Submitted:
25 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methodology
Discussion
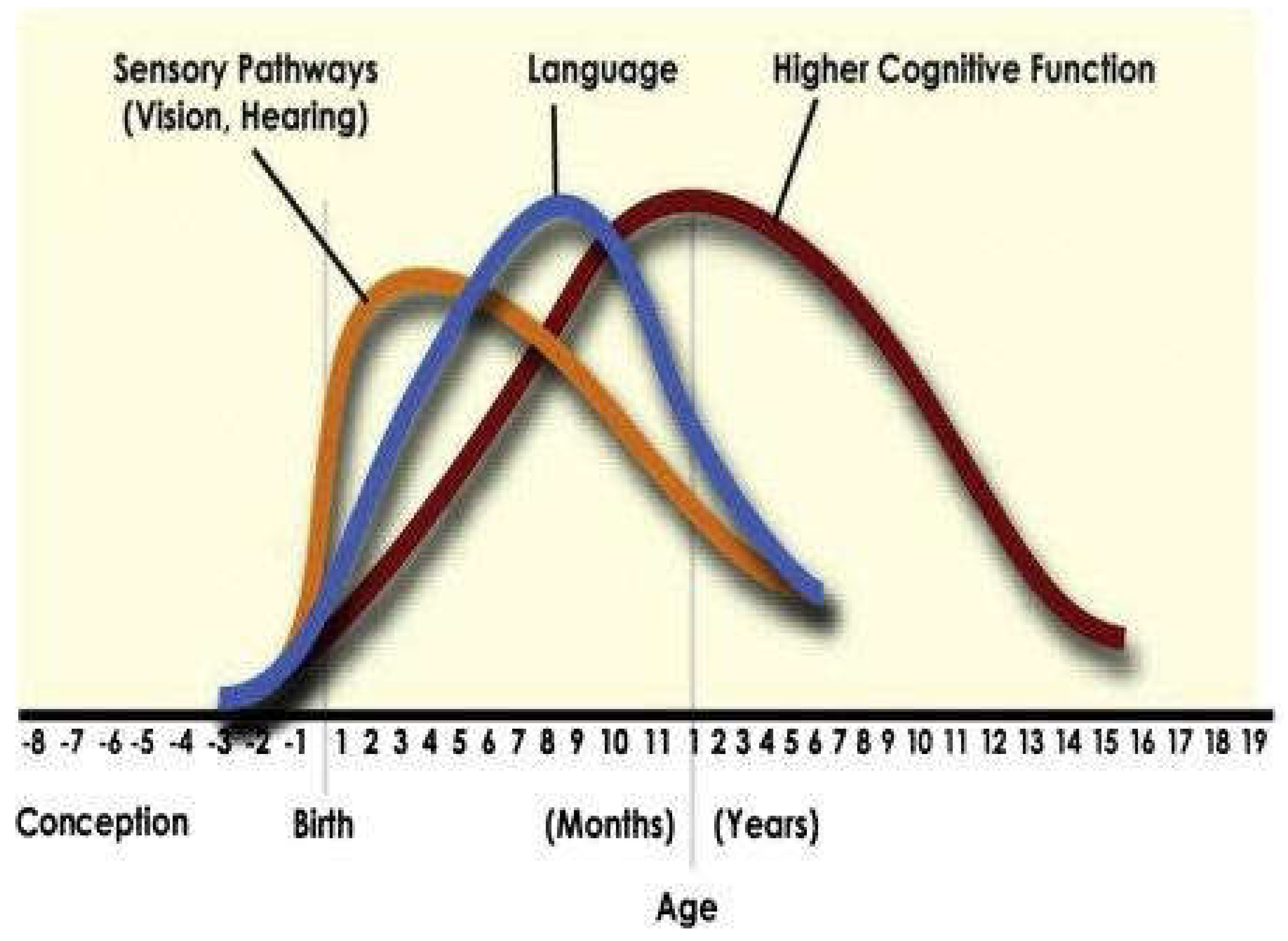
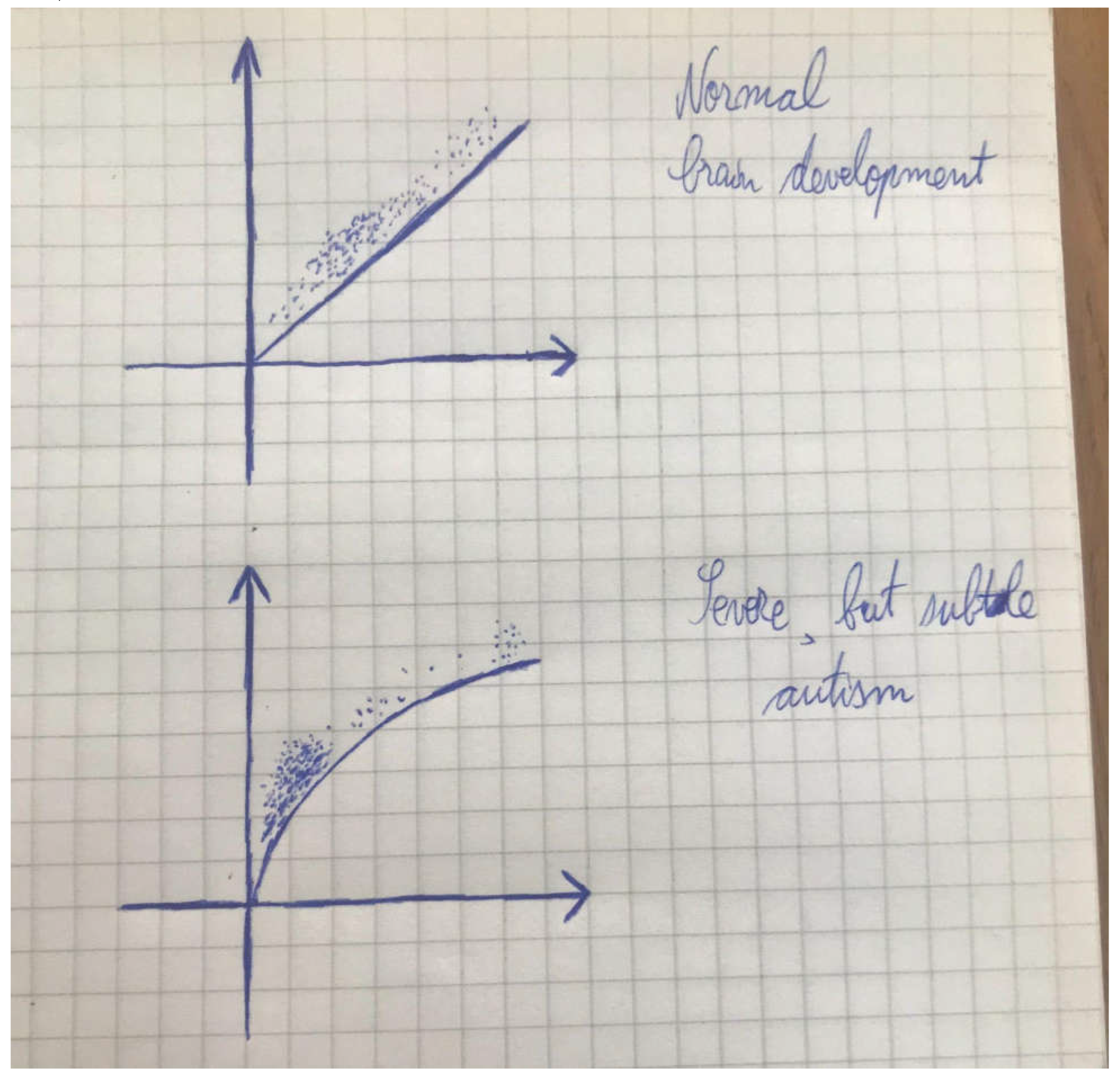
A More Widespread Incidence of Neurodevelopmental Delays Places an Impact upon the Average Age of Full Brain Maturity and Discernment
- Gradual separation of hundreds of millions of people from their natural origins, through a mass relocation into hyper-industrialised megalopolises, mostly by international means.
- Increasing technology abuse and dependence in young children. Risking a gradual, but certain replacement of manual work with automated, robotics-mediated work, leading gradually to a general loss of human workforce.
- The performance of genetic “plagiarism” against natural origins, through increasingly widespread clinical practices of molecular cloning and creation of GMOs, with the good intention of minimising human and animal suffering.
- Malthus versus Dr. Verhulst: Disregarding the other side of the scientific debate regarding the theme of overpopulation and projected inevitability of reaching resource insufficiency. Namely, Dr. Pierre Verhulst, a mathematician who obtained his PhD University diploma at the age of 25, stated that the natural environment is fully capable of self-regulation and self-preservation, including human and animal population management. Such a theory seemingly demolishes the foundations feeding unnecessary anxiety regarding projected insufficiency of vital resources, as well as efforts to develop methodologies that may be considerably controversial in nature, made under the heretical perception that it would be less risky than allowing the human population to solely rely upon resources that have not undergone artificially-induced replication.
- Direct and indirect manipulation of the human genome, such as gene therapy using foreign genetic information, as well as an increasing manipulation of the environment respectively.
- Widespread consumption of junk food, fast food and unhealthy beverages by young children. Fast food often contains various hormones, which may play a considerable role in creating or amplifying delays in neurogenesis and neurological development in children, potentially affecting them for many years after.
- A significant decrease of social interest in civilizational values and unspoken rules that primarily contributed to the establishment and maintenance of society.
- Incomplete availability of resources and logistical pathways to offer immunomodulatory treatment for pregnant mothers experiencing moderate and severe infectious diseases. Insufficient clinical focus upon the importance of natural immunity in prophylaxis and early treatment of infectious diseases of concern.
- Potentially major factor: The administration of 28-32 vaccine doses or more, including one or more doses of the experimental COVID-19 vaccine using the genetic information of a viral protein with potential characteristics of a superantigen, in babies aged 0-2, who are in critical stages or neuro-immunological development. Moreover, the increase of infant vaccine doses, from 12-15 in the 1980s, to about 30 or locally 35 by 2015, and to 40-45 doses and locally more from 2022, has been accompanied by an insufficient focus upon the potential roles of early innate immune activation in the vaccinological combat against infectious diseases of individual and public health concern, and applications of early innate immune activation into vaccinology would promote neurogenesis and neuroprotection more substantially. The number of administered childhood vaccine doses has recently risen from 45 in 2015 to 76 in 2022, in the United States of America. The administration of vaccines that contain multiple antigens each may strengthen the pressurising effects upon the brain to develop, thereby raising the possibility and extent of the ulterior onset of neurodevelopmental delays. The addition of heavy metals, including mercury, as a vaccine adjuvant in rather many cases can only further amplify such effects of neuro-immunology-related developmental delays and damages.
- Hyper-automation and lack of healthy work and study environments, leading to privation from essential human contact and direct instruction and mentorship during key stages of neuronal growth.
- Increased repetitive patterns in the individual and collective thinking and behaviour caused by the gradual, but certain separation of humankind from her natural roots.
- Polarisation of resource-based, economic, financial, academic and professional power, turning the world, not even into a bipolar arena, where the disadvantaged matter in society, but outright into a unipolar arena, where the disadvantaged are treated like they are nonexistent, reducing the extent of middle class to its extinction and separating society into the poor class, consisting of over 99% of the world’s population, and the rich class, consisting of less than 1% of the world’s population. Placing much of the blame of the corrupt leadership on the poorer segments of society, instead of using the gained multi-billion financial resources to support the poor in conducting themselves adequately to support the reconstruction of Nature and simultaneously the reunification of humankind with Nature, instead of punishing her by separating her into hyper-industrialised environments by means of psychological and financial force.
- Manipulation of healthy educational patterns, by allowing children and teenagers to be exposed to environmental factors that would induce delays in the development of healthy cognitive function and behaviour.
- Manipulation of imagery via social media and television. Prioritisation of unhealthy thinking and behaviours.
- Manipulation of frequencies (i.e. the change of the music frequency from 432 Hz to 440 Hz in 1938), making people more irritable, angrier and more aggressive, thereby increasing levels of testosterone, which will increase the incidence of excess testosterone, which in turn will ultimately increase the incidence of serious neurodevelopmental delays.
- Manipulation of music, including the usage of negative subliminal messages, indirectly or directly increasing the epigenetic stimulation of testosterone synthesis and ultimately leading to an excess of aggression, anger and hopelessness.
- An exponential increase of the incidences of serious early childhood trauma, causing neurological damage and often major neurodevelopmental delays.
- The widespread usage of genetically-modified organisms (GMOs), which is promoted by the currents of Malthusianism and Cornucopianism. Notably, Dr. Pierre Verhulst, who obtained his PhD in biology at the age of 25, theorised that the natural environment is fully capable of self-regulation, meaning that the human population is equally manageable by Nature. Likewise, any artificial attempt of population growth control may represent a scientifically heretical approach, leading to increased risks of inducing unprecedented harm to major fragments of the human civilization in the end.
- Manipulation of the climate, such as an attempt to block ultraviolet light from the sun in the atmosphere in the name of combating heat waves during the summer.
- A substantially decreased use and availability of natural remedies in healthcare settings with a progressively heavier reliance on pharmaceuticals instead.
- An increasingly widespread administration of medical drugs that often bring controversial health outcomes.
- Sedentary lifestyle induced by many, if not all listed factors above.
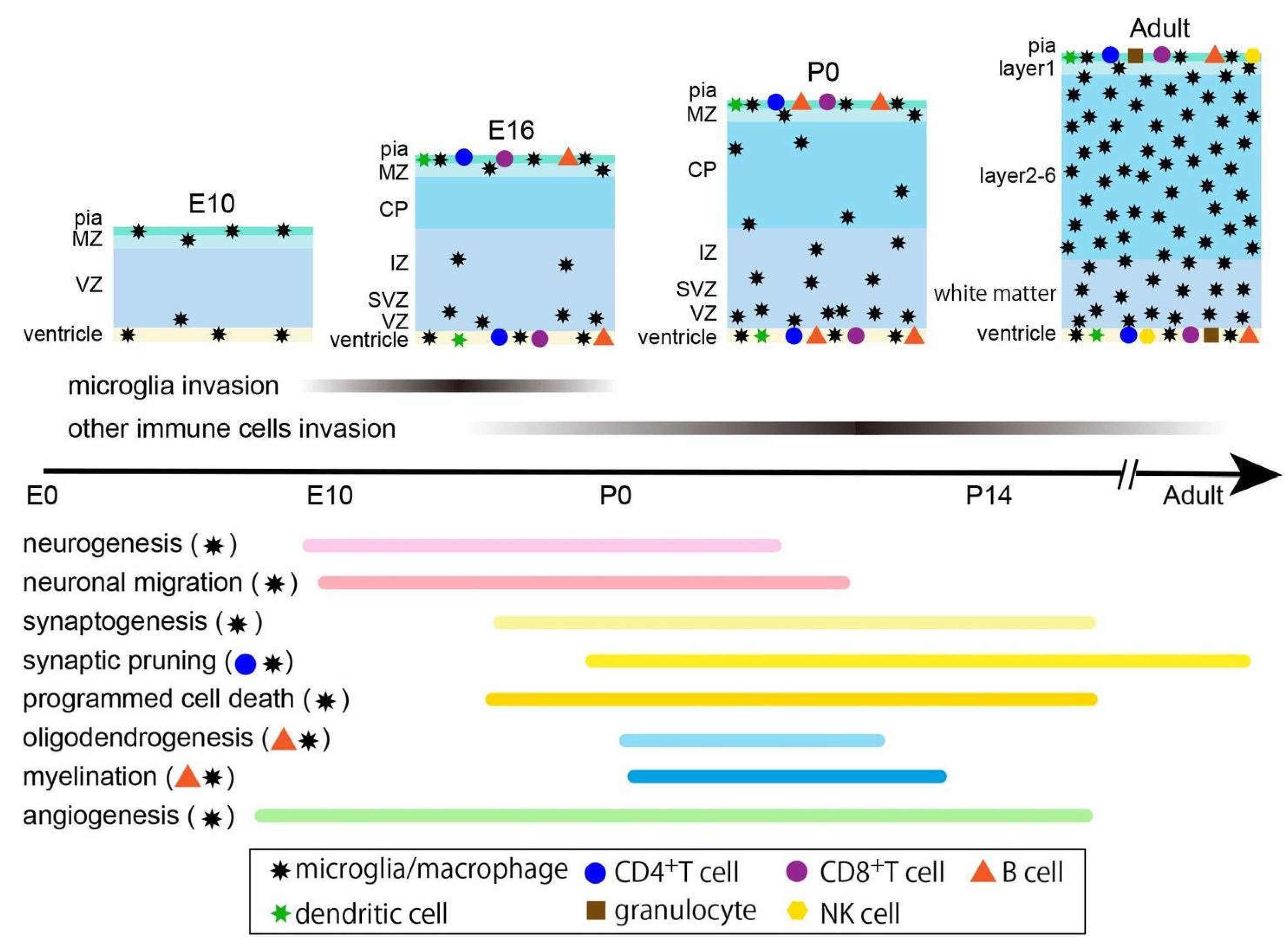
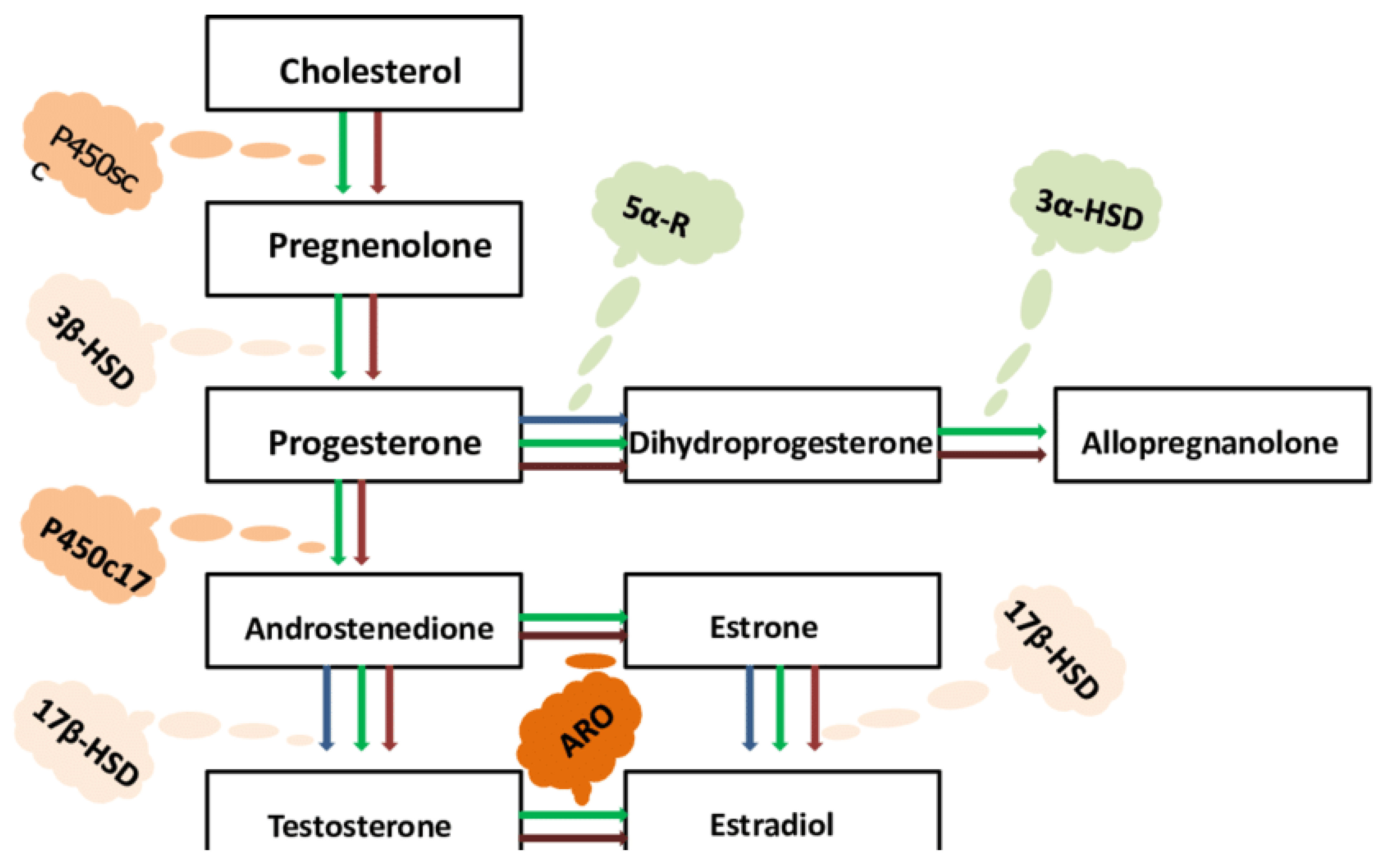
Conclusion
References
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2020, 21, 83–100. [Google Scholar] [CrossRef]
- Yang, J.; Qi, F.; Gu, H.; Zou, J.; Yang, Y.; Yuan, Q.; Yao, Z. Neonatal BCG vaccination of mice improves neurogenesis and behavior in early life. Brain Res. Bull. 2016, 120, 25–33. [Google Scholar] [CrossRef]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef]
- Baines, K.J.; Hillier, D.M.; Haddad, F.L.; Rajakumar, N.; Schmid, S.; Renaud, S.J. Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats. Front. Immunol. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Denes, A.; Miyan, J.A. Brain-immune interactions in health and disease. Front. Neurosci. 2014, 8, 382. [Google Scholar] [CrossRef]
- Kamimura, D.; Yamada, M.; Harada, M.; Sabharwal, L.; Meng, J.; Bando, H.; Ogura, H.; Atsumi, T.; Arima, Y.; Murakami, M. The gateway theory: bridging neural and immune interactions in the CNS. Front. Neurosci. 2013, 7, 204. [Google Scholar] [CrossRef]
- Geenen, V.; Bodart, G.; Henry, S.; Michaux, H.; Dardenne, O.; Charlet-Renard, C.; Martens, H.; Hober, D. Programming of neuroendocrine self in the thymus and its defect in the development of neuroendocrine autoimmunity. Front. Neurosci. 2013, 7, 187. [Google Scholar] [CrossRef]
- Goyal, D.K.; Miyan, J.A. Neuro-Immune Abnormalities in Autism and Their Relationship with the Environment: A Variable Insult Model for Autism. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Owen, A.M.; Hernandez, A.; Patil, N.K.; Williams, D.L.; Bohannon, J.K. Innate Immune Memory and the Host Response to Infection. J. Immunol. 2022, 208, 785–792. [Google Scholar] [CrossRef]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Kloc, M.; Kubiak, J.Z.; Zdanowski, R.; Ghobrial, R.M. Memory Macrophages. Int. J. Mol. Sci. 2022, 24, 38. [Google Scholar] [CrossRef]
- Taylor, M. W. (2014). Interferons. Viruses and Man: A History of Interactions, 101–119. [CrossRef]
- Chandwani, M.N.; Creisher, P.S.; O'Donnell, L.A. Understanding the Role of Antiviral Cytokines and Chemokines on Neural Stem/Progenitor Cell Activity and Survival. Viral Immunol. 2019, 32, 15–24. [Google Scholar] [CrossRef]
- Borsini, A.; Cattaneo, A.; Malpighi, C.; Thuret, S.; A Harrison, N.; A Zunszain, P.; Pariante, C.M. MRC ImmunoPsychiatry Consortium Interferon-Alpha Reduces Human Hippocampal Neurogenesis and Increases Apoptosis via Activation of Distinct STAT1-Dependent Mechanisms. Int. J. Neuropsychopharmacol. 2017, 21, 187–200. [Google Scholar] [CrossRef]
- Borsini, A.; Pariante, C.M.; Zunszain, P.A.; Hepgul, N.; Russell, A.; Zajkowska, Z.; Mondelli, V.; Thuret, S. The role of circulatory systemic environment in predicting interferon-alpha–induced depression: The neurogenic process as a potential mechanism. Brain, Behav. Immun. 2019, 81, 220–227. [Google Scholar] [CrossRef]
- Su, K.-P.; Lai, H.-C.; Peng, C.-Y.; Su, W.-P.; Chang, J.P.-C.; Pariante, C.M. Interferon-alpha-induced depression: Comparisons between early- and late-onset subgroups and with patients with major depressive disorder. Brain, Behav. Immun. 2019, 80, 512–518. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Kuo, R.-L.; Huang, H.-I. Activation of type I interferon antiviral response in human neural stem cells. Stem Cell Res. Ther. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Bhat, H.; Lang, K.S.; Hardt, C.; Lang, J. Interferon in the CNS. Neurosignals 2019, 27, 44–53. [Google Scholar] [CrossRef]
- Owens, T.; Khorooshi, R.; Wlodarczyk, A.; Asgari, N. Interferons in the central nervous system: A few instruments play many tunes. Glia 2013, 62, 339–355. [Google Scholar] [CrossRef]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef]
- Raftopoulou, S.; Rapti, A.; Karathanasis, D.; Evangelopoulos, M.E.; Mavragani, C.P. The role of type I IFN in autoimmune and autoinflammatory diseases with CNS involvement. Front. Neurol. 2022, 13, 1026449. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.; Lee, R.V.; Weinstein, J.R. Microglial Interferon Signaling and White Matter. Neurochem. Res. 2017, 42, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, J.; Pariante, C.M.; Borsini, A. The innate immune system and neurogenesis as modulating mechanisms of electroconvulsive therapy in pre-clinical studies. J. Psychopharmacol. 2020, 34, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Nettis, M.A.; Pariante, C.M. Is there neuroinflammation in depression? Understanding the link between the brain and the peripheral immune system in depression. International review of neurobiology 2020, 152, 23–40. [Google Scholar] [CrossRef]
- Marques, A.H.; Cizza, G.; Sternberg, E. Interações imunocerebrais e implicações nos transtornos psiquiátricos. Rev. Bras. de Psiquiatr. 2007, 29, s27–s32. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, J.D.; Kemper, C.; Woodruff, T.M. The Complement Receptor C5aR2: A Powerful Modulator of Innate and Adaptive Immunity. J. Immunol. 2019, 202, 3339–3348. [Google Scholar] [CrossRef]
- Yu, S.; Wang, D.; Huang, L.; Zhang, Y.; Luo, R.; Adah, D.; Tang, Y.; Zhao, K.; Lu, B. The complement receptor C5aR2 promotes protein kinase R expression and contributes to NLRP3 inflammasome activation and HMGB1 release from macrophages. J. Biol. Chem. 2019, 294, 8384–8394. [Google Scholar] [CrossRef]
- Hernandez, M.X.; Namiranian, P.; Nguyen, E.; Fonseca, M.I.; Tenner, A.J. C5a Increases the Injury to Primary Neurons Elicited by Fibrillar Amyloid Beta. ASN Neuro 2017, 9. [Google Scholar] [CrossRef]
- Pamies, D.; Sartori, C.; Schvartz, D.; González-Ruiz, V.; Pellerin, L.; Nunes, C.; Tavel, D.; Maillard, V.; Boccard, J.; Rudaz, S.; et al. Neuroinflammatory Response to TNFα and IL1β Cytokines Is Accompanied by an Increase in Glycolysis in Human Astrocytes In Vitro. Int. J. Mol. Sci. 2021, 22, 4065. [Google Scholar] [CrossRef]
- Dhungana, H.; Rolova, T.; Savchenko, E.; Wojciechowski, S.; Savolainen, K.; Ruotsalainen, A.-K.; Sullivan, P.M.; Koistinaho, J.; Malm, T. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J. Neuroinflammation 2013, 10, 102–102. [Google Scholar] [CrossRef]
- Mäkinen, E.; Lensu, S.; Honkanen, M.; Laitinen, P.; Wikgren, J.; Koch, L.G.; Britton, S.L.; Kainulainen, H.; Pekkala, S.; Nokia, M.S. Rats bred for low intrinsic aerobic exercise capacity link obesity with brain inflammation and reduced structural plasticity of the hippocampus. Brain, Behav. Immun. 2021, 97, 250–259. [Google Scholar] [CrossRef] [PubMed]
- A Rudick, R.; Ransohoff, R.M. Biologic effects of interferons: relevance to multiple sclerosis. Multiple sclerosis 1995, 1 (Suppl 1), S12–S16. [Google Scholar] [PubMed]
- Javed, A.; Reder, A.T. Therapeutic role of beta-interferons in multiple sclerosis. Pharmacol. Ther. 2006, 110, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Luo, Z.; Zhang, Y.; Lv, L.; Zhao, J.; Sui, B.; Huang, F.; Cui, M.; Fu, Z.F.; et al. Interferon-λ Attenuates Rabies Virus Infection by Inducing Interferon-Stimulated Genes and Alleviating Neurological Inflammation. Viruses 2020, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zuo, Z.; Yang, J.; Hu, S.; Yang, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. J. Neuroinflammation 2017, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zuo, Z.; Hu, S.; Xia, Y.; Song, D.; Kong, J.; Yang, Y.; Wu, Y.; Wang, X.; Yang, J.; et al. An enriched environment restores hepatitis B vaccination-mediated impairments in synaptic function through IFN-γ/Arginase1 signaling. Brain, Behav. Immun. 2018, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, F.; Yang, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z. Neonatal hepatitis B vaccination impaired the behavior and neurogenesis of mice transiently in early adulthood. Psychoneuroendocrinology 2016, 73, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Xing, Z.; Zhang, H.; Wen, Y.; Qi, F.; Zuo, Z.; Xu, J.; Yao, Z. IL-4 mediates the delayed neurobehavioral impairments induced by neonatal hepatitis B vaccination that involves the down-regulation of the IL-4 receptor in the hippocampus. Cytokine 2018, 110, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yang, J.; Xia, Y.; Yuan, Q.; Guo, K.; Zou, J.; Yao, Z. A(H1N1) vaccination recruits T lymphocytes to the choroid plexus for the promotion of hippocampal neurogenesis and working memory in pregnant mice. Brain, Behav. Immun. 2016, 53, 72–83. [Google Scholar] [CrossRef]
- Xia, Y.; Qi, F.; Zou, J.; Yang, J.; Yao, Z. Influenza vaccination during early pregnancy contributes to neurogenesis and behavioral function in offspring. Brain, Behav. Immun. 2014, 42, 212–221. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; van Praag, H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain, Behav. Immun. 2015, 45, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Yee, B.K.; Knuesel, I.; Feldon, J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain, Behav. Immun. 2007, 22, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Moon, H.M.; Su, J.; Palmer, T.D.; Courchesne, E.; Pramparo, T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol. Psychiatry 2017, 23, 1001–1013. [Google Scholar] [CrossRef]
- Haddad, F.L.; Patel, S.V.; Schmid, S. Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020, 113, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.A.; Mustafin, Z.S.; Lashin, S.A.; Kochetov, A.V. Abnormal mTOR Activity in Pediatric Autoimmune Neuropsychiatric and MIA-Associated Autism Spectrum Disorders. Int. J. Mol. Sci. 2022, 23, 967. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2016, 42, 284–298. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Stevens, H.E.; Jones, K.L. Prenatal Stress, Maternal Immune Dysregulation, and Their Association With Autism Spectrum Disorders. Curr. Psychiatry Rep. 2018, 20, 1–12. [Google Scholar] [CrossRef]
- McLellan, J.; Kim, D.H.J.; Bruce, M.; Ramirez-Celis, A.; Van de Water, J. Maternal Immune Dysregulation and Autism–Understanding the Role of Cytokines, Chemokines and Autoantibodies. Front. Psychiatry 2022, 13, 834910. [Google Scholar] [CrossRef]
- Jones, K.L.; Van de Water, J. Maternal autoantibody related autism: mechanisms and pathways. Mol. Psychiatry 2018, 24, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, D.Q.; Shah, A.; Jhin, A.; Noel-MacDonnell, J.; Hecht, P.; Ferguson, B.J.; Bruce, D.; Tilley, M.; Talebizadeh, Z. microRNAs and Gene–Environment Interactions in Autism: Effects of Prenatal Maternal Stress and the SERT Gene on Maternal microRNA Expression. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef]
- Boktor, J.C.; Adame, M.D.; Rose, D.R.; Schumann, C.M.; Murray, K.D.; Bauman, M.D.; Careaga, M.; Mazmanian, S.K.; Ashwood, P.; Needham, B.D. Global metabolic profiles in a non-human primate model of maternal immune activation: implications for neurodevelopmental disorders. Mol. Psychiatry 2022, 27, 4959–4973. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Nørgaard-Pedersen, B.; Thorsen, P.; Mortensen, E.L.; Hougaard, D.M. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. World J. Biol. Psychiatry 2011, 14, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.W.; Larsen, N.; Mortensen, E.L.; Atladóttir, H.; Nørgaard-Pedersen, B.; Bonefeld-Jørgensen, E.C.; Grove, J.; Hougaard, D.M. Neonatal levels of cytokines and risk of autism spectrum disorders: An exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J. Neuroimmunol. 2012, 252, 75–82. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Bonefeld-Jørgensen, E.C.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, E.L. Neonatal chemokine levels and risk of autism spectrum disorders: Findings from a Danish historic birth cohort follow-up study. Cytokine 2013, 61, 370–376. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Pearce, B.D.; Larsen, N.; Greaves-Lord, K.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, E.L.; Grove, J. Amniotic Fluid MMP-9 and Neurotrophins in Autism Spectrum Disorders: An Exploratory Study. Autism Res. 2012, 5, 428–433. [Google Scholar] [CrossRef]
- Missault, S.; Van Den Eynde, K.; Vanden Berghe, W.; Fransen, E.; Weeren, A.; Timmermans, J.-P.; Kumar-Singh, S.; Dedeurwaerdere, S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain, Behav. Immun. 2014, 42, 138–146. [Google Scholar] [CrossRef]
- Han, V.X.; Jones, H.F.; Patel, S.; Mohammad, S.S.; Hofer, M.J.; Alshammery, S.; Maple-Brown, E.; Gold, W.; Brilot, F.; Dale, R.C. Emerging evidence of Toll-like receptors as a putative pathway linking maternal inflammation and neurodevelopmental disorders in human offspring: A systematic review. Brain, Behav. Immun. 2021, 99, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, S.; Dalman, C.; Karlsson, H.; Gardner, R. Association of maternal diabetes with neurodevelopmental disorders: autism spectrum disorders, attention-deficit/hyperactivity disorder and intellectual disability. Leuk. Res. 2020, 50, 459–474. [Google Scholar] [CrossRef]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of Prenatal Maternal Anemia With Neurodevelopmental Disorders. JAMA Psychiatry 2019, 76, 1294–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chan, A.Y.L.; Coghill, D.; Ip, P.; Lau, W.C.Y.; Simonoff, E.; Brauer, R.; Wei, L.; Wong, I.C.K.; Man, K.K.C. Association Between Prenatal Exposure to Antipsychotics and Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, Preterm Birth, and Small for Gestational Age. JAMA Intern. Med. 2021, 181, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Lawlor, D.A.; Larsson, H.; Montgomery, S. Association Between Hypertensive Disorders of Pregnancy and Neurodevelopmental Outcomes Among Offspring. JAMA Pediatr. 2021, 175, 577–585. [Google Scholar] [CrossRef]
- Bergdolt, L.; Dunaevsky, A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2018, 175, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Bauman, M.D. Primate Models as a Translational Tool for Understanding Prenatal Origins of Neurodevelopmental Disorders Associated With Maternal Infection. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2022, 7, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, R.M.; Iosif, A.-M.; Ryan, A.M.; Funk, L.H.; Murai, T.; Chen, S.; Lesh, T.A.; Rowland, D.J.; Bennett, J.; Hogrefe, C.E.; et al. Maternal Immune Activation during Pregnancy Alters Postnatal Brain Growth and Cognitive Development in Nonhuman Primate Offspring. J. Neurosci. 2021, 41, 9971–9987. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.D.; Iosif, A.-M.; Smith, S.E.; Bregere, C.; Amaral, D.G.; Patterson, P.H. Activation of the Maternal Immune System During Pregnancy Alters Behavioral Development of Rhesus Monkey Offspring. Biol. Psychiatry 2013, 75, 332–341. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, P.; Han, W.; Luo, Y.; Li, Y.; Yang, Y.; Xia, H.; Chen, Z.; Chen, Q.; Wang, H.; et al. Maternal Prenatal Inflammation Increases Brain Damage Susceptibility of Lipopolysaccharide in Adult Rat Offspring via COX-2/PGD-2/DPs Pathway Activation. Int. J. Mol. Sci. 2022, 23, 6142. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Zhang, J.; Luo, Y.; Han, W.; Wang, H.; Xia, H.; Chen, Z.; Yang, Y.; Chen, Q.; et al. Maternal Inflammation Exaggerates Offspring Susceptibility to Cerebral Ischemia–Reperfusion Injury via the COX-2/PGD2/DP2 Pathway Activation. Oxidative Med. Cell. Longev. 2022, 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Careaga, M.; Murai, T.; Bauman, M.D. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biol. Psychiatry 2016, 81, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.K.; Forghany, R.; Smith, S.E.; Patterson, P.H.; McAllister, A.K.; Schumann, C.M.; Bauman, M.D. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain, Behav. Immun. 2015, 48, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Careaga, M.; Van de Water, J.; McAllister, K.; Bauman, M.D.; Ashwood, P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain, Behav. Immun. 2016, 63, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Missault, S.; Van Den Eynde, K.; Vanden Berghe, W.; Fransen, E.; Weeren, A.; Timmermans, J.-P.; Kumar-Singh, S.; Dedeurwaerdere, S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain, Behav. Immun. 2014, 42, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.Y.; Lee, O.P.E.; Leong, M.C. Vitamin C deficiency as an unusual cause of pulmonary hypertension and refusal to walk. Cardiol. Young- 2020, 31, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Vuillermot, S.; Luan, W.; Meyer, U.; Eyles, D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol. Autism 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gáll, Z.; Székely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011, 25, 2167–2179. [Google Scholar] [CrossRef]
- Saurman, V.; Margolis, K.G.; Luna, R.A. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig. Dis. Sci. 2020, 65, 818–828. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.L., Patel, S.V., & Schmid, S. (2020). Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neuroscience and biobehavioral reviews, 113, 546–567. https://doi.org/10.1016/j.neubiorev.2020.04.012Delorme, T.C., Srivastava, L.K., & Cermakian, N. (2021). Altered circadian rhythms in a mouse model of neurodevelopmental disorders based on prenatal maternal immune activation. Brain, behavior, and immunity, 93, 119–131. https://doi.org/10.1016/j.bbi.2020.12.030.
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.S.; Polesel, M.; Richetto, J.; Meyer, U.; Weber-Stadlbauer, U. Mouse models of maternal immune activation: Mind your caging system! Brain, Behav. Immun. 2018, 73, 643–660. [Google Scholar] [CrossRef]
- Smolders, S.; Notter, T.; Smolders, S.M.; Rigo, J.-M.; Brône, B. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain, Behav. Immun. 2018, 73, 51–65. [Google Scholar] [CrossRef]
- de Cossío, L.F.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain, Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, T.; Shimada, H.; Sakata-Haga, H.; Iizuka, H.; Hatta, T. Molecular mechanisms underlying the models of neurodevelopmental disorders in maternal immune activation relevant to the placenta. Congenit. Anomalies 2018, 59, 81–87. [Google Scholar] [CrossRef]
- Meyer, U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef]
- Massarali, A.; Adhya, D.; Srivastava, D.P.; Baron-Cohen, S.; Kotter, M.R. Virus-Induced Maternal Immune Activation as an Environmental Factor in the Etiology of Autism and Schizophrenia. Front. Neurosci. 2022, 16, 834058. [Google Scholar] [CrossRef]
- Cheng, M.H.; Zhang, S.; Porritt, R.A.; Rivas, M.N.; Paschold, L.; Willscher, E.; Binder, M.; Arditi, M.; Bahar, I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. 2020, 117, 25254–25262. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 941009. [Google Scholar] [CrossRef]
- Porritt, R.A.; Paschold, L.; Rivas, M.N.; Cheng, M.H.; Yonker, L.M.; Chandnani, H.; Lopez, M.; Simnica, D.; Schultheiß, C.; Santiskulvong, C.; Van Eyk, J.; Fasano, A.; Bahar, I.; Binder, M.; Arditi, M. Identification of a unique TCR repertoire, consistent with a superantigen selection process in Children with Multi-system Inflammatory Syndrome. bioRxiv the preprint server for biology 2020, 2020.11.09.372169. [Google Scholar] [CrossRef]
- Wong, H.; Hoeffer, C. Maternal IL-17A in autism. Exp. Neurol. 2018, 299, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Tian, J.; Zhu, B.; Zhang, Y.; Yang, K.; Ling, Y.; Hu, Y. IL-17 and IFN-γ production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. European review for medical and pharmacological sciences 2012, 16, 2029–36. [Google Scholar] [PubMed]
- Shen, H.; Wang, Y.; Chen, C.Y.; Frencher, J.; Huang, D.; Yang, E.; Ryan-Payseur, B.; Chen, Z.W. Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vγ2Vδ2 T cells after Mycobacterium tuberculosis infection or vaccination. Eur. J. Immunol. 2014, 45, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, T.M.; Saunders, B.M.; Ryan, A.A.; Britton, W.J. Mycobacterium bovis BCG-Specific Th17 Cells Confer Partial Protection against Mycobacterium tuberculosis Infection in the Absence of Gamma Interferon. Infect. Immun. 2010, 78, 4187–4194. [Google Scholar] [CrossRef] [PubMed]
- Burl, S.; Adetifa, U.J.; Cox, M.; Touray, E.; Ota, M.O.; Marchant, A.; Whittle, H.; McShane, H.; Rowland-Jones, S.L.; Flanagan, K.L. Delaying Bacillus Calmette-Guérin Vaccination from Birth to 4 1/2 Months of Age Reduces Postvaccination Th1 and IL-17 Responses but Leads to Comparable Mycobacterial Responses at 9 Months of Age. J. Immunol. 2010, 185, 2620–2628. [Google Scholar] [CrossRef]
- Freches, D.; Romano, M.; Korf, H.; Renauld, J.-C.; Van Snick, J.; Uyttenhove, C.; Huygen, K. Increased Pulmonary Tumor Necrosis Factor Alpha, Interleukin-6 (IL-6), and IL-17A Responses Compensate for Decreased Gamma Interferon Production in Anti-IL-12 Autovaccine-Treated, Mycobacterium bovis BCG-Vaccinated Mice. Clin. Vaccine Immunol. 2011, 18, 95–104. [Google Scholar] [CrossRef]
- Pitt, J.M.; Stavropoulos, E.; Redford, P.S.; Beebe, A.M.; Bancroft, G.J.; Young, D.B.; O’garra, A. Blockade of IL-10 Signaling during Bacillus Calmette-Guérin Vaccination Enhances and Sustains Th1, Th17, and Innate Lymphoid IFN-γ and IL-17 Responses and Increases Protection to Mycobacterium tuberculosis Infection. Journal of immunology 2012, 189, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Bergdolt, L.; Dunaevsky, A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2018, 175, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.D.; Van de Water, J. Translational opportunities in the prenatal immune environment: Promises and limitations of the maternal immune activation model. Neurobiol. Dis. 2020, 141, 104864–104864. [Google Scholar] [CrossRef] [PubMed]
- Ashe, P.C., Berry, M.D., & Boulton, A.A. (2001). Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Progress in neuro-psychopharmacology & biological psychiatry, 25(4), 691–707. https://doi.org/10.1016/s0278-5846(01)00159-2Rund B. R. (2009). Is schizophrenia a neurodegenerative disorder?. Nordic journal of psychiatry, 63(3), 196–201. https://doi.org/10.1080/08039480902767286.
- Kochunov, P.; Hong, L.E. Neurodevelopmental and Neurodegenerative Models of Schizophrenia: White Matter at the Center Stage. Schizophr. Bull. 2014, 40, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.S.; Phillips, M.R.; Yang, L.H.; Kegeles, L.S.; Susser, E.S.; Lieberman, J.A. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophr. Res. 2022, 243, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-L.; Lin, H.-Y.; Tung, Y.-H.; Hwang, T.-J.; Chen, C.-L.; Wu, C.-S.; Shang, C.-Y.; Hwu, H.-G.; Tseng, W.-Y.I.; Liu, C.-M.; et al. Neurodevelopmental model of schizophrenia revisited: similarity in individual deviation and idiosyncrasy from the normative model of whole-brain white matter tracts and shared brain-cognition covariation with ADHD and ASD. Mol. Psychiatry 2022, 27, 3262–3271. [Google Scholar] [CrossRef] [PubMed]
- de Mooij, S.M.; Henson, R.N.; Waldorp, L.J.; Kievit, R.A. Age Differentiation within Gray Matter, White Matter, and between Memory and White Matter in an Adult Life Span Cohort. J. Neurosci. 2018, 38, 5826–5836. [Google Scholar] [CrossRef]
- Cropley, V.L.; Klauser, P.; Lenroot, R.K.; Bruggemann, J.; Sundram, S.; Bousman, C.; Pereira, A.; Di Biase, M.A.; Weickert, T.W.; Weickert, C.S.; et al. Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am. J. Psychiatry 2017, 174, 286–295. [Google Scholar] [CrossRef]
- Fletcher, E.; Gavett, B.; Harvey, D.; Farias, S.T.; Olichney, J.; Beckett, L.; DeCarli, C.; Mungas, D. Brain volume change and cognitive trajectories in aging. Neuropsychology 2018, 32, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chiappelli, J.; Kochunov, P.; Regenold, W.T.; Rapoport, S.I.; Hong, L.E. Is Schizophrenia a Neurodegenerative Disease? Evidence from Age-Related Decline of Brain-Derived Neurotrophic Factor in the Brains of Schizophrenia Patients and Matched Nonpsychiatric Controls. Neurodegener. Dis. 2014, 15, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lasoń, W.; Jantas, D.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef]
- Hollander, E.; Wang, A.T.; Braun, A.; Marsh, L. Neurological considerations: Autism and Parkinson's disease. Psychiatry Res. 2009, 170, 43–51. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. North Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-S.; Lin, P.-Y.; Liu, C.-H.; Su, H.-C.; Tsai, K.-J. Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Deneubourg, C.; Ramm, M.; Smith, L.J.; Baron, O.; Singh, K.; Byrne, S.C.; Duchen, M.R.; Gautel, M.; Eskelinen, E.-L.; Fanto, M.; et al. The spectrum of neurodevelopmental, neuromuscular and neurodegenerative disorders due to defective autophagy. Autophagy 2021, 18, 496–517. [Google Scholar] [CrossRef]
- Young, H.K.; Barton, B.A.; Waisbren, S.; Dale, L.P.; Ryan, M.M.; Webster, R.I.; North, K.N. Cognitive and Psychological Profile of Males With Becker Muscular Dystrophy. J. Child Neurol. 2007, 23, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Koeks, Z.; Hellebrekers, D.M.; van de Velde, N.M.; Alleman, I.; Spitali, P.; van Duyvenvoorde, H.A.; Verschuuren, J.J.; Hendriksen, J.G.; Niks, E.H. The neurocognitive profile of adults with Becker muscular dystrophy in the Netherlands. J. Neuromuscul. Dis. 2022, 9, 543–553. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Fratzl-Zelman, N.; O'Sullivan, R.; Bull, M.; Peel, N.F.; Pollitt, R.C.; Jones, R.; Milne, E.; Smith, K.; Roschger, P.; et al. Novel PLS3 variants in X-linked osteoporosis: Exploring bone material properties. Am. J. Med Genet. Part A 2018, 176, 1578–1586. [Google Scholar] [CrossRef]
- Yousefi, B.; Kokhaei, P.; Mehranfar, F.; Bahar, A.; Abdolshahi, A.; Emadi, A.; Eslami, M. The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neurosci. Biobehav. Rev. 2021, 132, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O'Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Fang, P., Kazmi, S.A., Jameson, K.G., & Hsiao, E.Y. (2020). The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell host & microbe, 28(2), 201–222. https://doi.org/10.1016/j.chom.2020.06.008Sasmita A. O. (2019). Modification of the gut microbiome to combat neurodegeneration. Reviews in the neurosciences, 30(8), 795–805. https://doi.org/10.1515/revneuro-2019-0005.
- Fang, X. Potential role of gut microbiota and tissue barriers in Parkinson's disease and amyotrophic lateral sclerosis. Int. J. Neurosci. 2015, 126, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation With Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evidence-Based Integr. Med. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Alfonsetti, M.; Castelli, V.; D’angelo, M. Are We What We Eat? Impact of Diet on the Gut–Brain Axis in Parkinson’s Disease. Nutrients 2022, 14, 380. [Google Scholar] [CrossRef]
- Moustafa, S.A.; Mohamed, S.; Dawood, A.; Azar, J.; Elmorsy, E.; Rizk, N.A.M.; Salama, M. Gut brain axis: an insight into microbiota role in Parkinson’s disease. Metab. Brain Dis. 2021, 36, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson's disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- Gonatopoulos-Pournatzis, T.; Niibori, R.; Salter, E.W.; Weatheritt, R.J.; Tsang, B.; Farhangmehr, S.; Liang, X.; Braunschweig, U.; Roth, J.; Zhang, S.; et al. Autism-Misregulated eIF4G Microexons Control Synaptic Translation and Higher Order Cognitive Functions. Mol. Cell 2020, 77, 1176–1192. [Google Scholar] [CrossRef]
- Gonatopoulos-Pournatzis, T.; Wu, M.; Braunschweig, U.; Roth, J.; Han, H.; Best, A.J.; Raj, B.; Aregger, M.; O’hanlon, D.; Ellis, J.D.; et al. Genome-wide CRISPR-Cas9 Interrogation of Splicing Networks Reveals a Mechanism for Recognition of Autism-Misregulated Neuronal Microexons. Mol. Cell 2018, 72, 510–524. [Google Scholar] [CrossRef]
- Irimia, M.; Weatheritt, R.J.; Ellis, J.D.; Parikshak, N.N.; Gonatopoulos-Pournatzis, T.; Babor, M.; Quesnel-Vallières, M.; Tapial, J.; Raj, B.; O’hanlon, D.; et al. A Highly Conserved Program of Neuronal Microexons Is Misregulated in Autistic Brains. Cell 2014, 159, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Quesnel-Vallières, M.; Irimia, M.; Cordes, S.P.; Blencowe, B.J. Essential roles for the splicing regulator nSR100/SRRM4 during nervous system development. Genes Dev. 2015, 29, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; Irimia, M.; Braunschweig, U.; Sterne-Weiler, T.; O’hanlon, D.; Lin, Z.-Y.; Chen, G.I.; Easton, L.E.; Ule, J.; Gingras, A.-C.; et al. A Global Regulatory Mechanism for Activating an Exon Network Required for Neurogenesis. Mol. Cell 2014, 56, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; O'Hanlon, D.; Vessey, J.P.; Pan, Q.; Ray, D.; Buckley, N.J.; Miller, F.D.; Blencowe, B.J. Cross-Regulation between an Alternative Splicing Activator and a Transcription Repressor Controls Neurogenesis. Mol. Cell 2011, 43, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Saso, A.; Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 2017, 39, 627–642. [Google Scholar] [CrossRef]
- Chaudhari, T. Vaccinations in the newborn. Best Pr. Res. Clin. Obstet. Gynaecol. 2020, 76, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Sakala, I.G.; Eichinger, K.M.; Petrovsky, N. Neonatal vaccine effectiveness and the role of adjuvants. Expert Rev. Clin. Immunol. 2019, 15, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Clemens, E.A.; Alexander-Miller, M.A. Understanding Antibody Responses in Early Life: Baby Steps towards Developing an Effective Influenza Vaccine. Viruses 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Julien, J.-P.; Rivest, S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002, 3, 216–227. [Google Scholar] [CrossRef]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2009, 58, 253–263. [Google Scholar] [CrossRef]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, Inflammation, and Innate Immunity. Neurol. Clin. 2006, 24, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.d.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2020, 1866, 165823–165823. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The role of IL-6 in mediating the anti-inflammatory effects of exercise. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2006, 57 (Suppl 10), 43–51. [Google Scholar] [PubMed]
- Hsu, C.-J.; Wong, L.-C.; Lee, W.-T. Immunological Dysfunction in Tourette Syndrome and Related Disorders. Int. J. Mol. Sci. 2021, 22, 853. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.L.; Swedo, S.E. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int. J. Neuropsychopharmacol. 2001, 4, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bellato, A.; Norman, L.; Idrees, I.; Ogawa, C.Y.; Waitt, A.; Zuccolo, P.F.; Tye, C.; Radua, J.; Groom, M.J.; Shephard, E. A systematic review and meta-analysis of altered electrophysiological markers of performance monitoring in Obsessive-Compulsive Disorder (OCD), Gilles de la Tourette Syndrome (GTS), Attention-Deficit/Hyperactivity disorder (ADHD) and Autism. Neurosci. Biobehav. Rev. 2021, 131, 964–987. [Google Scholar] [CrossRef] [PubMed]
- Kurlan, R. Tourette's syndrome and ‘PANDAS’ Will the relation bear out? Neurology 1998, 50, 1530–1534. [Google Scholar] [CrossRef]
- Trifiletti, R.R.; Packard, A.M. Immune mechanisms in pediatric neuropsychiatric disorders. Tourette's syndrome, OCD, and PANDAS. Child and adolescent psychiatric clinics of North America 1999, 8, 767–75. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Suhas, S. Neurocognitive deficits in obsessive–compulsive disorder: A selective review. Indian J. Psychiatry 2019, 61, 30–S36. [Google Scholar] [CrossRef]
- Cox, D.J.; Field, R.H.; Williams, D.G.; Baran, M.; Bowie, A.G.; Cunningham, C.; Dunne, A. DNA sensors are expressed in astrocytes and microglia in vitro and are upregulated during gliosis in neurodegenerative disease. Glia 2015, 63, 812–825. [Google Scholar] [CrossRef]
- Elizalde-Díaz, J.P.; Miranda-Narváez, C.L.; Martínez-Lazcano, J.C.; Martínez-Martínez, E. The relationship between chronic immune response and neurodegenerative damage in long COVID-19. Front. Immunol. 2022, 13, 1039427. [Google Scholar] [CrossRef] [PubMed]
- Zengeler, K.E.; Lukens, J.R. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat. Rev. Immunol. 2021, 21, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015, 1617, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 2018, 48, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, B.; Oler, E.; Armistead, B.; Elsayed, N.A.; Weinberger, D.R.; Bernier, R.; Burd, I.; Kapur, R.; Jacobsson, B.; Wang, C.; Mysorekar, I.; Rajagopal, L.; Adams Waldorf, K.M. The fetal origins of mental illness. American journal of obstetrics and gynecology 2019, 221, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry — novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Prenatal factors associated with autism spectrum disorder (ASD). Reprod. Toxicol. 2015, 56, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Buerki, S.F.; Kurz, J.; Mohaupt, M.G. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. Int. J. Mol. Sci. 2021, 22, 12324. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.P.; Fontes-Dantas, F.L.; da Poian, A.T.; Clarke, J.R. SARS-CoV-2-associated cytokine storm during pregnancy as a possible risk factor for neuropsychiatric disorder development in post-pandemic infants. Neuropharmacology 2021, 201, 108841–108841. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Kim, G.H.; Tan, J.-W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.-Y.; Xu, H.; Lee, S.-H.; Do, N.-Y.; et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Eberl, G. A new age for (mucosal) NeuroImmunology. Mucosal Immunol. 2022, 15, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, L.; Servettini, I.; Caramia, M.; Catacuzzeno, L.; Franciolini, F.; D’adamo, M.C.; Pessia, M. Update on the implication of potassium channels in autism: K+ channelautism spectrum disorder. Front. Cell. Neurosci. 2015, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.H.; Guo, S.Z.; Iyer, V.G. Agents which block potassium-chloride cotransport prevent sound-triggered seizures in post-ischemic audiogenic seizure-prone rats. Brain Res. 2000, 864, 134–137. [Google Scholar] [CrossRef]
- Martel, P.; Leo, D.; Fulton, S.; Bérard, M.; Trudeau, L.-E. Role of Kv1 Potassium Channels in Regulating Dopamine Release and Presynaptic D2 Receptor Function. PLOS ONE 2011, 6, e20402. [Google Scholar] [CrossRef]
- Fung, L.K.; Libove, R.A.; Phillips, J.; Haddad, F.; Hardan, A.Y. Brief Report: An Open-Label Study of the Neurosteroid Pregnenolone in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2014, 44, 2971–2977. [Google Scholar] [CrossRef]
- A Geier, D.; Geier, M.R. A clinical trial of combined anti-androgen and anti-heavy metal therapy in autistic disorders. Neuro endocrinology letters 2006, 27, 833–8. [Google Scholar] [PubMed]
- Palomba, S.; Orio, F.; Falbo, A.; Oppedisano, R.; Tolino, A.; Zullo, F. Tibolone reverses the cognitive effects caused by leuprolide acetate administration, improving mood and quality of life in patients with symptomatic uterine leiomyomas. Fertil. Steril. 2008, 90, 165–173. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Neurosteroids and Ischemic Stroke: Progesterone a Promising Agent in Reducing the Brain Injury in Ischemic Stroke. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 191–205. [Google Scholar] [CrossRef]
- Medical article indicating a possible efficacy of leuprolide acetate against Alzheimer’s Disease; available at: https://www.neurologyadvisor.
- Schober, J.M.; Kuhn, P.J.; Kovacs, P.G.; Earle, J.H.; Byrne, P.M.; Fries, R.A. Leuprolide Acetate Suppresses Pedophilic Urges and Arousability. Arch. Sex. Behav. 2005, 34, 691–705. [Google Scholar] [CrossRef]
- Briken, P.; Berner, W.; Noldus, J.; Nika, E.; Michl, U. Therapie mit dem LHRH-Agonisten Leuprorelinacetat bei Paraphilien und sexuell aggressiven Impulshandlungen. Der Nervenarzt 2000, 71, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Bourreau, E.; Jung-Testas, I.; Robel, P.; E Baulieu, E. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc. Natl. Acad. Sci. 1987, 84, 8215–8219. [Google Scholar] [CrossRef]
- Testas, I.J.; Hu, Z.Y.; Baulieuf, E.E.; Robel, P. Neurosteroids: Biosynthesis of Pregnenolone and Progesterone in Primary Cultures of Rat Glial Cells*. Endocrinology 1989, 125, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Máčová, L.; Bičíková, M.; Ostatníková, D.; Hill, M.; Stárka, L. Vitamin D, Neurosteroids and Autism. Physiol. Res. 2017, 66, S333–S340. [Google Scholar] [CrossRef]
- Maguire, J. Neurosteroid Deficiency Associated with Epilepsy. Epilepsy Curr. 2016, 16, 108–109. [Google Scholar] [CrossRef]
- Siracusano, M.; Riccioni, A.; Abate, R.; Benvenuto, A.; Curatolo, P.; Mazzone, L. Vitamin D Deficiency and Autism Spectrum Disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef]
- Cannell, J.J. Autism and vitamin D. Med. Hypotheses 2008, 70, 750–759. [Google Scholar] [CrossRef]
- Stubbs, G.; Henley, K.; Green, J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses 2016, 88, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Elnazir, B.; Greally, P.; Coghlan, D. Blunted serum 25(OH)D response to vitamin D3supplementation in children with autism. Nutr. Neurosci. 2020, 23, 537–542. [Google Scholar] [CrossRef]
- Grant, W.B. Vitamin D and health in the Mediterranean countries. Hormones 2018, 18, 23–35. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroid replacement therapy for catamenial epilepsy, postpartum depression and neuroendocrine disorders in women. J. Neuroendocr. 2021, 34, e13028–e13028. [Google Scholar] [CrossRef] [PubMed]
- Carp, T.; Metoudi, M.; Brown, B.; Ojha, V. Low-Dose Interferon I and III-Based Nasal Sprays: A Good-Looking COVID-19 Vaccine Candidate and a Therapy of the Future? Preprints.org 2022, 2022120155. [Google Scholar] [CrossRef]
- Carp, T.N. Countering and tackling advanced first-line immune evasion represents the most feasible and precise approach to control and eradicate rabies.
- Carp, T.N. Applied Theory of Relativity into Human and Animal Biology and Psychology? Preprints. 2024. [Google Scholar] [CrossRef]
- Calamassi, D.; Vigni, M.L.L.; Fumagalli, C.; Gheri, F.; Pomponi, G.P.; Bambi, S. The Listening to music tuned to 440 Hz versus 432 Hz to reduce anxiety and stress in emergency nurses during the COVID-19 pandemic: a double-blind, randomized controlled pilot study. Acta bio-medica : Atenei Parmensis 2022, 93, e2022149. [Google Scholar] [CrossRef]
- Calamassi, D.; Pomponi, G.P. Music Tuned to 440 Hz Versus 432 Hz and the Health Effects: A Double-blind Cross-over Pilot Study. EXPLORE 2019, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Calamassi, D.; Lucicesare, A.; Pomponi, G.P.; Bambi, S. Music tuned to 432 Hz versus music tuned to 440 Hz for improving sleep in patients with spinal cord injuries: a double-blind cross-over pilot study. Acta bio-medica : Atenei Parmensis 2020, 91((12-S)), e2020008. [Google Scholar] [CrossRef] [PubMed]
- The ALSUntangled Group ALSUntangled, No. 23: The Rife Machine and retroviruses. Amyotroph. Lateral Scler. Front. Degener. 2013, 15, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Blizard, R. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016, 101, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Carter, C. The barrier, airway particle clearance, placental and detoxification functions of autism susceptibility genes. Neurochem. Int. 2016, 99, 42–51. [Google Scholar] [CrossRef]
- Wong, C.T.; Wais, J.; Crawford, D.A. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur. J. Neurosci. 2015, 42, 2742–2760. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Potential for Early-Life Immune Insult Including Developmental Immunotoxicity in Autism and Autism Spectrum Disorders: Focus on Critical Windows of Immune Vulnerability. J. Toxicol. Environ. Heal. Part B 2008, 11, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. Developmental Immunotoxicology: Focus on Health Risks. Chem. Res. Toxicol. 2008, 22, 17–23. [Google Scholar] [CrossRef] [PubMed]
- van De Sande, M.M.H.; van Buul, V.J.; Brouns, F.J.P.H. Autism and nutrition: the role of the gut–brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Kawicka, A.; Regulska-Ilow, B. How nutritional status, diet and dietary supplements can affect autism. A review. Roczniki Panstwowego Zakladu Higieny 2013, 64, 1–12. [Google Scholar] [PubMed]
- Hsiao, E.Y. Gastrointestinal Issues in Autism Spectrum Disorder. Harv. Rev. Psychiatry 2014, 22, 104–111. [Google Scholar] [CrossRef]
- Bellato, A.; Norman, L.; Idrees, I.; Ogawa, C.Y.; Waitt, A.; Zuccolo, P.F.; Tye, C.; Radua, J.; Groom, M.J.; Shephard, E. A systematic review and meta-analysis of altered electrophysiological markers of performance monitoring in Obsessive-Compulsive Disorder (OCD), Gilles de la Tourette Syndrome (GTS), Attention-Deficit/Hyperactivity disorder (ADHD) and Autism. Neurosci. Biobehav. Rev. 2021, 131, 964–987. [Google Scholar] [CrossRef]
- Lutein, Brain, and Neurological Functions. (2015). Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease, 41–47. [CrossRef]
- The Urban Child Institute (2011), Memphis’ Education Funding Misses Best Chance For Impact. https://urbanchildinstitute.org/articles/perceptions/memphis-education-funding-misses-best-chance-for-impact.
- Daniel Garisto (2022), The Universe Is Not Locally Real and the Physics Nobel Prize Winners Proved It, available at: https://www.scientificamerican.com/article/the-universe-is-not-locally-real-and-the-physics-nobel-prize-winners-proved-it/.
- Transcranial Magnetic Stimulation: https://neuromodec.org/what-is-transcranial-magnetic-stimulation-tms/.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




