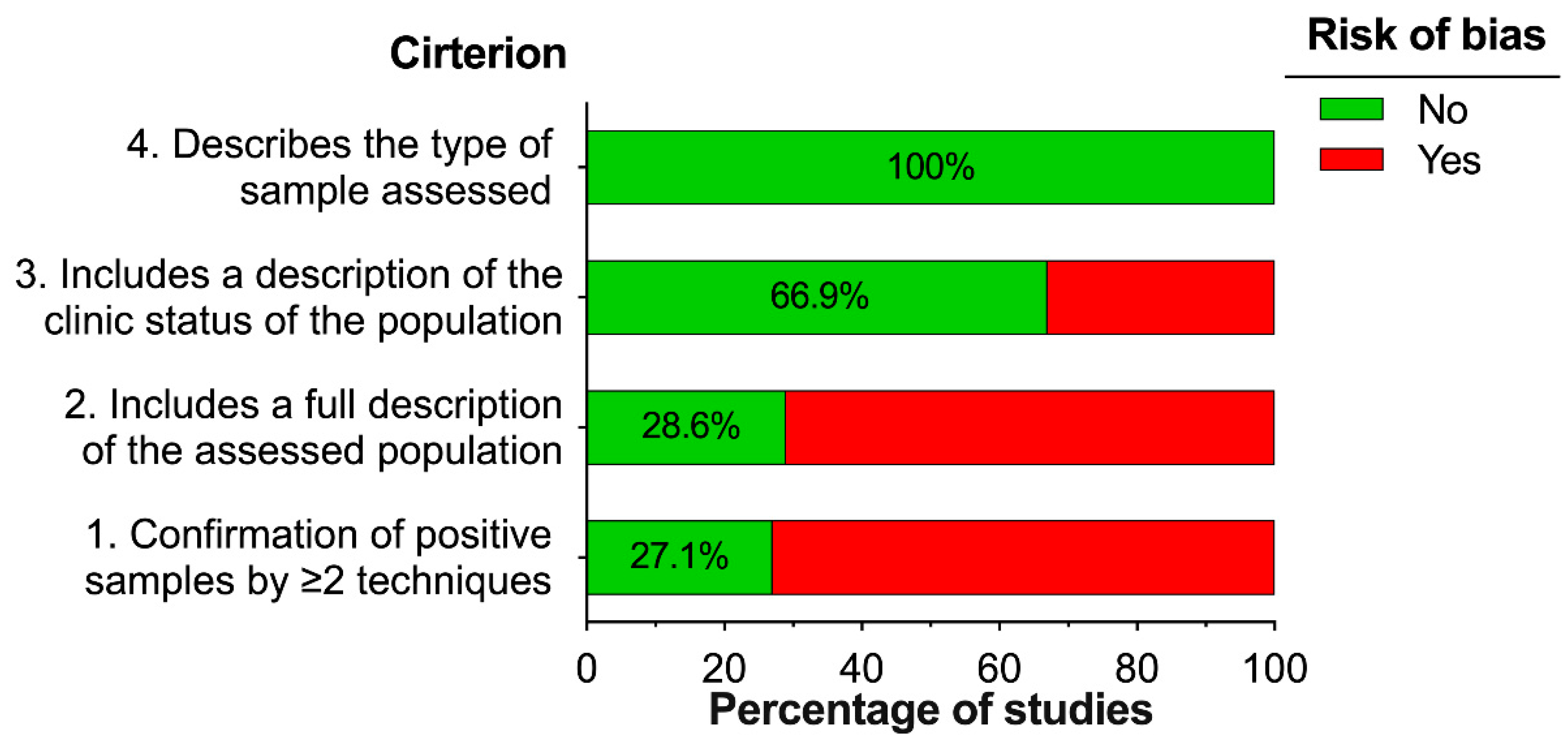
2.6. Risk of Bias Assessment
2.7. Summary of Evidence and Statistical Analysis of Data
3. Results
3.1. Study Selection
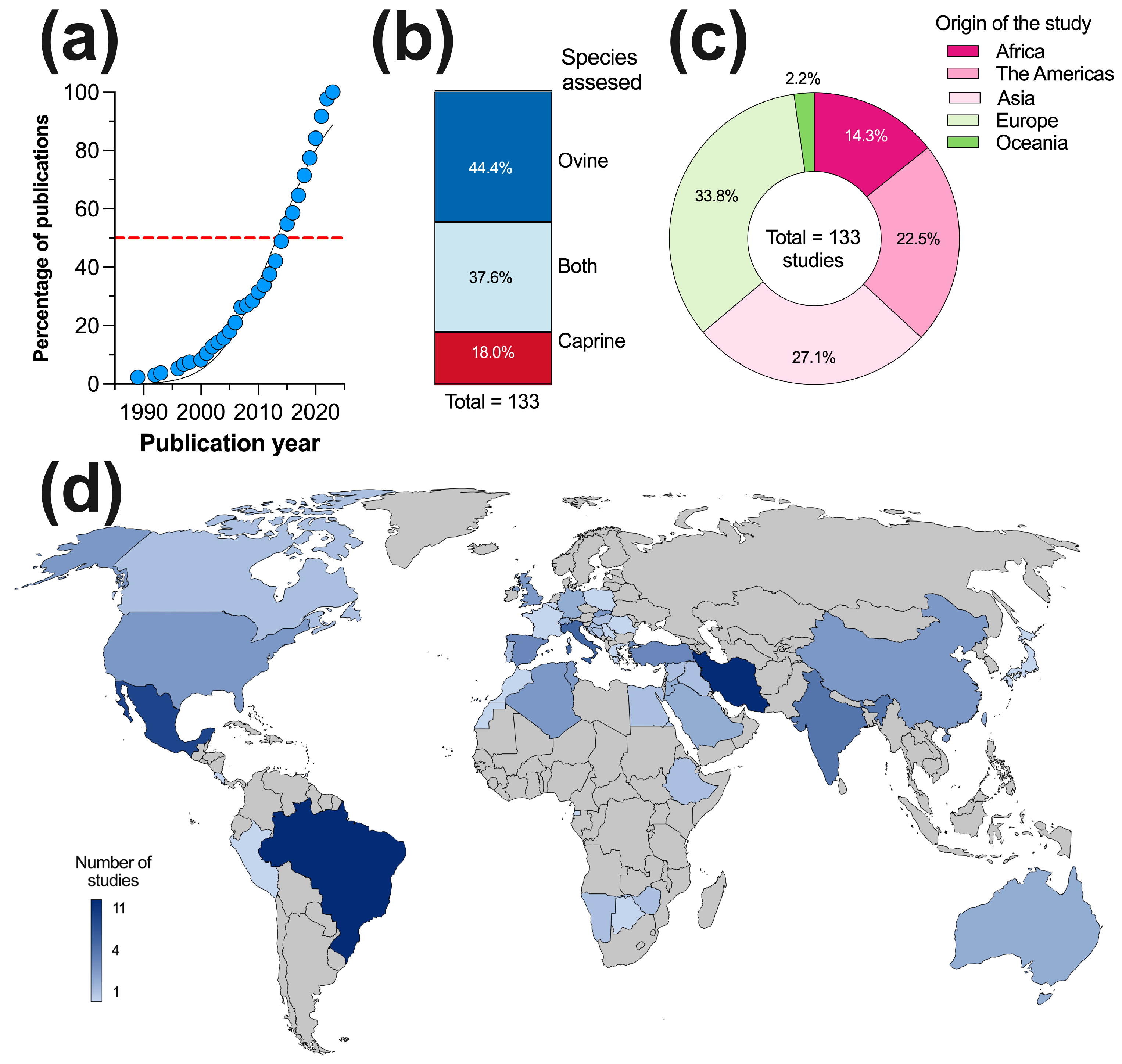
3.2. General Characteristics of the Studies
3.3. Risk of Bias of Individual Studies
3.4. Individual Characteristics of the Studies Evaluating Sheep
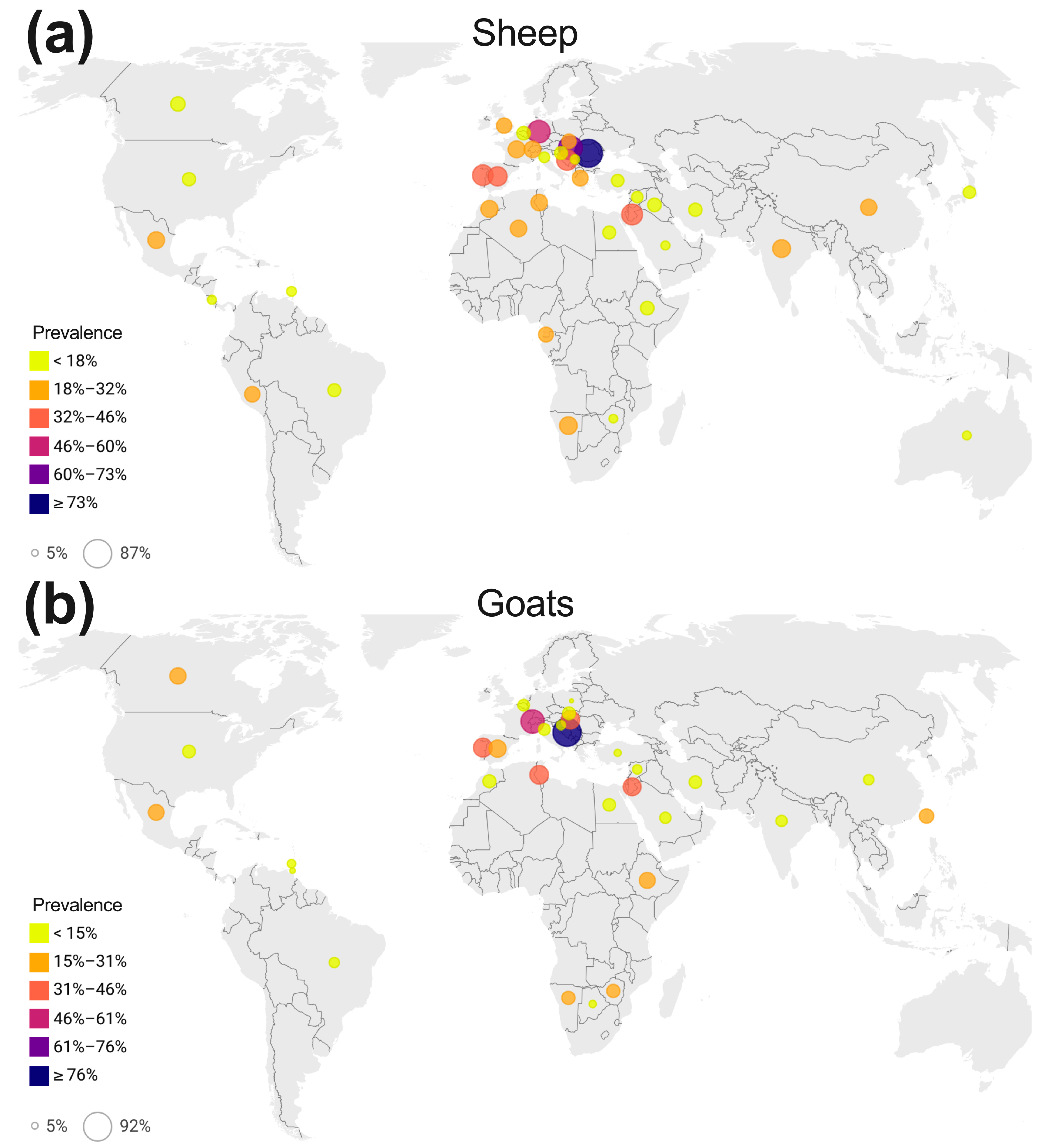
3.5. Global, Regional, and National Prevalence of C. abortus in Sheep
3.5.1. Additional Analysis of the Prevalence Estimations of C. abortus in Sheep
3.6. Individual Characteristics of the Studies That Evaluated Goats
3.7. Global, Regional, and National Prevalence of C. abortus in Goats
3.7.1. Additional Analysis of the Prevalence Estimations of C. abortus in Goats
4. Discussion
5. Limitations
6. Conclusions
7. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Organization for Animal Health (OIE). Enzootic Abortion of Ewes (Ovine Chlamydiosis) (infection with Chlamydia abortus). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.07.05_ENZ_ABOR.pdf (accessed on 10 Marzo 2020).
- Selim, A. Chlamydophila abortus infection in small ruminants: A review. Asian J. Anim. Vet. Adv 2016, 11, 587–593. [Google Scholar] [CrossRef]
- Rodolakis, A.; Laroucau, K. Chlamydiaceae and chlamydial infections in sheep or goats. Vet Microbiol 2015, 181, 107–118. [Google Scholar] [CrossRef]
- Kauffold, J.; Henning, K.; Bachmann, R.; Hotzel, H.; Melzer, F. The prevalence of chlamydiae of bulls from six bull studs in Germany. Anim Reprod Sci 2007, 102, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kauffold, J.; Wehrend, A.; Sigmarsson, H.; Hoopsa, M. Chlamydia and Chlamydophilia in small ruminants and other farm animals. Clinical Theriogenology 2014, 6, 255–260. [Google Scholar]
- Longbottom, D.; Coulter, L.J. Animal chlamydioses and zoonotic implications. J Comp Pathol 2003, 128, 217–244. [Google Scholar] [CrossRef]
- Rodolakis, A.; Yousef Mohamad, K. Zoonotic potential of Chlamydophila. Vet Microbiol 2010, 140, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Essig, A.; Longbottom, D. Chlamydia abortus: new aspects of infectious abortion in sheep and potential risk for pregnant women. Current clinical microbiology reports 2015, 2, 22–34. [Google Scholar] [CrossRef]
- Hovingh, E. Abortions in dairy cattle I: Common causes of abortions. 2009.
- Longbottom, D. Chlamydial infections of domestic ruminants and swine: new nomenclature and new knowledge. Vet J 2004, 168, 9–11. [Google Scholar] [CrossRef]
- Robertson, A.; Handel, I.; Sargison, N.D. General evaluation of the economic impact of introduction of Chlamydia abortus to a Scottish sheep flock. Veterinary Record Case Reports 2018, 6, e000689. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane handbook for systematic reviews of interventions; John Wiley & Sons: 2019.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Van Driel, M.L.; De Sutter, A.; De Maeseneer, J.; Christiaens, T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epid 2009, 62, 838–844.e833. [Google Scholar] [CrossRef]
- Chaidez-Ibarra, M.A.; Velazquez, D.Z.; Enriquez-Verdugo, I.; Castro Del Campo, N.; Rodriguez-Gaxiola, M.A.; Montero-Pardo, A.; Diaz, D.; Gaxiola, S.M. Pooled molecular occurrence of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry: A systematic review and meta-analysis. Transbound Emerg Dis 2022, 69, 2499–2511. [Google Scholar] [CrossRef]
- Diaz, D.; Hernandez-Carreno, P.E.; Velazquez, D.Z.; Chaidez-Ibarra, M.A.; Montero-Pardo, A.; Martinez-Villa, F.A.; Canizalez-Roman, A.; Ortiz-Navarrete, V.F.; Rosiles, R.; Gaxiola, S.M.; Jimenez-Trejo, F. Prevalence, main serovars and anti-microbial resistance profiles of non-typhoidal Salmonella in poultry samples from the Americas: A systematic review and meta-analysis. Transbound Emerg Dis 2022, 69, 2544–2558. [Google Scholar] [CrossRef]
- StataCorp. Stata MEta-analysis Reference Manual. Stata: Release 18. Statistical Software 2023, 429.
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J Epidemiol Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.; Mavridis, D.; Salanti, G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health 2014, 17, 53–57. [Google Scholar] [CrossRef]
- Diaz, D.; Rosiles, R.J.; Urias-Castro, C.J.; Rodriguez-Gaxiola, M.A.; Gaxiola, S.M.; Montero-Pardo, A. Systematic review and meta-analysis of the efficacy of reproductive management practices used to induce resumption of ovarian cyclical activity in anestrous does. Prev Vet Med 2019, 169, 104709. [Google Scholar] [CrossRef] [PubMed]
- Romo-Barron, C.B.; Diaz, D.; Portillo-Loera, J.J.; Romo-Rubio, J.A.; Jimenez-Trejo, F.; Montero-Pardo, A. Impact of heat stress on the reproductive performance and physiology of ewes: a systematic review and meta-analyses. Int J Biometeorol 2019, 63, 949–962. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research synthesis methods 2017, 8, 5–18. [Google Scholar] [CrossRef]
- Sánchez-Perez, J.N.; Félix-Leyva, B.J.; Velázquez, D.Z.; Rosiles, J.R.; Montero-Pardo, A.; Strappini, A.C.; Gallo, C.; Robles-Estrada, J.C.; Portillo-Loera, J.J.; Diaz, D. Prevalence, risk factors, and main characteristics of bruises in cattle: A meta-analysis in the American continent. Veterinaria México OA 2022, 9. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Reinhold, P.; Sachse, K.; Kaltenboeck, B. Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet J 2011, 189, 257–267. [Google Scholar] [CrossRef]
- Longbottom, D.; Entrican, G.; Wheelhouse, N.; Brough, H.; Milne, C. Evaluation of the impact and control of enzootic abortion of ewes. The Veterinary Journal 2013, 195, 257–259. [Google Scholar] [CrossRef]
- CABI. Pathogen, Invasive Species Compendium: Chlamydophila abortus. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.89292#toDistributionMaps (accessed on 30 October).
- Laroucau, K.; Vorimore, F.; Bertin, C.; Mohamad, K.Y.; Thierry, S.; Hermann, W.; Maingourd, C.; Pourcel, C.; Longbottom, D.; Magnino, S. Genotyping of Chlamydophila abortus strains by multilocus VNTR analysis. Veterinary microbiology 2009, 137, 335–344. [Google Scholar] [CrossRef]
- Osman, K.; Ali, H.; ElJakee, J.; Galal, H. Chlamydophila psittaci and Chlamydophila pecorum infections in goats and sheep in Egypt. Revue Scientifique et Technique-OIE 2011, 30, 939. [Google Scholar] [CrossRef]
- Dorsch, M.A.; Cantón, G.J.; Driemeier, D.; Anderson, M.L.; Moeller, R.B.; Giannitti, F. Bacterial, protozoal and viral abortions in sheep and goats in South America: A review. Small Ruminant Research 2021, 205, 106547. [Google Scholar] [CrossRef]
- Turin, L.; Surini, S.; Wheelhouse, N.; Rocchi, M.S. Recent advances and public health implications for environmental exposure to Chlamydia abortus: from enzootic to zoonotic disease. Vet Res 2022, 53, 37. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; Guyatt, G.H. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, M.S.; Wattegedera, S.; Meridiani, I.; Entrican, G. Protective adaptive immunity to Chlamydophila abortus infection and control of ovine enzootic abortion (OEA). Veterinary microbiology 2009, 135, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Milne, C.E.; Gunn, G.J.; Entrican, G.; Longbottom, D. Epidemiological modelling of chlamydial abortion in sheep flocks. Veterinary microbiology 2009, 135, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wheelhouse, N.; Longbottom, D.; O’Donovan, J. Chlamydia in cases of bovine abortion in Ireland. Vet Rec 2014, 174, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.; Entrican, G.; Wattegedera, S.; Buxton, D.; McKendrick, I.J.; Longbottom, D. Antibody responses to recombinant protein fragments of the major outer membrane protein and polymorphic outer membrane protein POMP90 in Chlamydophila abortus-infected pregnant sheep. Clinical and Vaccine Immunology 2005, 12, 770–777. [Google Scholar] [CrossRef]
- O’Neill, L.M.; O’Driscoll, A.; Markey, B. Comparison of three commercial serological tests for the detection of Chlamydia abortus infection in ewes. Ir Vet J 2018, 71, 13. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, D.; Sait, M.; Livingstone, M.; Laroucau, K.; Sachse, K.; Harris, S.R.; Thomson, N.R.; Seth-Smith, H.M. Genomic evidence that the live Chlamydia abortus vaccine strain 1B is not attenuated and has the potential to cause disease. Vaccine 2018, 36, 3593–3598. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.; Wattegedera, S.R.; Palarea-Albaladejo, J.; Aitchison, K.; Corbett, C.; Sait, M.; Wilson, K.; Chianini, F.; Rocchi, M.S.; Wheelhouse, N.; et al. Efficacy of Two Chlamydia abortus Subcellular Vaccines in a Pregnant Ewe Challenge Model for Ovine Enzootic Abortion. Vaccines (Basel) 2021, 9, 898. [Google Scholar] [CrossRef]
- Kohlhoff, S.A.; Hammerschlag, M.R. Treatment of Chlamydial infections: 2014 update. Expert opinion on pharmacotherapy 2015, 16, 205–212. [Google Scholar] [CrossRef]
- Bommana, S.; Polkinghorne, A. Mini Review: Antimicrobial Control of Chlamydial Infections in Animals: Current Practices and Issues. Front Microbiol 2019, 10, 113. [Google Scholar] [CrossRef]
- Longbottom, D.; Livingstone, M. Vaccination against chlamydial infections of man and animals. Vet J 2006, 171, 263–275. [Google Scholar] [CrossRef]
- Vargas-Bello-Perez, E.; Díaz, C.A.L.; Ruiz-Romero, R.A.; Chay-Canul, A.J.; Lee-Rangel, H.A.; Gonzalez-Ronquillo, M.; Ghavipanje, N.; Tajonar, K. A brief update on sheep production in Mexico: challenges and perspectives. Tropical and Subtropical Agroecosystems 2023, 26. [Google Scholar] [CrossRef]
- Navarro, J.A.; Ortega, N.; Buendia, A.J.; Gallego, M.C.; Martinez, C.M.; Caro, M.R.; Sanchez, J.; Salinas, J. Diagnosis of placental pathogens in small ruminants by immunohistochemistry and PCR on paraffin-embedded samples. Vet Rec 2009, 165, 175–178. [Google Scholar] [CrossRef]
- Kerr, K.; Entrican, G.; McKeever, D.; Longbottom, D. Immunopathology of Chlamydophila abortus infection in sheep and mice. Research in veterinary science 2005, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Burnard, D.; Polkinghorne, A. Chlamydial infections in wildlife-conservation threats and/or reservoirs of ’spill-over’ infections? Vet Microbiol 2016, 196, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Acha, P.N.; Szyfres, B. Zoonoses and communicable diseases common to man and animals; Pan American Health Org: 2003; Volume 580.
- Barbosa, M.A.; Salazar, F.; Fernandez, P.; Montes de Oca, R. Deteccción de anticuerpos serológicos contra Chlamydophila abortus en dos grupos de personas expuestas a riesgo en explotaciones ovinas en Xalatlaco, México. Trop Sub Agroecosystems 2013, 16, 483–486. [Google Scholar]
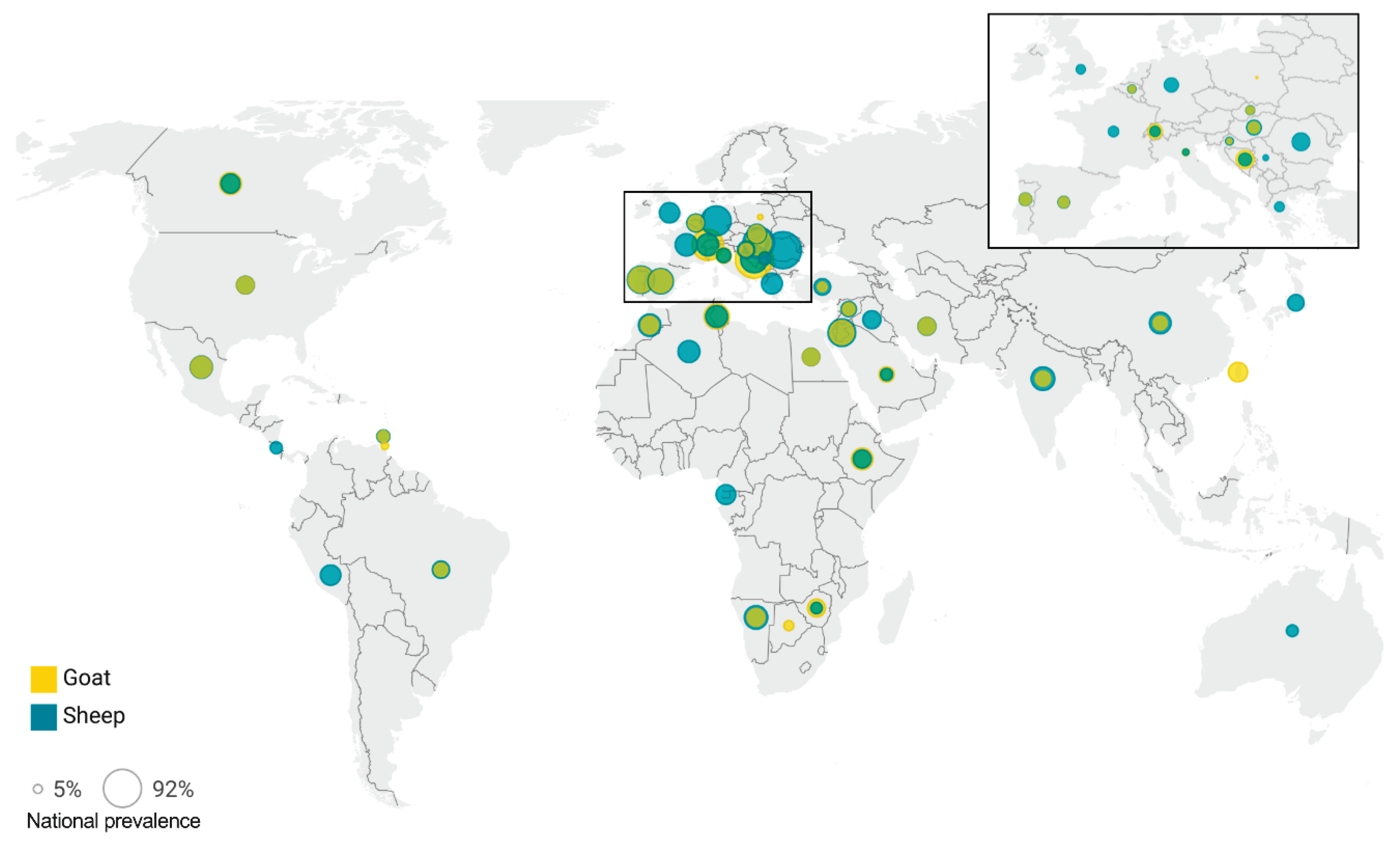
| WHO Region | Sheep | Goats | ||||
|---|---|---|---|---|---|---|
| Country | Studies | Positive/Total | Prevalence (95% CI) | Studies | Positive/Total | Prevalence (95% CI) |
| Global | 109 | 11,235 / 81,788 | 20.1 (17.8 – 22.4) | 74 | 2,729 / 22,696 | 14.4 (11.6 – 17.4) |
| Sub-Saharan Africa | 5 | 531 / 2333 | 17.1 (9.8 – 25.9) | 7 | 670 / 5341 | 15.4 (7.5 – 25.4) |
| Botswana | - | - | - | 1 | 57 / 1799 | 3.2 (2.4 – 4.0) |
| Equatorial Guinea | 1 | 199 / 1002 | 19.9 (17.4 – 22.4) | - | - | - |
| Ethiopia | 2 | 52 / 346 | 16.0 (2.5 – 37.5) | 2 | 71 / 383 | 24.8 (2.4 – 59.5) |
| Namibia | 1 | 277 / 922 | 30.0 (27.1 – 33.0) | 2 | 86 / 1076 | 15.6 (2.9 – 35.8) |
| Zimbabwe | 1 | 3 / 63 | 4.7 (0.6 – 11.7) | 2 | 157 / 898 | 15.5 (5.6 – 29.1) |
| North America | 5 | 230 / 2617 | 16.2 (7.6 – 27.3) | 2 | 97 / 471 | 19.7 (9.8 –32.1) |
| Canada | 2 | 52 / 609 | 18.4 (8.1 – 31.7) | 1 | 67 / 260 | 25.8 (20.6 – 31.3) |
| USA | 3 | 127 / 2008 | 14.9 (3.8 – 31.4) | 1 | 30 / 211 | 14.2 (9.8 – 19.3) |
| Latin America and the Caribbean | 15 | 1358 / 10,174 | 15.5 (12.1. – 19.3) | 14 | 398 / 3690 | 12.3 (7.3 – 18.3) |
| Brazil | 8 | 403 / 2716 | 14.0 (10.1 – 18.5) | 6 | 212 / 2656 | 8.0 (5.3 – 11.2) |
| Costa Rica | 1 | 19 / 359 | 5.2 (3.2 – 7.9) | - | - | - |
| Grenada | 1 | 24 / 355 | 6.8 (4.4 – 9.6) | 1 | 10 / 209 | 4.8 (2.2 – 8.2) |
| Mexico | 4 | 710 / 5806 | 26.4 (13.7 – 41.3) | 6 | 175 / 736 | 24.1 (8.6 – 43.9) |
| Peru | 1 | 202 / 938 | 21.5 (19.0 – 24.2) | - | - | - |
| Trinidad and Tobago | - | - | - | 1 | 1 / 89 | 1.1 (0.0 – 4.8) |
| South Asia | 4 | 134 / 1020 | 30.6 (7.1 – 61.1) | 5 | 101 / 1235 | 10.4 (0.09 – 26.6) |
| India | 4 | 134 / 1020 | 30.6 (7.1 – 61.1) | 5 | 101 / 1235 | 10.4 (0.09 – 26.6) |
| East Asia and Pacific | 7 | 937 / 4425 | 14.0 (7.4 – 22.2) | 5 | 306 / 2174 | 13.6 (5.3 – 24.4) |
| Australia | 3 | 135 / 987 | 4.9 (0.0 – 25.2) | 1 | 0 / 5 | 0.0 (0.0 – 31.7) |
| China | 3 | 769 / 3171 | 24.7 (14.5 – 36.6) | 1 | 77 / 911 | 8.5 (6.7 – 10.4) |
| Japan | 1 | 33 / 267 | 12.4 (8.7 – 16.6) | - | - | - |
| Taiwan | - | - | - | 3 | 229 / 1258 | 19.0 (7.1 – 35.0) |
| Europe and Central Asia | 41 | 5757 / 47,107 | 24.8 (20.2 – 29.6) | 22 | 518 / 5221 | 17.6 (10.6 – 25.7) |
| Belgium | 1 | 15 / 95 | 15.8 (9.1 – 23.9) | 1 | 1 / 9 | 11.1 (0.0 – 41.8) |
| Bosnia-Herzegovia | 1 | 77 / 178 | 43.3 (36.0 – 50.6) | 1 | 11 / 12 | 91.7 (67.6 – 100) |
| Croatia | 2 | 47 / 353 | 14.1 (8.2 – 21.3) | 2 | 13 / 236 | 6.1 (1.1 – 14.1) |
| France | 1 | 116 / 430 | 27.0 (22.9 – 31.3) | - | - | - |
| Germany | 3 | 334 / 1833 | 53.3 (12.1 – 91.9) | - | - | - |
| Greece | 1 | 106 / 464 | 22.8 (19.1 – 26.8) | - | - | - |
| Hungary | 2 | 157 / 305 | 60.1 (31.6 – 85.3) | 2 | 17 / 81 | 35.3 (0.2 – 8.4) |
| Italy | 6 | 664 / 13,178 | 8.4 (3.7 – 14.7) | 5 | 116 / 1874 | 11.2 (0.2 – 30.3) |
| Poland | - | - | - | 1 | 2 / 918 | 0.2 (0.0 – 0.7) |
| Portugal | 2 | 38 / 88 | 43.2 (32.8 – 53.8) | 2 | 41 / 110 | 36.6 (24.6 – 49.4) |
| Romania | 1 | 40 / 46 | 87.0 (75.5 – 95.4) | 1 | 0 / 10 | 0.0 (0.0 – 16.5) |
| Serbia | 1 | 33 / 552 | 6.0 (4.1 – 8.1) | - | - | - |
| Slovakia | 4 | 2614 / 22,013 | 18.5 (7.7 – 32.7) | 2 | 109 / 1261 | 14.4 (2.1 – 34.6) |
| Spain | 4 | 950 / 4280 | 36.4 (8.7 – 70.5) | 2 | 198 / 673 | 29.4 (23.0 – 36.3) |
| Switzerland | 3 | 179 / 884 | 26.2 (15.6 – 38.4) | 1 | 9 / 15 | 60.0 (33.8 – 83.7) |
| Turkey | 5 | 106 / 746 | 12.8 (8.8 – 17.4) | 2 | 1 / 22 | 2.8 (0.0 – 26.3) |
| United Kingdom | 4 | 281 / 1662 | 21.1 (2.1 – 52.2) | - | - | - |
| Middle East and North Africa | 32 | 2288 / 14,112 | 18.2 (14.5 – 22.2) | 19 | 639 / 4564 | 14.3 (9.6 – 19.7) |
| Algeria | 4 | 220 / 934 | 25.7 (15.2 – 38.0) | - | - | - |
| Egypt | 2 | 117 / 836 | 13.9 (11.7 – 16.4) | 1 | 17 / 123 | 13.8 (8.2 – 20.5) |
| Iran | 10 | 453 / 1230 | 15.2 (9.4 – 22.0) | 9 | 317 / 1901 | 13.8 (7.6 – 21.2) |
| Iraq | 2 | 59 / 210 | 16.0 (0.0 – 51.3) | - | - | - |
| Jordan | 3 | 480 / 2062 | 44.5 (14.9 – 76.4) | 2 | 98 / 747 | 32.8 (0.0 – 84.3) |
| Morocco | 1 | 55 / 202 | 27.2 (21.3 – 33.6) | 1 | 16 / 106 | 15.1 (8.8 – 22.6) |
| Palestine | 1 | 385 / 2608 | 14.8 (13.4 – 16.2) | - | - | - |
| Saudi Arabia | 3 | 222 / 2771 | 5.4 (0.6 – 14.6) | 3 | 173 / 1500 | 10.3 (0.2 – 31.4) |
| Syria | 2 | 101 / 978 | 10.3 (8.5 – 12.3) | 1 | 9 / 142 | 6.3 (2.8 – 11.0) |
| Tunisia | 4 | 196 / 1244 | 25.5 (6.1 – 52.0) | 2 | 9 / 45 | 35.8 (0.0 – 93.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





