Submitted:
05 February 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
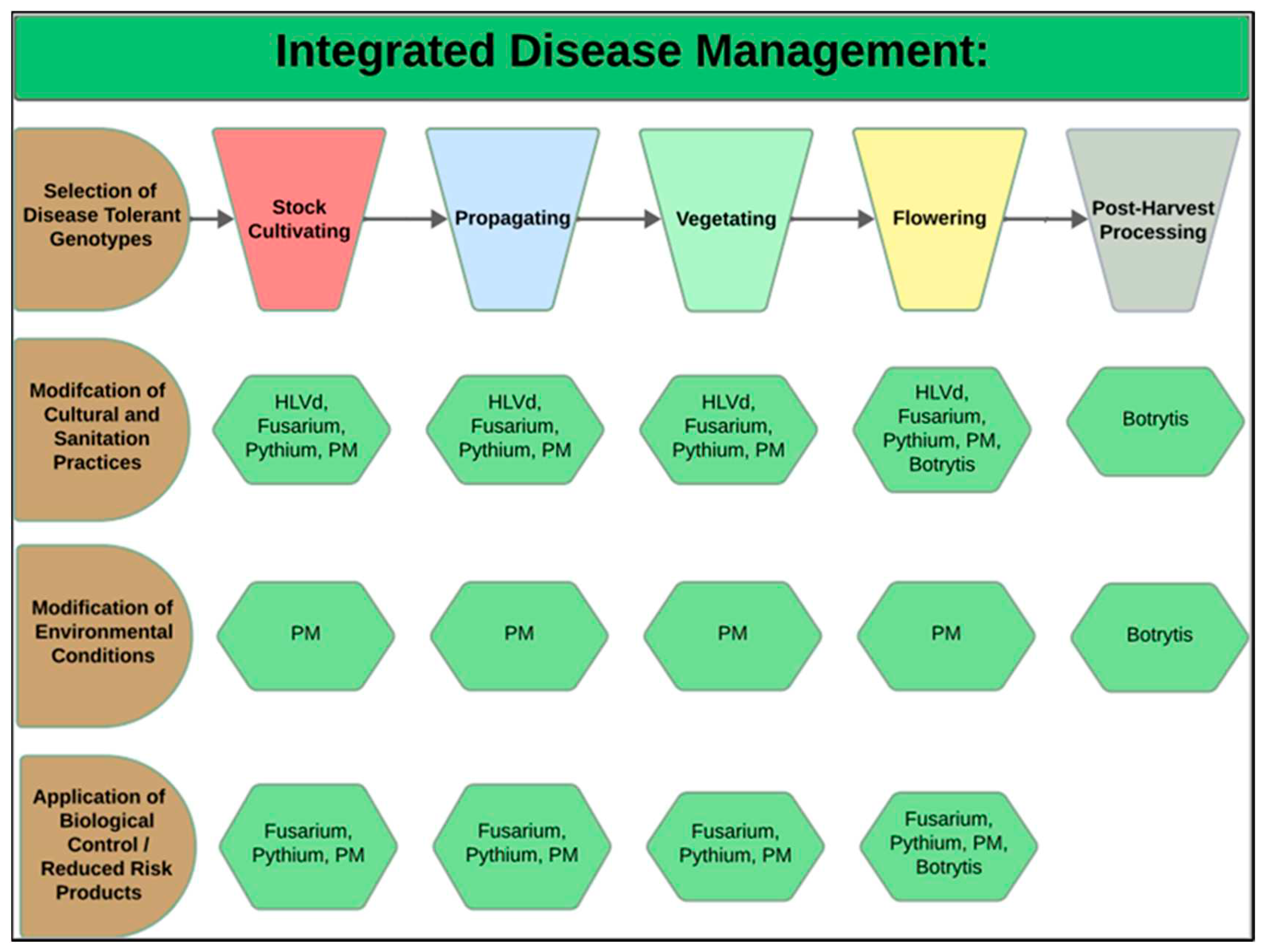
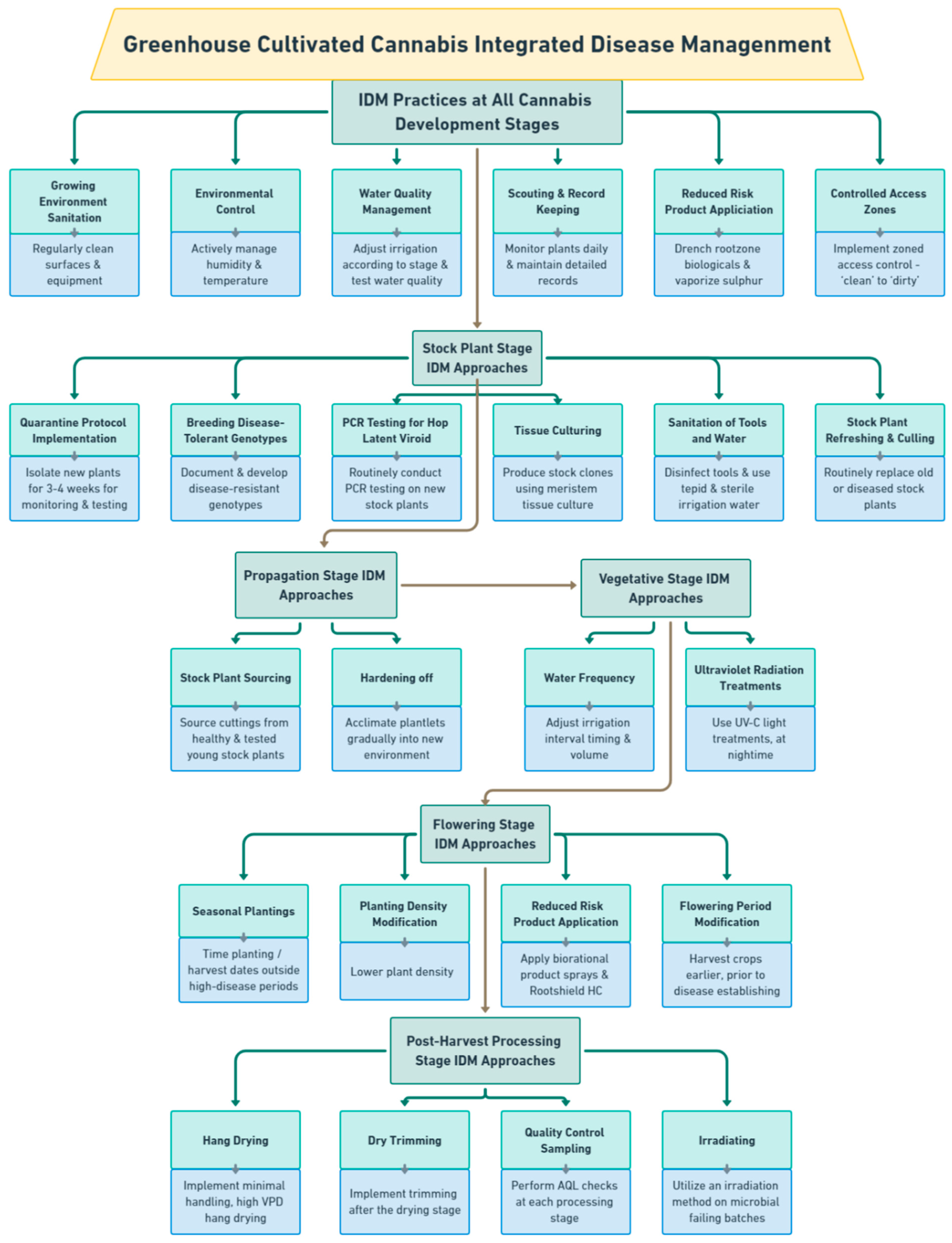
2. Cannabis Pathogens: Symptoms and Management Approaches at Different Stages of Growth
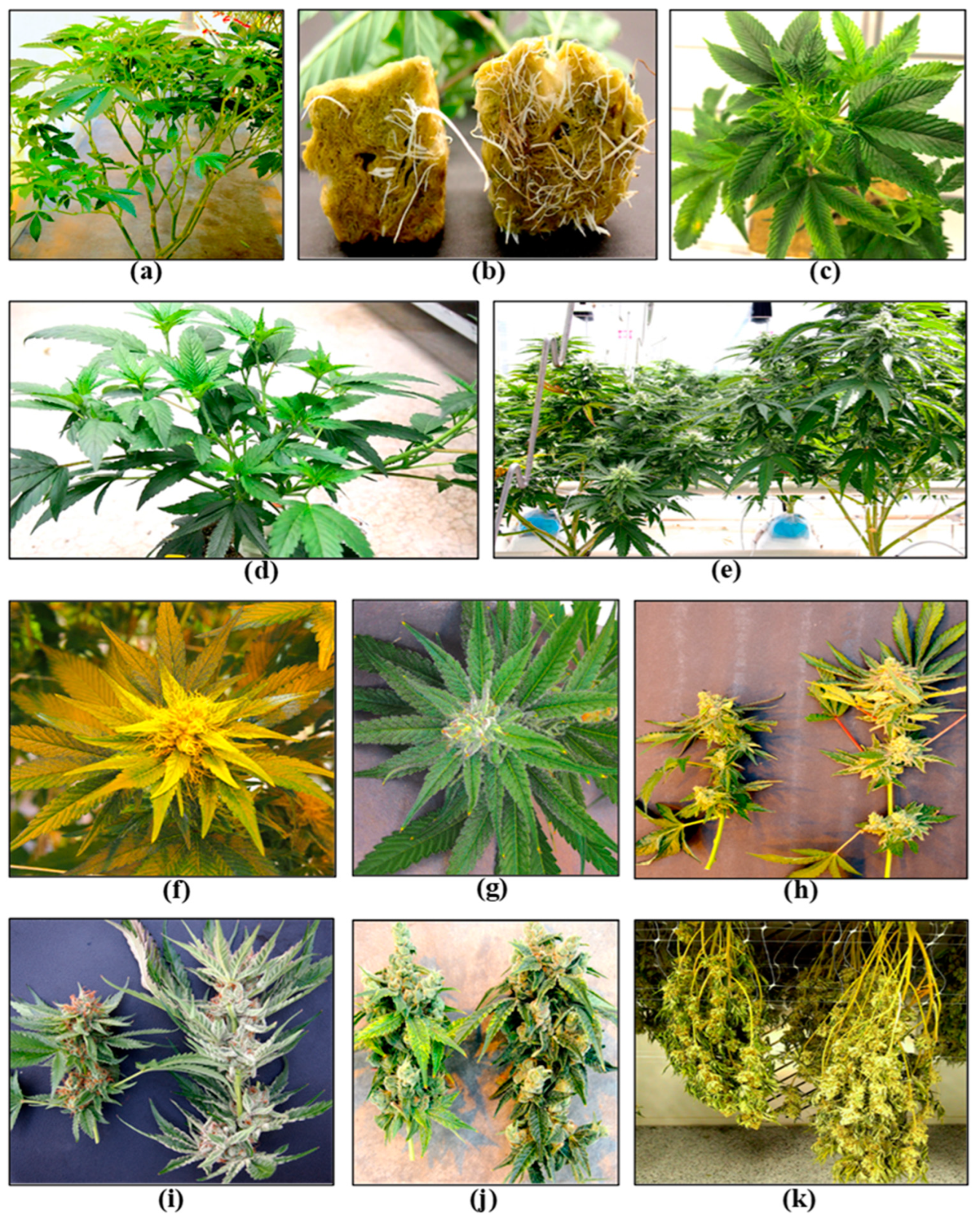
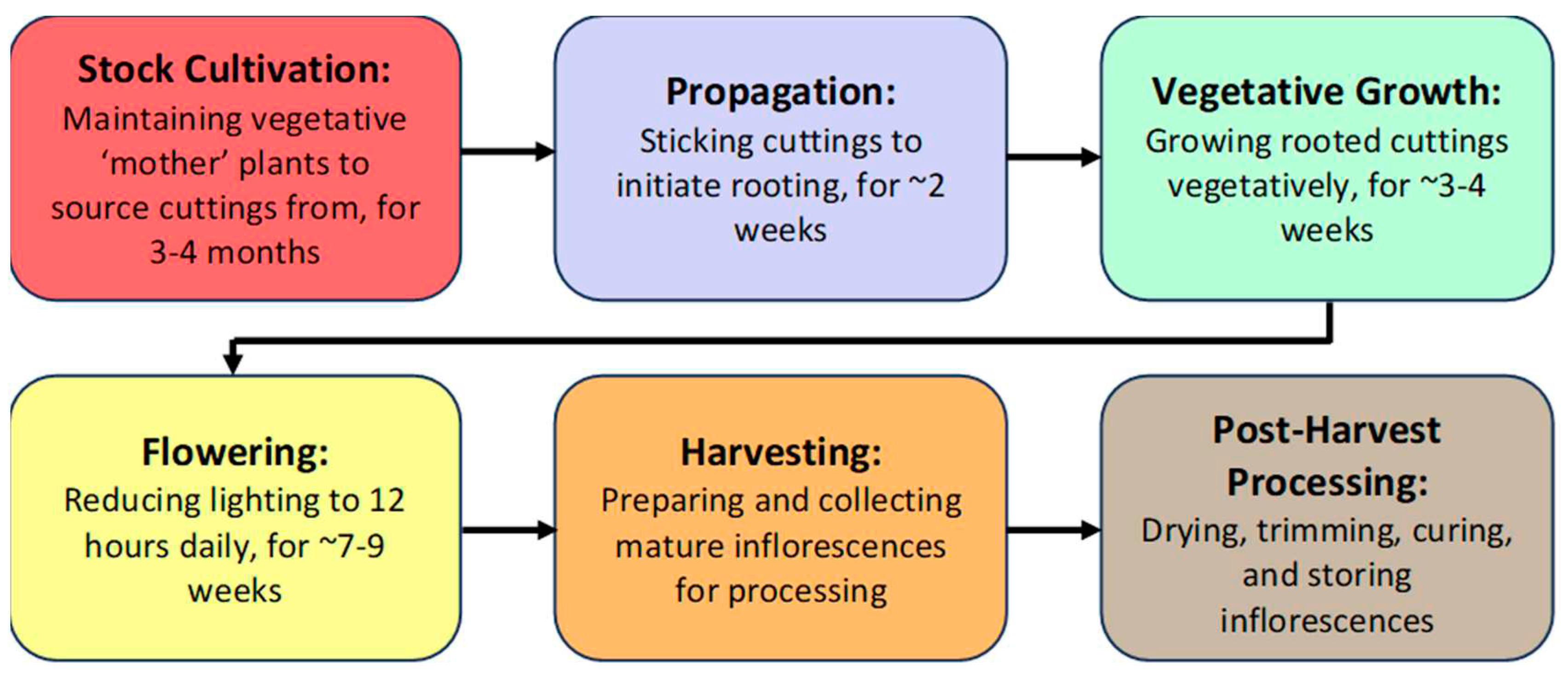
2.1. Stock Cultivation Stage
2.2. IDM Approaches at Stock Cultivation Stage
2.2.1. Biosecurity and Quarantine Inspection
2.2.2. Cultural and Environmental Management
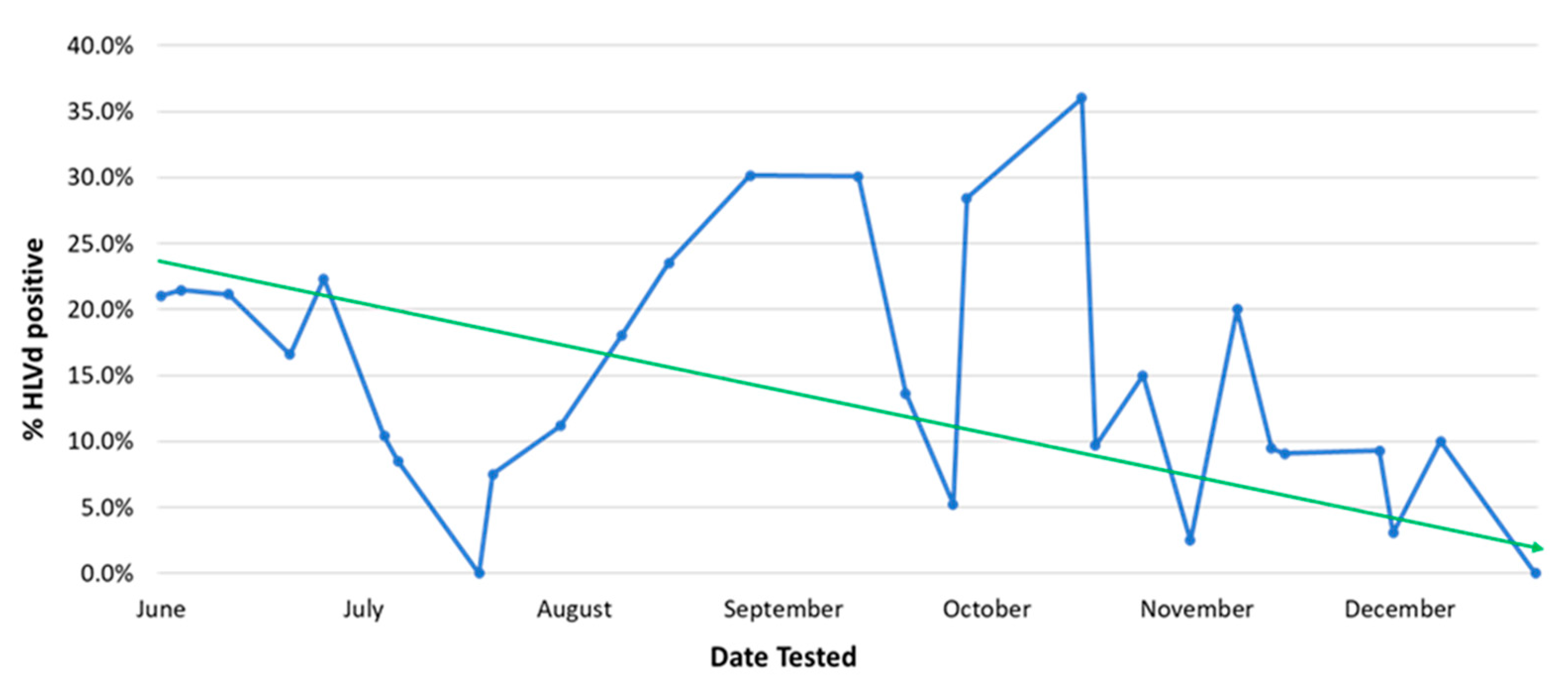
2.2.3. Testing for Pathogen Presence and Eradication
2.2.4. Sanitary Practices
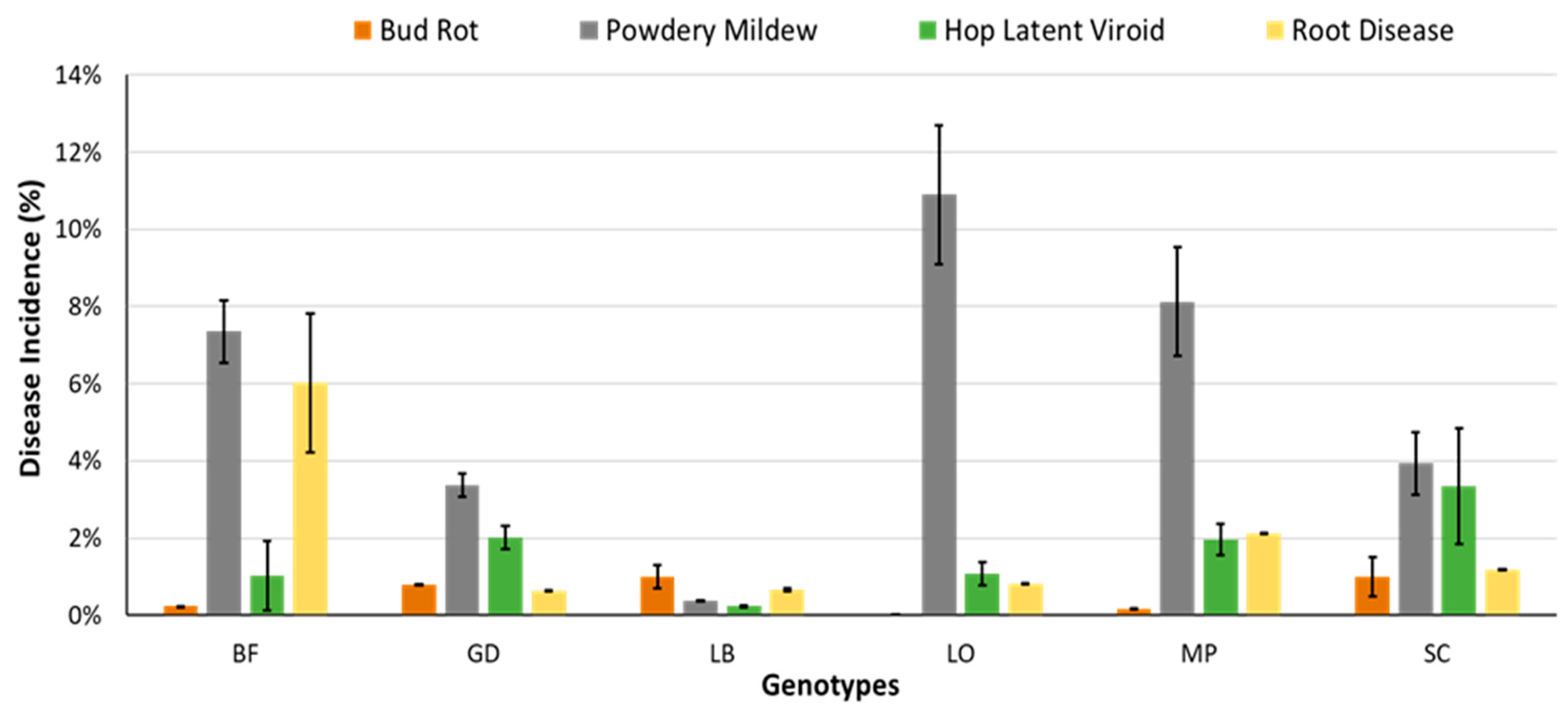
2.2.5. Utilizing Disease Tolerant Genotypes
2.3. Propagation Stage
2.4. Propagation Stage IDM Approaches
2.4.1. Cultural and Environmental Management
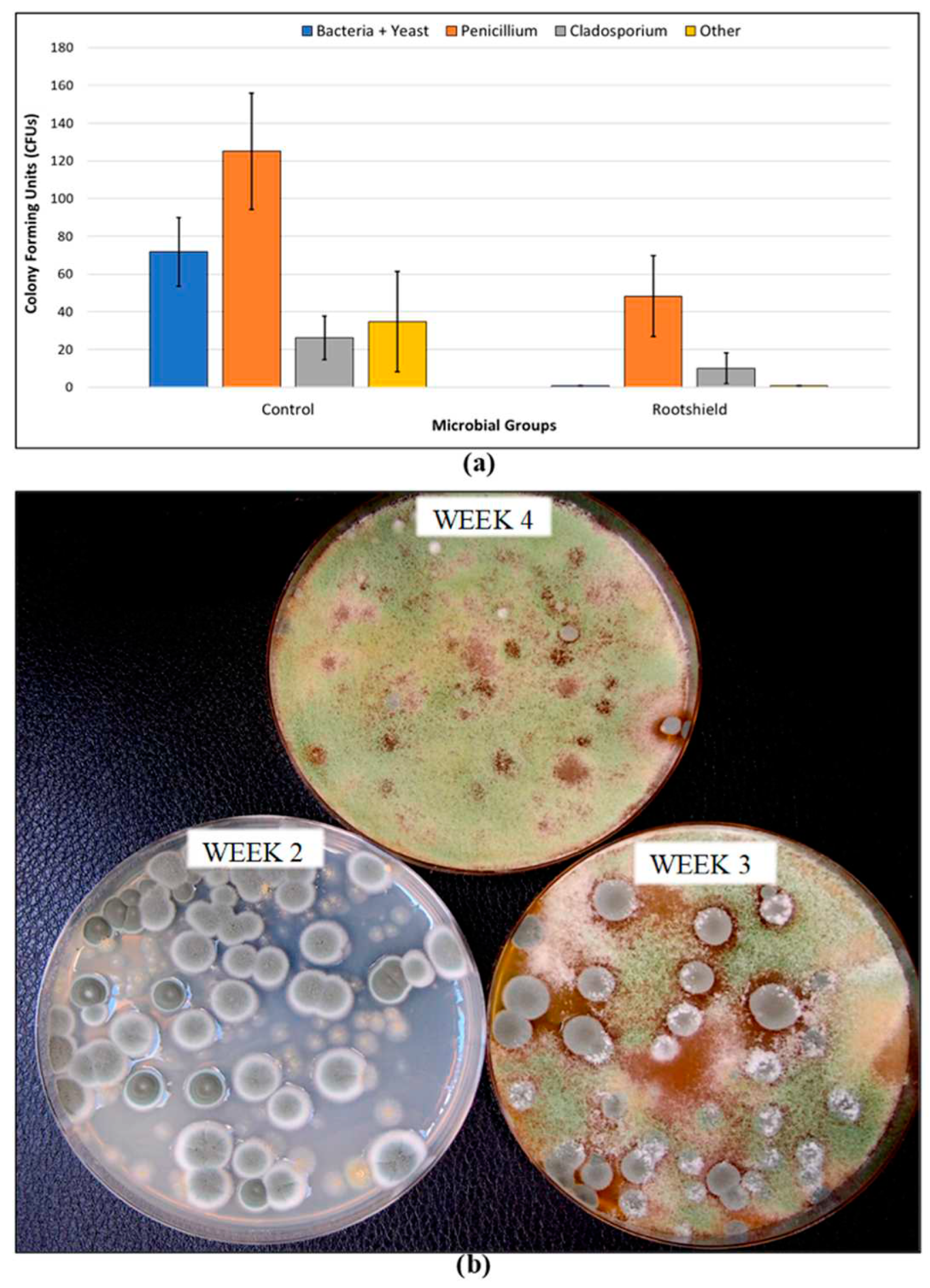
2.4.2. Application of Biological Control Agents
2.5. Vegetative Growth Stage
2.6. Vegetative Growth Stage IDM Approaches
2.6.1. Cultural and Environmental Management
2.6.2. Application of Biological Control Agents
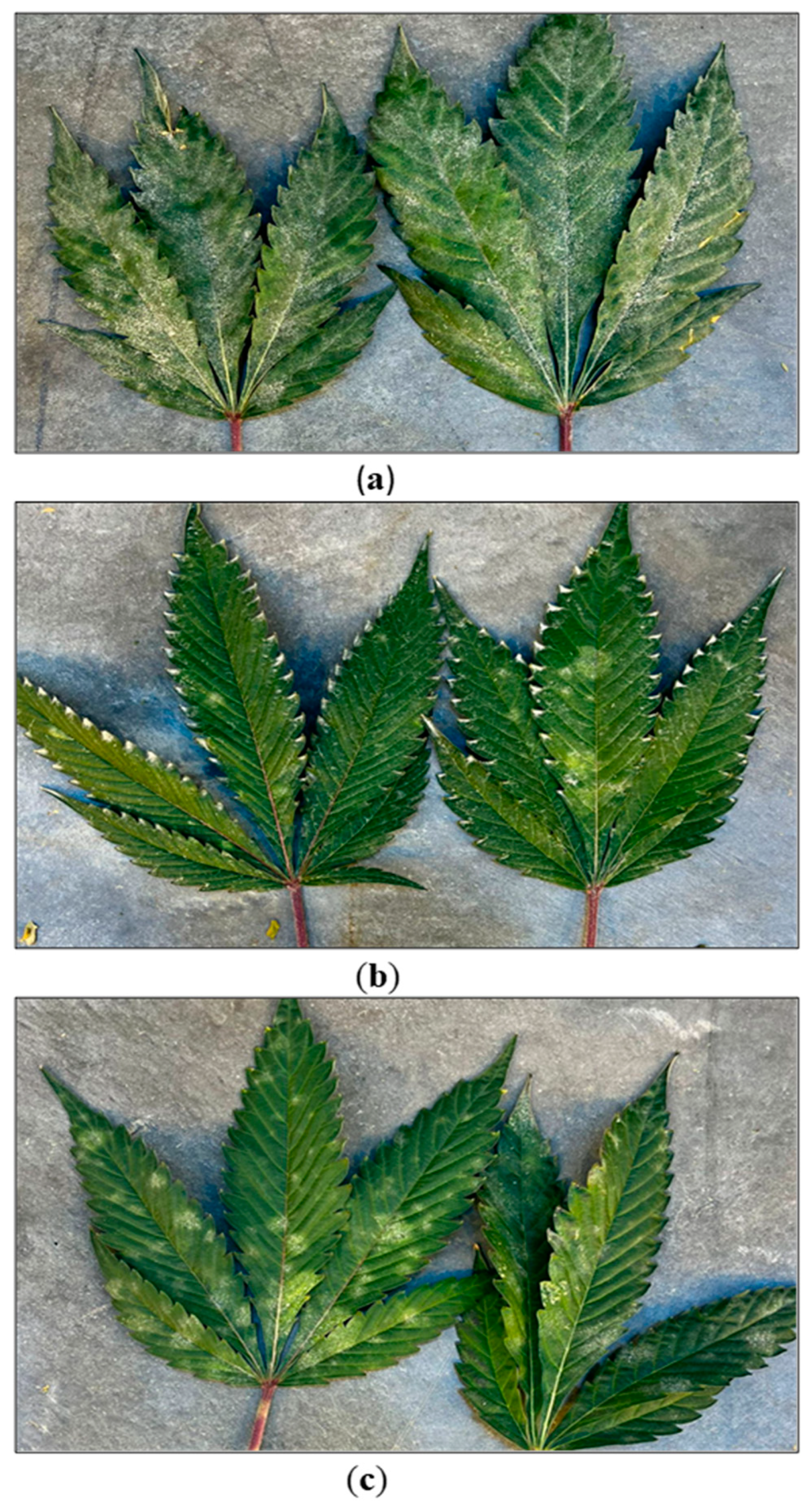
2.7. Flowering Stage
2.8. Flowering stage IDM approaches
2.8.1. Cultural and Environmental Management
2.8.2. Utility of Disease Tolerant Genotypes
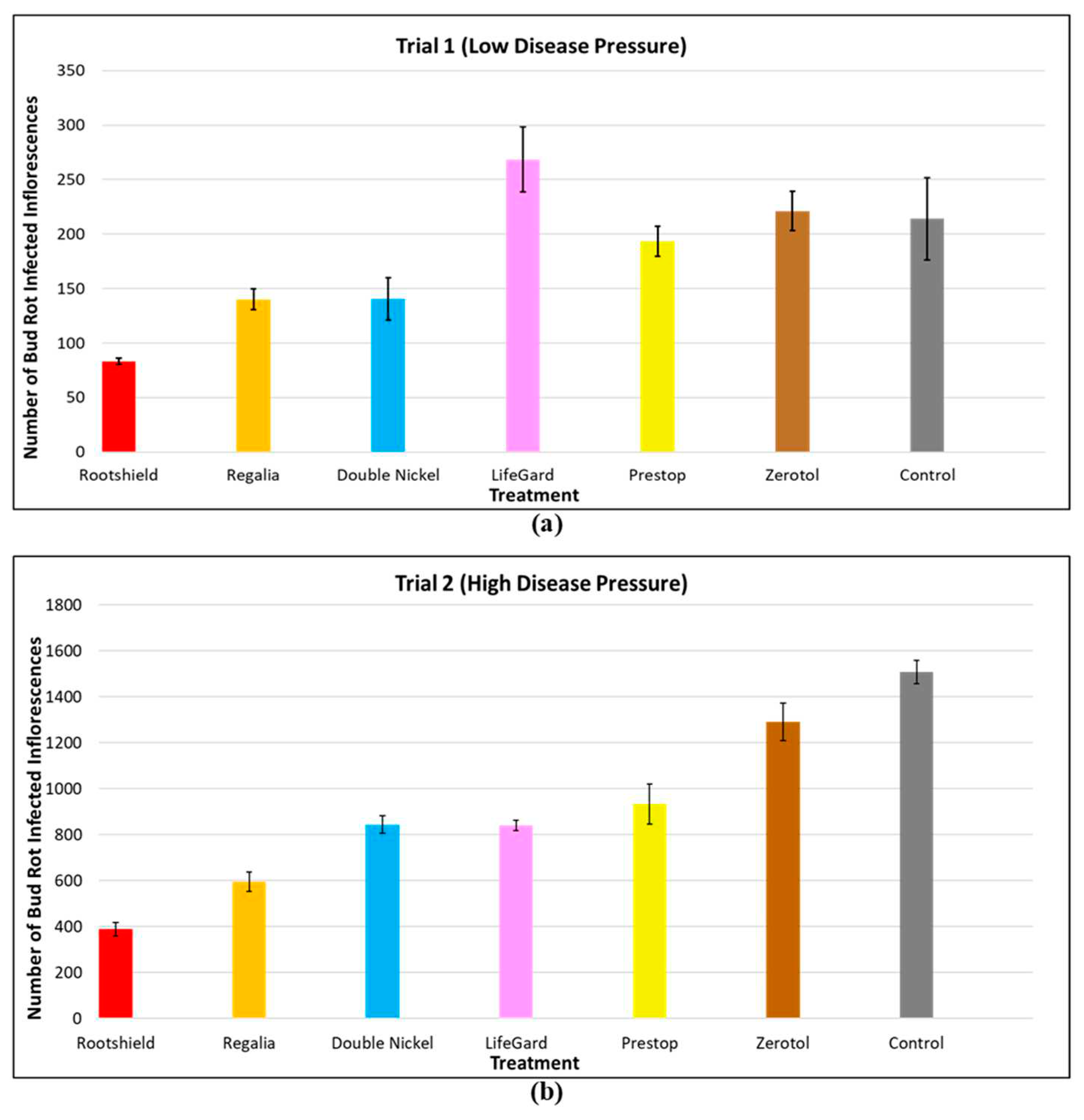
2.8.3. Application of Biological Control Agents.
2.8.4. Application of Reduced-Risk Products
2.9. Post-Harvest IDM Approaches
2.10. Future Potential Areas for IDM Development for Cannabis
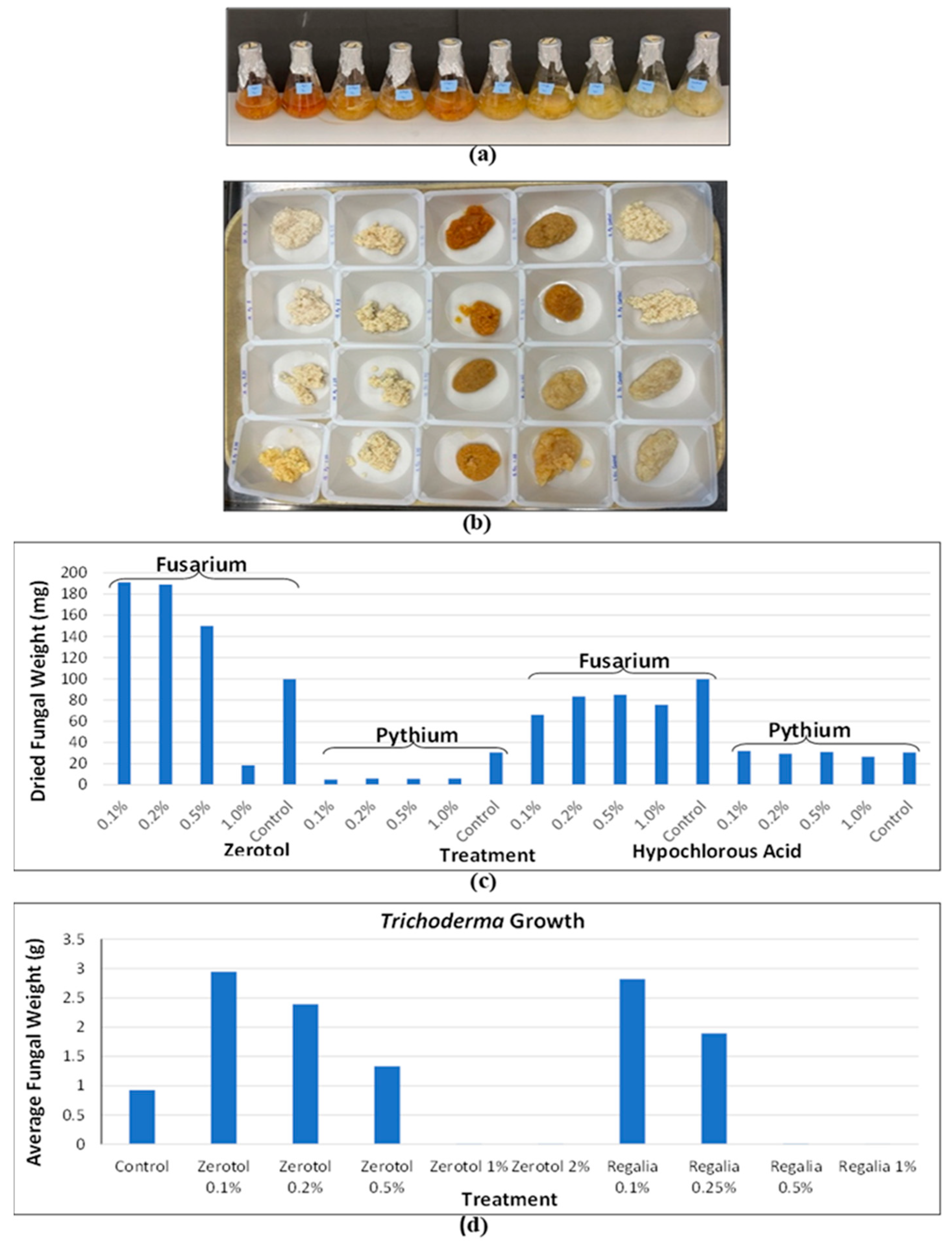
2.10.1. Evaluation of Endophytes and Microbial Antagonists in Cannabis
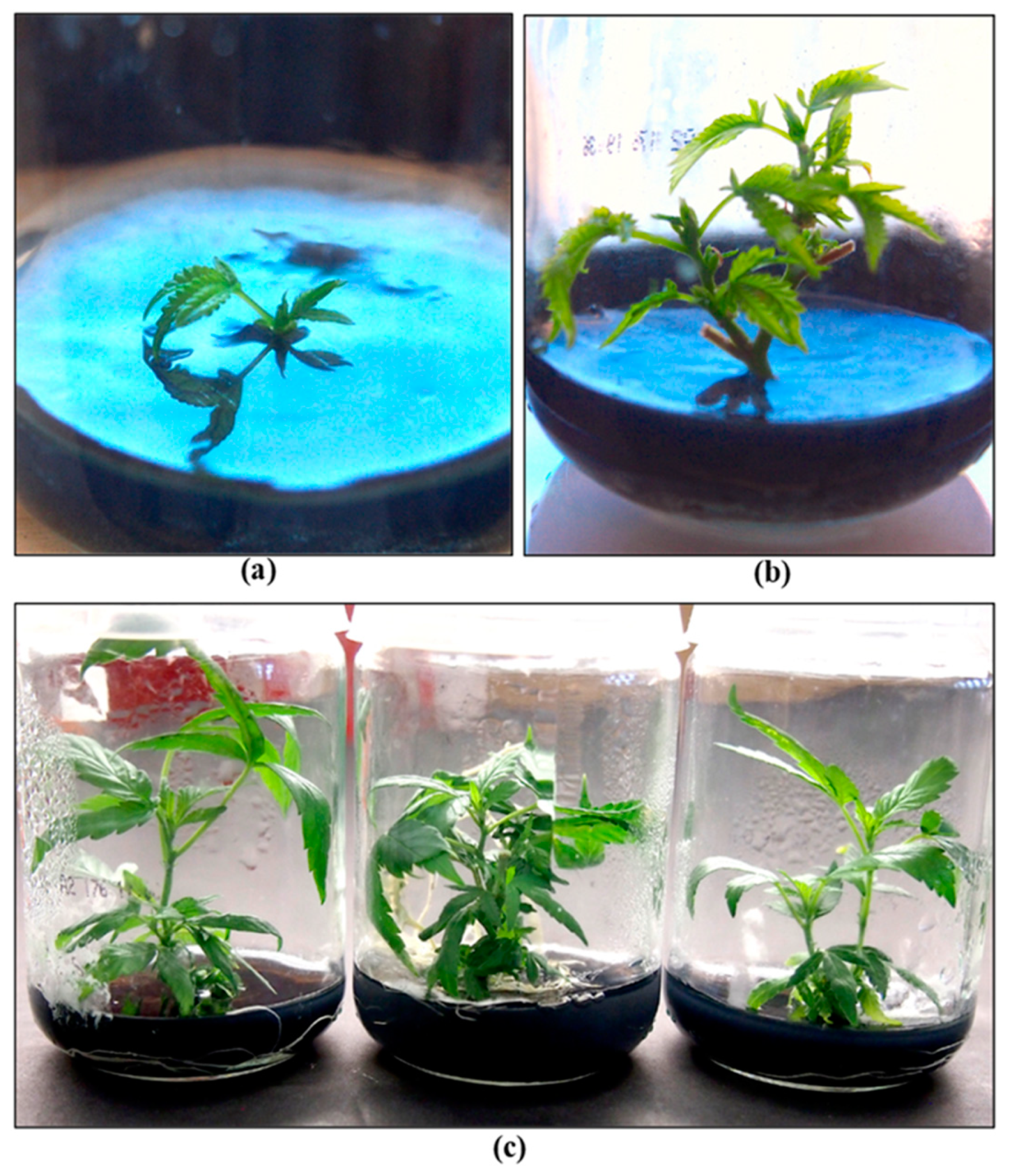
2.10.2. Tissue Culture Applications for Cannabis
2.10.3. Pathogen Control Product Registration for Cannabis
2.10.4. Nutrient Supplements for Cannabis Disease Suppression
2.10.5. Alternative Technologies for Cannabis Disease Detection
2.10.6. Induction of Plant Defence Responses in Cannabis
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Clercq: P. Integrated pest and disease management in greenhouse crops. In Developments in Plant Pathology. R. Albajes, M.L. Gullino, J.C. van Lenteren and Y. Elad, Eds.; Kluwer Academic Publishers, Dordrecht, The Netherlands, 2000.
- Razdan, V.K.; Sabitha, M. Integrated disease management: Concepts and practices. In Integrated Pest Management. R. Peshin and A.K. Dhawan, Eds.; Springer: Netherlands, 2009; pp. 369–389.
- Nicot, P.C.; Gullino, M.L.; Albajes, R. Integrated pest and disease management in greenhouse crops. Springer International Publishing, 2020.
- Wang, S. Diagnosing hemp and cannabis crop diseases. Oxfordshire, UK; Boston, MA: CABI, CAB International, 2021.
- Scott, C.; Punja, Z.K.; Sabaratnam, S. Diseases of cannabis in British Columbia. BC Ministry of Agriculture. 2021. Retrieved from https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/plant-health/diseases_of_cannabis_in_british_columbia.pdf.
- Punja, Z.K.; Scott, C. Management of diseases on cannabis in controlled environment production. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 216–252.
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag. Sci. 2021. 77, 3857–3870. [CrossRef]
- Awasthi, L.P. Biopesticides in organic farming: recent advances. CRC Press: Taylor & Francis, Boca Raton. FL, USA, 2021.
- Grof, C.P.L. Cannabis, from plant to pill. Brit. J. Clin. Pharmacol. 2018. 84, 2463–2467. [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy. 2021. 13, 546–561.
- Mihalyov, P.D.; Garfinkel, A.R. Discovery and genetic mapping of PM1, a powdery mildew resistance gene in Cannabis sativa L. Front. Agron. 2021. 3, 720215. [CrossRef]
- Stack, G.M.; Cala, A.R.; Quade, M.A.; Toth, J.A.; Monserrate, L.A.; Wilkerson, D.G.; Carlson, C.H.; Mamerto, A.; Michael, T.P.; Crawford, S.; Smart, C.; Smart, L.B. Genetic mapping, identification, and characterization of a candidate susceptibility gene for powdery mildew in Cannabis sativa L. Mol. Plant-Microbe Interact. 2024. 37(1). [CrossRef]
- Sirangelo, T.M.; Ludlow, R.A.; Spadafora, N.D. Molecular mechanisms underlying potential pathogen resistance in Cannabis sativa. Plants (Basel). 2023. 12, 2764. [CrossRef]
- Zheng, Y. Handbook of Cannabis Production in Controlled Environments. CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022.
- Fleming, H.; Chamberlain, Z.; Zager, J.J.; Lange, B.M. Controlled environments for cannabis cultivation to support "omics" research studies and production. Methods Enzym. 2023. 680, 353–380.
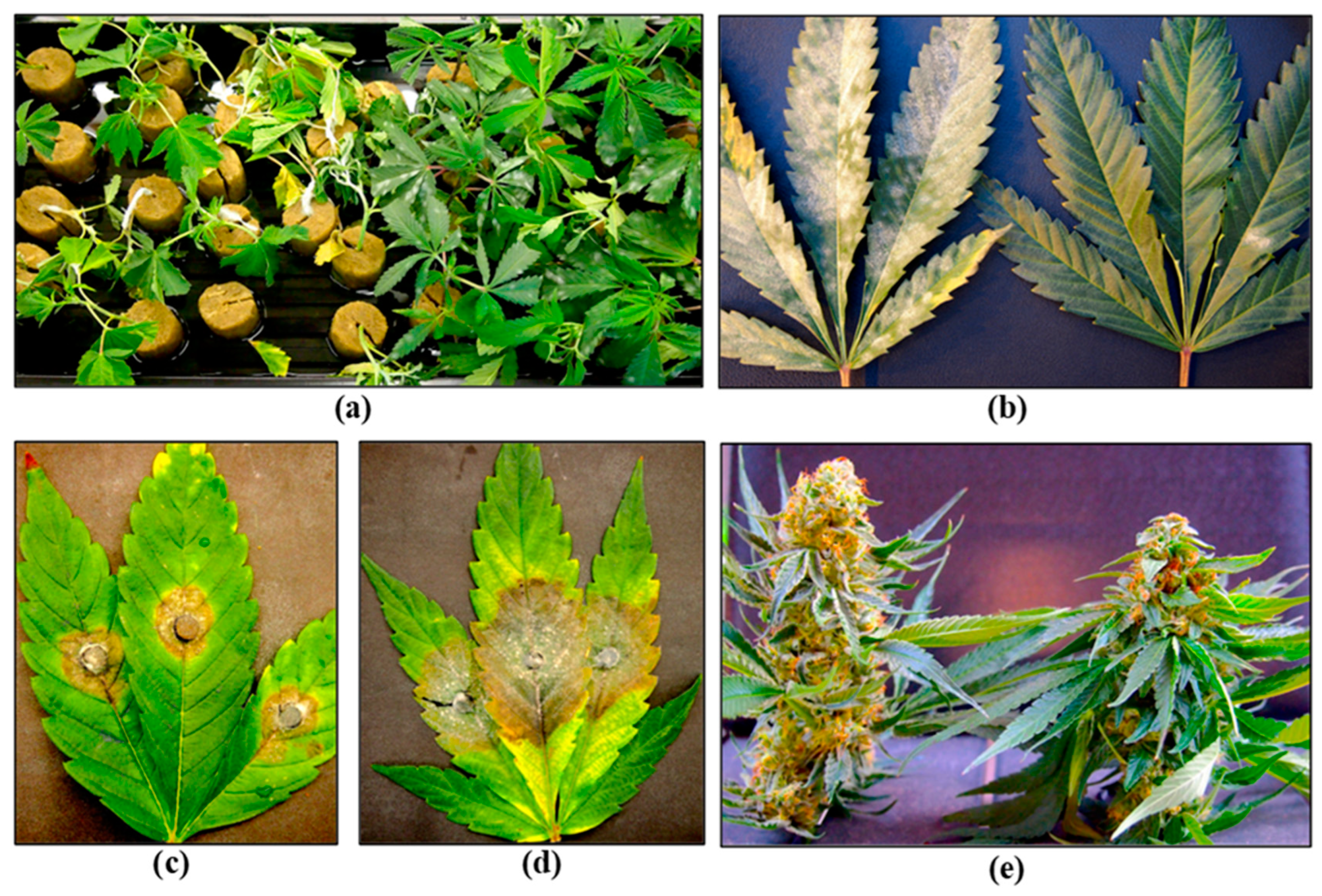
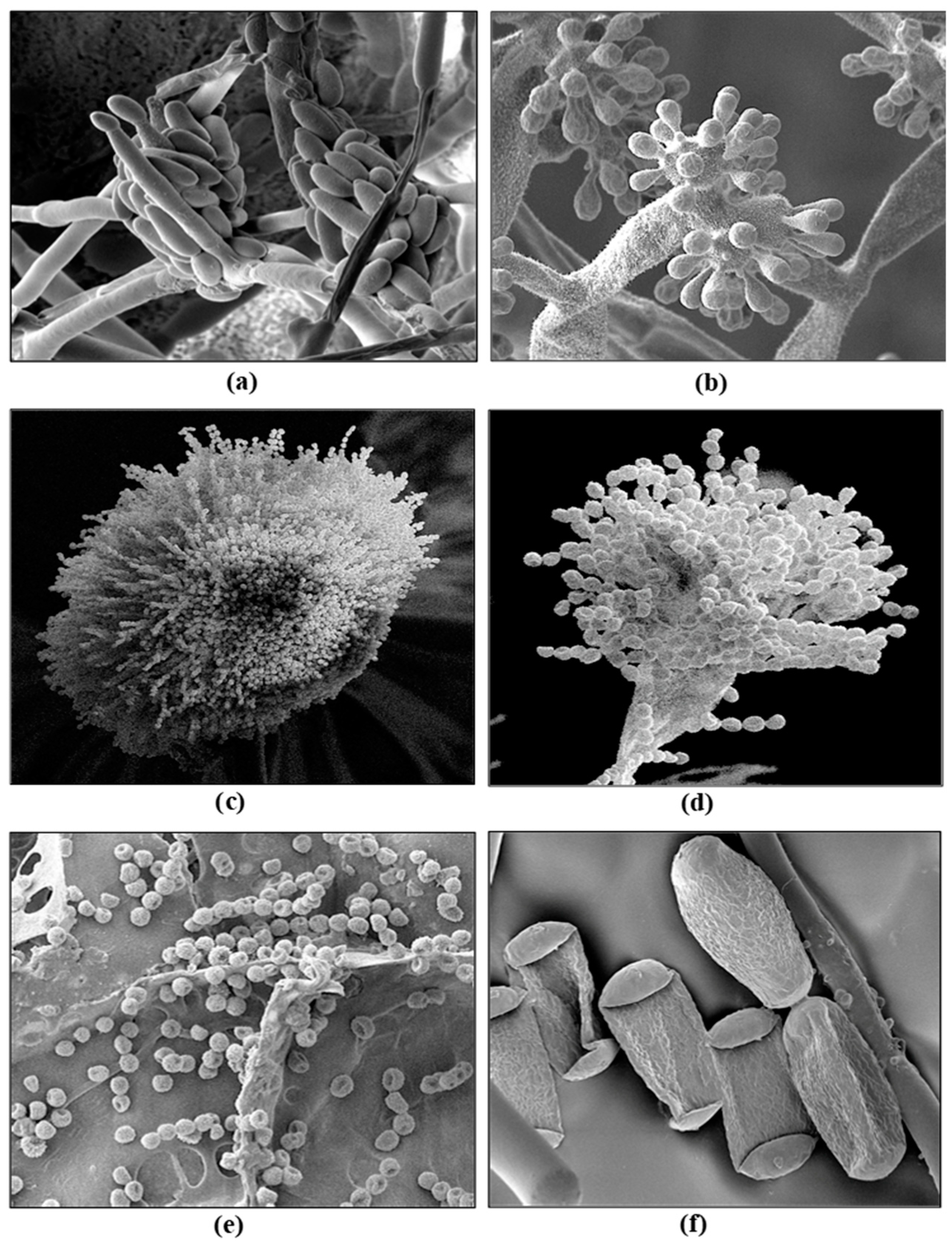
- Punja, Z.K. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018. 40, 514–527. [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019. 10, 1120. [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Fungal pathogens affecting the production and quality of medical cannabis in Israel. Plants (Basel) 2020. 9, 882. [CrossRef]
- Punja, Z.K.; Ni, L.; Lung, S.; Buirs, L. Total yeast and mold levels in high THC-containing cannabis (Cannabis sativa L.) inflorescences are influenced by genotype, environment, and pre-and post-harvest handling practices. Front. Microbiol. 2023. 14, 1192035. [CrossRef]
- Gwinn, K.D.; Leung, M.C.K.; Stephens, A.B.; Punja, Z.K. Fungal and mycotoxin contaminants in cannabis and hemp flowers: implications for consumer health and directions for further research. Front. Microbiol. 2023. 14, 1278189. [CrossRef]
- Punja, Z.K.; Rodriguez, G. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018. 40, 498–513. [CrossRef]
- Punja, Z.K.; Ni, L.; Roberts, A. The Fusarium solani species complex infecting cannabis (Cannabis sativa L., marijuana) plants and a first report of Fusarium (Cylindrocarpon) lichenicola causing root and crown rot. Can. J. Plant Pathol. 2021. 43, 567–581. [CrossRef]
- Punja, Z.K. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L.) plants in commercial greenhouse production. Can. J. Plant Pathol. 2021. 43(2), 216–235. [CrossRef]
- Punja, Z.K.; Scott, C.; Lung, S. Several Pythium species cause crown and root rot on cannabis (Cannabis sativa L.) plants grown under commercial greenhouse conditions. Can. J. Plant Pathol. 2022. 44(1), 66–81. [CrossRef]
- Adkar-Purushothama, C.R.; Sano, T.; Perreault, J.-P. Hop latent viroid: A hidden threat to the cannabis industry. Viruses 2023. 15(3), 681. [CrossRef]
- Punja, Z.K.; Wang, K.; Lung, S.; Buirs, L. Symptomology, prevalence, and impact of hop latent viroid on greenhouse-grown cannabis (Cannabis sativa L.) plants in Canada. Can. J. Plant Pathol. 2024. 19(1), 1-24. [CrossRef]
- Atallah, O.O.; Yassin, S.M.; Verchot, J. New insights into hop latent viroid detection, infectivity, host range, and transmission. Viruses. 2024. 16, 30. [CrossRef]
- Kruidhof, H.M.; Elmer, W.H. Cultural methods for greenhouse pest and disease management. In Integrated Pest and Disease Management in Greenhouse Crops. M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 285–330.
- Van Lenteren, J.C.; Nicot, P.C. Integrated pest management methods and considerations concerning implementation in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops. M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 177–193.
- Stasiak, M.; Dixon, M. Growing facilities and environmental control. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 14-40.
- Konstantinidou, P.C.; Gilardi, G.; Gard, B.; Gullino, M.L. Vegetable and herb disease management in protected culture. In Handbook of Vegetable and Herb Diseases. Springer, Cham: Edinburgh, Scotland. 2022; pp. 1-50.
- Sirangelo, T.M. Nlr- and mlo-based resistance mechanisms against powdery mildew in Cannabis sativa. Plants (Basel). 2023. 13(1), 105. [CrossRef]
- Roberts, A.J.; Punja, Z.K. Pathogenicity of seedborne Alternaria and Stemphylium species and stem-infecting Neofusicoccum and Lasiodiplodia species to cannabis. Can. J. Plant Pathol. 2022. 44(2), 250–269. [CrossRef]
- Punja, Z.K.; Ni, L. The bud rot pathogens infecting cannabis (Cannabis sativa L., marijuana) inflorescences: symptomology, species identification, pathogenicity and biological control. Can. J. Plant Pathol. 2021. 43(6), 827–854. [CrossRef]
- Mahmoud, M.; BenRejeb, I.; Punja, Z.K.; Buirs, L.; Jabaji, S. Understanding bud rot development, caused by Botrytis cinerea, on cannabis (Cannabis sativa L.) plants grown under greenhouse conditions. Botany. 2023, 101(7), 200–231. [CrossRef]
- Balthazar, C.; Cantin, G.; Novinscak, A.; Joly, D.L.; Filion, M. Expression of putative defense responses in cannabis primed by Pseudomonas and/or Bacillus strains and infected by Botrytis cinerea. Front. Plant Sci. 2020, 11, 572112. [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Cannabis seedlings inherit seed-borne bioactive and anti-fungal endophytic Bacilli. Plants (Basel). 2022, 11(16), 2127. [CrossRef]
- Jones, M.; Monthony, A.S. Cannabis propagation. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 41–90.
- Munkvold, G.P.; Gullino, M.L. Seed and propagative material. In Integrated Pest and Disease Management in Greenhouse Crops; M. Gullino, R. Albajes, P. Nicot, Eds.; Springer, Cham: Edinburgh, Scotland. 2020; pp. 331–354.
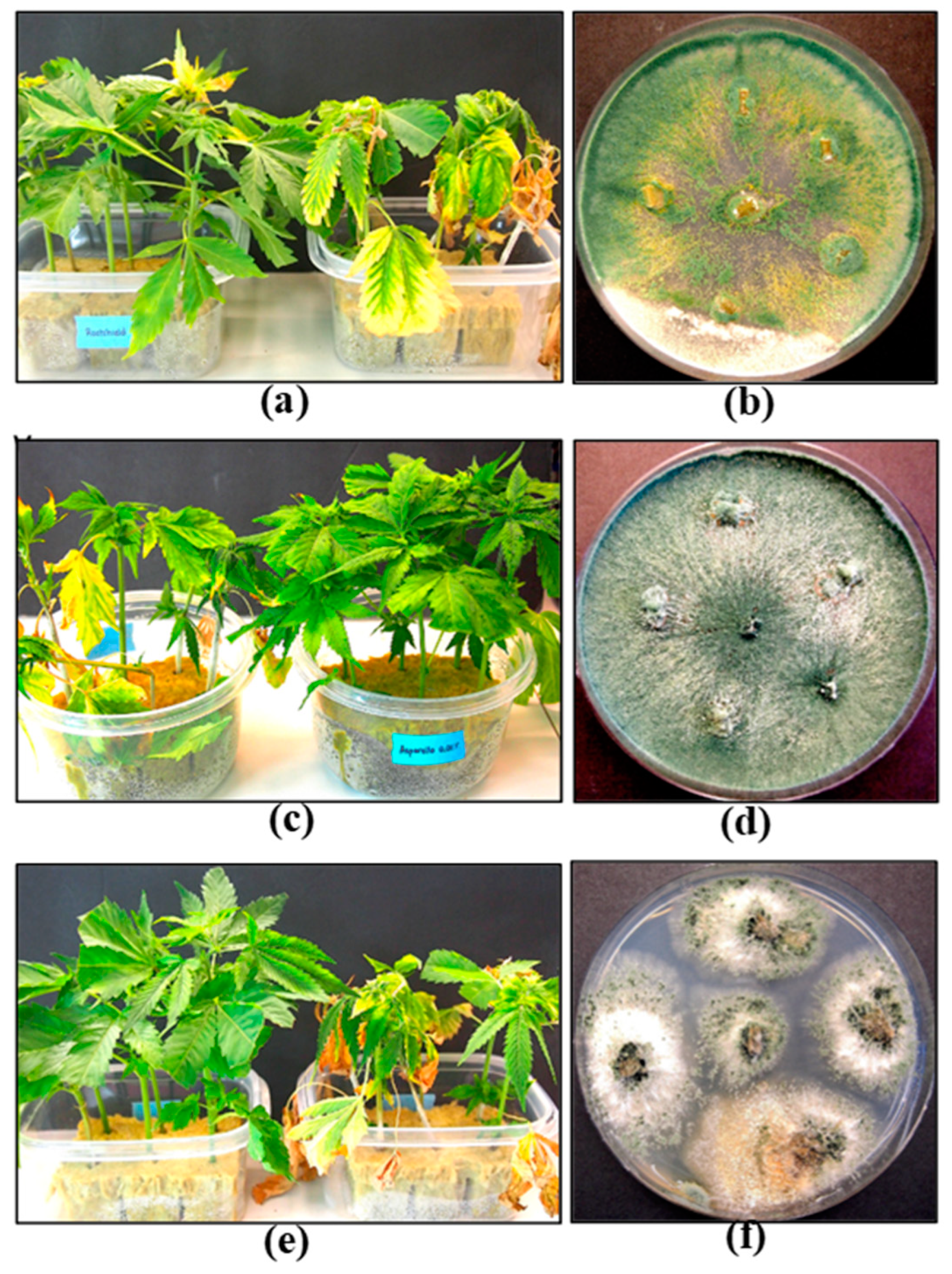
- Scott, C.; Punja, Z.K. Biological control of Fusarium oxysporum causing damping-off and Pythium myriotylum causing root and crown rot on cannabis (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2023. 45(3), 238–252. [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient management in substrate systems. In Plant Nutrition of Greenhouse Crops; C. Sonneveld and W. Voogt, Eds.; Springer: Netherlands: 2009; pp. 277–312.
- Zheng, Y. Rootzone management in cannabis production. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 163–188.
- Scott, C.; Punja, Z.K. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Can. J. Plant Pathol. 2020. 43(3), 394–412. [CrossRef]
- Janisiewicz, W.J.; Takeda, F.; Nichols, B.; Glenn, D.M.; Jurick II, W.M.; Camp, M.J. Use of low-dose UV-C irradiation to control powdery mildew caused by Podosphaera aphanis on strawberry plants. Can. J. Plant Pathol. 2016. 38(4), 430–439. [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016. 105, 1–11. [CrossRef]
- Darras, A.I.; Demopoulos, V.; Bali, I.; Tiniakou, C. Photomorphogenic reactions in geranium stimulated by brief exposures of ultraviolet-C irradiation. Plant Growth Regul. 2012. 68(3), 343–350.
- Bridgen, M.P. Using ultraviolet-c (UV-C) irradiation on greenhouse ornamental plants for growth regulation. Acta Hortic. 2016. 1134, 49-56. [CrossRef]
- Zheng, Y.; Llewellyn, D. Lighting and CO2 in cannabis production. In Handbook of Cannabis Production in Controlled Environments; Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 163-186.
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Is twelve hours really the optimum photoperiod for promoting flowering in indoor-grown cultivars of Cannabis sativa ? Plants (Basel). 2023. 12(14), 2605. [CrossRef]
- Punja, Z.K. The diverse mycoflora present on dried cannabis (Cannabis sativa L., marijuana) inflorescences in commercial production. Can. J. Plant Pathol. 2021. 43(1), 88–100. [CrossRef]
- Matzneller, P.; Gutierrez, J.D.; Caplan, D. Canopy management. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 109-125.
- Caplan, D.; Matzneller, P.; Gutierrez, J.D. Harvest and post-harvest. In Handbook of Cannabis Production in Controlled Environments. Y. Zheng, Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2022; pp. 292-310.
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010. 72(1), 1–13. [CrossRef]
- Mahmud, M.S.; Zaman, Q.U.; Esau, T.J.; Chang, Y.K.; Price, G.W.; Prithiviraj, B. Real-time detection of strawberry powdery mildew disease using a mobile machine vision system. Agronomy (Basel). 2020. 10(7), 1027. [CrossRef]
- Anagnostis, A.; Tagarakis, A.C.; Asiminari, G.; Papageorgiou, E.; Kateris, D.; Moshou, D.; Bochtis, D. A deep learning approach for anthracnose infected trees classification in walnut orchards. Comput. Electron. Agric. 2021, 182, 105998. [CrossRef]
- Fountas, S.; Malounas, I.; Athanasakos, L.; Avgoustakis, I.; Espejo-Garcia, B. AI-assisted vision for agricultural robots. AgriEngineering. 2022. 4(3), 674–694. [CrossRef]
- Shakeel, Q.; Bajwa, R.T.; Rashid, I.; Aslam, H.U.; Iftikhar, Y.; Mubeen, M.; Li, G.; & Wu, M. Concepts and applications of infrared thermography for plant disease measurement. In Trends in Plant Disease Assessment. I. Ul Haq and S. Ijaz, Eds.; Springer: Singapore, 2022; pp. 109–125.
- Elad, Y. Mechanisms involved in the biological control of Botrytis cinerea incited diseases. Eur. J. Plant Pathol. 1996. 102(8), 719–732. [CrossRef]
- Vos, C.M.F.; De Cremer, K.; Cammue, B.P.A.; De Coninck, B. Toolbox of Trichoderma spp. in the biocontrol of Botrytis cinerea disease. Mol. Plant Pathol. 2015. 16(4), 400–412. [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000. 19(8), 709–714. [CrossRef]
- Ahmed, M.F.A. Evaluation of some biocontrol agents to control Thompson seedless grapevine powdery mildew disease. Egypt. J. Biol. Pest Control 2018. 28(1), 1–7. [CrossRef]
- Esawy, A.A.; Elsharkawy, M.M.; Omara, R.I.; Khalifa, M.A.F.; Fadel, F.M.; El-Naggar, M.M. Biological control of Golovinomyces cichoracearum, the causal pathogen of sunflower powdery mildew. Egypt. J. Biol. Pest Control 2021. 31(1), 1–10. [CrossRef]
- Konstantinidou-Doltsinis, S.; Schmit, A. Impact of treatment with plant extracts from Reynoutria sachalinensis (F. Schmidt) Nakai on intensity of powdery mildew severity and yield in cucumber under high disease pressure. Crop Prot. 1998. 17(8), 649–656. [CrossRef]
- Avila-Adame, C.; Tan, E.; Campbell, B.; Huang, H.; Fernandez, L.; Koivunen, M.; Marrone, P. MOI-106: A new alternative for controlling fungal plant pathogens in ornamentals and edible crops. Phytopathology 2008. 98(6).
- Esquivel-Cervantes, L.F.; Tlapal-Bolaños, B.; Tovar-Pedraza, J.M.; Pérez-Hernández, O.; Leyva-Mir, S.G.; Camacho-Tapia, M. Efficacy of biorational products for managing diseases of tomato in greenhouse production. Plants (Basel) 2022. 11(13), 1638. [CrossRef]
- Margaritopoulou, T.; Toufexi, E.; Kizis, D.; Balayiannis, G.; Anagnostopoulos, C.; Theocharis, A.; Rempelos, L.; Troyanos, Y.; Leifert, C.; Markellou, E. Reynoutria sachalinensis extract elicits SA-dependent defense responses in courgette genotypes against powdery mildew caused by Podosphaera xanthii. Sci. Rep. 2020. 10(1), 3354. [CrossRef]
- Abdu-Allah, G.A.M.; Abo-Elyousr, K.A.M. Effect of certain plant extracts and fungicides against powdery mildew disease of grapevines in upper Egypt. Arch. Phytopathol. Plant Prot. 2017. 50(19-20), 957–969.
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. 2nd ed. 2018; Springer, Cham: Edinburgh, Scotland.
- Hazekamp, A. Evaluating the effects of gamma-irradiation for decontamination of medicinal cannabis. Front. Pharmacol. 2016. 7(108). [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Effects of cold plasma, gamma and e-beam irradiations on reduction of fungal colony forming unit levels in medical cannabis inflorescences. J. Cannabis Res. 2020. 2(1), 12. [CrossRef]
- Majumdar, C.G.; ElSohly, M.A.; Ibrahim, E.A.; Elhendawy, M.A.; Stanford, D.; Chandra, S.; Wanas, A.S.; Radwan, M.M. Effect of gamma irradiation on cannabinoid, terpene, and moisture content of cannabis biomass. Molecules (Basel) 2023. 28(23), 7710. [CrossRef]
- Dhillon, G.S.; Hukkeri, S.; Nightingale, D.; Callaway, J. Evaluation of different techniques to decontaminate medical cannabis and their effect on cannabinoid content. ACS Agric. Sci. Technol. 2022. 2(6), 1126–1133. [CrossRef]
- Frink, S.; Marjanovic, O.; Tran, P.; Wang, Y.; Guo, W.; Encarnacion, N.; Alcantara, D.; Moezzi, B.; Vrdoljak, G. Use of X-ray irradiation for inactivation of Aspergillus in cannabis flower. PloS One 2022, 17(11), 277649-277649. [CrossRef]
- Gautam, A.K.; Kant, M.; Thakur, Y. Isolation of endophytic fungi from Cannabis sativa and study their antifungal potential. Arch. Phytopathol. 2013. 46(6), 627–635. [CrossRef]
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013. 60(1), 137–151. [CrossRef]
- Taghinasab, M.; Jabaji, S. Cannabis microbiome and the role of endophytes in modulating the production of secondary metabolites: An overview. Microorganisms (Basel). 2020. 8(3), 355. [CrossRef]
- Balthazar, C.; Novinscak, A.; Cantin, G.; Joly, D.L.; Filion, M. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other cannabis fungal pathogens. Phytopathology 2022. 112(3), 549–560. [CrossRef]
- Lyu, D.; Backer, R.; Robinson, W.G.; Smith, D.L. Plant growth-promoting rhizobacteria for cannabis production: Yield, cannabinoid profile and disease resistance. Front Microbiol. 2019. 10(1761). [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions between Bacillus spp., Pseudomonas spp. and Cannabis sativa promote plant growth. Front. Microbiol. 2021. 12, 715758. [CrossRef]
- Scott, M.; Rani, M.; Samsatly, J.; Charron, J.-B.; Jabaji, S. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: Identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can. J. Microbiol. 2018. 64(10), 664–680. [CrossRef]
- Gabriele, M.; Vitali, F.; Chelucci, E.; Chiellini, C. Characterization of the cultivable endophytic bacterial community of seeds and sprouts of Cannabis sativa L. and perspectives for the application as biostimulants. Microorganisms (Basel). 2022. 10(9), 1742. [CrossRef]
- Kusari, P.; Kusari, S.; Lamshöft, M.; Sezgin, S.; Spiteller, M.; Kayser, O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl. Microbiol. Biotechnol. 2014. 98(16), 7173–7183. [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases – Review and future prospects. Biol. Control. 2016. 103, 62–68. [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001. 52, 487–511. [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27(12), 3622–3633. [CrossRef]
- Holmes, J.E.; Lung, S.; Collyer, D.; Punja, Z.K. Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front. Plant Sci. 2021. 12, 732344. [CrossRef]
- Punja, Z.K.; Scott, C. Organically grown cannabis (Cannabis sativa L.) plants contain a diverse range of culturable epiphytic and endophytic fungi in inflorescences and stem tissues. Botany. 2023. 101(7), 255–269. [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The entomopathogenic fungi Metarhizium brunneum and Beauveria bassiana promote systemic immunity and confer resistance to a broad range of pests and pathogens in tomato. Phytopathology. 2022. 112(4), 784–793. [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: modifiers of plant disease. Plant Mol. Biol. 2016. 90, 645–655. [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K. Use of endophytes as biocontrol agents. Fung. Biol. Reviews. 2019. 33(2), 133-148. [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; Bhowmik, P. Medical cannabis and industrial hemp tissue culture: present status and future potential. Front. Plant Sci. 2021. 12, 627240–627240. [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants. 2021. 10(1), 185. [CrossRef]
- Slack, S.A.; Tufford, L.A. Meristem culture for virus elimination. In Plant Cell, Tissue and Organ Culture. O.L. Gamborg and G.C. Phillips, Eds.; Springer: Berlin, Germany, 1995; pp. 117-128.
- Rao, C. K. Adoption of tissue culture in horticulture : A study of banana-growing farmers from a South-Indian state. Cambridge Scholars Publishing. 2014.
- Sharma, N.; Sharma, L.; Singh, B.P. Propagation. In Strawberries. R.M. Sharma, R. Yamdagni, A.K. Dubey, and V. Pandey, Eds.; CRC Press: United Kingdom, 2019; pp. 179–192.
- Nehra, N.S.; Kartha, K.K. Meristem and shoot tip culture: requirements and applications. In Plant Cell and Tissue Culture; I.K. Vasil and T.A. Thorpe, Eds.; Springer: Dordrecht, Netherlands, 1994; pp. 37-70.
- De Jesús Romo-Paz, F.; Folgado, R.; Delgado-Aceves, L.; Zamora-Natera, J.F.; Portillo, L. Tissue culture of Physalis angulata L. (Solanaceae): techniques for micropropagation and germplasm long-term preservation. Plant Cell Tissue Organ Cult. 2021. 144(1), 73–78. [CrossRef]
- Zapata, C. J.; Miller, C. J.; Smith, R. H. An in vitro procedure to eradicate potato viruses X, Y, and S from Russet Norkotah and two of its strains. In Vitro Cell. Dev. Biol. Plant 1995. 31(3), pp. 153–159. [CrossRef]
- Bhojwani, S. S.; Dantu, P. K. Production of virus-free plants. In Plant Tissue Culture: An Introductory Text. Springer India, 2013; pp. 227–243.
- Wang, Q.; Cuellar, W. J.; Rajamäki, M.-L.; Hirata, Y.; Valkonen, J. P. Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol. Plant Pathol. 2008. 9(2), 237–250. [CrossRef]
- Singh, V.; Adil, S.; Quraishi, A. Elimination of BBTV via a systemic in vitro electrotherapy approach. J. Virol. Methods 2022. 300, 114367–114367. [CrossRef]
- Kanwar, J.; Kaul, M. K.; Naruka, I. S.; Singh, P. P. In-vitro micrografting technique in sweet orange (Citrus sinensis) cv. Blood Red to produce virus free plants. Indian J. Agric. Sci. 2019. 89(3), 494-499. [CrossRef]
- Akinrinlola, R. J.; Hansen, Z. R. Efficacy of organic fungicides against hemp powdery mildew caused by Golovinomyces ambrosiae in a greenhouse in Tennessee. Plant Dis. 2023. 107(6), 1867–1873. [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K. P.; Keswani, C.; Minkina, T.; Srivastava, A. K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022. 13, 883970–883970. [CrossRef]
- Datnoff, E. L.; Elmer, W. H.; Rodrigues, F. A. Mineral Nutrition and Plant Disease. Second Edition. Amer. Phytopathol. Soc. St. Paul, Minnesota, 2023.
- Lamichhane, J. R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J. B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018. 38(3), 1–18. [CrossRef]
- Flemming, C. A.; Trevors, J. T. Copper toxicity and chemistry in the environment: A review. Water Air Soil Pollut. 1989. 44(1), 143–158. [CrossRef]
- Mayton, H.; Amirkhani, M.; Loos, M.; Johnson, B.; Fike, J.; Johnson, C.; Myers, K.; Starr, J.; Bergstrom, G. C.; Taylor, A. Evaluation of industrial hemp seed treatments for management of damping-off for enhanced stand establishment. Agric. (Basel). 2022. 12(5), 591. [CrossRef]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Plaza Pérez, C. D.; De La Torre-Roche, R.; Zuverza-Mena, N.; Haynes, C. L.; White, J. C.; Hamers, R. J. Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): Role of particle morphology, composition and dissolution behavior. ACS Sustain. Chem. Eng. 2018. 6(11), 14847–14856. [CrossRef]
- Shen, Y.; Borgatta, J.; Ma, C.; Elmer, W.; Hamers, R. J.; White, J. C. Copper nanomaterial morphology and composition control foliar transfer through the cuticle and mediate resistance to root fungal disease in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2020. 68(41), 11327–11338. [CrossRef]
- Freires, A.L.A.; Figueiredo, F.R.A.; Alves, T.R.C.; Barroso, K.A.; da Silva, I.V.P.; Silva, J. L.S.; de Almeida Nogueira, G.; Melo, N.J.A.; Júnior, R.S.; Negreiros, A.M.P.; de Queiroz Ambrósio, M.M. Alternative products in the management of powdery mildew (Podosphaera xanthii) in melon. Trop. Plant Pathol. 2022. 47(5), 608–617. [CrossRef]
- Newman, S.; Roll, M.; Harkrader, R. Naturally occurring compounds for controlling powdery mildew of greenhouse roses. HortScience. 1999. 34(4), 686–689. [CrossRef]
- Mmbaga, M. T.; Sauve, R. J. Management of powdery mildew in flowering dogwood in the field with biorational and conventional fungicides. Can. J. Plant Sci. 2004, 84(3), 837–844. [CrossRef]
- Aleksic, G.; Milicevic, Z.; Kuzmanovic, S.; Starovic, M.; Stevanovic, M.; Delibasic, G.; Zivkovic, S. Efficacy of copper citrate in grapevine disease control. Pestic. Fitomed. 2019. 34(2), 103–109. [CrossRef]
- Sakr, N. Silicon control of bacterial and viral diseases in plants. J. Plant Prot. Res. 2016. 56(4), 331–336. [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H. Y. H. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020. 168, 104641. [CrossRef]
- Dixon, E.; Leonberger, K.; Amsden, B.; Szarka, D.; Munir, M.; Payee, W.; Datnoff, L.; Tubana, B.; Gauthier, N. Suppression of hemp powdery mildew using root-applied silicon. Plant Health Prog. 2022. 23(3), 260–264. [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Ehret, D.L.; Menzies, J.G. Distribution of silicon in cucumber leaves during infection by the powdery mildew fungus (Sphaerotheca fuliginea). Can. J. Bot. 1991. 69(1), 140–146. [CrossRef]
- Liang, Y.; Sun, W.; Si, J.; Romheld, V. Effects of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005. 54(5), 678–685. [CrossRef]
- Shetty, R.; Jensen, B.; Shelton, D.; Jørgensen, K.; Pedas, P.; Jørgensen, H.J.L. Site-specific, silicon-induced structural and molecular defence responses against powdery mildew infection in roses. Pest Manag. Sci. 2021. 77(10). [CrossRef]
- Shetty, R.; Jensen, B.; Shetty, N.P.; Hansen, M.; Hansen, C.W.; Starkey, K.R.; Jørgensen, H.J.L. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 2012. 61(1), 120-131. [CrossRef]
- Kanto, T.; Miyoshi, A.; Ogawa, T.; Maekawa, K.; Aino, M. Suppressive effect of potassium silicate on powdery mildew of strawberry in hydroponics. J. Gen. Plant Pathol. 2004. 70(4), 207. [CrossRef]
- Liu, B.; Davies, K.; Hall, A. Silicon builds resilience in strawberry plants against both strawberry powdery mildew Podosphaera aphanis and two-spotted spider mites Tetranychus urticae. PloS One. 2020. 15(12). [CrossRef]
- Reekie, H.; Punja, Z.K. Calcium and plant disease. In Mineral Nutrition and Plant Disease – Second Edition. Datnoff, E.L., Elmer, W.H., Rodrigues, F.A., Eds.; Amer. Phytopathol. Soc., St. Paul, Minnesota, 2023.
- Elad, Y.; Volpin, H. Reduced development of grey mould (Botrytis cinerea) in bean and tomato plants by calcium nutrition. J. Phytopathol. 1993, 139(2), 146–156. [CrossRef]
- Volpin, H.; Elad, Y. Influence of calcium nutrition on susceptibility of rose flowers to Botrytis blight. Phytopathology 1991. 81(11), 1390. [CrossRef]
- Yermiyahu, U.; Shamai, I.; Peleg, R.; Dudai, N.; Shtienberg, D. Reduction of Botrytis cinerea sporulation in sweet basil by altering the concentrations of nitrogen and calcium in the irrigation solution. Plant Pathol. 2006. 55(4), 544-552. [CrossRef]
- Traversari, S.; Cacini, S.; Galieni, A.; Nesi, B.; Nicastro, N.; Pane, C. Precision agriculture digital technologies for sustainable fungal disease management of ornamental plants. Sustainability. 2021, 13(7), 3707. [CrossRef]
- Berdugo, C.A.; Zito, R.; Paulus, S.; Mahlein, A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2014. 63(6), 1344-1356. [CrossRef]
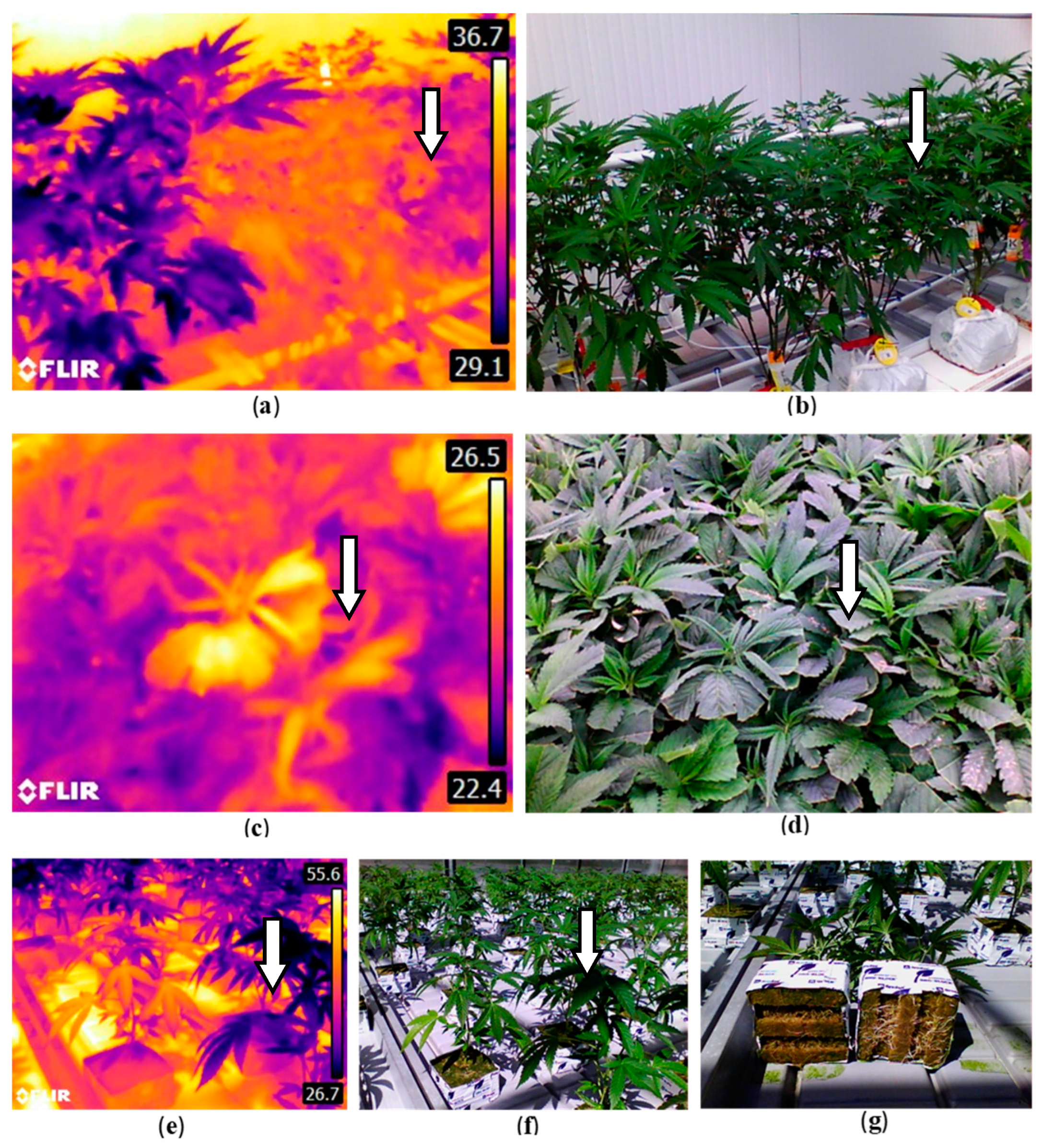
- Xu, H.; Zhu, S.; Ying, Y.; Jiang, H. Early detection of plant disease using infrared thermal imaging. SPIE Conference Proceedings 2006. 6381(1), 638110-638117.
- Vagelas, I.; Papadimos, A.; Lykas, C. Pre-symptomatic disease detection in the vine, chrysanthemum, and rose leaves with a low-cost infrared sensor. Agronomy. 2021. 11(9), 1682. [CrossRef]
- Lindenthal, M.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology. 2005. 95(3), 233-240. [CrossRef]
- Liaghat, S.; Mansor, S.; Ehsani, R.; Shafri, H.Z.M.; Meon, S.; Sankaran, S. Mid-infrared spectroscopy for early detection of basal stem rot disease in oil palm. Comput. Electron. Agric. 2014. 101, 48-54. [CrossRef]
- Morelli, M.; Bahar, O.; Papadopoulou, K.K.; Hopkins, D.L.; Obradović, A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020. 11(1312). [CrossRef]
- Sharma, A.K.; Sharma, P. Eds. Trichoderma – Host-pathogen interactions and applications. Springer Nature, Singapore Pte. Limited. 79 pp.
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: our best fungal allies in the biocontrol of plant diseases –A review. Plants (Basel). 2023 12(3):432. [CrossRef]





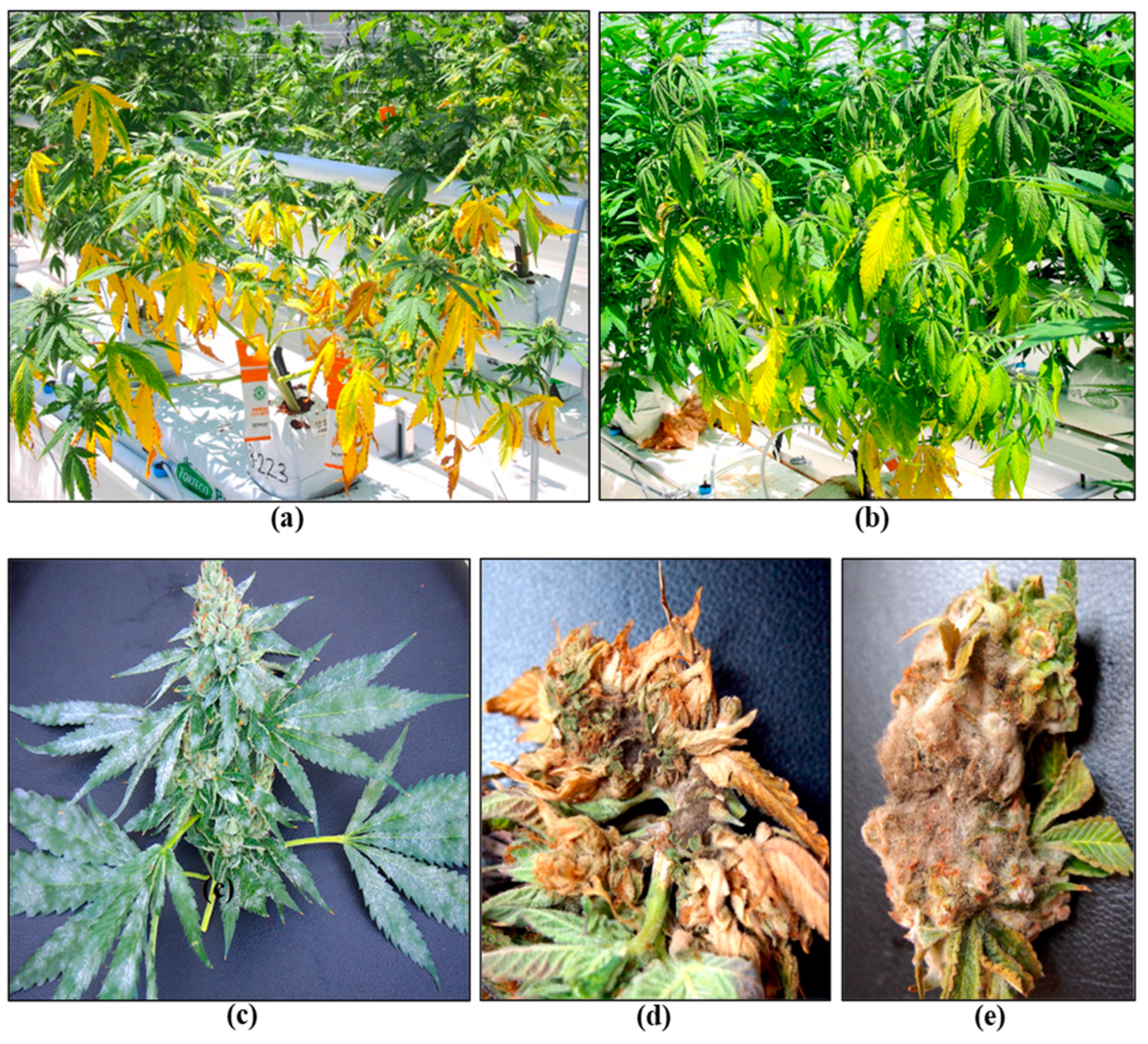
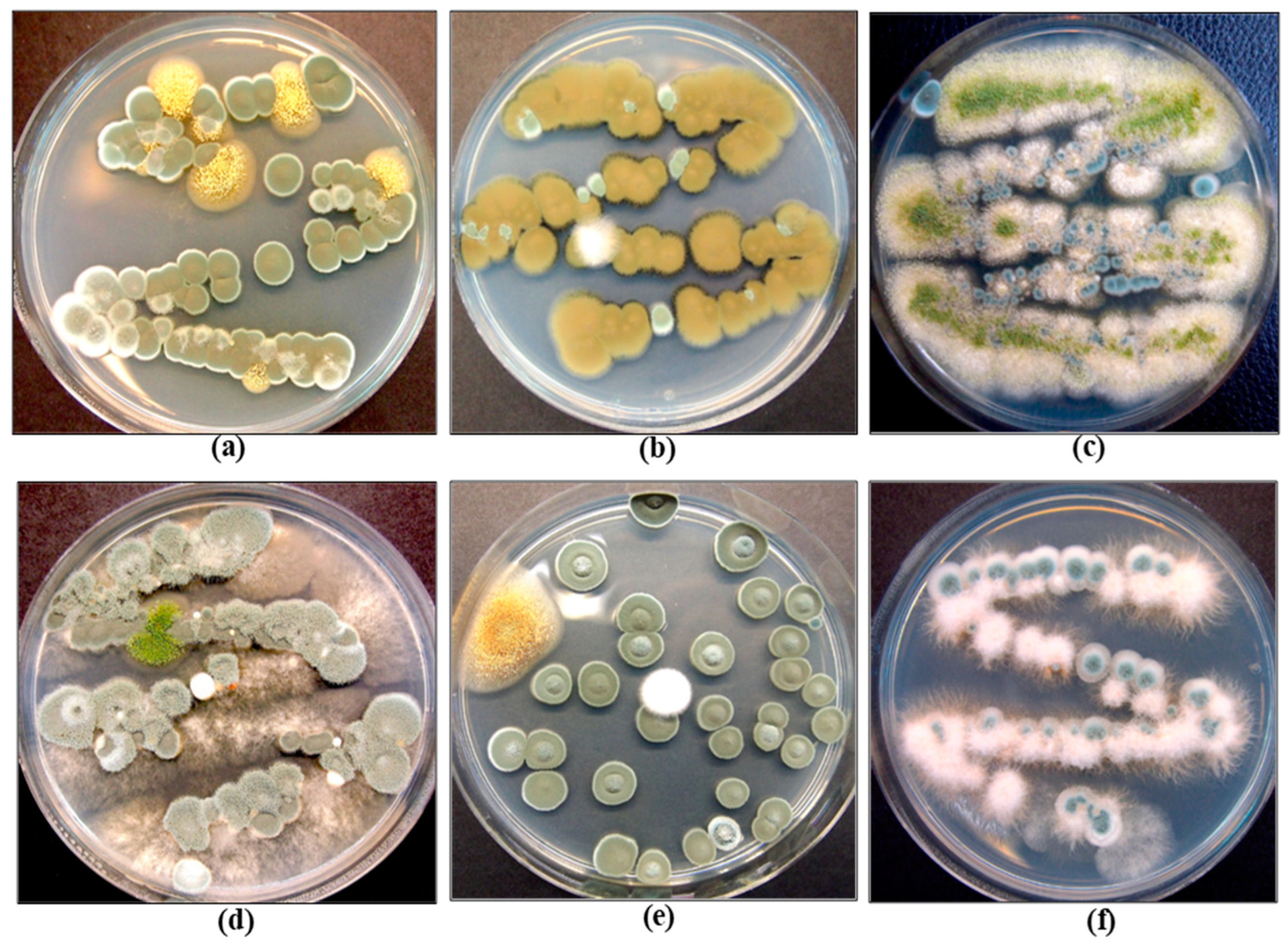
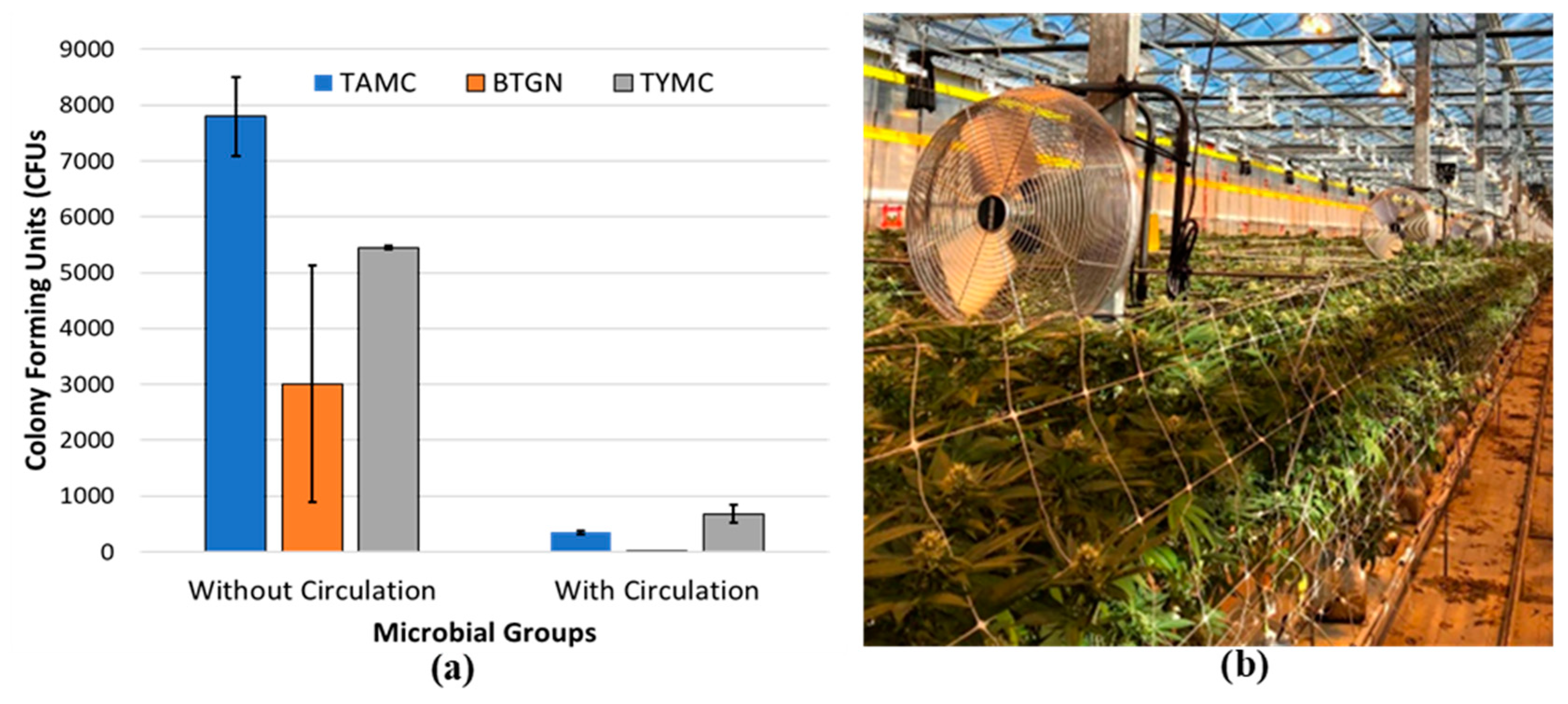
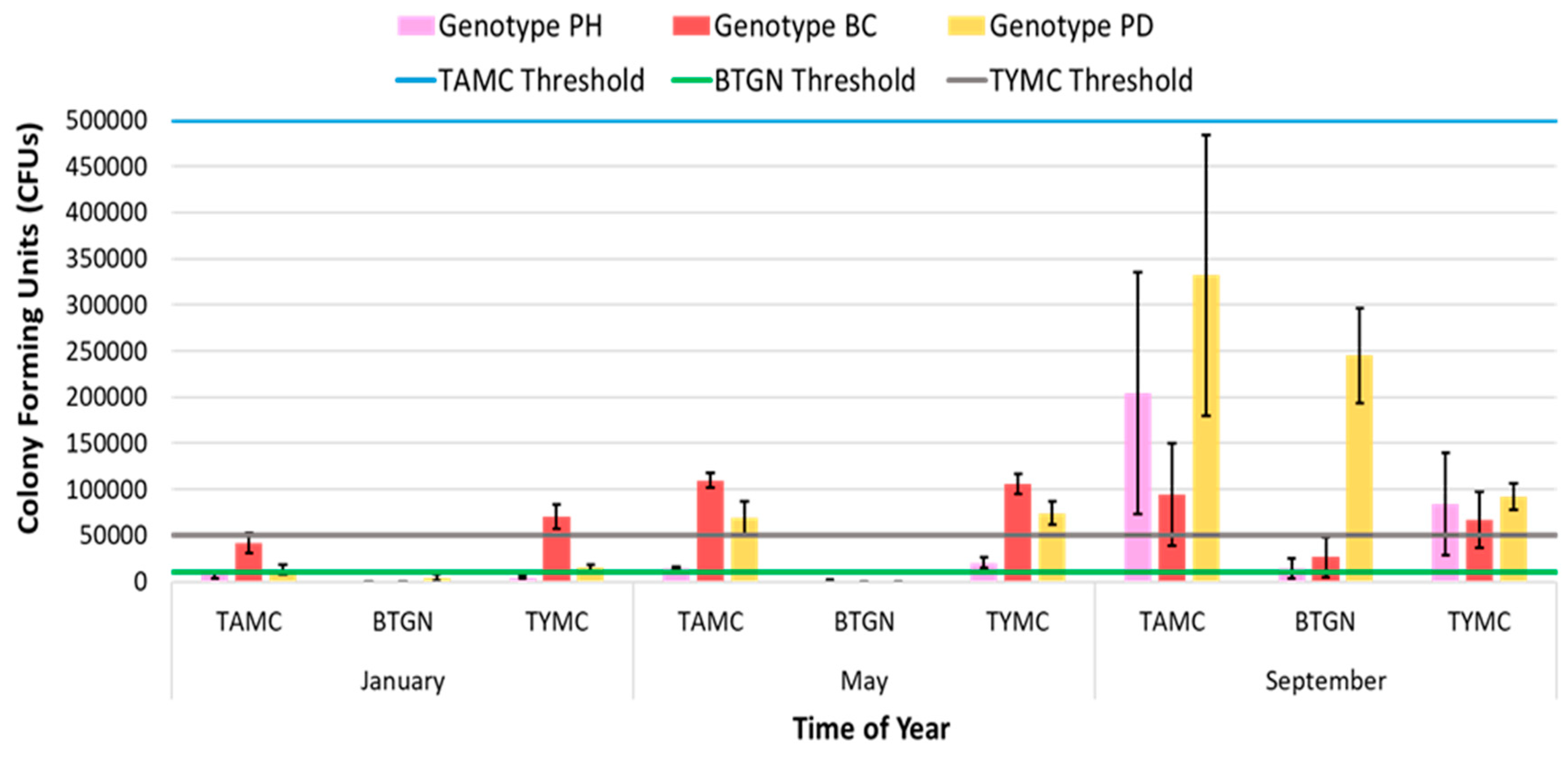
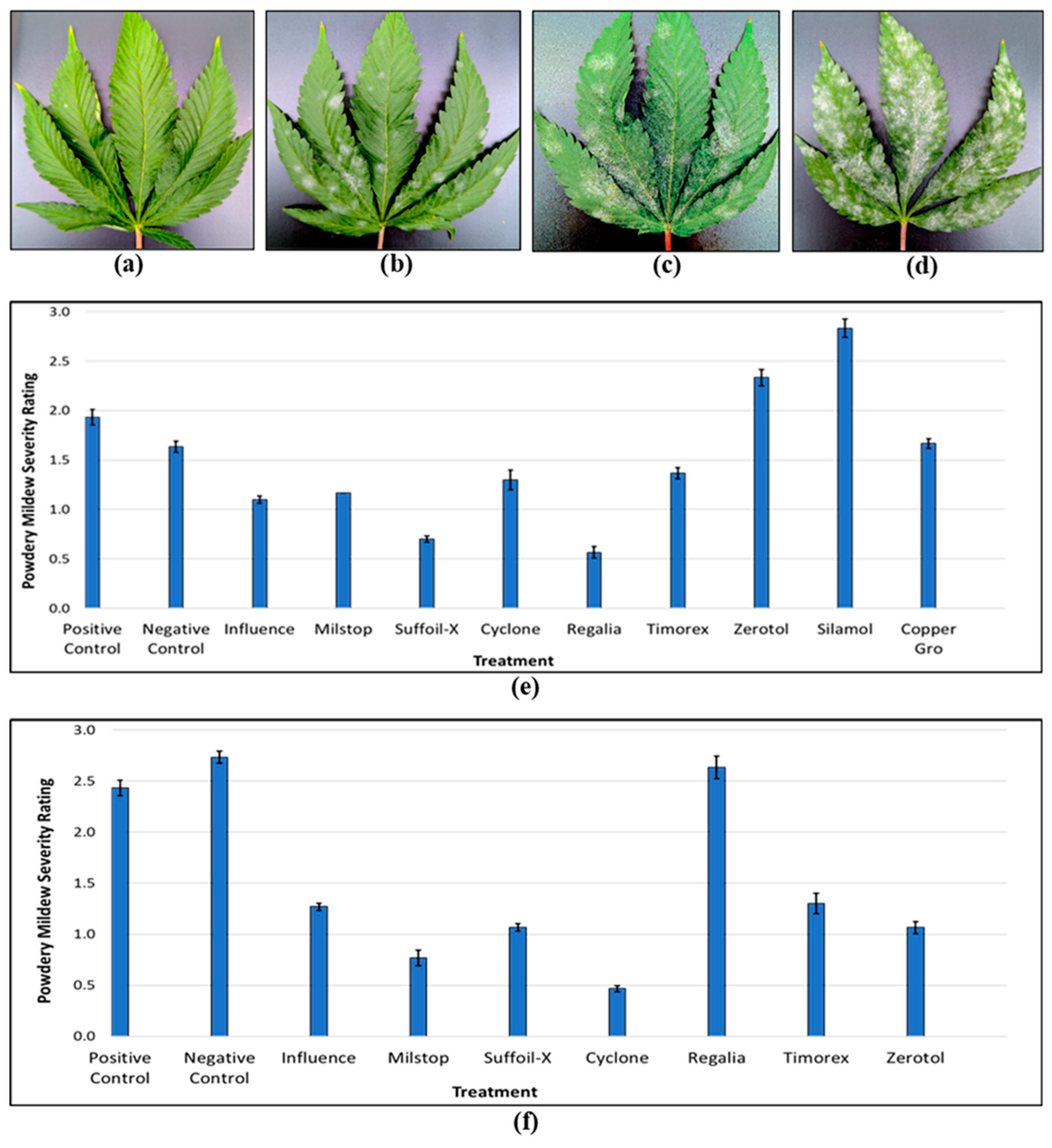
| HLVd Stunting Disease | Fusarium/Pythium Root & Crown Rot | Botrytis Bud Rot | Powdery Mildew | |
| Prevention | Test propagative materials and stock plants; utilize pathogen-free planting materials. | Test propagative materials and stock plants; utilize pathogen-free planting materials. | Reduce canopy humidity by adjusting planting density and enhancing air circulation. | Maintain an even climate, above 21°C, and vaporize sulfur nightly. |
| Sanitation | Clean equipment and bench surfaces; destroy diseased plants. | Clean equipment and bench surfaces; actively remove dead or diseased tissues. | Fog growing environment with reduced risk products prior to planting. | Fog growing environment with reduced risk products prior to planting. |
| Protection | Isolate propagative materials and stock plants in controlled access areas. | Apply Trichoderma harzianum and Gliocladium cantenulatum as a drench to rooted cuttings and plants. | Apply Rootshield HC® on developing inflorescences, from day 14 to day 28 of flowering. | Preventatively spray reduced risk products such as Suffoil-X, Regalia Maxx, on susceptible genotypes. |
| Monitoring | Scout regularly for symptoms; routinely sample water and suspect plants. | Scout regularly for symptoms; routinely sample water and suspect plants. | Conduct daily scouting for bud rot from the sixth week of flowering onwards. | Conduct weekly scouting at all plant development stages. |
| Eradication | Immediately remove and safely dispose of diseased plants at all stages of growth. | Immediately remove and safely dispose of diseased plants at all stages of growth. | Remove and dispose infected inflorescences; perform post-drying bud rot severity checks. | Remove infected leaves and dispose; spot spray reduced risk products. |
| Genotype Selection | Avoid highly susceptible genotypes; evaluate tolerant genotypes. | Avoid highly susceptible genotypes; evaluate tolerant genotypes. | Avoid planting highly susceptible genotypes during Botrytis-prone periods; evaluate tolerant genotypes. | Avoid highly susceptible genotypes; evaluate tolerant genotypes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





