Introduction
Visceral leishmaniasis (VL) is a severe, neglected tropical disease and a serious global public health problem, endemic in 92 countries, particularly in Brazil, East Africa, and India [
1,
2]. In 2020, Brazil represented over 90% of the VL cases in the region of Americas, with an average of
1.492 cases reported annually [
3].
Leishmania (L.) species are known to cause VL in both humans and animal reservoir hosts, with
L. infantum and
L. donovani as main etiologic agents [
1]. In Brazil, VL is caused by the obligate intracellular protozoan
L. infantum (synonym of
L. chagasi). Transmission to man and dogs occurs usually through the bite of female infected phlebotomine sandflies [
4]. Domestic dogs (
Canis familiaris) represent the major rural and urban reservoir of
L. infantum and can also develop the disease. CanL shows clinical variability, ranging from asymptomatic (low parasite burden) to severely ill dogs (high parasite burden). Infected dogs are a major source of
L. infantum and thus represent a risk factor for spreading the disease to humans [
5,
6,
7].
Diagnosis of CanL remains a major challenge due to the variable clinical manifestations and the large number of asymptomatic dogs. CanL diagnosis through positive parasitological testing involves the detection of amastigotes in aspirates and smears of bone marrow, spleen, liver, lymph nodes and biopsies of either intact or injured skin [
8]. Despite the high specificity, the diagnostic outcome depends on not only the training and ability of the observer, but also on the parasite load and the type of immune response developed by the dog. Thus, serological tests are often used as an alternative to parasitological diagnosis, demonstrating anti-
Leishmania antibodies, mainly of IgG isotype, to detect infected animals [
9]. However, some asymptomatic dogs cannot be detected by conventional serological tests, while through molecular diagnostics, such as PCR, the percentage of sero-negative, asymptomatic dogs is considerably high in endemic areas [
10].
ELISA and immunochromatographic rapid tests (IRT) are the most used serological methods for the diagnosis of CanL, often based on whole parasite antigens. To reduce cross-reactions with other endemic infections such as trypanosomiasis and Ehrlichiosis, efforts have been made to synthesize recombinant proteins from immunodominant
Leishmania antigens, rather than using crude
Leishmania antigens [
11].
Recombinant antigens commonly used for diagnosis of CanL are rK39 and rK28. The rK39 is a kinesin-related protein from L.
infantum consisting of 6.5 copies of the 39 amino acid (AA) tandem repeats (TR). AA sequences of rk39 are similar between
L. donovani and
L. infantum and provide good sensitivity for the diagnosis of symptomatic CanL cases [
12,
13]. The recombinant chimeric protein rK28 generated by the fusion of rK39, rK9 and rK26 from
L. donovani has an identical AA sequence between
L. donovani and
L. infantum and has been shown to provide high accuracy in the detecting symptomatic dogs particular in regions where sensitivity to rK39 is low [
14,
15].
The Dual Path Platform (DPP®, Biomanguinhos, FIOCRUZ-RJ), a rapid test based on lateral flow (LF) technology, detects antibodies against the rK28 fusion protein and is recommended for CanL screening in Brazil [
15]. Although the DPP test is not sensitive enough to reliably detect asymptomatic dogs infected with
L. infantum, its high sensitivity in symptomatic dogs makes it useful for confirming clinically suspected cases [
16].
Recently, a new diagnostic antigen, rKLi8.3, containing 8.3 TR motifs from a
L. infantum strain from Sudan, was identified by comparison of kinesin sequences from several VL strains, together with computational analysis of structural requirements of TRs for optimal B cell antigenicity. The rKLi8.3 based ELISA showed increased sensitivity and specificity in different VL and CanL endemic areas compared to the rK39 and rK28 ELISAs [
17,
18].
Here, we investigated whether the diagnostic performance of VL-infected animals with low antibody titers can be improved by including an additional step with rabbit anti-canine IgG antibodies in the rKLi8.3-ELISA. The assay relied on the use of protein A/G, which is a molecule that binds to the Fc portion of IgGs from many mammalian species [
19,
20]. The results demonstrate that addition of an amplifying anti-IgG incubation is able to enhance the detection of rKLi8.3-specific antibodies particularly in low clinical score dogs.
Materials and methods
Serum samples
A total of 38 dog serum samples were obtained from the Protozoology Laboratory, Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, RJ, Brazil. Samples were collected from Leishmania-infected dogs from areas endemic for leishmaniasis in the state of Mato Grosso, Brazil. Diagnosis was based on the presence of amastigotes in the spleen. All dogs underwent a clinical examination by two veterinarians to assess common clinical signs of CanL: dermatitis, onychogryphosis, conjunctivitis, weight loss, alopecia and lymphadenopathy [
21]. The severity of each clinical sign was rated on a 0 (not present), 1 (mild), 2 (moderate), and 3 (severe) scale, as per [
22]. The CanL samples were further divided into two groups based on clinical score: the low clinical score group (CanL-L) with clinical scores ranging from 0-5 points and the high clinical score group (CanL-H) with clinical scores ranging from 6-18 points. Additionally, two control groups were included in the study. The first control group comprised 21 healthy dogs from Rio de Janeiro, RJ, Brazil, with no previous history of leishmaniasis and tested negative with the rapid immunochromatographic test TR-DPP® BioManguinhos (H-Ct); the infection control group (I-Ct) consisted of 37 dogs diagnosed with other infections, including acute trypanosomiasis (n=20), anaplasmosis (n = 3), ehrlichiosis (n = 4), babesiosis (n = 6) and toxoplasmosis (n = 4). I-Ct samples were obtained from the Department of Parasitology at the Federal University of Minas Gerais, and the Protozoology Laboratory (FIOCRUZ-RJ) for Toxoplasma samples. Trypanosomiasis samples were obtained from dogs experimentally infected with
Trypanosoma cruzi during the acute phase and were obtained from the Laboratory of Chagas Disease at the Federal University of Ouro Preto-MG. All samples were stored at -20ºC until further evaluation.
Ethical Issues
Samples from dogs diagnosed with CanL were obtained from necropsies performed by the Zoonosis Control Center in Cuiabá - MT, Brazil according to the recommendations of the Ministry of Health. There was no animal experimentation, therefore, no need for a license according to the guidance of the Ethics Committee on Animal Use of the Oswaldo Cruz Foundation and the Brazilian law 11794/08. The access to genetic heritage is registered and certified in SISGEN under number A76E438.
rKLi8.3 recombinant protein
The rKLi8.3 recombinant protein was expressed and purified from an
L. infantum kinesin, at the Institute for Medical Microbiology, Philipps University of Marburg, Marburg, Germany as described [
17]. In brief, the kinesin gene (8.3 tandem repeats) was amplified by polymerase chain reaction (PCR) and cloned into the pCR 2.1-TOPO (Invitrogen Life Technologies, USA) pQ41 bacterial expression plasmid vector (Qiagen GmbH, Germany), containing an N-terminal histidine tag. Escherichia coli HB101 (Promega, Germany) were transformed with recombinant plasmids and subcloned into the pET28a(1) expression vector. The plasmids were transformed into BL21(DE3)
E. coli (Sigma-Aldrich, Germany), and bacterial lysates were purified by Ni21 affinity chromatography and ÄKTA Prime (GE Healthcare, USA). The impact of the number of TR on B-cell antigenicity was analyzed using the prediction program of linear B-cell epitopes, BepiPred 1.0. Purity and size were verified by gel electrophoresis and western blotting with anti-His antibodies and sera from patients with VL [
17].
Sero-diagnostic test systems
DPP® (Biomanguinhos/Fiocruz, Rio de Janeiro, Brazil) is an immunochromatographic test recommended for screening and diagnosis of CanL by the Brazilian Ministry of Health. It is based on rK28 protein, a chimeric protein generated by the fusion of rK39, rK9 and rK26 kinesin-related recombinant antigens from
L. donovani [
16]. DPP was obtained from Biomanguinhos/Fiocruz as a donation for research purposes.
Enzyme-linked immunosorbent assay (ELISA), was used as indirect rKLi8.3 based ELISA (rKLi8.3-ELISA), manufactured by Gold Standard Diagnostics Frankfurt (GSD Frankfurt) Germany. Dog sera were diluted 1/100 in phosphate buffer (10mM), pH7.2 and incubated for 1 hour at 37oC. Wells were then washed with phosphate buffer (0.2M), pH7.2 and, in indicated experiments, a new step involving incubation with rabbit anti-dog IgG (1:500, Invitrogen, USA) was included. After incubation at 25oC for 30 min, the plates were washed three times and the conjugate formed by protein A/G linked to peroxidase (NovaTec) was added. After incubation for 30 minutes at room temperature and subsequent washing, the substrate (TMB, NovaTec) was added. The reaction was stopped by adding of 2N H2SO4 (NovaTec) and the readings performed with an ELISA Spectramax-190 reader (Molecular Devices, Sunnyvale, CA, USA) at 450nm. The results were expressed by NovaTec Units (NTU), calculated by the formula NTU = Sx10/Cut-off, where S is the average optical density value of the duplicate test samples and Cut-off is the mean absorbance value of the Cut-off Control determinants (NovaTec Immunodiagnostica GMBH).
rKLi8.3 based immunochromatographic lateral flow test (LFT, INgezim® Leishma CROM, GSD Madrid, Spain) using protein A/G as capture reagent was used for rapid detection of VL-specific antibodies. A total volume of 10 µL of serum was added to the application zone. After the sample was completely absorbed, 150 µl of running buffer (Tris-HCl, pH 7.5) was added and the results were read after 10 min. According to the manufacturer’s protocols, valid LFT results (strong internal control band) were performed once. If the control band was weak or absent, the LFT were repeated.
Results
Dogs with parasitologically confirmed CanL cases were divided into low clinical score (CanL-L, n=19) and high clinical score animals (CanL-H, n=19) and classified according to the number of parasites in the spleen (
Table 1). The control groups consisted of 21 uninfected, healthy dogs (H-Ct) and 37 dogs diagnosed with other infections (I-Ct). Prior to testing CanL-specific IgG antibodies with the rKLi8.3 ELISA and by LFT (INgezim®), all serum samples were pre-tested with the Dual Path Platform (DPP®-CanL; Biomanguinhos/Fiocruz, Rio de Janeiro, Brazil).
The number of DPP positive dogs was higher in the CanL-H than in the CanL-L group (
Table 1). LFT and ELISA showed a sensitivity and specificity similar to DPP. LFT and rKLi8.3-ELISA were negative for all H-Ct (Table S1) while CanL-H dogs were highly positive (84,2%). Interestingly, several infected but low clinical score dogs (approx. 50%) were neither tested positive by DPP and LFT nor by rKLi8.3-ELISA (
Table 1).
To assess the concordance between LFT and DPP rapid tests as well as rKLi8.3-ELISA, serial dilutions of positive sera were comparatively tested with above mentioned tests.
Table 2 shows that all three test systems gave the same results of CanL positivity. The high antibody titer (1:4096) of the CanL-H 256 serum sample measured by DPP and LFT appears to correlate with increased parasite load rather than clinical score (7 versus 12) compared to CanL-H 285. On the other hand, the CanL-L 251 serum with lowest clinical score (0) but high parasite load had the lowest antibody titer (1:16) in DPP and LFT. These results suggest a moderate positive correlation between parasite load, clinical score and serum antibody titer. They also demonstrate the high efficacy of the LFT, which is comparable to DPP in the serological diagnosis of CanL. rKLi8.3-ELISAs were similar to DPP and LFT in diagnosing CanL, but as expected, reveled an increased sensitivity to detect CanL as seen by highly diluted serum samples (
Table 2).
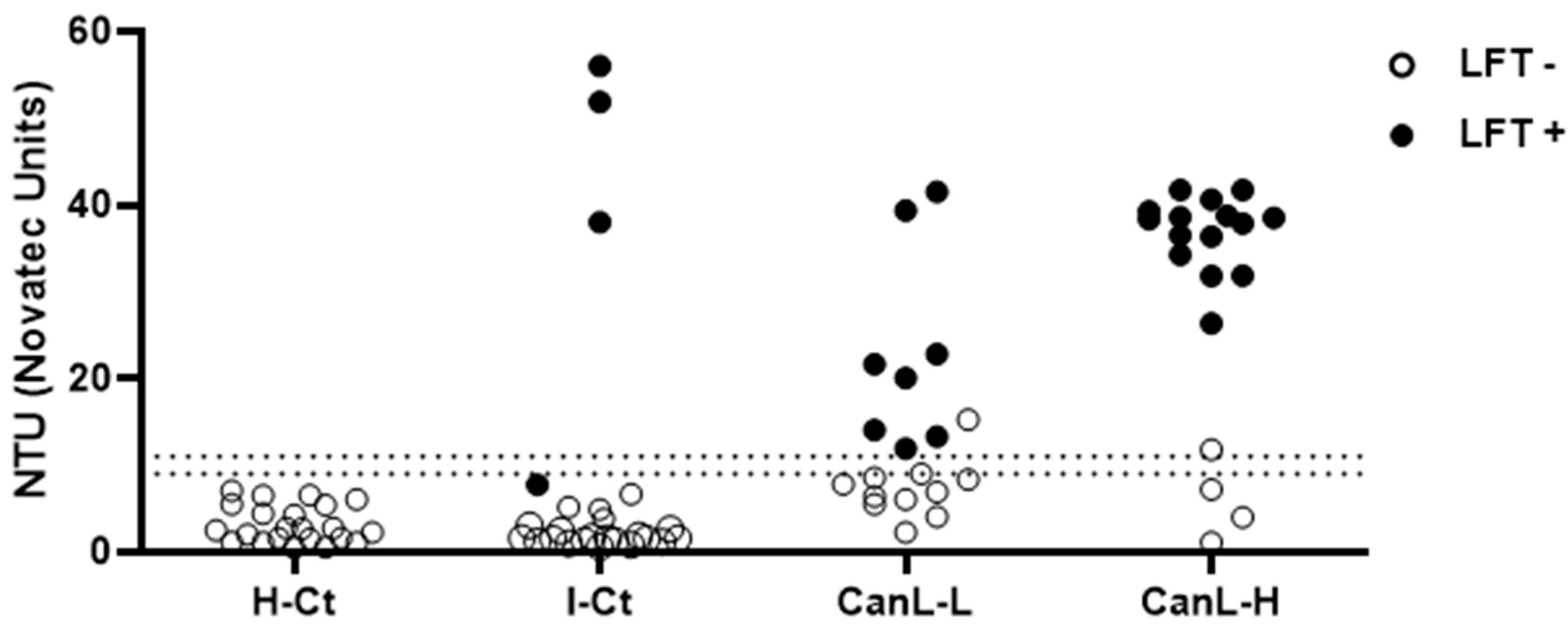
Significant amounts of rKLi8.3-specific antibodies were detected by ELISA and LFT in the infected CanL group but not in the H-Ct group (
Figure 1). As expected, the number of positive sera was higher in the CanL-H group (16+/19) compared to the CanL-L group (9+/19). Within the infected control group (I-Ct) three sera were tested positive in ELISA (3+/37) and four in LFT but all H-Ct sera (n=21) were negative. In general, sera that were positive by ELISA were also positive by LFT, with only three exceptions, one in the I-Ct group and one in both the CanL-L and CanL-H groups (
Figure 1).
As the amount of
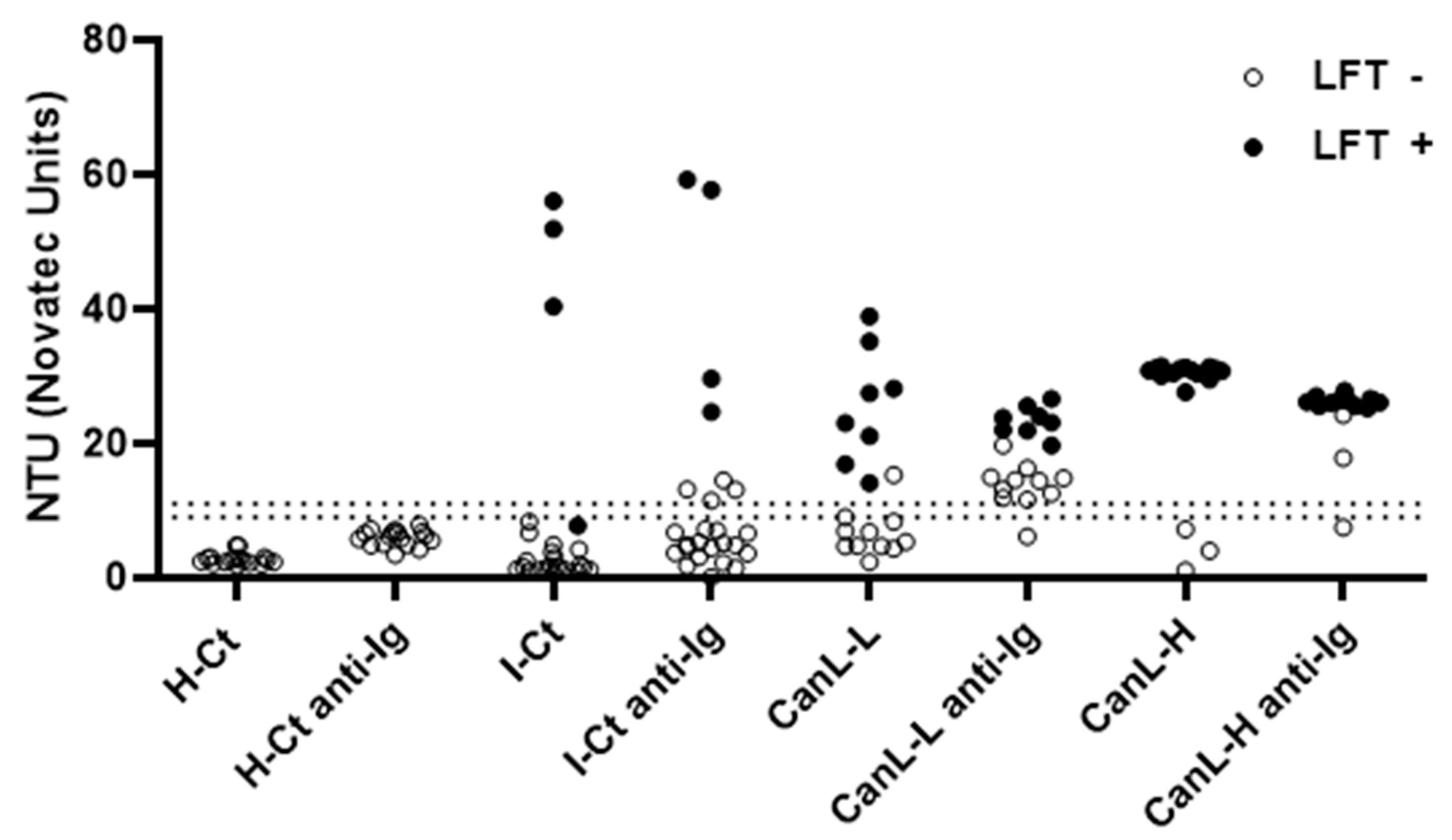
Leishmania-specific antibodies in CanL-asymptomatic dogs is often very low, we wondered whether we could enhance the sensitivity of the rKLi8.3-ELISA by adding an additional rabbit anti-dog IgG to the system, after serum incubation. Addition of rabbit anti-dog IgG antibodies further increased the sensitivity of rKLi8.3-ELISA at higher dilutions as shown in
Table 2. To further study whether the increase in sensitivity goes along with reduced specificity, we retested the sera shown in
Figure 1 with or without addition of the anti-IgG step.
Figure 2 demonstrates that sensitivity of the indirect rKLi8.3-ELISA increased without significantly influencing its specificity. All serum samples collected from healthy dogs remained negative. However, five out of thirty-four serum samples from the I-CT control group (14.7%) became positive after the anti-IgG addition. Interestingly, 51 sera from European dogs diagnosed with other infections did not show any positivity for CanL when tested by DPP, LFT or rKLi8.3-ELISA (data not shown). In addition, showing that the difficulty of detecting the CanL in dogs with low antibody titers is reversible, in the CanL-L group, 9 out of 10 previously negative sera became positive with the anti-IgG, and in the CanL-H group, two of the three negative sera became positive in the rKLi8.3-ELISA plus anti-IgG (
Figure 2).
Discussion
L. infantum-infected dogs, who reach a prevalence of 80% in some endemic areas, are the major reservoir of the parasite in urban areas, and thus play a key role in the transmission cycle of
Leishmania to humans [
5,
7]. In fact, an increase in CanL has been observed to precede an increase in human cases of leishmaniasis [
23], highlighting the need of early diagnosis of infected dogs. The serodiagnosis of CanL was investigated using the recently described rKLi8.3 antigen in the form of a Protein A/G-based ELISA and two point of care tests (POC), rKLi8.3-LFT and DPP. Our results show that the diagnostic accuracy of both rKLi8.3-ELISA and LFT was similar to that of DPP which is based on the rK9/rK26/rK39 antigens. Similarly, ELISAs using the rK39 and rK26 antigens gave good results in terms of diagnosing symptomatic dogs but not asymptomatic animals [
12].
Both POCs, the rKLi8.3-LFT and DPP showed very high sensitivity and specificity, with no false positive reaction in sera from healthy control animals, but moderate sensitivity in detecting asymptomatic CanL, represented by the low clinical score studied group. Although no significant differences were observed between the accuracy of LFT and DPP for detecting CanL, the rKLi8.3-LFT showed some advantages: The device is smaller and easier to perform, it is faster, and most importantly, the LFT results are more stable, as it remains unchanged for several days.
Interestingly, although the rKLi8.3 antigen was derived from a Sudanese
L. infantum strain, the rKLi8.3 ELISA and LFT showed high specificity and no false-positive reactions with sera from healthy control animals from Brazil. However, similar to DPP, which is based on the rK28 fusion protein, dogs with low clinical score CanL were difficult to detect. Although early diagnosis of CanL is critical for disease control, CanL dogs with low clinical scores, comparable to asymptomatic dogs, often represent the early stages of infection, when seroconversion has not yet occurred or is low. In these cases,
Leishmania-specific antibodies are absent or very low making serological diagnosis impossible or very difficult [
12,
24,
25]. As many asymptomatic, seronegative animals may develop symptoms with the time of infection and/or changes in their nutritional or immune status, they could become highly infectious to their sandfly vectors and thus also contribute to the transmission cycle of CanL [
26]. Thus, early and simple detection of CanL-infected asymptomatic dogs is highly desirable for the disease control.
As an alternative to enzyme conjugated antibodies, the
S. aureus protein A (PA) and streptococcal protein G (PG) have been applied in the diagnosis of many infectious diseases with the advantage of having affinity for immunoglobulins from various animal species. Evidence for a higher binding affinity of both PA and PG to IgG promoting the detection of lower amounts of antibodies than anti-IgG conjugates has been demonstrated in the diagnosis of diverse infectious diseases [
27,
28].
We were surprised to see that the addition of a second, rabbit anti-dog IgG antibody was able to significantly increase the sensitivity of the rKLi8.3-ELISA in detecting low clinical score CanL without altering the specificity. Then, this anti-dog IgG amplifies the sensing of the rKLi8.3-ELISA, promoting the detection of lower amounts of anti-L. infantum antibodies.
It is worth noting that three sera from the I-CT group were strongly positive in the rKLi8.3 ELISA and LFT. In agreement, these three sera also tested positive in the DPP (data not shown). However, in previous experiments, we tested the specificity of both assays with 52 sera from dogs that were infected with 7 different pathogens including,
Babesia,
Ehrlichia canis,
Anaplasma,
Giardia duodenalis,
Dirofilaria repens,
Toxocara and
Anclystoma duodenalis. None of these sera reacted with rKLi8.3, demonstrating the high specificity of this diagnostic antigen [
17]. We therefore assume that the three I-CT animals may also have been co-infected with
L. infantum but this was masked by another infection. Indeed, few sera (15%) from the other infections group turned positive with anti-IgG, which may reflect the presence of cross-reaction or previous exposure to
L. infantum, deserving further studies.
Several studies have shown that the diagnosis of
Leishmania infection in asymptomatic animals is problematic because there is no gold standard and both serological and parasitological methods have inherent limitations [
29]. In asymptomatic animals a low level of humoral reactivity can result in low antibody concentrations and borderline titers which can lead to false negative or positive results due to cross-reactivity [
10,
29]. In addition, the diagnostic performance of assays targeting kinesin-derived proteins may vary according to the geographical region examined and, in the case of CanL, according to the clinical signs [
30,
31].
In conclusion, our results highlight the usefulness of both rKLi8.3-ELISA and LFT in the diagnosis of CanL and demonstrate that the rKLi8.3-ELISA modified by the addition of an anti-IgG step, greatly enhances the ability to detect CanL in dogs with low antibody titers without reducing its specificity in healthy dogs or dogs infected with other diseases.
Author Contributions
All authors had access to the data in this study and take responsibility for integrity and accuracy of data analysis. The authors contribution was as follows: Conceptualization: R.M., U.S., H.C.T.; methodology: G.P.C.V., P.M.S., E.E.O, A.L., D.H., C.A., F.M. M.L.; Formal analysis: H.C.T., R.M., R.P., U.L.; writing-original draft preparation, H.C.T., R.M., U.S.; writing—review and editing: U.S., R.P., H.C.T.; resources, F.M., H.C.T., A.L., C.A., R.P.; critical review of manuscript and data: All authors; supervision, H.C.T. and U.S.; project administration, U.S. and H.C.T.; funding acquisition, U.S., R.P., H.C.T.
Funding
This work was supported by Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq, Project 310313/2019-8 to HCT) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Project RED-00313-16 to HCT). F.N.M. and GPCV received fellowships from CNPq. The work was also funded by the Loewe Center Druid (Project C4 to U.S.) within the Hessian Excellence Program.
Acknowledgments
We are grateful for the cooperation of the staff of the Zoonosis Control Center – MT, Brazil; We thank Dr. Ricardo T. Fujiwara (UFMG, Brazil) for the kind donation of sera from dogs with Babesia, Ehrlichia canis and Anaplasma.
Conflicts of Interest
U.S. and R.M. are inventors on a patent application related to the use of rKLi8.3 that has been filed by the Philipps-University Marburg (EP22152398.8). The title of patent application: Diagnostic test for high sensitive detection of antibodies from visceral Leishmaniasis patients. A.L., D.H. are employees of Gold Standard Diagnostics Frankfurt and C.A. is employed by Gold Standard Diagnostics, Madrid and were involved in the development of rKLi8.3 ELISA and Lateral flow test. The author(s) R.P and F.N.M. declared to be MDPI guest editors at the time of submission. This had no impact on the peer review process and the final decision.
References
- Alemayehu B, Alemayehu M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Science Journal. 2017, 11. [CrossRef]
- dos Santos PL, de Oliveira FA, Santos MLB, Cunha LCS, Lino MTB, de Oliveira MFS, et al. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLOS Neglected Tropical Diseases. 2016, 10, e0004375. [CrossRef]
- World Health Organization. Leishmaniasis [Internet]. Who.int. World Health Organization: WHO; 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- Peixoto HM, de Oliveira MRF, Romero GAS. Serological diagnosis of canine visceral leishmaniasis in Brazil: systematic review and meta-analysis. Tropical Medicine & International Health. 2014, 20, 334-52. [CrossRef]
- Reis AB, Martins-Filho OA, Teixeira-Carvalho A, Giunchetti RC, Carneiro CM, Mayrink W, et al. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Veterinary Immunology and Immunopathology [Internet]. 2009, 128, 87–95. [CrossRef] [PubMed]
- Leal GGA, Roatt BM, Aguiar-Soares RDO, Carneiro CM, Giunchetti RC, Teixeira-Carvalho A, et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Veterinary Parasitology. 2014, 205, 472-82. [CrossRef]
- Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ. Heterogeneities in Leishmania infantum Infection: Using skin parasite burdens to identify highly infectious dogs. PLoS Neglected Tropical Diseases. 2014, 8, e2583. [CrossRef]
- Figueiredo FB, Madeira MF, Nascimento LD, Abrantes TR, Mouta-Confort E, Passos RLS, et al. Canine visceral leishmaniasis: study of methods for the detection of IgG in serum and eluate samples. Revista do Instituto de Medicina Tropical de São Paulo. 2010, 52, 193-6. [CrossRef]
- do Rosario EY, Genaro O, França-Silva JCF, da Costa RT, Mayrink W, Reis AB, et al. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Memorias do Instituto Oswaldo Cruz. 2005, 100, 197–203. [CrossRef] [PubMed]
- Iniesta L, Fernández-Barredo S, Bulle B, Gómez MT, Piarroux R, Gállego M, et al. Diagnostic techniques to detect cryptic leishmaniasis in dogs. Clinical and Vaccine Immunology. 2002, 9, 1137-41. [CrossRef]
- Paz GF, Rugani JMN, Marcelino AP, Gontijo CMF. Implications of the use of serological and molecular methods to detect infection by Leishmania spp. in urban pet dogs. Acta Tropica. 2018, 182, 198–201. [CrossRef]
- Porrozzi R, da Costa MVS, Teva A, Falqueto A, Ferreira AL, dos Santos CD, et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clinical and Vaccine Immunology. 2007, 14, 544-8. [CrossRef]
- Venturin GL, Bragato JP, Silva KLO, de Lima VMF. Recombinant K28 antigen in ELISA in the diagnosis of canine visceral leishmaniosis. Parasite Immunology. 2015, 37, 670-3. [CrossRef]
- Lauricella MA, Maidana CG, Frias VF, Romagosa CM, Negri V, Benedetti R, et al. An rK28-Based immunoenzymatic assay for the diagnosis of canine visceral leishmaniasis in Latin America. American Journal of Tropical Medicine and Hygiene. 2016, 95, 92-8. [CrossRef]
- Dantas-Torres F, Sales KGS, da Silva LG, Otranto D, Figueredo LA. Level of agreement between two commercially available rapid serological tests and the official screening test used to detect Leishmania seropositive dogs in Brazil. The Veterinary Journal. 2018, 234, 102-4. [CrossRef]
- Grimaldi G, Teva A, Ferreira AL, dos Santos CB, Pinto IS, de-Azevedo CT, et al. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CANL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012, 106, 54-9. [CrossRef]
- Mahdavi R, Shams-Eldin H, Witt S, Latz A, Heinz D, Fresco-Taboada A, et al. Development of a novel enzyme-linked immunosorbent assay and lateral flow test system for improved serodiagnosis of visceral leishmaniasis in different areas of endemicity. Microbiology Spectrum. 2023, 11. [CrossRef]
- Mahdavi R, Martinkovic F, Shams-Eldin H, Pereira IE, Reis AB, Latz A, Heinz D, Aira C, Fresco-Taboada A, Abass E, Romero-Olmedo J, Teixeira HC, Steinhoff, U. Comparative study of a novel lateral flow rapid test with conventional serological test-systems for the diagnosis of Canine Leishmaniosis in Croatia and Brazil. Pathogens. 2023; preprint forthcoming.
- Lindmark R, Thorén-Tolling K, Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. Journal of Immunological Methods. 1983, 62, 1–13. [CrossRef] [PubMed]
- Dahlbom I, Agardh D, Hansson T. Protein A and protein G ELISA for the detection of IgG autoantibodies against tissue transglutaminase in childhood celiac disease. Clinica Chimica Acta. 2008, 395, 72-6. [CrossRef]
- Solano-Gallego L, Koutinas A, Miro G, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G: Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol 2009, 165, 1–18. [CrossRef]
- Quinnell RJ, Courtenay O, Davidson S, Garcez L, Lambson B, Ramos P, et al. Detection of Leishmania infantum by PCR, serology and cellular immune response in a cohort study of Brazilian dogs. Parasitology. 2001, 122. [CrossRef]
- Leite BMM, Solcà MS, Santos LCS, Coelho LB, Amorim LDAF, Donato LE, et al. The mass use of deltamethrin collars to control and prevent canine visceral leishmaniasis: A field effectiveness study in a highly endemic area. PLOS Neglected Tropical Diseases. 2018, 12, e0006496-6. [CrossRef]
- Vaish M, Bhatia A, Reed SG, Chakravarty J, Sundar S. Evaluation of rK28 antigen for serodiagnosis of visceral Leishmaniasis in India. Clinical Microbiology and Infection. 2012, 18, 81-5. [CrossRef]
- De Carvalho FLN, Riboldi EO, Bello GL, Ramos RR, Barcellos RB, Gehlen M, et al. Canine visceral leishmaniasis diagnosis: a comparative performance of serological and molecular tests in symptomatic and asymptomatic dogs. Epidemiology and Infection. 2018, 146, 571-6.
- Di Pietro S, Crinò C, Falcone A, Crupi R, Francaviglia F, Vitale F, et al. Parasitemia and its daily variation in canine leishmaniasis. Parasitology Research. 2020, 119, 3541-8. [CrossRef]
- Bhullar SS, Kashyap RS, Chandak NH, Purohit HJ, Taori GM, Daginawala HF. Protein A-based ELISA: its evaluation in the diagnosis of herpes simplex encephalitis. Viral Immunology. 2011, 24, 341-6. [CrossRef]
- Bezerra MF, Xavier CC, Almeida AMP., Reis CRS., Evaluation of a multi-species Protein A-ELISA assay for plague serologic diagnosis in humans and other mammal hosts. PLOS Neglected Tropical Diseases. 2022, 16, e0009805-3. [CrossRef]
- Otranto D, Paradies P, De Caprariis D, Stanneck D, Testini G, Grimm F, et al. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clinical and Vaccine Immunology. 2009, 16, 337-43. [CrossRef]
- Zijlstra EE, Nur Y, Desjeux P, Khalil EAG, El-hassan AM, Groen J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. 2001, 6, 108–13. [CrossRef]
- Siqueira WF, Cardoso MS, Clímaco MC, Silva ALT, Heidt B, Eersels K, et al. Serodiagnosis of leishmaniasis in asymptomatic and symptomatic dogs by use of the recombinant dynamin-1-like protein from Leishmania infantum: A preliminary study. Acta Tropica. 2023, 239, 106827-7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).