Submitted:
26 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physicochemical aspects of DES-based systems, kinetics of electrochemical processes and their impact on composite electrodeposition
2.1. Colloidal-chemical stability of plating baths based on DESs
2.2. Kinetics and mechanism of composite coating deposition from DESs
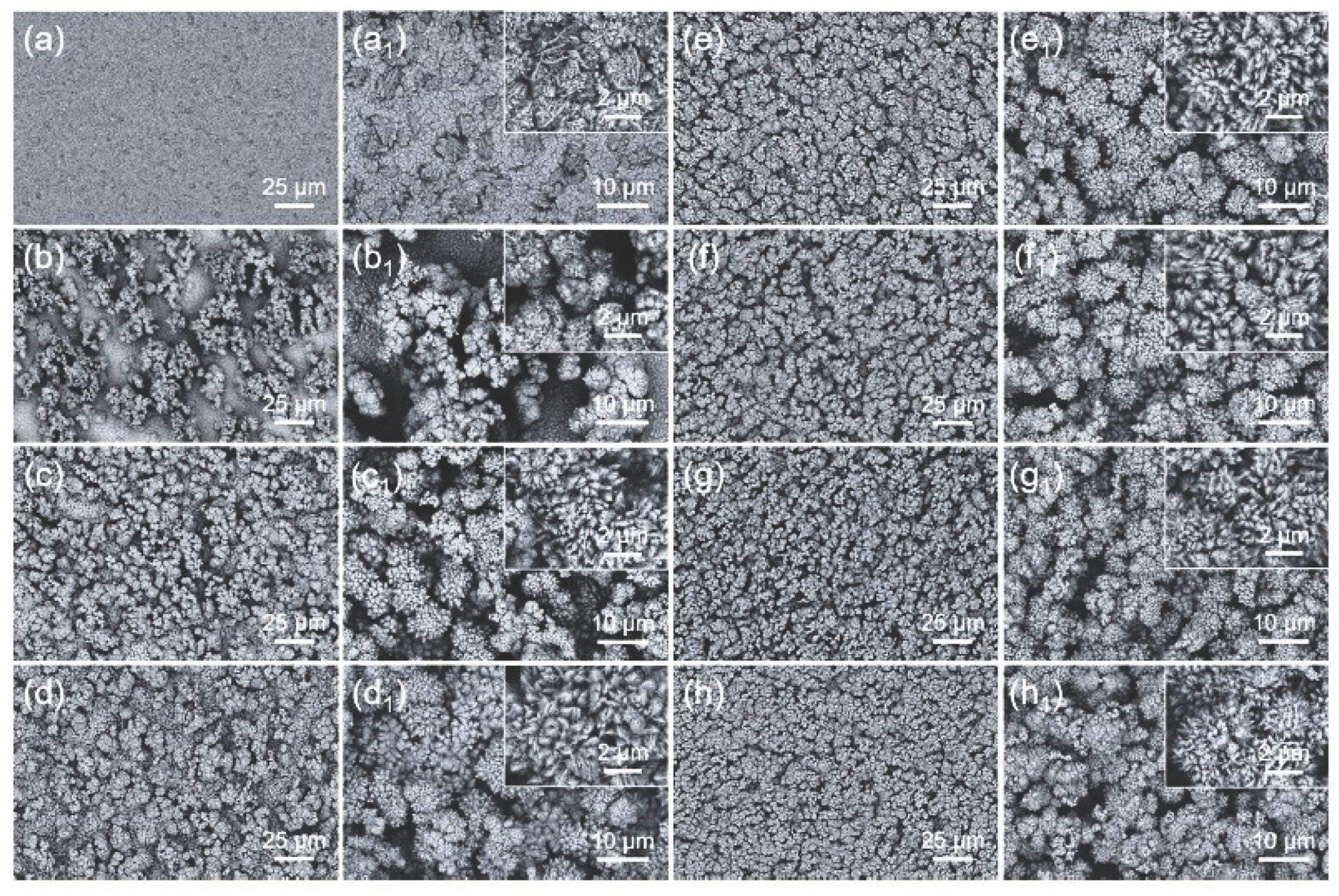
3. Case studies
3.1. Copper-based composites
3.2. Silver-based composites
3.3. Zinc-based composites
3.4. Tin-based composites
3.5. Nickel-based composites
3.6. Cobalt-based composites
3.7. Chromium-based composites
4. Conclusions and possible directions of future research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okonkwo, B.O.; Jeong, C.; Jang, C. Advances on Cr and Ni electrodeposition for industrial applications – a review. Coatings 2022, 12, 1555. [CrossRef]
- Larson, C.; Smith, J.R. Recent trends in metal alloy electrolytic and electroless plating research: A review. Trans. Inst. Met. Finish. 2011, 89, 333–341. [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern electroplating, 5th ed.; John Wiley & Sons, 2014. 752 p.
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [CrossRef]
- Walsh, F.C.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. Inst. Met. Finish. 2020, 98, 288–299. [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [CrossRef]
- Walsh, F.C.; Ponce de Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Trans. Inst. Met. Finish. 2014, 92, 83–98. [CrossRef]
- Musiani, M. Electrodeposition of composites: an expanding subject in electrochemical materials science. Electrochim. Acta 2000, 45, 3397–3402. [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; Gurkan, B.; Maginn, E.J.; Ragauskas, A.; Dadmun, M.; Zawodzinski, T.A.; Baker, G.A.; Tuckerman, M.E.; Savinell, R.F.; Sangoro, J.R. Deep eutectic solvents: a review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [CrossRef]
- Abbott, A.P.; Edler, K.J.; Page, A.J. Deep eutectic solvents – the vital link between ionic liquids and ionic solutions. J. Chem. Phys. 2021, 155, 150401. [CrossRef]
- Tome, L.I.N.; Baiao, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [CrossRef]
- Abbott, A.P.; Ryder, K.S.; König, U. Electrofinishing of metals using eutectic based ionic liquids. Trans. Inst. Met. Finish. 2008, 86, 196–204. [CrossRef]
- Smith, E.L. Deep eutectic solvents (DESs) and the metal finishing industry: where are they now? Trans. Inst. Met. Finish. 2013, 91, 241–248. [CrossRef]
- Abbott, A.P. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [CrossRef]
- Costa, J.G.d.R.d.; Costa, J.M.; Almeida Neto, A.F.d. Progress on electrodeposition of metals and alloys using ionic liquids as electrolytes. Metals 2022, 12, 2095. [CrossRef]
- Nam, N.N.; Do, H.D.K.; Trinh, K.T.L.; Lee, N.Y. Design strategy and application of deep eutectic solvents for green synthesis of nanomaterials. Nanomaterials 2023, 13, 1164. [CrossRef]
- Kityk, A.; Pavlik, V.; Hnatko, M. Exploring deep eutectic solvents for the electrochemical and chemical synthesis of photo and electrocatalysts for hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 39823–39853. [CrossRef]
- Danilov, F.I.; Protsenko, V.S. Electrodeposition of composite coatings using electrolytes based on deep eutectic solvents: A mini-review. Voprosy Khimii i Khimicheskoi Tekhnologii 2018, (1), 13–21.
- Abbott, A.P.; El Ttaib, K.; Frisch, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of copper composites from deep eutectic solvents based on choline chloride. Phys. Chem. Chem. Phys. 2009, 11, 4269–4277. [CrossRef]
- Higashitani, K.; Kondo, M.; Hatade, S. Effect of particle size on coagulation rate of ultrafine colloidal particles. J. Colloid Interface Sci. 1991, 142, 204–213. [CrossRef]
- Shchukin, E.D.; Pertsov, A.V.; Amelina, E.A.; Zelenev, A.S. Colloid and Surface Chemistry, Elsevier: Amsterdam, 2001.
- You, Y.-H.; Gu, C.-D.; Wang, X.-L.; Tu, J.-P. Electrochemical preparation and characterization of Ni–PTFE composite coatings from a non-aqueous solution without additives. Int. J. Electrochem. Sci. 2012, 7, 12440–12455. [CrossRef]
- Li, R.; Hou, Y.; Liu, B.; Wang, D.; Liang, J. Electrodeposition of homogenous Ni/SiO2 nanocomposite coatings from deep eutectic solvent with in-situ synthesized SiO2 nanoparticles. Electrochim. Acta 2016, 222, 1272–1280. [CrossRef]
- Li, R.; Hou, Y.; Liang, J. Electro-codeposition of Ni-SiO2 nanocomposite coatings from deep eutectic solvent with improved corrosion resistance. Appl. Surf. Sci. 2016, 367, 449–458. [CrossRef]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [CrossRef]
- Celis, J.P.; Roos, J.R.; Buelens, C. A mathematical model for the electrolytic codeposition of particles with a metallic matrix. J. Electrochem. Soc. 1987, 134, 1402–1408. [CrossRef]
- Fransaer, J.; Celis, J.P.; Roos, J.R. Analysis of the electrolytic codeposition of non-Brownian particles with metals. J. Electrochem. Soc. 1992, 139, 413–425. [CrossRef]
- Maurin, G.; Lavanant, A. Electrodeposition of nickel/silicon carbide composite coatings on a rotating disc electrode. J. Appl. Electrochem. 1995, 25, 1113–1121. [CrossRef]
- Hwang, B.J.; Hwang, C.S. Mechanism of codeposition of silicon carbide with electrolytic cobalt. J. Electrochem. Soc. 1993, 140, 979–984. [CrossRef]
- Vereecken, P.M.; Shao, I.; Searson, P.C. Particle codeposition in nanocomposite films. J. Electrochem. Soc., 2000, 147, 2572–2575. [CrossRef]
- Shao, I.; Vereecken, P.M.; Cammarata, R.C.; Searson, P.C. Kinetics of particle codeposition of nanocomposite. J. Electrochem. Soc. 2002, 149, C610–C614. [CrossRef]
- Berçot, P.; Peña-Muñoz, E.; Pagetti, J. Electrolytic composite Ni-PTFE coatings: an adaptation of Guglielmi’s model for the phenomena of incorporation. Surf. Coat. Technol. 2002, 157, 282–289. [CrossRef]
- Bahadormanesh, B.; Dolati, A. The kinetics of Ni-Co/SiC composite coatings electrodeposition. J. Alloys Compd. 2010, 504, 514–518. [CrossRef]
- Eroglu, D.; West, A.C. Mathematical modeling of Ni/SiC co-deposition in the presence of a cationic dispersant. J. Electrochem. Soc. 2013, 160, D354–D360. [CrossRef]
- Adamczyk, Z.; Jasczółt, K.; Michna, A.; Siwek, B.; Szyk-Warszyńska, L.; Zembala, M. Irreversible adsorption of particles on heterogeneous surfaces. Adv. Colloid Interface Sci. 2005, 118, 25–42. [CrossRef]
- Wojtaszczyk, P.; Bonet Avalos, J.; Rubi, J.M. Kinetics of particles adsorption processes driven by diffusion. Europhys. Lett. 1997, 40, 299–304. [CrossRef]
- Binks, B.P. Particles as surfactants – similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Kinetic model of composite coatings electrodeposition assuming irreversible adsorption of dispersed particles on a growing metal substrate. J. Electroanal. Chem. 2022, 918, 116463. [CrossRef]
- Atilhan, M.; Aparicio, S. Deep eutectic solvents on the surface of face centered cubic metals, J. Phys. Chem. C 2016, 120, 10400–10409. [CrossRef]
- Mamme, M.H.; Moors, S.L.C.; Terryn, H.A.; Deconinck, J.; Ustarroz, J.; De Proft, F. Atomistic insights into the electrochemical double layer of choline chloride-urea deep eutectic solvent: a clustered interfacial structuring. J. Phys. Chem. Lett. 2018, 9, 6296–6304. [CrossRef]
- Danilov, F.I.; Kityk, A.A.; Shaiderov, D.A.; Bogdanov, D.A.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–TiO2 composite coatings using electrolyte based on a deep eutectic solvent. Surf. Eng. Appl. Electrochem. 2019, 55, 138–149. [CrossRef]
- Bobrova, L.S.; Danilov, F.I.; Protsenko, V.S. Effects of temperature and water content on physicochemical properties of ionic liquids containing CrCl3⋅xH2O and choline chloride. J. Mol. Liq. 2016, 223, 48–53. [CrossRef]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 24604–24616. [CrossRef]
- Li, R.; Chu, Q.; Liang, J. Electrodeposition and characterization of Ni–SiC composite coatings from deep eutectic solvent. RSC Adv. 2015, 5, 44933–44942. [CrossRef]
- El Ttaib, K.; Benhmid, A. The study of the effect of surfactants on copper codeposition with SiC nano particulate from deep eutectic solvent ionic liquids (Ethaline). Int. Res. J. Pure Appl. Chem. 2024, 25, 22-27. [CrossRef]
- Abbott, A.P.; El Ttaib, K.; Frisch, G.; Ryder, K.S.; Weston, D. The electrodeposition of silver composites using deep eutectic solvents. Phys. Chem. Chem. Phys. 2012, 14, 2443–2449. [CrossRef]
- Marín-Sánchez, M.; Gracia-Escosa, E.; Conde, A.; Palacio, C.; García, I. Deposition of zinc–cerium coatings from deep eutectic ionic liquids. Materials 2018, 11, 2035. [CrossRef]
- Li, R.; Liang, J.; Hou, Y.; Chu, Q. Enhanced corrosion performance of Zn coating by incorporating graphene oxide electrodeposited from deep eutectic solvent. RSC Adv. 2015, 5, 60698–60707. [CrossRef]
- Costovici, S.; Pantazi, A.; Balan, D.; Cojocaru, A.; Visan, T.; Enachescu, M.; Anicai, L. Electrodeposition of tin-reduced graphene oxide composite from deep eutectic solvents based on choline chloride and ethylene glycol. Metals 2023, 13, 203. [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Bogdanov, D.A.; Korniy, S.A.; Danilov, F.I. Electrochemical synthesis of Ni/TiO2 composite coatings from deep eutectic solvent and electrocatalytic characteristics of deposits. Surf. Eng. Appl. Electrochem. 2022, 58, 440–450. [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical corrosion behavior of Ni–TiO2 composite coatings electrodeposited from a deep eutectic solvent-based electrolyte. Coatings 2022, 12, 800. [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Danilov, F.I. Preparation and characterization of Ni–TiO2 composites electrodeposited from an ethylene glycol-based deep eutectic solvent. Mater. Today Proc. 2022, 62, 7712–7716. [CrossRef]
- Danilov, F.I.; Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.A.; Vasil’eva, E.A.; Pramod Kumar, U.; Joseph Kennady, C. Electrodeposition of nanocrystalline nickel coatings from a deep eutectic solvent with water addition. Prot. Met. Phys. Chem. Surf. 2017, 53, 1131–1138. [CrossRef]
- Gu, C.D.; You, Y.H.; Yu, Y.L.; Qu, S.X.; Tu, J.P. Microstructure, nanoindentation, and electrochemical properties of the nanocrystalline nickel film electrodeposited from choline chloride–ethylene glycol. Surf. Coat. Technol. 2011, 205, 4928–4933. [CrossRef]
- Abbott, A.P.; El Ttaib, K.; Ryder, K.S.; Smith, E.L. Electrodeposition of nickel using eutectic based ionic liquids. Trans. Inst. Met. Finish. 2008, 86, 234–240. [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: a review. Environ. Chem. Lett. 2022, 20, 153–188. [CrossRef]
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of hydrogen as fuel: a trend of the 21st century. Energies 2022, 15, 311. [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: recent developments, limitations and new avenues for applications, J. Environ. Chem. Eng. 2016, 4, 4143–4164. [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl. Catal. A: Gen. 2010, 389, 1–8. [CrossRef]
- Liu, S.; Ji, R.; Liu, Y.; Zhang, F.; Jin, H.; Li, X.; Zheng, Q.; Lu, S.; Cai, B. Effects of boric acid and water on the deposition of Ni/TiO2 composite coatings from deep eutectic solvent. Surf. Coat. Technol. 2021, 409, 126834. [CrossRef]
- Martis, P.; Dilimon, V.S.; Delhalle, J.; Mekhalif, Z. Electro-generated nickel/carbon nanotube composites in ionic liquid. Electrochim. Acta 2010, 55, 5407–5410. [CrossRef]
- Liu, D.G.; Sun, J.; Gui, Z.X.; Song, K.J.; Luo, L.M.; Wu, Y.C. Super-low friction nickel based carbon nanotube composite coating electro-deposited from eutectic solvents. Diamond Relat. Mater. 2017, 74, 229–232. [CrossRef]
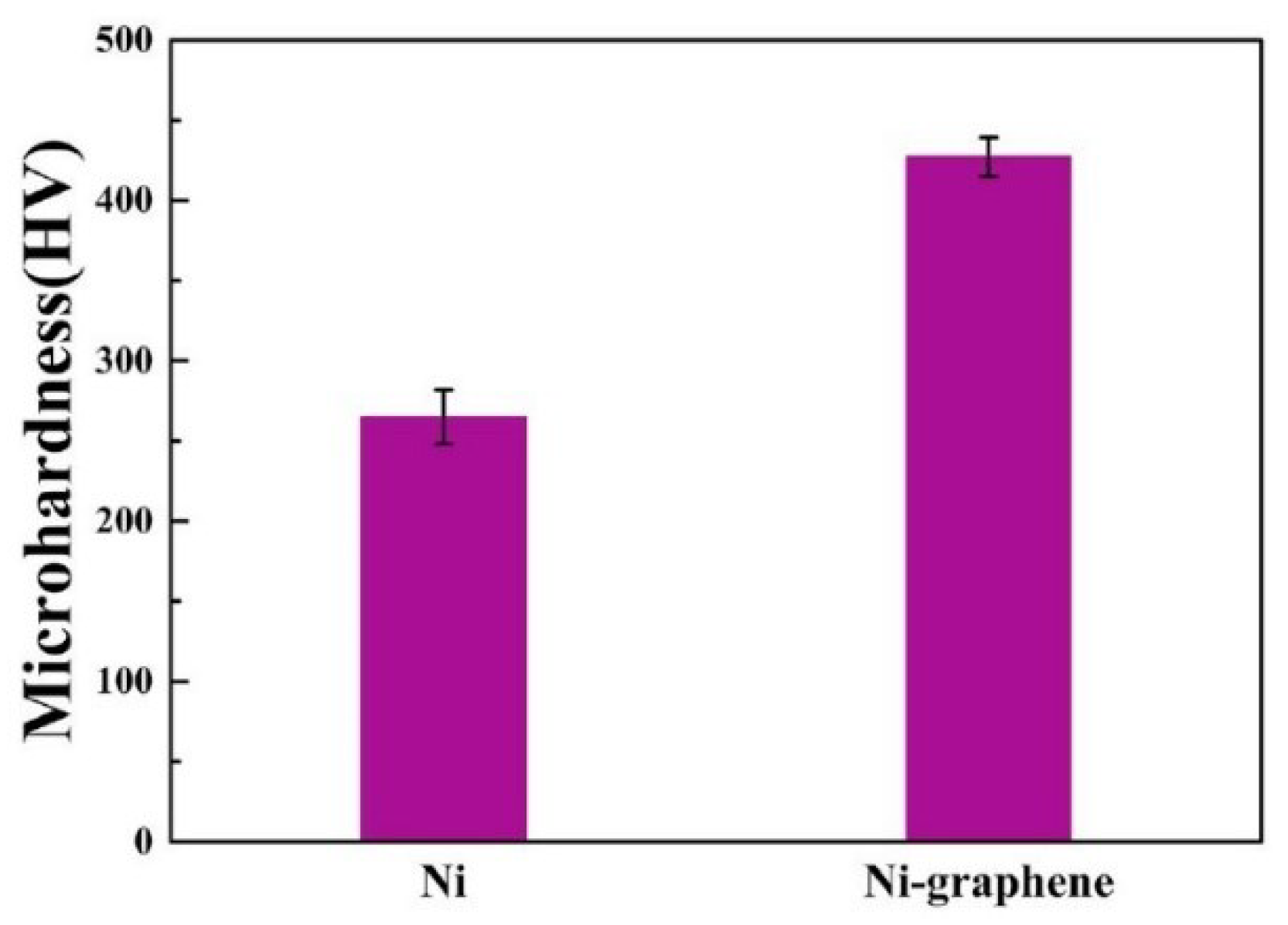
- Xiang, L.; Shen, Q.; Zhang, Y.; Bai, W.; Nie, C. One-step electrodeposited Ni-graphene composite coating with excellent tribological properties. Surf. Coat. Technol. 2019, 373, 38–46. [CrossRef]
- Wang, S.; Zou, X.; Shi, T.; Ding, K.; Pang, Z.; Huang, Y.; Tang, W.; Xu, Q.; Zhou, Z.; Lu, X. Facile electrodeposition of three-dimensional flower-like structure of nickel matrix composite electrodes for hydrogen evolution reaction. Appl. Surf. Sci. 2019, 498, 143768. [CrossRef]
- Cherigui, E.A.M.; Sentosun, K.; Bouckenooge, P.; Vanrompay, H.; Bals, S.; Terryn H., Ustarroz J. Comprehensive study of the electrodeposition of nickel nanostructures from deep eutectic solvents: self-limiting growth by electrolysis of residual water. J. Phys. Chem. C 2017, 121, 9337–9347. [CrossRef]
- Winiarski, J.; Niciejewska, A.; Ryl, J.; Darowicki, K.; Baśladyńska, S.; Winiarska, K.; Szczygieł, B. Ni/cerium molybdenum oxide hydrate microflakes composite coatings electrodeposited from choline chloride: Ethylene glycol deep eutectic solvent. Materials 2020, 13, 924. [CrossRef]
- Hou, Y.; Peng, Z.; Liang, J.; Fu, S. Ni–Ti nanocomposite coatings electro-codeposited from deep eutectic solvent containing Ti nanoparticles. J. Electrochem. Soc. 2020, 167, 042502. [CrossRef]
- Hou, Y.; Peng, Z.; Liang, J.; Liu, M. Ni-Al nanocomposite coating electrodeposited from deep eutectic solvent. Surf. Coat. Technol. 2021, 405, 126587. [CrossRef]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Electrodeposition of NiSn-rGO composite coatings from deep eutectic solvents and their physicochemical characterization. Metals 2020, 10, 1455. [CrossRef]
- Pereira, N.M.; Brincoveanu, O.; Pantazi, A.G.; Pereira, C.M.; Araújo, J.P.; Silva, A.F.; Enachescu, M.; Anicai, L. Electrodeposition of Co and Co composites with carbon nanotubes using choline chloride-based ionic liquids. Surf. Coat. Technol. 2017, 324, 451–462. [CrossRef]
- Maharaja, J.; Raja, M.; Mohan, S. Pulse electrodeposition of Cr–SWCNT composite from choline chloride based electrolyte. Surf. Eng. 2014, 30, 722–727. [CrossRef]
- Protsenko, V.S. Kinetics and mechanism of electrochemical reactions occurring during the chromium electrodeposition from electrolytes based on Cr(III) compounds: a literature review. Reactions 2023; 4, 398–419. [CrossRef]
- Mejía-Caballero, I.; Le Manh, T.; Aldana-González, J.; Arce-Estrada, E.M.; Romero-Romo, M.; Campos-Silva, I.; Ramírez-Silva, M.T.; Palomar-Pardavé, M. Electrodeposition of nanostructured chromium conglomerates from Cr(III) dissolved in a deep eutectic solvent: influence of forced convection. J. Electrochem. Soc. 2021, 168, 112512. [CrossRef]
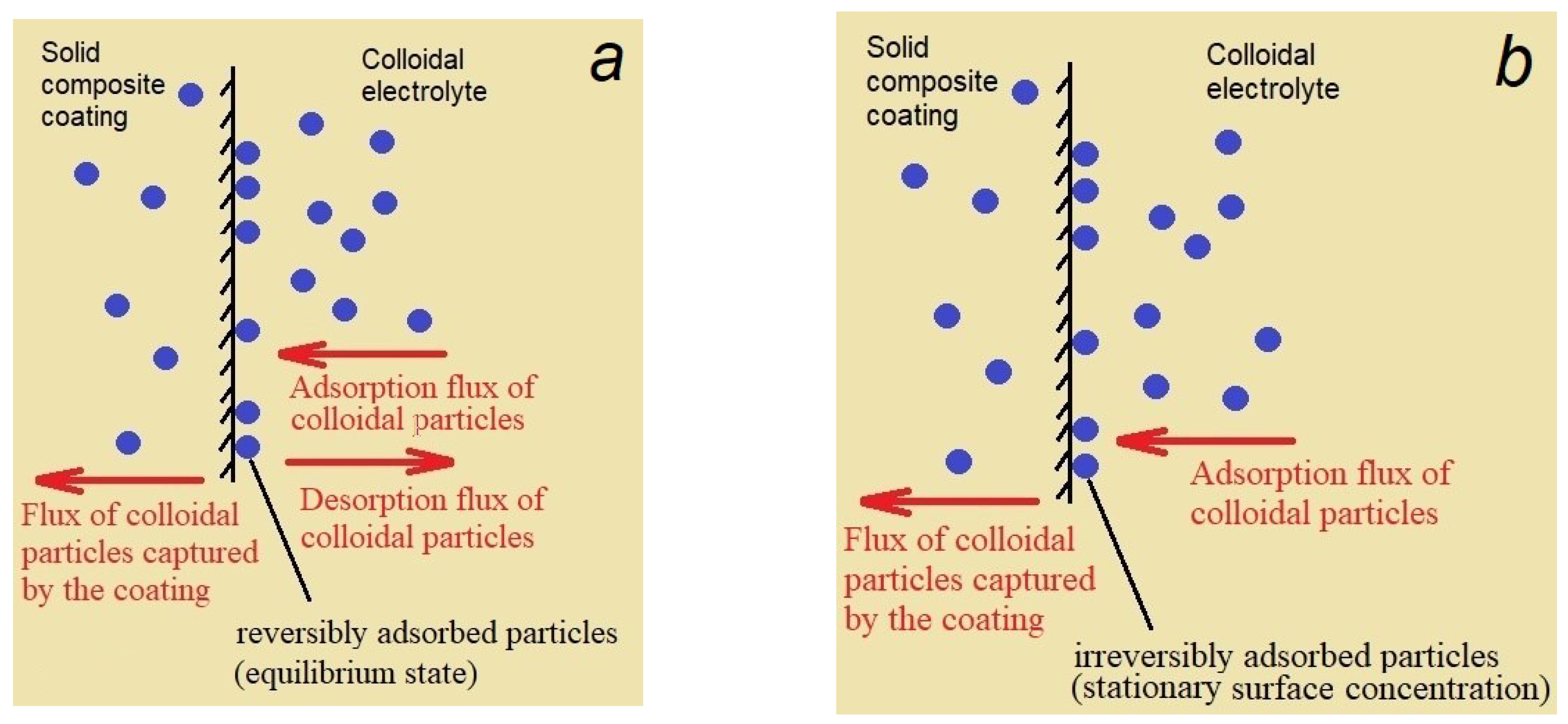
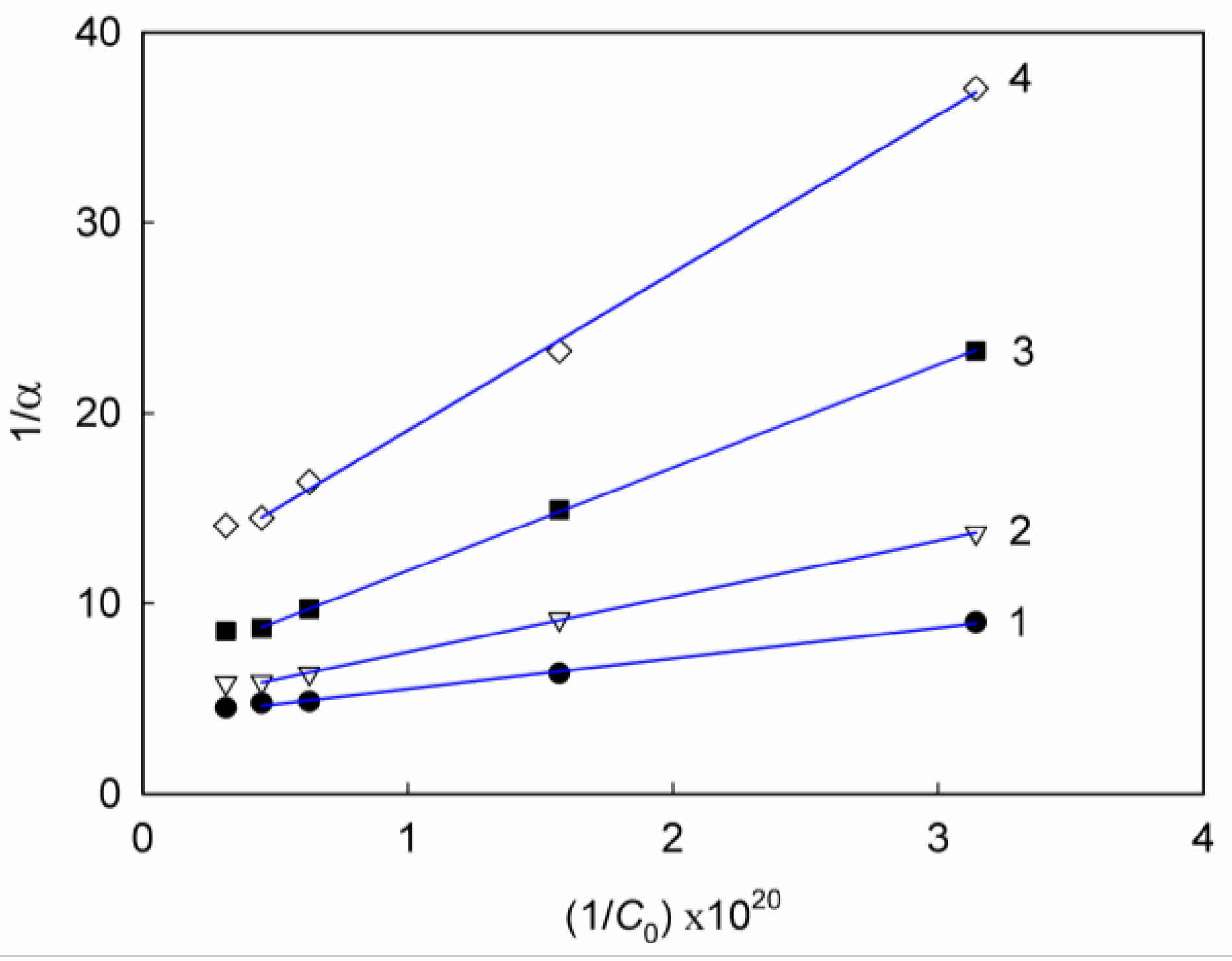
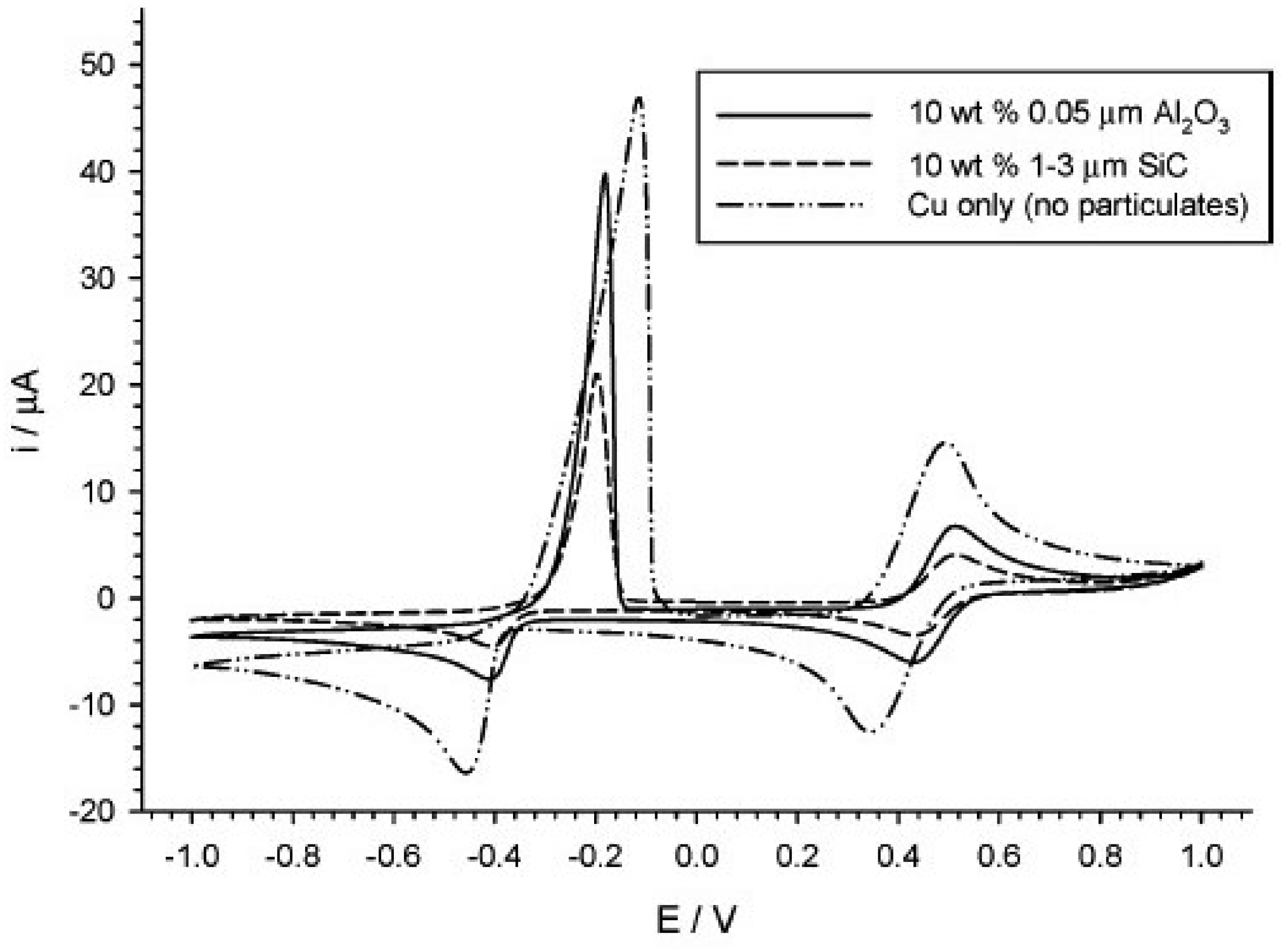
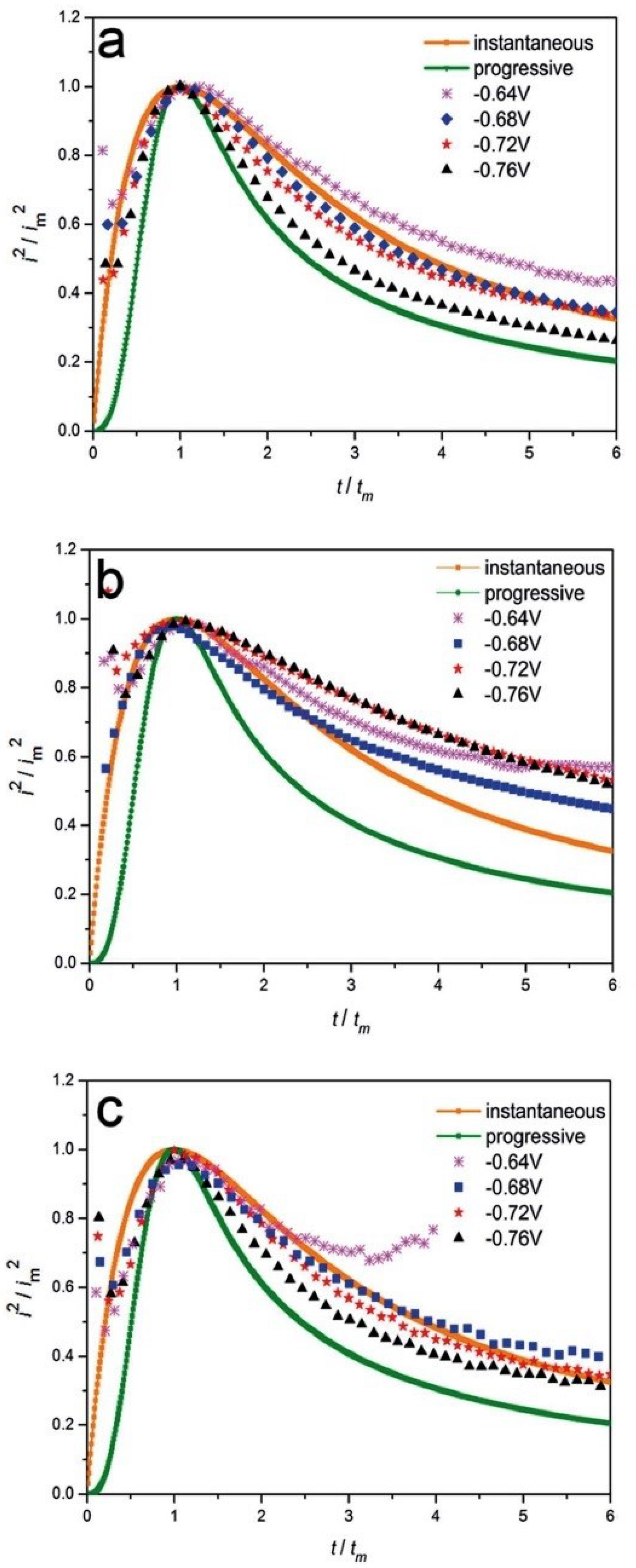
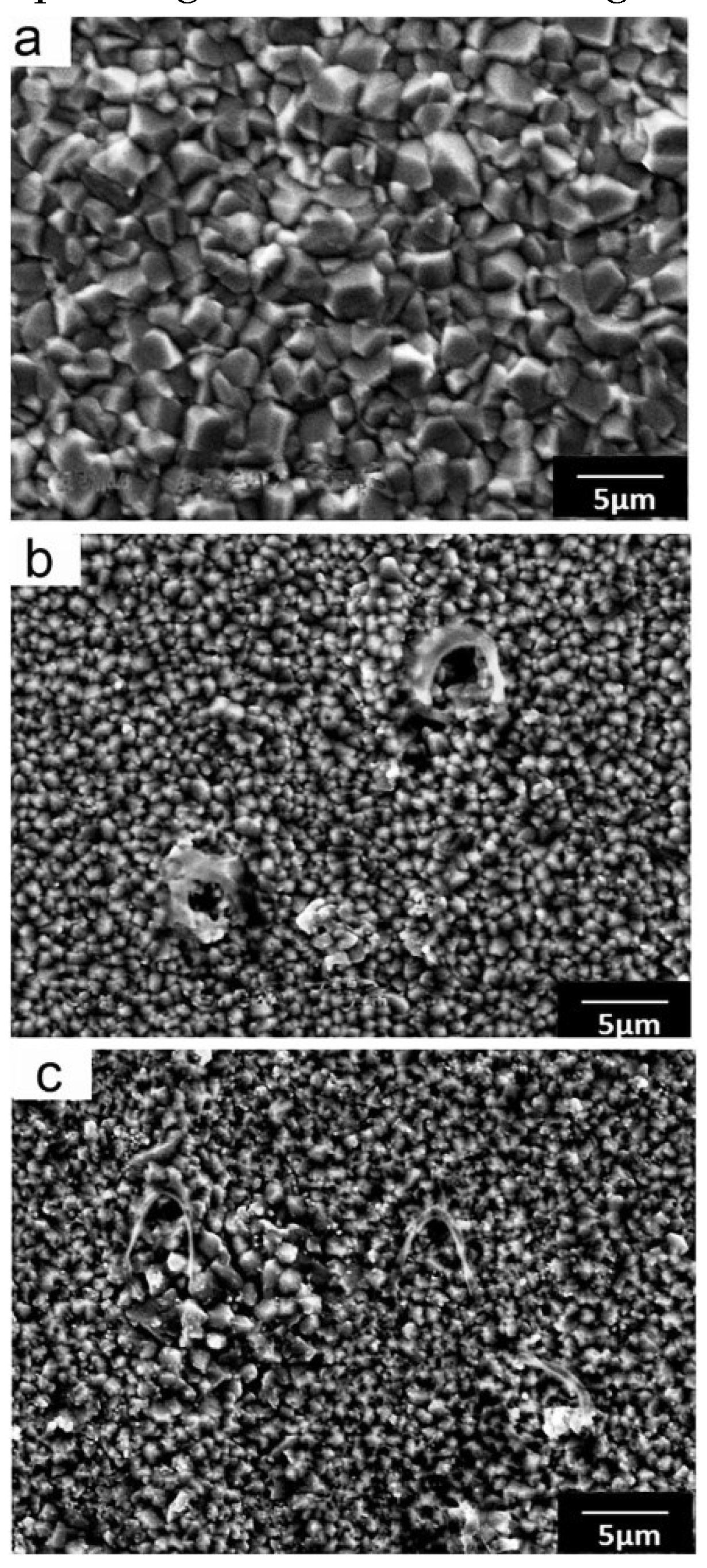


| Constituents of DES | Salt:hydrogen bond donor molar ratio | Viscosity, cP | Density, g/cm3 (25°C) | |
|---|---|---|---|---|
| Salt | Hydrogen bond donor | |||
| ChCl | urea | 1:2 | 750 (25°C) | 1.25 |
| ChCl | urea | 1:2 | 169 (40°C) | |
| ChCl | ethylene glycol | 1:2 | 36 (20°C) | |
| ChCl | ethylene glycol | 1:2 | 37 (25°C) | 1.12 |
| ChCl | ethylene glycol | 1:3 | 19 (20°C) | 1.12 |
| ChCl | ethylene glycol | 1:4 | 19 (20°C) | |
| ChCl | glucose | 1:1 | 34400 (50°C) | |
| ChCl | glycerol | 1:2 | 376 (20°C) | 1.18 |
| ChCl | glycerol | 1:2 | 259 (25°C) | |
| ChCl | glycerol | 1:3 | 450 (20°C) | 1.20 |
| ChCl | glycerol | 1:4 | 503 (20°C) | |
| ChCl | 1,4-butanediol | 1:3 | 140 (20°C) | |
| ChCl | 1,4-butanediol | 1:4 | 88 (20°C) | |
| ChCl | CF3CONH2 | 1:2 | 77 (40°C) | 1.342 |
| ChCl | ZnCl2 | 1:2 | 85000 (25°C) | |
| ChCl | xylitol | 1:1 | 5230 (30°C) | |
| ChCl | sorbitol | 1:1 | 12730 (30°C) | |
| ChCl | malonic acid | 1:2 | 1124 (25°C) | 1.25 |
| ZnCl2 | urea | 1:3.5 | 11340 (25°C) | 1.63 |
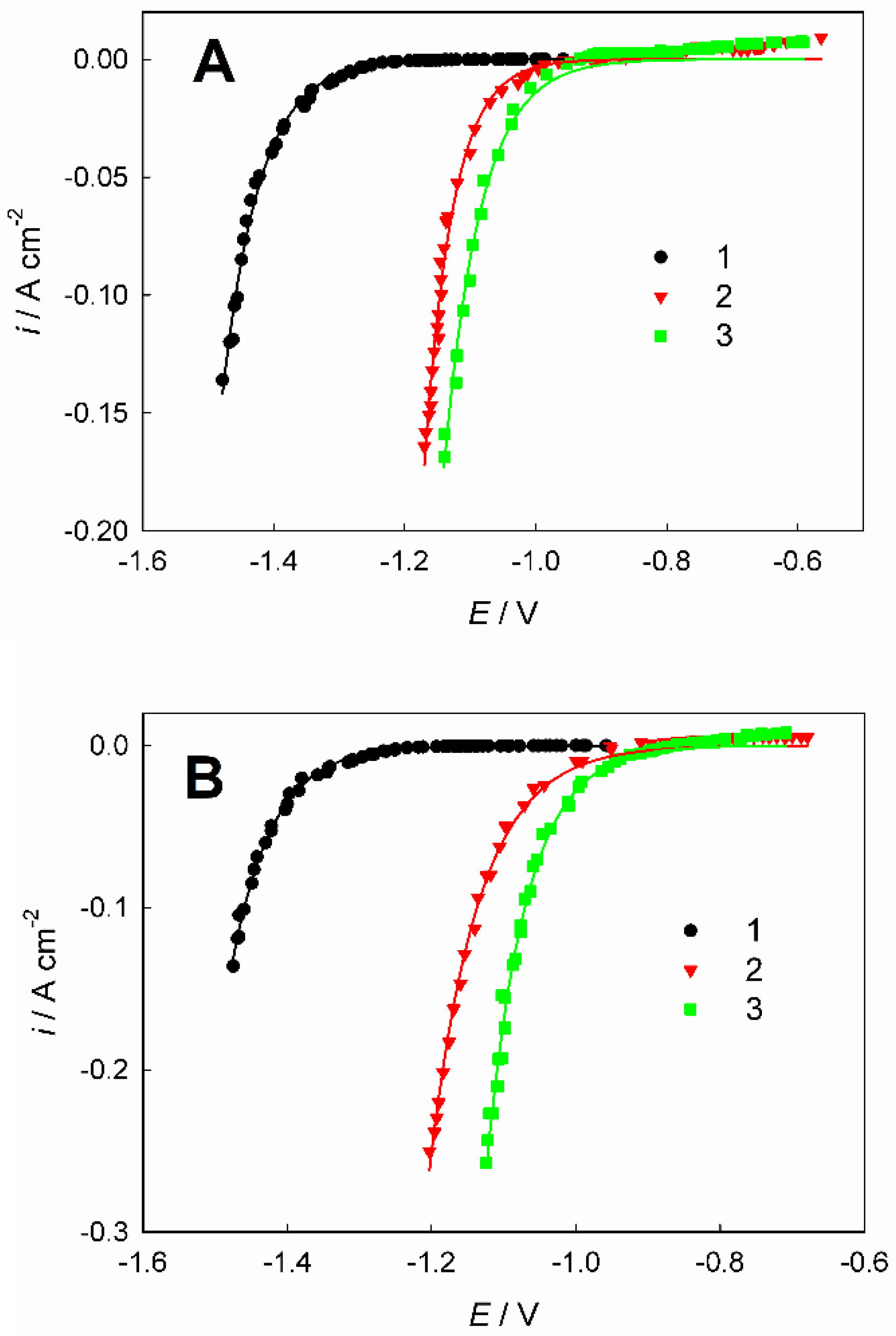
| Current density (A dm–2) | Parameter* | ||
| k (m s–1) | αmax | R2 | |
| 1 | 2.612×10–6 | 0.256 | 0.997 |
| 1.5 | 2.159×10–6 | 0.220 | 0.999 |
| 2 | 1.554×10–6 | 0.158 | 0.999 |
| 3 | 1.520×10–6 | 0.093 | 0.998 |
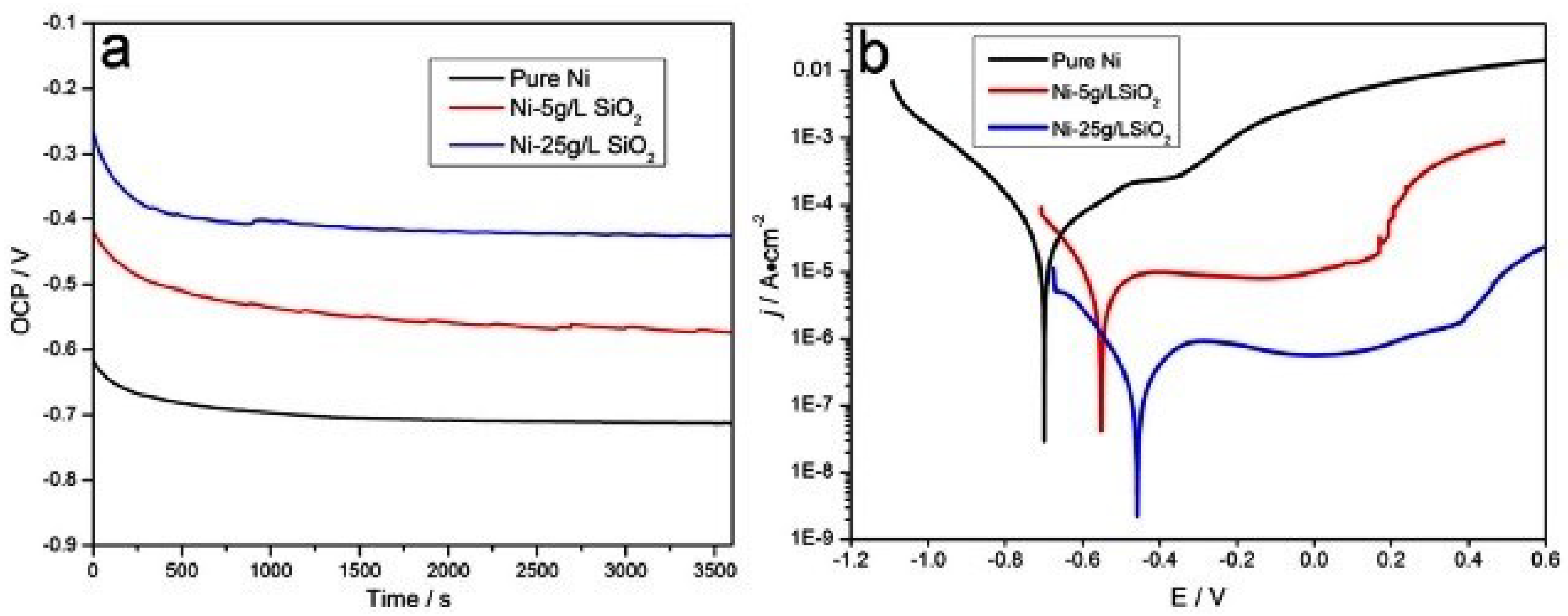
| Specimens | Ecorr (V vs. Ag/AgCl | icorr (A/cm2) |
|---|---|---|
| Pure Ni | –0.72 | 3.92×10–5 |
| Ni-5g/LSiO2 | –0.57 | 3.23×10–6 |
| Ni-25g/LSiO2 | –0.43 | 2.89×10–7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





