Submitted:
16 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of LNP-GA
2.3. LNP-GA characterization by size analysis, PDI, and Z-potential
2.4. Transmission electron microscopy (TEM)
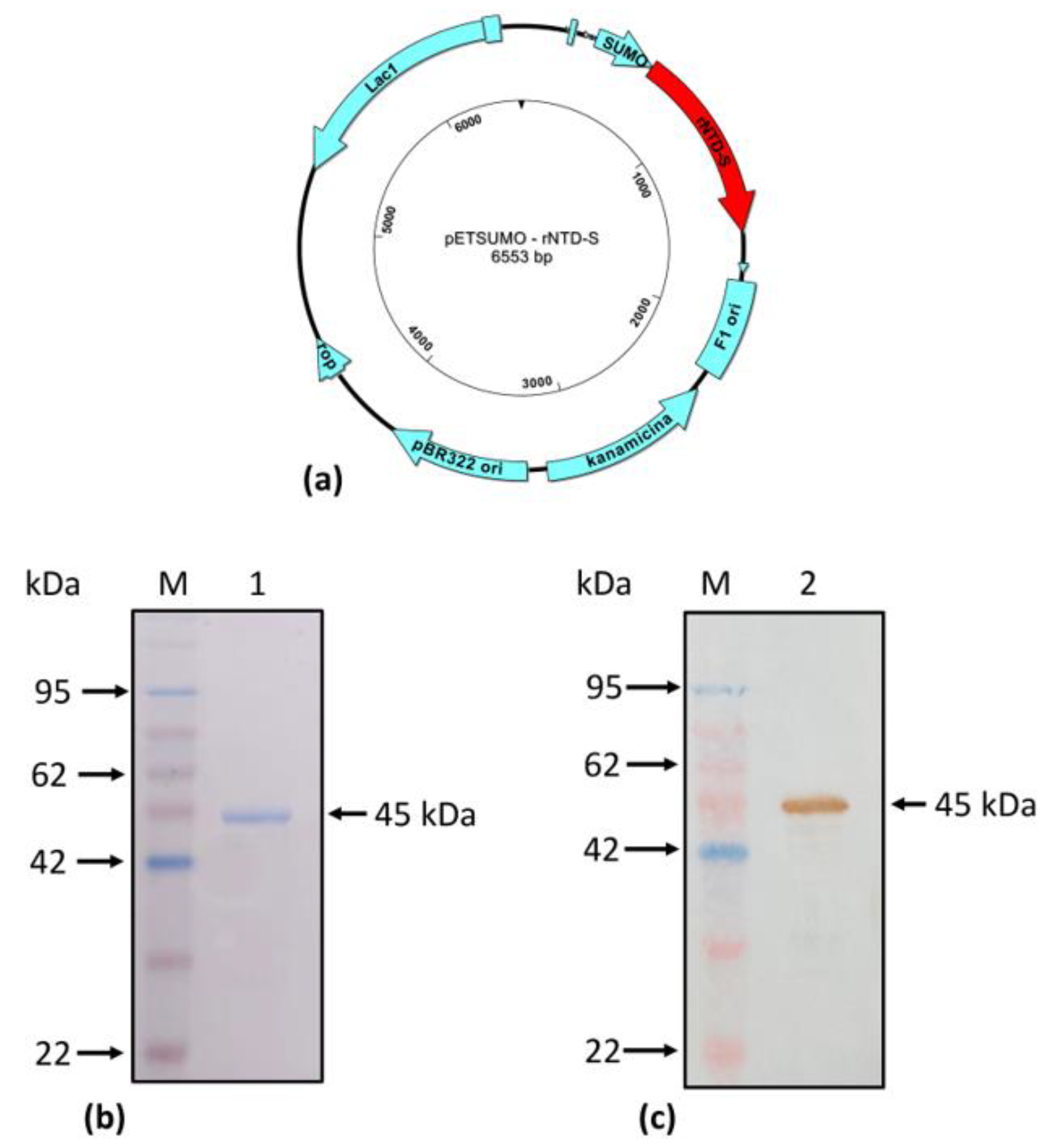
2.5. Production, expression, and purification of rNTD-S recombinant protein
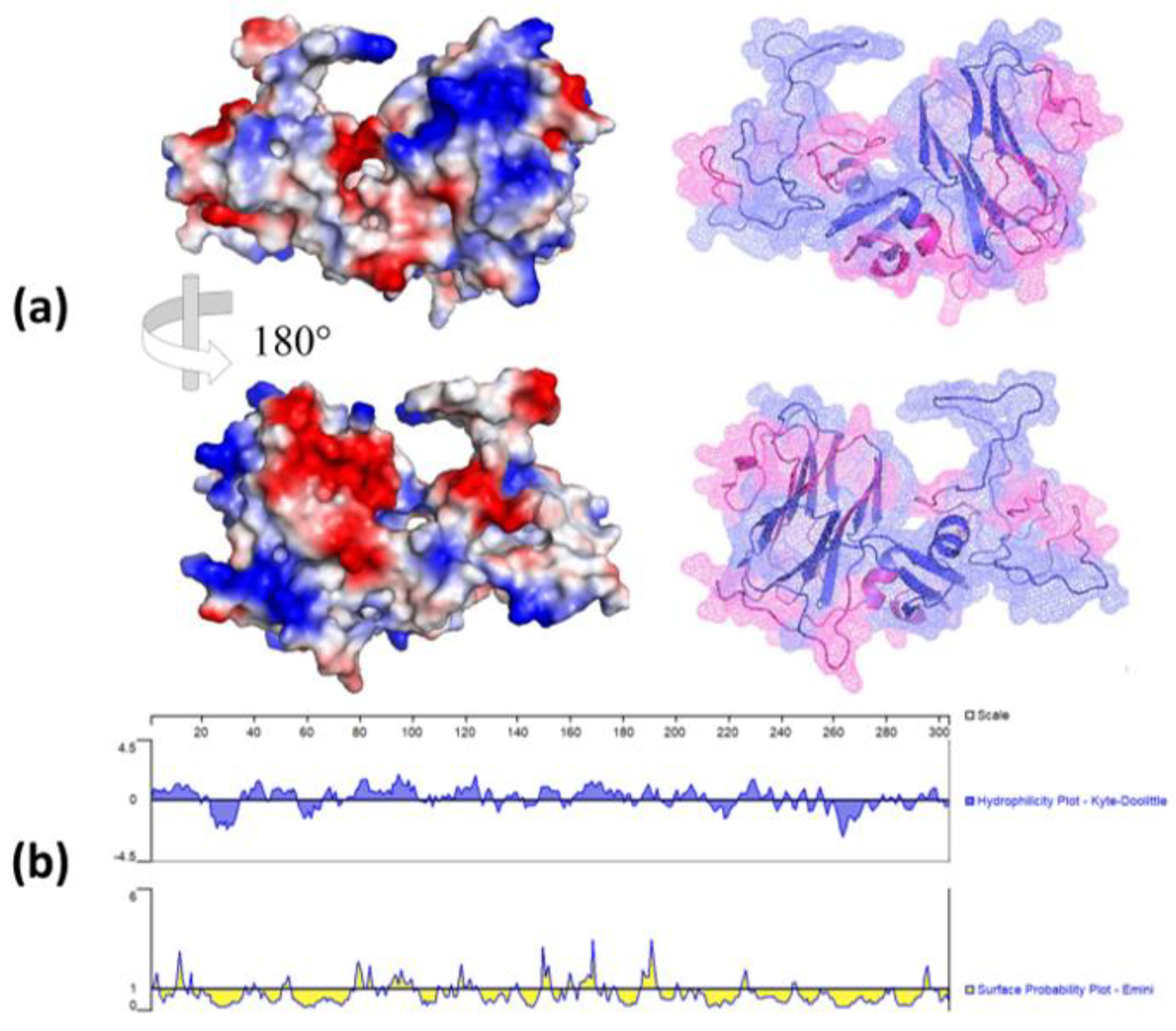
2.6. Structure and antigenic epitopes prediction of NTD-S protein
2.7. Immunogenicity evaluation of LNP-GA coupled to rNTD-S by mice immunization and tested by indirect enzyme-linked immunosorbent assay (iELISA)
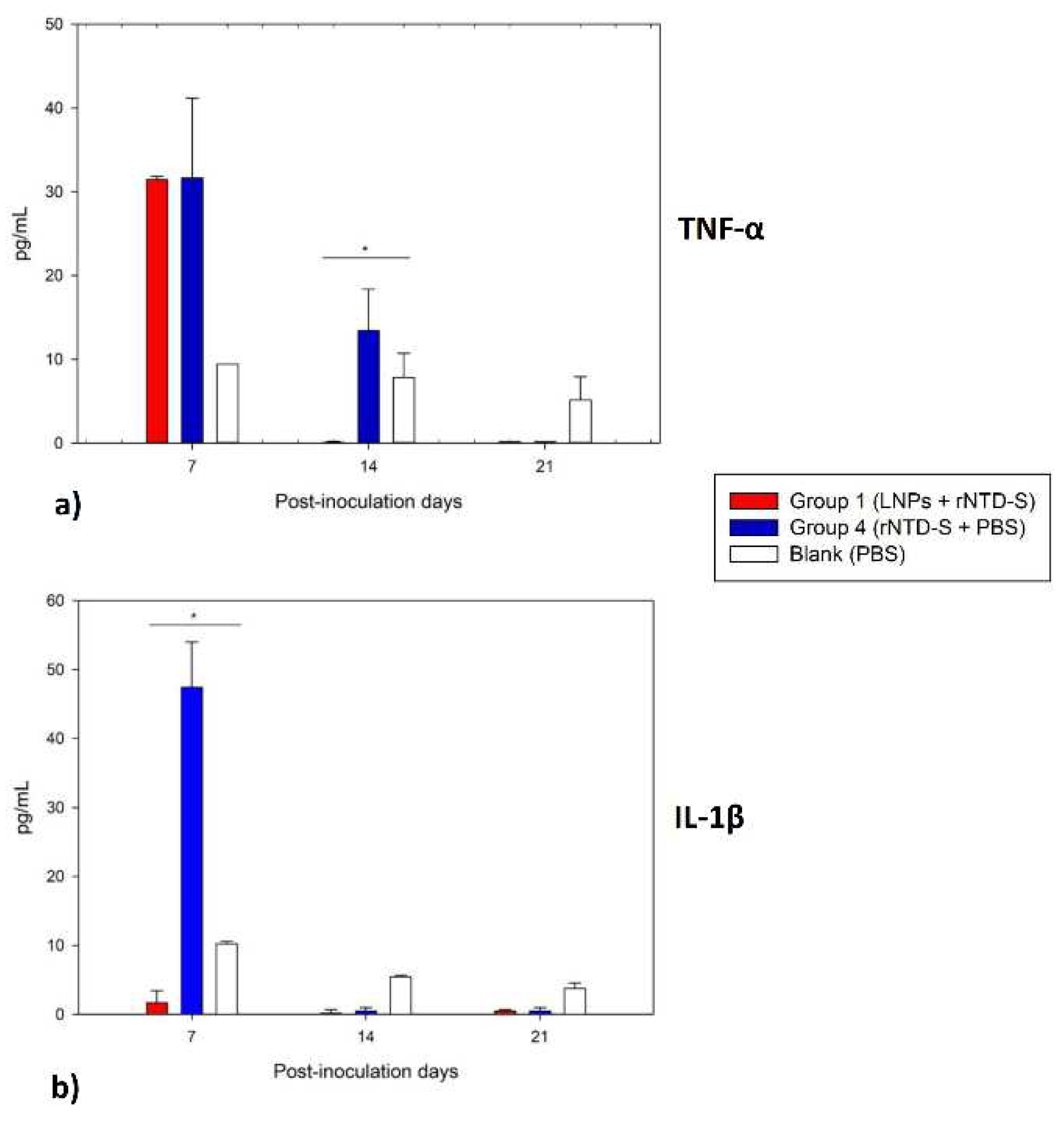
2.8. Determination of pro-inflammatory cytokines
3. Results
3.1. Characteristics of LNP-GA
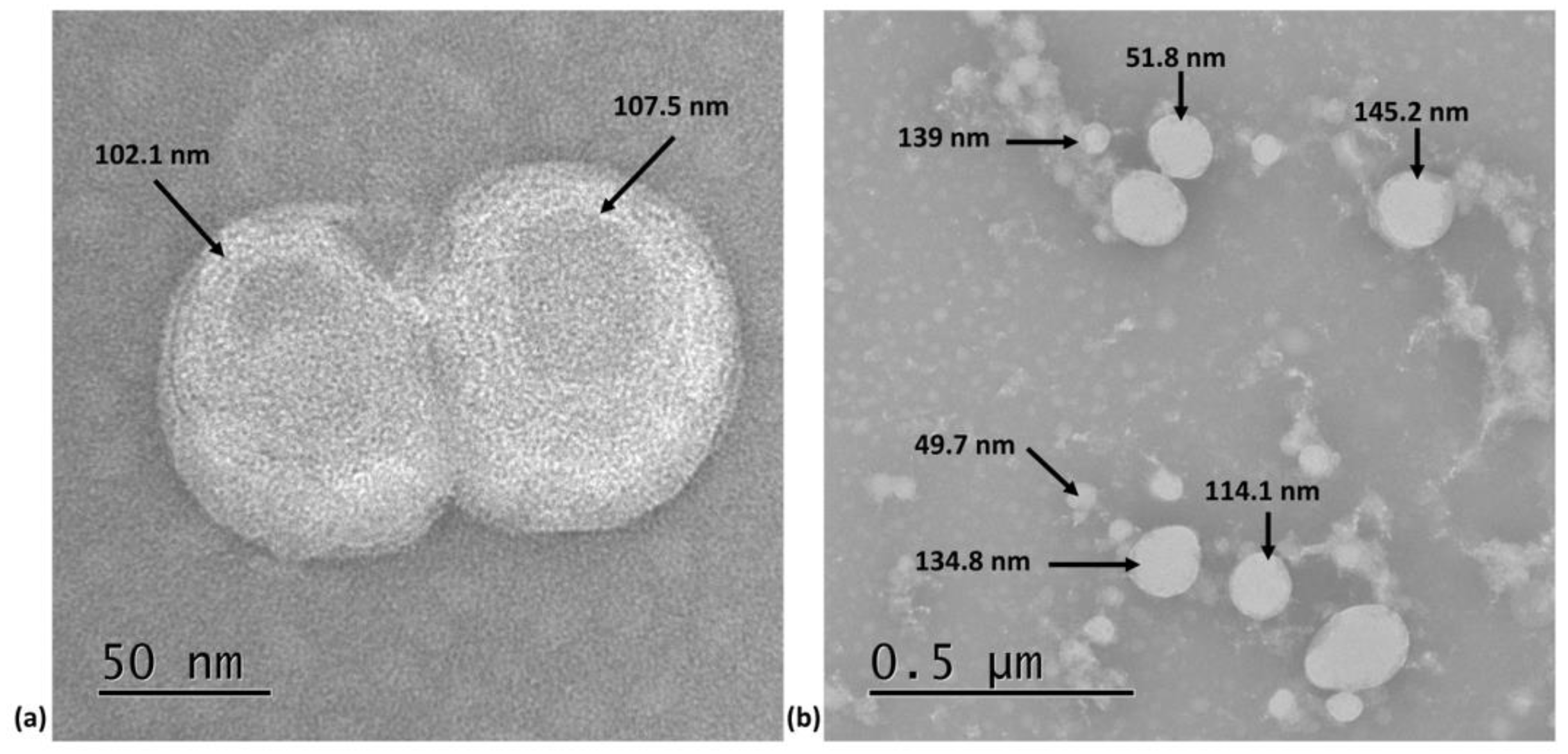
3.2. Assessment of LNP-GA by TEM
3.3. Production of recombinant NTD-S protein (rNTD-S)
3.4. Antigenic structural evaluation of NTD-S
3.5. Antibody response of immunized mice with rNTD-S coupled to LNP-GA as adjuvant
3.6. Potential anti-inflammatory effect study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: where are we headed? Porc. Health Manag. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus genes. 2012, 44, 167–177. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Yang, L.; Wang, A. A comprehensive view in the host factors and viral proteins associated with porcine epidemic diarrhea virus infection. Front. Microbiol. 2021, 12, 762358. [Google Scholar] [CrossRef]
- Lin, C.M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, B.H.; Lee, K.Y.; Jang, Y.S. N-Terminal Domain of the Spike Protein of Porcine Epidemic Diarrhea Virus as a New Candidate Molecule for a Mucosal Vaccine. Immune Netw. 2018, 18, 3. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, F.; Fan, B.; Muhammad, H.M.; Zou, Y.; Jiang, P. Development an indirect ELISA based on truncated S protein of porcine epidemic diarrhea virus. Can. J. Microbiol. 2015, 61, 811–817. [Google Scholar] [CrossRef]
- Deng, F.; Ye, G.; Liu, Q.; Navid, M.T.; Zhong, X.; Li, Y.; Peng, G. Identification and Comparison of Receptor Binding Characteristics of the Spike Protein of Two Porcine Epidemic Diarrhea Virus Strains. Viruses. 2016, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. Development of Lipid Nanoparticles for the Delivery of Macromolecules Based on the Molecular Design of pH-Sensitive Cationic Lipids. Chem. Pharm. Bull. 2021, 69, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.; Alsowinea, A. Approved and marketed nanoparticles for disease targeting and applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977. [Google Scholar] [CrossRef]
- Mashima, R.; Takada, S. Lipid Nanoparticles: A Novel Gene Delivery Technique for Clinical Application. Curr. Issues Mol. Biol. 2022, 44, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Chonn, A.; Cullis, P. Recent advances in liposomal drug-delivery systems. Curr. Opin. Biotechnol. 1995, 6, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.; Swidan, S.; El-Nabarawi, M.; Teaima, M. Lipid based nanoparticles as a novel treatment modality for hepatocellular carcinoma: a comprehensive review on targeting and recent advances. J. Nanobiotechnol. 2022, 20, 109. [Google Scholar] [CrossRef]
- Morein, B.; Simons, K. Subunit vaccines against enveloped viruses: Virosomes, micelles and other protein complexes. Vaccine, 1985, 3, 83–93. [Google Scholar] [CrossRef]
- Demana, P.H.; Davies, N.M.; Hook, S.; Rades, T. Quil A-lipid powder formulations releasing ISCOMs and related colloidal stuctures upon hydration. J Control Release. 2005, 103, 45–59. [Google Scholar] [CrossRef]
- Güçlü-Üstünda?, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Zelikman, M.V.; Kim, A.V.; Medvedev, N.N.; Selyutina, O.Y.; Polyakov, N.E. Structure of dimers of glycyrrhizic acid in water and their complexes with cholesterol: Molecular dynamics simulation. J. Struct. Chem. 2015, 56, 67–76. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.; Wang, D.; Hu, Y.; Guo, L.; Ruan, S.; Yuan, J. Immunological adjuvant efficacy of glycyrrhetinic acid liposome against Newcastle disease vaccine. Vaccine. 2011, 29, 9611–9617. [Google Scholar] [CrossRef]
- Castañeda-Montes, M.A.; Cuevas-Romero, J.S.; Cerriteño-Sánchez, J.L.; de María Ávila-De la Vega, L.; García-Cambrón, J.B.; Ramírez-Álvarez, H. Small ruminant lentivirus capsid protein (SRLV-p25) antigenic structural prediction and immunogenicity to recombinant SRLV- r p25-coupled to immunostimulatory complexes based on glycyrrhizinic acid. Biosci. Biotech. Bioch. 2022, 87, 267–278. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Younis, M.A.; Alsowinea, A.F.; Abdallah, E.M.; Abdel-Bakky, M.S.; Al-Subaiyel, A.; Tawfeek, H.M. Lipid nanoparticles technology in vaccines: Shaping the future of prophylactic medicine. Colloids Surf. B. 2023, 222, 113111. [Google Scholar] [CrossRef]
- Viegas, C.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J Drug Deliv Sci Technol. 2022, 103839. [Google Scholar] [CrossRef]
- Copland, M.J.; Davies, N.M.; Rades, T. Hydration of lipid films with an aqueous solution of Quil A: A simple method for the preparation of immune-stimulating complexes. Int. J. Pharm. 2000, 196, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Urbán-Morlán, Z.; Ganem-Rondero, A.; Melgoza-Contreras, L.M.; Escobar-Chávez, J.J.; Nava-Arzaluz, M.G.; Quintanar-Guerrero, D. Preparation and characterization of solid lipid nanoparticles containing cyclosporine by the emulsification-diffusion method. Int. J. Nanomed. 2010, 5, 611–620. [Google Scholar] [CrossRef]
- Cao, B.; Xu, H.; Mao, C. Transmission electron microscopy as a tool to image bioinorganic nanohybrids: The case of phage-gold nanocomposites. Microsc. Res. Techniq. 2011, 74, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lara-Romero, R.; Gómez-Núñez, L.; Cerriteño-Sánchez, J.L.; Márquez-Valdelamar, L.; Mendoza-Elvira, S.; Ramírez-Mendoza, H.; Rivera-Benítez, J.F. Molecular characterization of the spike gene of the porcine epidemic diarrhea virus in Mexico, 2013-2016. Virus genes. 2018, 54, 215–224. [Google Scholar] [CrossRef]
- Lara-Romero, R.; Cerriteño-Sánchez, J.L.; Mendoza-Elvira, S.; García-Cambrón, J.B.; Castañeda-Montes, M.A.; Pérez-Aguilar, J.M.; Cuevas-Romero, J.S. Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction. Viruses. 2022, 14, 1946. [Google Scholar] [CrossRef]
- García-González, E.; Cerriteño-Sánchez, J.L.; Cuevas-Romero, J.S.; García-Cambrón, J.B.; Castañeda-Montes, F.J.; Villaseñor-Ortega, F. Seroepidemiology Study of Porcine Epidemic Diarrhea Virus in Mexico by Indirect Enzyme-Linked Immunosorbent Assay Based on a Recombinant Fragment of N-Terminus Domain Spike Protein. Microorganisms. 2023, 11, 1843. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.; Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Bhandari, M.; Martini, O.; Sewall, L.M.; Bangaru, S.; Yoon, K.J.; Ward, A.B. Structure and immune recognition of the porcine epidemic diarrhea virus spike protein. Structure. 2021, 29, 385–392. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, W.; Han, C.; Sui, X.; Liu, C.; Ma, X.; Dong, Y. Preparation of Glycyrrhetinic Acid Liposomes Using Lyophilization Monophase Solution Method: Preformulation, Optimization, and In Vitro Evaluation. Nanoscale Res. Lett. 2018, 1-13, 324. [CrossRef]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar] [CrossRef]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009, 27, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Bosquez-Molina, E.; Guerrero-Legarreta, I.; Vernon-Carter, J.E. Moisture barrier properties and morphology of mesquite gum-candelilla wax based edible emulsion coatings. Food Res. Int. 2003, 36, 885–893. [Google Scholar] [CrossRef]
- Pashkina, E.; Evseenko, V.; Dumchenko, N.; Zelikman, M.; Aktanova, A.; Bykova, M.; Kozlov, V. Preparation and Characterization of a Glycyrrhizic Acid-Based Drug Delivery System for Allergen-Specific Immunotherapy. Nanomaterials. 2022, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Bentacur, B.; Jiménez, D.; Linares, B. Potencial Zeta (?) como criterio de optimización de dosificación de coagulante en planta de tratamiento de agua potable. Dyna. 2012, 79, 166–172. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods in molecular biology. Clifton, N.J. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterization, preparation, and recent innovation in clinical applications. J Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Fossum, C.; Hjertner, B.; Ahlberg, V.; Charerntantanakul, W.; McIntosh, K.; Fuxler, L.; Bengtsson, K.L. Early inflammatory response to the saponin adjuvant Matrix-M in the pig. Vet. Immunol. Immunop. 2014, 158, 53–61. [Google Scholar] [CrossRef]
- Deng, F.; Ye, G.; Liu, Q.; Navid, M.T.; Zhong, X.; Li, Y.; Peng, G. Identification and Comparison of Receptor Binding Characteristics of the Spike Protein of Two Porcine Epidemic Diarrhea Virus Strains. Viruses. 2016, 8, 55. [Google Scholar] [CrossRef]
- Magnusson, S.E.; Reimer, J.M.; Karlsson, K.H.; Lilja, L.; Bengtsson, K.L.; Stertman, L. Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine. 2013, 31, 1725–1733. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: a review of the recent advances. Ther. Adv. Vaccines Immunother. 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhong, J.Y.; Gao, E.N.; Yang, H. Effects of glycyrrhizin acid and licoric. flavonoids on LPS-induced cytokines expression in macrophage. Zhong Yao Cai. 2014, 39, 3841–3845. [Google Scholar]

| Sample | Mean Particle Size (nm)* | Polydispersion(PDI)* | Z-Potential (mV)** |
|---|---|---|---|
| Recombinant N-terminal domain of the PEDV spike protein (rNTD-S) | 2150.3 | 1.715 | -9.13 ± 4 |
| Glycyrrhizinic acid (GA) | 205.7 | 1.8 | -16.29 ± 7.32 |
| Glycyrrhizinic acid-based Lipid Nanoparticle (LNPs-GA) | 211.5 | 0.283 | -27.6 ± 9.19 |
| Glycyrrhizinic acid-based Lipid Nanoparticle (LNPs-GA) plus rNTD-S | 347.3 | 0.648 | -21.73 ± 8.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





