Submitted:
18 January 2024
Posted:
19 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Clinicopathological Features of 73 Patients with Laryngeal Cancer
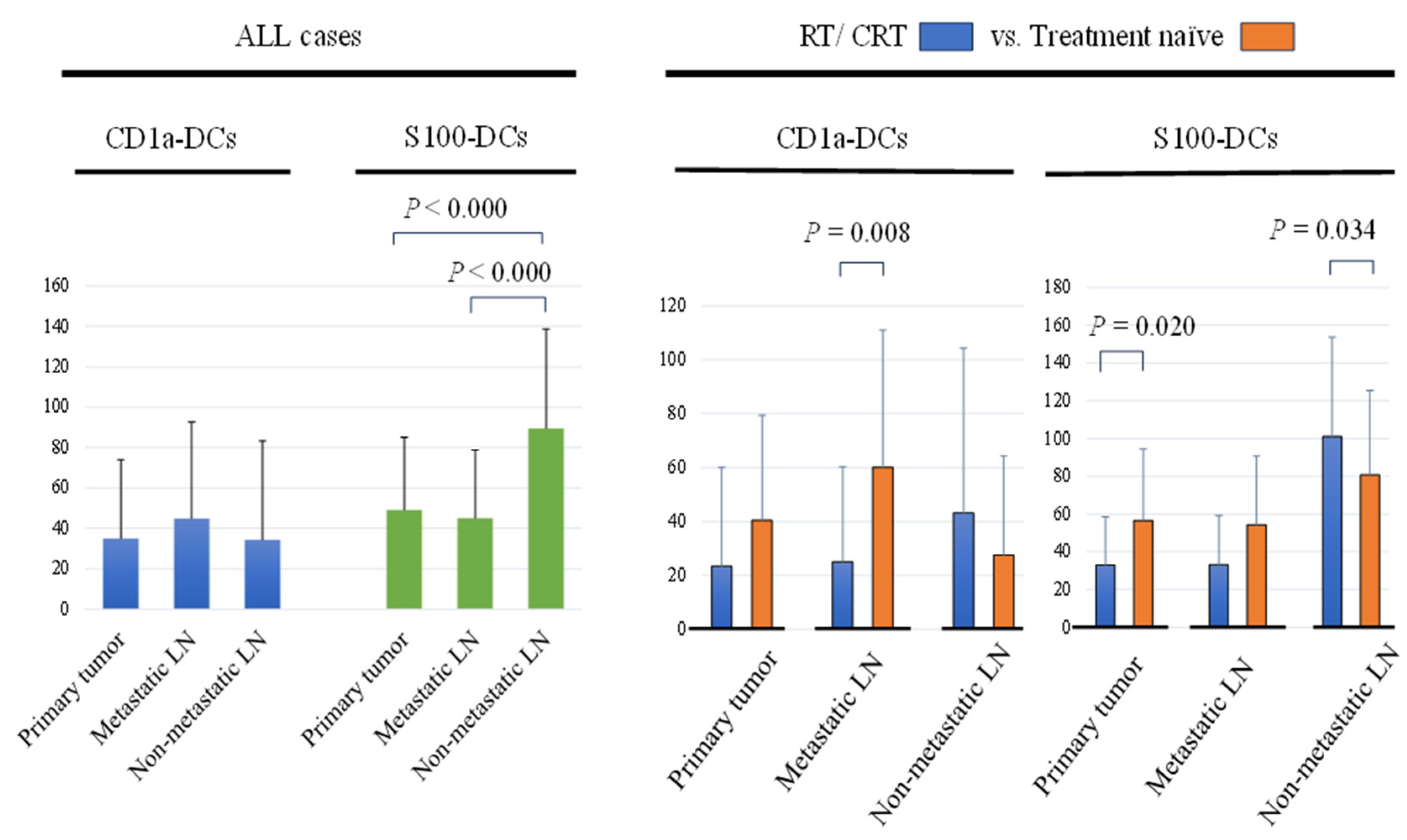
2.2. Assessment of DCs in Primary Tumor and Regional LNs
2.3. Comparison of DCs in Primary Tumor and Regional LNs between the RT/CRT and Treatment Naïve Groups
2.4. Clinicopathological Features per CD1a-DCs Infiltration in Primary Tumor, Metastatic LNs and Non-Metastatic LNs
2.5. Clinicopathological Features per S-100 DC Infiltration in Primary Tumor, Metastatic LNs and Non-Metastatic LNs
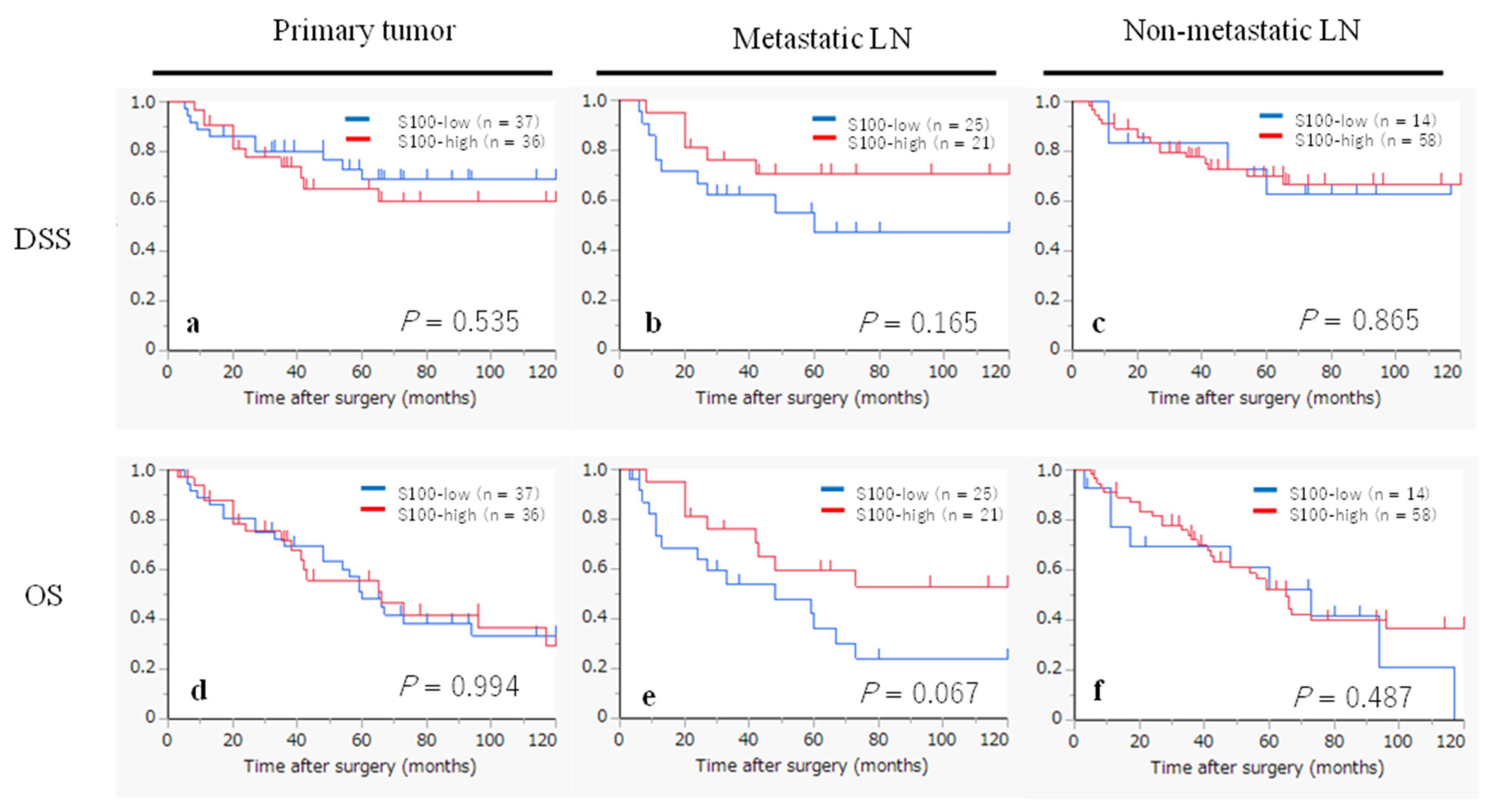
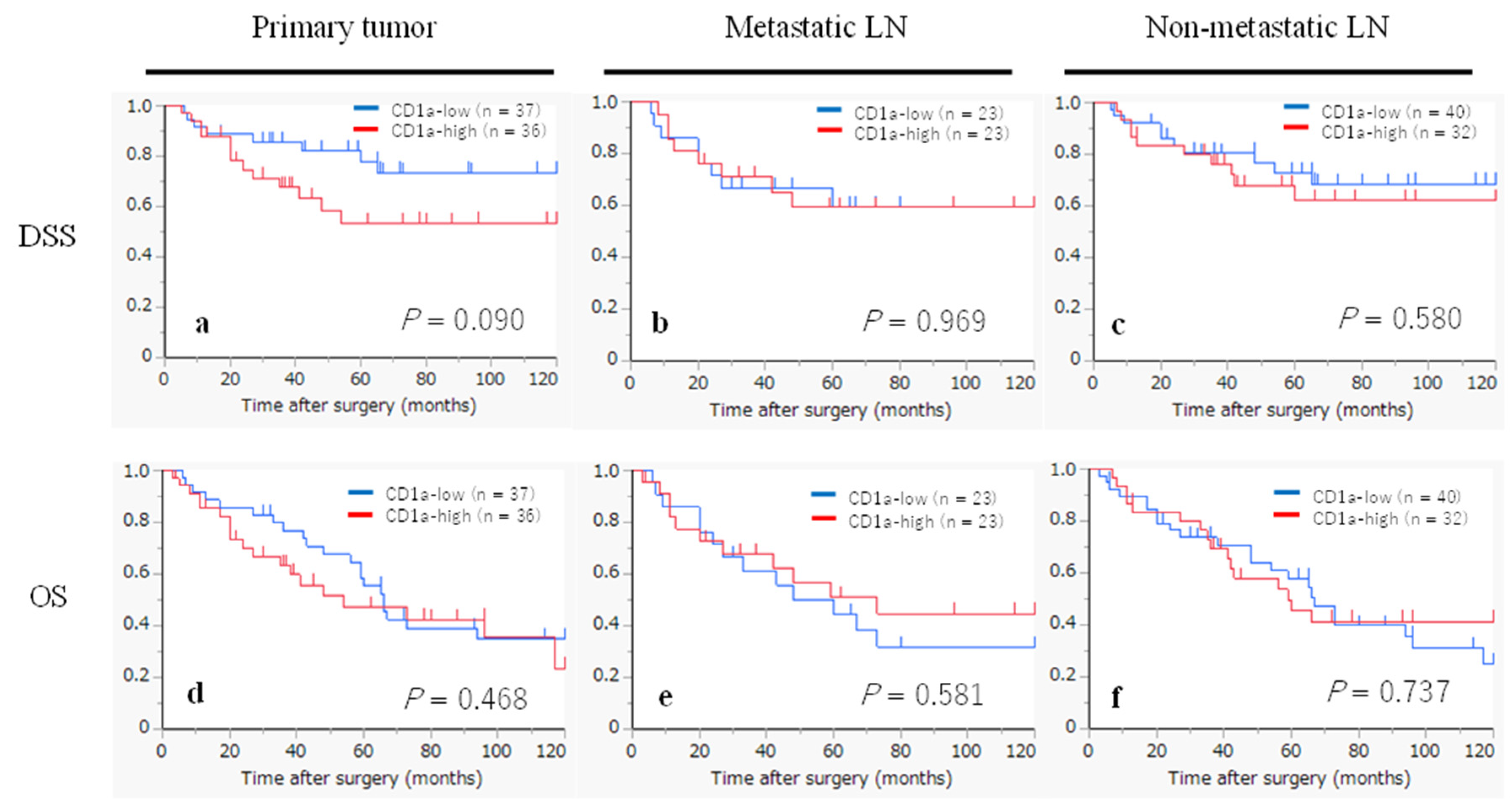
2.6. Kaplan-Meier Survival Curves According to the Infiltration of CD1a- and S100- DCs
2.7. Univariate Analyses for DSS and OS in All Patients (n = 73)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Assessment of CD1a- and S100-DCs
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; Colevas, A.D.; Eisele, D.W.; Fenton, M.; Foote, R.L.; Galloway, T.; Gillison, M.L.; Haddad, R.I.; Hicks, W.L.; Hitchcock, Y.J.; Jimeno, A.; Leizman, D.; Maghami, E.; Mell, L.K.; Mittal, B.B.; Pinto, H.A.; Ridge, J.A.; Rocco, J.W.; Rodriguez, C.P.; Shah, J.P.; Weber, R.S.; Weinstein, G.; Witek, M.; Worden, F.; Yom, S.S.; Zhen, W.; Burns, J.L.; Darlow, S.D. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Nibu, K.; Hayashi, R.; Asakage, T.; Ojiri, H.; Kimata, Y.; Kodaira, T.; Nagao, T.; Nakashima, T.; Fujii, T.; Fujii, H.; Homma, A.; Matsuura, K.; Monden, N.; Beppu, T.; Hanai, N.; Kirita, T.; Kamei, Y.; Otsuki, N.; Kiyota, N.; Zenda, S.; Omura, K.; Omori, K.; Akimoto, T.; Kawabata, K.; Kishimoto, S.; Kitano, H.; Tohnai, I.; Nakatsuka, T. Japanese Clinical Practice Guideline for Head and Neck Cancer. Auris Nasus Larynx. 2017, 44, 375–380. [Google Scholar] [CrossRef]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Glabbeke, M.V.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.K.; Lefebvre, J.L. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Maruo, T.; Zenda, S.; Shinozaki, T.; Tomioka, T.; Okano, W.; Sakuraba, M.; Tahara, M.; Hayashi, R. Comparison of salvage surgery for recurrent or residual head and neck squamous cell carcinoma. Jpn. J. Clin. Oncol. 2020, 50, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Homma, A.; Kiyota, N.; Tahara, M.; Hanai, N.; Asakage, T.; Matsuura, K.; Ogawa, T.; Saito, Y.; Sano, D.; Kodaira, T.; Motegi, A.; Yasuda, K.; Takahashi, S.; Tanaka, K.; Onoe, T.; Yokota, T.; Imamura, Y.; Ariizumi, Y.; Akimoto, T.; Hayashi, R. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck. Jpn. J. Clin. Oncol. 2021, 51, 173–179. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Coventry, B.J.; Lee, P.L.; Gibbs, D.; Hart, D.N.J. Dendritic cell density and activation status in human breast cancer- CD1a, CMRF-44, CMRF-56 and CD-83 expression. Br. J. Cancer. 2002, 86, 546–551. [Google Scholar] [CrossRef]
- Mori, L.; Libero, G.D. Presentation of lipid antigens to T cells. Immunol. Lett. 2008, 117, 1–8. [Google Scholar] [CrossRef]
- Hilly, O.; Rath-Wolfson, L.; Koren, R.; Mizrachi, A.; Hamzany, Y.; Bachar, G.; Shpitzer, T. CD1a-positive dendritic cell density predicts disease-free survival in papillary thyroid carcinoma. Pathol. Res. Pract. 2015, 211, 652–656. [Google Scholar] [CrossRef]
- Kai, K.; Tanaka, T.; Ide, T.; Kawaguchi, A.; Noshiro, H.; Aishima, S. Immunohistochemical analysis of the aggregation of CD1a-positive dendritic cells in resected specimens and its association with surgical outcomes for patients with gallbladder cancer. Transl. Oncol. 2021, 14, 100923. [Google Scholar] [CrossRef]
- Giorello, M.B.; Matas, A.; Marenco, P.; Davies, K.M. , Borzone, F.R.; Calcagno, M.D.L.; Garcia-Rivello, H; Wernicke, A.; Martinez, L.M.; Labovsky, V.; Chasseing, N.A. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer. 2021, 28, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Coventry, B.J.; Morton, J. CD1a- positive infiltrating-dendritic cell density and 5-year survival from human breast cancer. Br. J. Cancer. 2003, 89, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.V.; Ananiev, J.R.; Vlaykova, T.I.; Yovchev, Y.; Tsoneva, V.; Manolova, I.M. Role of dendritic cell in progression and clinical outcome of colon cancer. Int. J. Colorectal Dis. 2012, 27, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Hilly, O.; Strenov, Y.; Rath-Wolfson, L.; Hod, R.; Shkedy, Y.; Mizrachi, A.; Koren, R.; Shpitzer, T. The predictive of dendritic cells in early squamous cell carcinoma of tongue. Pathol. Res. Pract. 2016, 212, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Malietzis, G.; Lee, G.H.; Jenkins, J.T.; Bernardo, D.; Moorghen, M.; Knight, S.C.; Al-Hassi, H.O. Prognostic Value of the Tumour-Infiltrating Dendritic Cells in Colorectal Cancer: A Systematic Review. Cell Commun. Adhes. 2015, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nagorsen, D.; Voigt, S.; Berg, E.; Stein, H.; Thiel, E.; Loddenkemper, C. Tumor-Infiltrating macrophages and dendritic cells in human colorectal cancer: Relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J. Transl. Med. 2007, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Minesaki, A.; Kai, K.; Kuratomi, Y.; Aishima, S. Infiltration of CD1a-positive dendritic cells in advanced laryngeal cancer correlates with unfavorable outcomes post-laryngectomy. BMC Cancer. 2021, 21, 973. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Gedikoglu, G.; Celik, A.; Onerci, M.; Turan, E. Prognostic significance of Langerhans cell infiltration in cancer of the larynx. Otolaryngol. Head Neck Surg. 2005, 132, 309–316. [Google Scholar] [CrossRef]
- Gallo, O.; Libonati, G.A.; Gallina, E.; Fini-Storchi, O.; Giannini, A.; Urso, C.; Bondi, R. Langerhans cells related to prognosis in patients with laryngeal carcinoma. Arch. Otolaryngol. Head Neack Surg. 1991, 117, 1007–1010. [Google Scholar] [CrossRef]
- Karakök, M.; Bayazit, Y.A.; Ucak, R.; Ozer, E.; Kanlikama, M.; Mumbuc, S.; Sari, I. Langerhans cell related inflammatory reaction in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2003, 30, 81–84. [Google Scholar] [CrossRef]
- Esteban, F.; Ruiz-Cabello, F.; Gonzalez-Moles, M.A.; Lopez-Gonzalez, M.A.; Funez, R.; Redondo, M. Clinical Significance of Langerhans Cells in Squamous Cell Carcinoma of the larynx. J. Oncol. 2012, 2012, 753296. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Ceausu, A.R.; Gaje, N.P.; Raica, M. Profile and Potential Significance of Dendritic Cells in Head and Neck Squamous Cell Carcinoma. Cancer Diagn. Progn. 2022, 6, 758–763. [Google Scholar] [CrossRef]
- Descamps, G.; Furgiuele, S.; Mhaidly, N.; Journe, F.; Saussez, S. Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer. Cancers. 2022, 14, 5560. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; Spasic, M.; Giurintano, J.P.; Chan, V.; Daud, A.I.; Ha, P.; Ye, C.J.; Roberts, E.W.; Krummel, M.F. Unleashing Type-2 Dendritic cells to Drive Protective Antitumor CD4+ Tcell Immunity. Cell. 2019, 177, 556–571. [Google Scholar] [CrossRef]
- Gerhard, G.M.; Bill, R.; Messemaker, M.; Klein, A.M.; Pettet, M.J. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J. Exp. Med. 2021, 218, e20200264. [Google Scholar] [CrossRef]
- Gardner, A.; Pulido, A.D.M.; Ruffell, B. Dendritic cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Suzuki, S.; Honma, K.; Matsuyama, T.; Suzuki, K.; Toriyama, K.; Ichinose, A.; Yamamoto, K.; Suematsu, T.; Nakamura, M.; Yui, K.; Kumatori, A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8α⁻dendritic cell development. Proc. Natl. Acad. Sci. USA. 2004, 101, 8981–8986. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Charry, A.; Maxwell, T.; Lopez, J.A. Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol. Cell Biol. 2005, 83, 451–461. [Google Scholar] [CrossRef]
- Menetrier-Caux, C.; Montmain, G.; Dieu, M.C.; Bain, C.; Favrot, M.C.; Caux, C.; Blay, J.Y. Inhibition of the Differentiation of Dendritic Cells From CD34+ Progenitors by Tumor Cells: Role of Interleukin-6 and Macrophage Colony-Stimulating Factor. Blood, 1998, 92, 4778–4791. [Google Scholar] [CrossRef]
- Park, S.J.; Nakagawa, T.; Kitamura, H.; Atsumi, T.; Kamon, H.; Sawa, S.; Kamimura, D.; Ueda, N.; Iwakura, Y.; Ishihara, K.; Murakami, M.; Hirano, T. IL-6 Regulates In Vivo Dendritic Cell Differentiation through STAT3 Activation. J. Immunol. 2004, 173, 3844–3854. [Google Scholar] [CrossRef]
- Buelens, C.; Verhasselt, V.; Groote, D.D.; Thielemans, K.; Goldman, M.; Willems, F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony stimulating factor. Eur. J. Immunol. 1997, 27, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Beckebaum, S.; Zhang, X.; Chen, X.; Yu, Z.; Frilling, A.; Dworacki, G.; Grosse-Wilde, H.; Broelsch, C.E.; Gerken, G.; Cicinnati, V.R. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clin. Cancer Res. 2004, 10, 7260–7269. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Chappell, D.B.; Apolloni, E.; Cabrelle, A.; Wang, M.; Hwu, P.; Restifo, N.P. Unopposed Production of Granulocyte- Macrophage Colony-Stimulating Factor by Tumors Inhibits CD8+ T cell Responses by Dysregulating Antigen-Presenting Cell Maturation. J. Immunol. 1999, 162, 5728–5737. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages In Vivo. Blood, 1998, 92, 4150–4166. [Google Scholar] [CrossRef] [PubMed]
- Baysal, H.; Siozopoulou, V.; Zaryouh, H.; Hermans, C.; Lau, H.W.; Lambrechts, H.; Fransen, E.; Pauw, I.D.; Jacobs, J.; Peeters, M.; Pauwels, P.; Vermorken, J.B.; Smits, E.; Lardon, F.; Waele, J.D.; Wouters, A. The prognostic impact of the immune signature in head and neck squamous cell carcinoma. Front. Immunol. 2022, 13, 1001161. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Askari, A.; Malietzis, G.; Bernardo, D.; Clark, S.K.; Knight, S.C.; Al-Hassi, H.O. The role of CD40 expression in dendritic cells in cancer biology; a systemic review. Curr. Cancer Drug Targets. 2014, 14, 610–620. [Google Scholar] [CrossRef]
- Almond, B.; Resser, J.R.; Lindman, B.; Nadaf, S.; Clark, J.I.; Kwon, E.D.; Carbone, D.P.; Gabrilovich, D.I. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 2000, 5, 1755–66. [Google Scholar]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; Yang, H.; Amigorena, S.; Ryffel, B.; Barrat, F.J.; Saftig, P.; Levi, F.; Lidereau, R.; Nogues, C.; Mira, J.P.; Chompret, A.; Joulin, V.; Clavel-Chapelon, F.; Bourhis, J.; Andre, F.; Delaloge, S.; Tursz, T.; Kroemer, G.; Zitvogel, L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L.; Hou, Y.; Meng, X.; Huang, X.; Rao, E.; Zheng, W.; Mauceri, H.; Mack, M.; Xu, M.; Fu, Y.X.; Weichselbaum, R.R. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017, 8, 1736. [Google Scholar] [CrossRef] [PubMed]
- Widau, R.C.; Parekh, A.D.; Ranck, M.C.; Golden, D.W.; Kumar, K.A.; Sood, R.F.; Pitroda, S.P.; Liao, Z.; Huang, X.; Darga, T.E.; Xu, D.; Huang, L.; Andrade, J.; Roizman, B.; Weichselbaum, R.R.; Khodarev, N.N. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc. Natl. Acad. Sci. USA. 2014, 111, E484–491. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liang, H.; Rao, E.; Zheng, W.; Huang, X.; Deng, L.; Zhang, Y.; Yu, X.; Xu, M.; Mauceri, H.; Arina, A.; Weichselbaum, R.R.; Fu, Y.X. Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity. 2018, 49, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; Metivier, D.; Larochette, N.; Endert, P.V.; Ciccosanti, F.; Piacentini, M.; Zitvogel, L.; Kroemer, G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]

| Age, years (mean ± SD) | 68.9 ± 9.4 |
| Sex | |
| Male / Female | 70 (95.9%) / 3 (4.1%) |
| Smoking habit | |
| Never / Ex / Current | 8 (11.0%) / 15 (20.5%) / 50 (68.5%) |
| Alcohol abuse | |
| (-) / (+) | 22 (30.1%) / 51 (69.9%) |
| Subsite | |
| Glottis / Supraglottis / Subglottis | 36 (49.3%) / 36 (49.3%) / 1 (1.4%) |
| Histology and differentiation | |
| Well / Mode / Poor / Carcinosarcoma | 33 (45.2%) / 35 (47.9%) / 4 (5.5%) / 1 (1.4%) |
| Primary T stage | |
| T1 / T2 / T3 / T4 | 8 (11.0%) / 21 (28.8%) / 21 (28.8%) / 23 (31.5%) |
| N | |
| (-) / (+) | 27 (63.0%) / 46 (37.0%) |
| Primary M stage | |
| M0 / M1 | 71 (97.3%) / 2 (2.7%) |
| Stage | |
| I / II / III / IV | 5 (6.8%) / 11 (15.1%) / 16 (21.9%) / 41 (56.2%) |
| Treatment background | |
| Surgery without RT / CRT | 42 (57.5%) |
| Surgery after RT | 2 (2.7%) |
| Surgery after CRT | 29 (39.7%) |
| Primary tumor (n = 73) |
Metastatic LN (n = 46) |
Non-metastatic LN (n = 72*) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD1a- low (n = 37) | CD1a- high (n = 36) | P | CD1a -low (n = 23) | CD1a- high (n = 23) | P | CD1a-low (n = 40) | CD1a- high (n = 32) | P | ||
| Age, years (mean ± SD) |
67.1 ± 8.9 | 70.5 ± 9.7 | 0.124 | 68.3 ± 9.5 | 67.5 ± 9.8 | 0.772 | 70.6 ± 8.4 | 66.6 ± 10.3 | 0.074 | |
| Sex | ||||||||||
| Male | 36 (97.3%) | 34 (94.4%) | 0.615 | 23 (100.0%) | 20 (87.0%) | 0.233 | 39 (97.5%) | 30 (93.8%) | 0.582 | |
| Female | 1 (2.7%) | 2 (5.6%) | 0 (0.0%) | 3 (13.0%) | 1 (2.5%) | 2 (6.3%) | ||||
| Primary T stage | ||||||||||
| T1/2 | 17 (46.0%) | 12 (33.3%) | 0.341 | 12 (52.2%) | 10 (43.5%) | 0.768 | 19 (47.5%) | 10 (31.3%) | 0.227 | |
| T3/4 | 20 (54.1%) | 24 (66.7%) | 11 (47.8%) | 13 (56.5%) | 21 (52.5%) | 22 (68.8%) | ||||
| N | ||||||||||
| N (-) | 12 (32.4%) | 15 (41.7%) | 0.473 | 0 (0.0%) | 0 (0.0%) | n/a | 14 (35.0%) | 13 (40.6%) | 0.634 | |
| N (+) | 25 (67.6%) | 21 (58.3%) | 23 (100%) | 23 (100%) | 26 (65.0%) | 19 (59.4%) | ||||
| Primary M stage | ||||||||||
| M0 | 36 (97.3%) | 35 (97.2%) | 1.000 | 22 (95.7%) | 22 (95.7%) | 1.000 | 40 (100.0%) | 30 (93.8%) | 0.194 | |
| M1 | 1 (2.7%) | 1 (2.8%) | 1 (4.4%) | 1 (4.4%) | 0 (0.0%) | 2 (6.3%) | ||||
| Timing of the resected samples | ||||||||||
| After RT or CRT | 16 (43.2%) | 7 (19.4%) | 0.043 | 14 (60.9%) | 6 (26.1%) | 0.036 | 18 (45.0%) | 12 (37.5%) | 0.632 | |
| Treatment naïve** | 21 (56.8%) | 29 (80.6%) | 9 (39.1%) | 17 (73.9%) | 22 (55.0%) | 20 (62.5%) | ||||
| Primary tumor (n = 73) |
Metastatic LN (n = 46) |
Non-metastatic LN (n = 72*) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S100-low (n = 37) | S100-high (n = 36) | P | S100-low (n = 25) | S100- high (n = 21) | P | S100- low (n = 14) | S100- high (n = 58) | P | |
| Age, years (mean ± SD) | 68.0 ± 9.3 | 69.7 ± 9.6 | 0.445 | 68.5 ± 10.5 | 67.2 ± 8.3 | 0.654 | 72.1 ± 9.3 | 68.0 ± 9.4 | 0.150 |
| Sex | |||||||||
| Male | 36 (97.3%) | 34 (94.4%) | 0.615 | 24 (96.0%) | 19 (90.5%) | 0.585 | 13 (92.9%) | 56 (96.6%) | 0.483 |
| Female | 1 (2.7%) | 2 (5.6%) | 1 (4.0%) | 2 (9.5%) | 1 (7.1%) | 2 (3.5%) | |||
| Primary T stage | |||||||||
| T1/2 | 15 (40.5%) | 14 (38.9%) | 1.000 | 7 (28.0%) | 15 (71.4%) | 0.007 | 5 (35.7%) | 24 (41.4%) | 0.770 |
| T3/4 | 22 (59.5%) | 22 (61.1%) | 18 (72.0%) | 6 (28.6%) | 9 (64.3%) | 34 (58.6%) | |||
| N | |||||||||
| N (-) | 15 (40.5%) | 12 (33.3%) | 0.630 | 0 (0.0%) | 0 (0.0%) | n/a | 5 (35.7%) | 22 (37.9%) | 1.000 |
| N (+) | 22 (59.5%) | 24 (66.7%) | 25 (100%) | 21 (100%) | 9 (64.3%) | 36 (62.1%) | |||
| Primary M stage | |||||||||
| M0 | 36 (97.3%) | 35 (97.2%) | 1.000 | 23 (92.0%) | 21 (100.0%) | 0.493 | 12 (85.7%) | 58 (100.0%) | 0.036 |
| M1 | 1 (2.7%) | 1 (2.8%) | 2 (8.0%) | 0 (0.0%) | 2 (14.3%) | 0 (0.0%) | |||
| Timing of resected samples | |||||||||
| After CRT or RT | 17 (45.9%) | 6 (16.7%) | 0.011 | 15 (60.0%) | 5 (23.8%) | 0.019 | 3 (21.4%) | 27 (46.6%) | 0.131 |
| Treatment naïve** | 20 (54.1%) | 30 (83.3%) | 10 (40.0%) | 16 (76.2%) | 11 (78.6%) | 31 (53.5%) | |||
| Characteristic | n | DSS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 0.683 | 0.783 | |||
| ≤68years | 38 | 1 | 1 | ||
| >68years | 35 | 0.84 (0.35-1.98) | 1.09 (0.59-2.03) | ||
| Sex | 0.473 | 0.470 | |||
| Female | 3 | 1 | 1 | ||
| Male | 70 | 0.48 (0.06-3.58) | 0.59 (0.14-2.47) | ||
| T stage | 0.109 | 0.031 | |||
| T1/T2 | 29 | 1 | 1 | ||
| T3/T4 | 44 | 2.18 (0.84-5.63) | 2.09 (1.07-4.08) | ||
| N stage | 0.025 | 0.037 | |||
| N0 | 27 | 1 | 1 | ||
| N1-3 | 46 | 2.96 (1.14-7.67) | 1.97 (1.04-3.72) | ||
| M stage | 0.050 | 0.179 | |||
| M0 | 71 | 1 | 1 | ||
| M1 | 2 | 4.32 (1.00-18.69) | 2.68 (0.64-11.25) | ||
| CD1a-DCs in primary tumor | 0.098 | 0.472 | |||
| low | 37 | 1 | 1 | ||
| high | 36 | 2.11 (0.87-5.12) | 1.25 (0.67-2.35) | ||
| CD1a-DCs in metastatic LN | 0.969 | 0.586 | |||
| low | 23 | 1 | 1 | ||
| high | 23 | 0.98 (0.36-2.61) | 0.80 (0.36-1.79) | ||
| CD1a-DCs in non-metastatic LN | 0.582 | 0.739 | |||
| low | 40 | 1 | 1 | ||
| high | 32 | 1.28 (0.53-3.08) | 0.90 (0.47-1.70) | ||
| S100-DCs in primary tumor | 0.538 | 0.994 | |||
| low | 37 | 1 | 1 | ||
| high | 36 | 1.31 (0.55-3.09) | 1.00 (0.54-1.87) | ||
| S100-DCs in metastatic LN | 0.177 | 0.077 | |||
| low | 25 | 1 | 1 | ||
| high | 21 | 0.50 (0.18-1.37) | 0.47 (0.21-1.08) | ||
| S100-DCs in non-metastatic LN | 0.866 | 0.490 | |||
| low | 14 | 1 | 1 | ||
| high | 58 | 0.91 (0.30-2.73) | 0.77 (0.36-1.62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





