Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Interventions
- Intervention group (B): 200 mg/kg/24h - 48h - 30 min i.v. infusion of VitC in 50 ml of normal saline every 6 hours, under UV protection.
- Control group (A): 30 min i.v. infusion of an equal volume of normal saline every 6 hours, under UV protection.
2.4. Outcome
2.5. Sample Size
2.6. Randomization and Masking
2.7. Statistical Methods
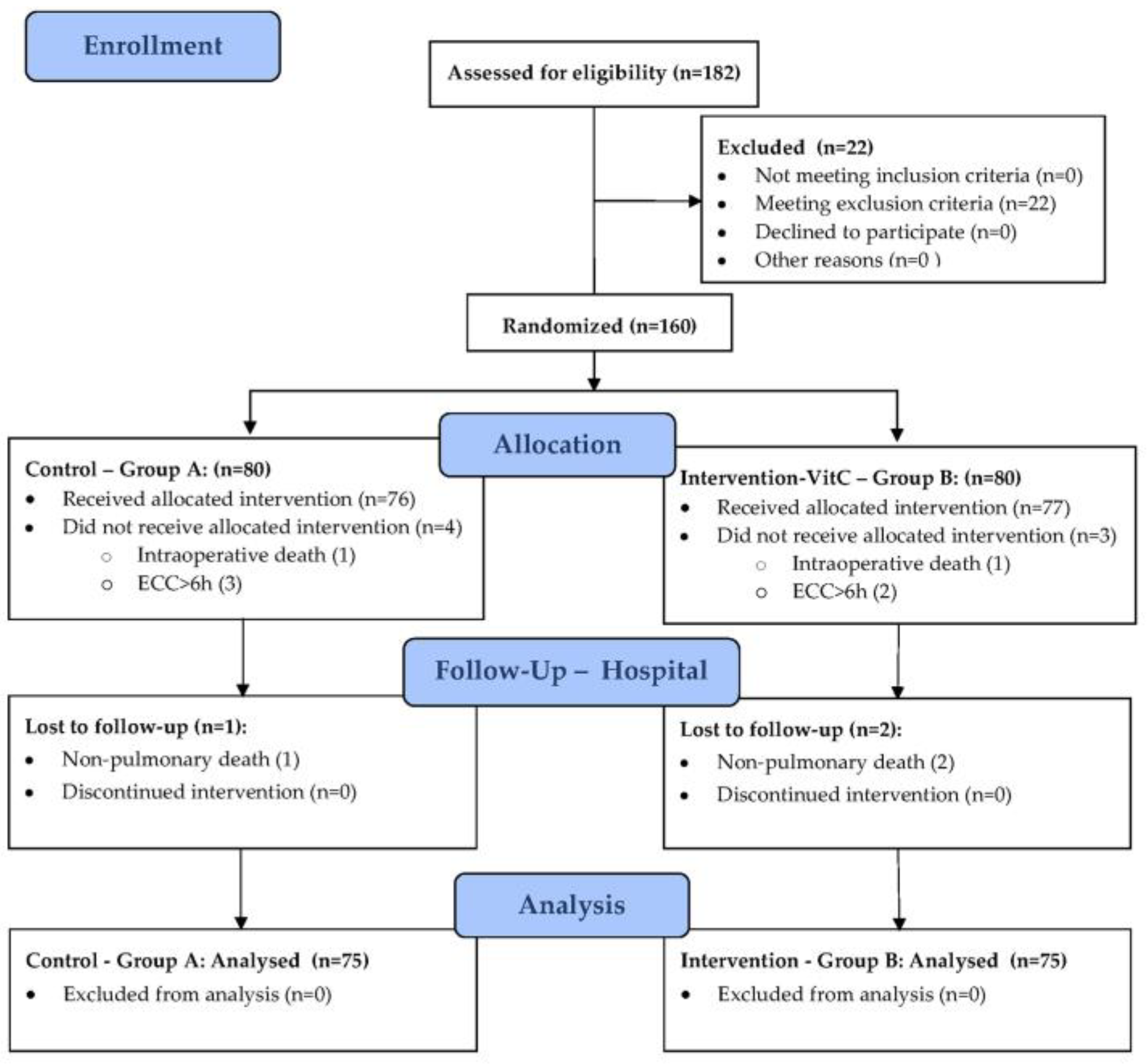
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J Cardiothorac Vasc Anesth 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am J Respir Crit Care Med 2015, 192, 958–964. [Google Scholar] [CrossRef]
- McGuinness, J.; Bouchier-Hayes, D.; Redmond, J.M. Understanding the inflammatory response to cardiac surgery. Surgeon 2008, 6, 162–171. [Google Scholar] [CrossRef]
- Semler, M.W.; Wheeler, A.P. Systemic inflammatory response syndrome after cardiac surgery: time for a change. Chest 2014, 145, 1181–1182. [Google Scholar] [CrossRef]
- Warltier, David C. ; Laffey, John G.; Boylan, John F.; Cheng, Davy C.H. The Systemic Inflammatory Response to Cardiac Surgery: Implications for the Anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- Joseph, D.; Puttaswamy, R.K.; Krovvidi, H. Non-respiratory functions of the lung. Continuing Education in Anaesthesia Critical Care & Pain 2013, 13, 98–102. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br J Anaesth 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Fischer, M.-O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Nigro Neto, C.; Kunstyr, J.; et al. Postoperative Pulmonary Complications After Cardiac Surgery: The VENICE International Cohort Study. Journal of Cardiothoracic and Vascular Anesthesia 2022, 36, 2344–2351. [Google Scholar] [CrossRef]
- Brown, P.P.; Kugelmass, A.D.; Cohen, D.J.; Reynolds, M.R.; Culler, S.D.; Dee, A.D.; Simon, A.W. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg 2008, 85, 1980–1986. [Google Scholar] [CrossRef]
- Abbott, T.E.F.; Fowler, A.J.; Pelosi, P.; Gama de Abreu, M.; Møller, A.M.; Canet, J.; Creagh-Brown, B.; Mythen, M.; Gin, T.; Lalu, M.M.; et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018, 120, 1066–1079. [Google Scholar] [CrossRef]
- Mali, S.; Haghaninejad, H. Pulmonary complications following cardiac surgery. Arch Med Sci Atheroscler Dis 2019, 4, e280–e285. [Google Scholar] [CrossRef] [PubMed]
- Naveed, A.; Azam, H.; Murtaza, H.G.; Ahmad, R.A.; Baig, M.A.R. Incidence and risk factors of Pulmonary Complications after Cardiopulmonary bypass. Pak J Med Sci 2017, 33, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Ballmer, P.E.; Reinhart, W.H.; Jordan, P.; Bühler, E.; Moser, U.K.; Gey, K.F. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations. J Thorac Cardiovasc Surg 1994, 108, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; O’Neill, L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Yamazaki, E. Vitamin C requirement in surgical patients. Current Opinion in Clinical Nutrition & Metabolic Care 2010, 13, 669–676. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Hess, A.S.; McCarthy, D.P.; DeCamp, M.M. Power for the Sickest: Vitamin C for Vasoplegia after Cardiac Surgery. J Cardiothorac Vasc Anesth 2020, 34, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis)interprete the clinical evidence. Redox Biol 2020, 34, 101532. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Borgs, C.; Fitzner, C.; Stoppe, C. Perioperative Vitamin C and E levels in Cardiac Surgery Patients and Their Clinical Significance. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Clasen, K.C.; Wendt, S.; Majoros Á, G.; Stoppe, C.; Adhikari, N.K.J.; Heyland, D.K.; Benstoem, C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mangoush, O.; Nakamura, K.; Al-Ruzzeh, S.; Athanasiou, T.; Chester, A.; Amrani, M. Effect of ascorbic acid on endothelium-dependent vasodilatation of human arterial conduits for coronary artery bypass grafting. Eur J Cardiothorac Surg 2003, 24, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Zhang, H.; Zhu, H.; Zhang, N.; Liu, J. Effect of Intravenous Injection of Vitamin C on Postoperative Pulmonary Complications in Patients Undergoing Cardiac Surgery: A Double-Blind, Randomized Trial. Drug Des Devel Ther 2020, 14, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Ascorbic acid and the common cold. Am J Clin Nutr 1971, 24, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Coppock, D.; Violet, P.C.; Vasquez, G.; Belden, K.; Foster, M.; Mullin, B.; Magee, D.; Mikell, I.; Shah, L.; Powers, V.; et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov 2020, 5, 100028. [Google Scholar] [CrossRef] [PubMed]
- PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Intravenous Vitamin C. Bethesda, MD: National Cancer Institute. Updated <06/17/2022>. Available at: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq. Accessed <12/10/2023>. [PMID: 26389504].
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Dingchao, H.; Zhiduan, Q.; Liye, H.; Xiaodong, F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg 1994, 42, 276–278. [Google Scholar] [CrossRef]
- Knuf, K.M.; Maani, C.V.; Cummings, A.K. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper Med (Lond) 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Lawrence, V.A.; Theroux, J.F.; Tuley, M.R. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med 1992, 152, 967–971. [Google Scholar] [CrossRef]
- Costa Leme, A.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; De Santis Santiago, R.R.; Osawa, E.A.; Pinheiro de Almeida, J.; Gerent, A.M.; Franco, R.A.; Zanetti Feltrim, M.I.; et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. Jama 2017, 317, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.; Helders, P.J.; Favié, N.J.; De Bie, R.A.; Brutel de la Riviere, A.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. Jama 2006, 296, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Huang, W.; Zhang, X.; Guo, Y.; Yu, P. Comparison of Tools for Postoperative Pulmonary Complications After Cardiac Surgery. J Cardiothorac Vasc Anesth 2023, 37, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, V.M.; Nikolić, N.; Radovanović Nenadić, U.; Öcal, A.; E, K.N.; Zečević, M. Preparedness and Preventive Behaviors for a Pandemic Disaster Caused by COVID-19 in Serbia. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Cheng, C.; Wei, X. Incidence of postoperative pulmonary complications in patients undergoing minimally invasive versus median sternotomy valve surgery: propensity score matching. Journal of Cardiothoracic Surgery 2021, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Hardman, J.; Sabaté, S.; Langeron, O.; Abreu, M.G.; Gallart, L.; Belda, J.; Markstaller, K.; Pelosi, P.; Mazo, V. PERISCOPE study: predicting post-operative pulmonary complications in Europe. Eur J Anaesthesiol 2011, 28, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 2004, 8, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015, 32, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, E.; Gologorsky, A.; Salerno, T.A. Lung-Centered Open Heart Surgery: A Call for a Paradigm Change. Front Cardiovasc Med 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Vaughan-Sarrazin, M.; Rosenthal, G.E.; Girotra, S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Yuqiang Wang; Yaxin Zhou; Zeruxin Luo; Wei Huang; Xiu Zhang; Yingqiang Guo; Yu, P. Comparison of Tools for Postoperative Pulmonary Complication Following Cardiac surgery. PREPRINT (Version 1) 2022, Research Square. [CrossRef]
- Hickey, S.; Roberts, H. Evolution and Deficiency. In Ascorbate: The Science of Vitamin C, Hickey, S., Roberts, H., Eds.; Lulu.com: 2004; pp. 66-72.
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.J. The discovery of vitamin C. Ann Nutr Metab 2012, 61, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Richards, E. Introduction. In Vitamin C and Cancer: Medicine or Politics?; Palgrave Macmillan UK: 1991; pp. 1-14.
- Li, C.C. [Changes of creatine phosphokinase and malondialdehyde in the serum and clinical use of large doses of vitamin C following open heart surgery]. Zhonghua Wai Ke Za Zhi 1990, 28, 16–17. [Google Scholar] [PubMed]
- Iizuka, Y.; Yoshinaga, K.; Takahashi, K.; Oki, S.; Chiba, Y.; Sanui, M.; Kimura, N.; Yamaguchi, A. Association between Plasma Ascorbic Acid Levels and Postoperative Delirium in Older Patients Undergoing Cardiovascular Surgery: A Prospective Observational Study. J Cardiovasc Dev Dis 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Nabzdyk, C.S.; Bittner, E.A. Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 2018, 7, 52–61. [Google Scholar] [CrossRef]
| Perioperative Parameters | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. Demographic and Anthropometric | |||
| Age (years) | 66.9±8.7 | 66.3±8.6 | 0.672 (t) |
| Male gender | 59 (78.7%) | 55 (73.3%) | 0.444 (chi) |
| BMI (kg/m2) | 28.4±3.9 | 26.7±3.3 | 0.005 (t) |
| 2. CVD Risk | |||
| HTA | 75 (100%) | 73 (97.3%) | 0.497 (fet) |
| DM | 36 (48%) | 36 (48%) | 1.000 (fet) |
| HLP | 72 (96%) | 68 (90.7%) | 0.190 (chi) |
| Smoking | 52 (69.3%) | 52 (69.3%) | 1.000 (chi) |
| 3. CV Status and Comorbidities | |||
| Recent MI | 41 (54.7%) | 34 (45.3%) | 0.253 (chi) |
| AP | 61 (81.3%) | 58 (77.3%) | 0.545 (chi) |
| TAs (mmHg) | 145.8±26.3 | 139.8±16.9 | 0.100 (t) |
| TAd (mmHg) | 81.9±13.1 | 77.3±11.6 | 0.022 (t) |
| EF-LV (%) | 46.5±9.1 | 48.4±8.0 | 0.187 (t) |
| HR (beats/min) | 70.4±9.3 | 69.5±9.7 | 0.565 (t) |
| Sinus | 68 (90.7%) | 66 (88%) | 0.597 (chi) |
| AF | 8 (10.7%) | 9 (12%) | 0.979 (chi) |
| CVD | 11 (14.7%) | 5 (6.7%) | 0.113 (chi) |
| CRF | 23 (30.7%) | 12 (16%) | 0.034 (chi) |
| COVID-19 | 22 (29.3%) | 17 (22.7%) | 0.352 (chi) |
| 4. Pulmonary status (PPC Score) | |||
| 0 | 20 (26.7%) | 25 (33.3%) | 0.373 (chi) |
| 1 | 55 (73.3%) | 50 (66.7%) | |
| 5. ASA Score | |||
| 3 | 65 (86.7%) | 66 (88.0%) | 0.806 (chi) |
| 4 | 10 (13.3%) | 9 (12.0%) | |
| 6. Surgery | |||
| CABG | 53 (70.7%) | 49 (65.3%) | 0.484 (chi) |
| Aortic valve | 9 (12%) | 13 (17.3%) | 0.356 (chi) |
| Mitral valve | 2 (2.7%) | 4 (5.3%) | 0.681 (fet) |
| Combined | 11 (14.7%) | 9 (12%) | 0.631 (chi) |
| Duration of surgery (min) | 245.7±40.2 | 219.9±45.0 | <0.001 (t) |
| ECC time (min) | 86.8±27.3 | 80.7±19.1 | 0.114 (t) |
| ACC time (min) | 55.6±20.7 | 56.3±15.4 | 0.799 (t) |
| Primary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. PPC Incidence | |||
| PPC ≥ 3 (n,%) | 45 (60.0%) | 10 (13.3%) | <0.001 (chi) |
| 2. PPC Severity | |||
| PPC severity score | 3 (2) | 1 (1) | <0.001 (mw) |
| Grade 0 (n,%) | 5 (6.7%) | 14 (18.7%) | <0.001 (mw) |
| Grade 1 (n,%) | 3 (4.0%) | 31 (41.3%) | |
| Grade 2 (n,%) | 22 (29.3%) | 20 (26.7%) | |
| Grade 3 (n,%) | 23 (30.7%) | 7 (9.3%) | |
| Grade 4 (n,%) | 18 (24.0%) | 3 (4.0%) | |
| Grade 5 (n,%) | 4 (5.3%) | 0 | |
| 3. PPC Types | |||
| Pneumonia (n,%) | 32 (42.7%) | 13 (17.3%) | <0.001 (chi) |
| Pneumothorax (n,%) | 10 (13.3%) | 3 (4%) | 0.042 (chi) |
| Pleural effusion (n,%) | 51 (68%) | 38 (50.7%) | 0.031 (chi) |
| Re-intubation (n,%) | 16 (21.3%) | 2 (2.7%) | <0.001 (chi) |
| Secondary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* | ||
|---|---|---|---|---|---|
| 1. Pulmonary oxygenation and ventilation | |||||
| Horowitz index (PaO2/FiO2) 48h | 268.9±112.6 | 312.6±107.4 | 0.008 (t) | ||
| Alveolar–arterial gradient (A-aDO2) 48h | 17.3±5.4 | 17.9±4.7 | 0.432 (t) | ||
| Total MV time (h) | 5.2±1.6 | 5.4±1.2 | 0.493 (t) | ||
| 2. Inflammatory markers (48h) ** | |||||
| Procalcitonin | 0.3 (0.6) | 0.5 (0.8) | 0.032 (mw) | ||
| C-reactive protein | 167.4 (82.9) | 95 (56.2) | <0.001 (mw) | ||
| Leucocytes | 13.3±3.3 | 12.4±3.4 | 0.102 (t) | ||
| Neutrophils | 80.6±5.5 | 80.5±5.1 | 0.881 (t) | ||
| Lymphocytes | 12±4.6 | 12.6±4 | 0.418 (t) | ||
| Sedimentation rate | 22 (18) | 20 (6) | 0.023 (mw) | ||
| Fibrinogen | 5.1±1.4 | 5.2±1 | 0.775 (t) | ||
| Albumin | 31.8±3.5 | 31.4±3.2 | 0.479 (t) | ||
| D-dimer | 0.5 (0.5) | 0.6 (0.4) | 0.877 (mw) | ||
| Ferritin | 202 (277) | 200 (104) | 0.287 (mw) | ||
| 3. Postoperative complications (non-pulmonary) | |||||
| PONV | 20 (26.7%) | 30 (40.0%) | 0.083 (chi) | ||
| Delirium | 22 (29.3%) | 16 (21.3%) | 0.260 (chi) | ||
| Transfusion | 32 (42.7%) | 36 (48%) | 0.512 (chi) | ||
| Acute renal failure | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
| Wound infection | 15 (20%) | 5 (6.7%) | 0.016 (chi) | ||
| CPR | 4 (5.3%) | 0 (0%) | 0.120 (fet) | ||
| 4. Renal function(48h) ** | |||||
| GFR < 60mL/min | 24 (32%) | 10 (13.3%) | 0.006 (chi) | ||
| Creatinine | 99.6±44.6 | 87.8±27.5 | 0.054 (t) | ||
| Urea | 7.1±3 | 6.3±1.9 | 0.041(t) | ||
| 5. Postoperative organ dysfunction | |||||
| SOFA score | 4 (2) | 4 (1) | 0.132 (mw) | ||
| ASA/SOFA ratio | 1.0 (0.4) | 0.8 (0.25) | 0.190 (mw) | ||
| 6. ICU outcome measures | |||||
| ICU re-admission | 15 (20%) | 4 (5.3%) | 0.007 (chi) | ||
| ICU stay | 48 (24) | 32 (24) | <0.001 (mw) | ||
| 7. Hospital outcome measures | |||||
| Hospital stay | 8 (2) | 8 (2) | 0.092 (mw) | ||
| Hospital mortality | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





