Introduction
Antibiotic stewardship (AMS) is assigned to good antibiotic use
, i.e., a lower antibiotic consumption (AC) to fight antimicrobial resistance and improve care [
1]. The program for AMS includes several tools among which organization, process and outcome measures must be in place in the present decade [
2]. However, previous studies showed that the benefit of each tool is difficult to identify, even if specific means have been reported more efficient than others [
3,
4]. For example, audits and feedback are the most efficient way to improve the quality of antibiotic prescriptions, followed by pre-prescription authorization [
5].
The core elements of AMS program have been put in place in many countries, being associated with both a reduction of AC and better care [
2,
3,
6,
7]. Accordingly, AC was in a decreasing phase these last years in France, as illustrated by successive European reports on antimicrobial consumptions: expressed as Defined Daily Dose (DDD) per 1000 inhabitants per day, total consumption of antimicrobials for systemic use was 25.7 in 2012 and 21.5 in 2021 [
8].
Undoubtedly the main challenge of the AMS policies is to keep persistent their benefits over time. Because clinical practices varied according to new health technologies, renewal of medical teams, and economic and resources constraints, it is possible to consider a progressive loss of efficiency of AMS tools. This is why the audits of the appropriateness of antimicrobial use should be done regularly [
9]. Moreover, major difficulty of health care system such as the COVID-19 pandemic can alter the performance of the AMS tools already in place. Previous reports showed that this pandemic was associated with an increase of consumption of some drugs such as azithromycin or doxycycline, but also of broad-spectrum antibiotics, notably during the first waves of the pandemic [
10,
11]. Because AC is subjected to both internal and external factors to the hospitals, we aimed to determine the current relationship between AC with both AMS tools and COVID-19 pandemic.
Results
Main characteristics of institutions
Records of AC via Consores® were provided by the institutions between April and June 2023, and involved 30 hospitals. The latter had medical and surgical activities in 23 cases (77%), surgical activities only in 6 cases (20%) and one institution had medical activity exclusively. Fifteen institutions (47%) had an emergency department and thirteen (43%) had an intensive care unit. The median [range] number of beds was 156 [45-300].
Antimicrobial consumption
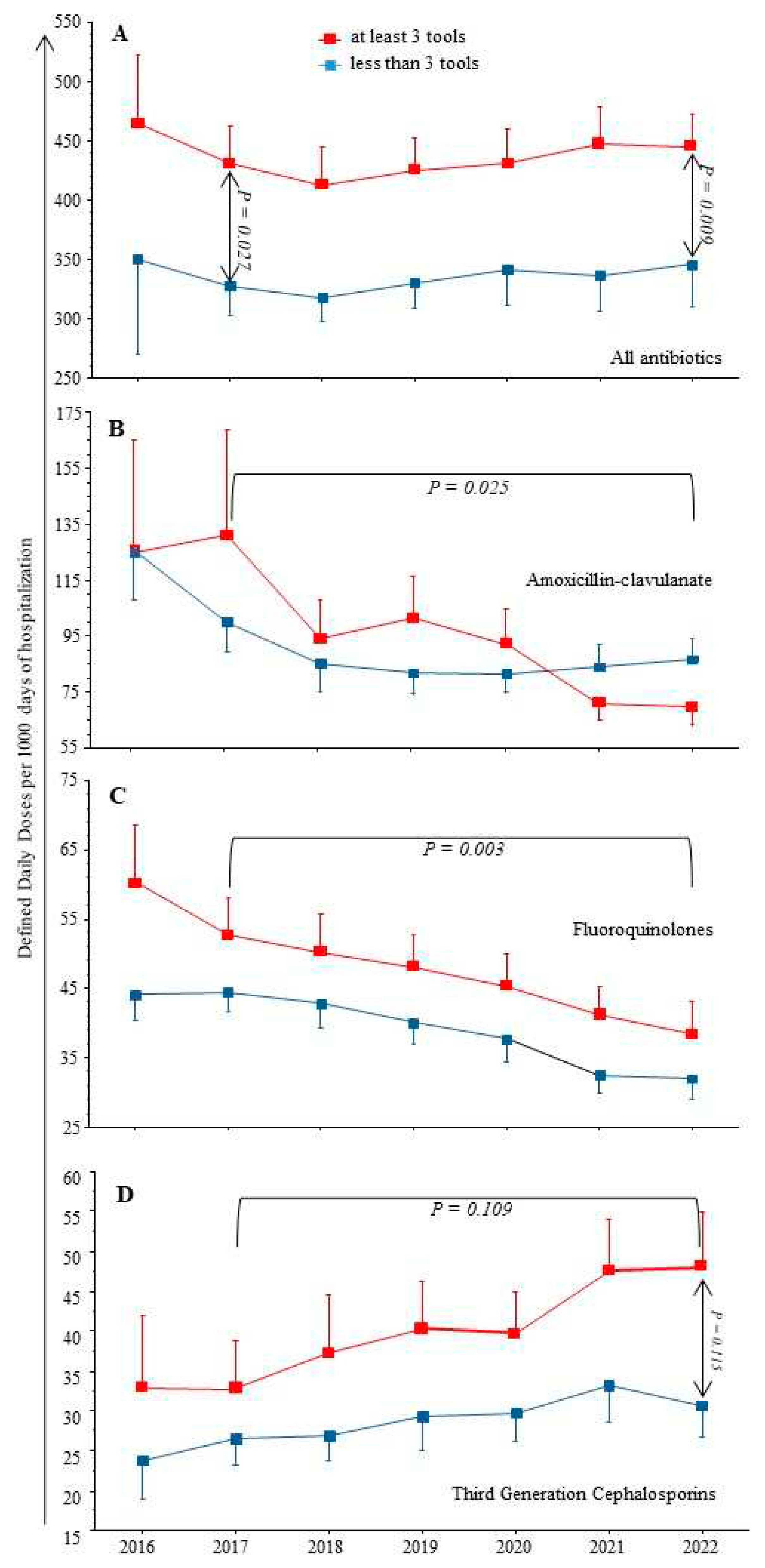
As shown in
Figure 1, from 2017 to 2022 the total AC were stable (means): from 390 to 405 DDD/1000 DH, but with a significant decrease of FQ and AMC, from 50 to 36 (p = 0.003) and 112 to 77 (p = 0.025) respectively. In contrast, we observed a trend toward increased TGC consumption on the same period (p = 0.109), and a significant increase of piperacillin/tazobactam use over the study period from 9 to 21 (p < 0.001).
Comparing institutions with ≥ 3 AMS tools already in place at the beginning of the study period, to other hospitals with ≤ 2 AMS tools, the latter had always significantly lower AC, whatever the drug considered (see
Figure 1).
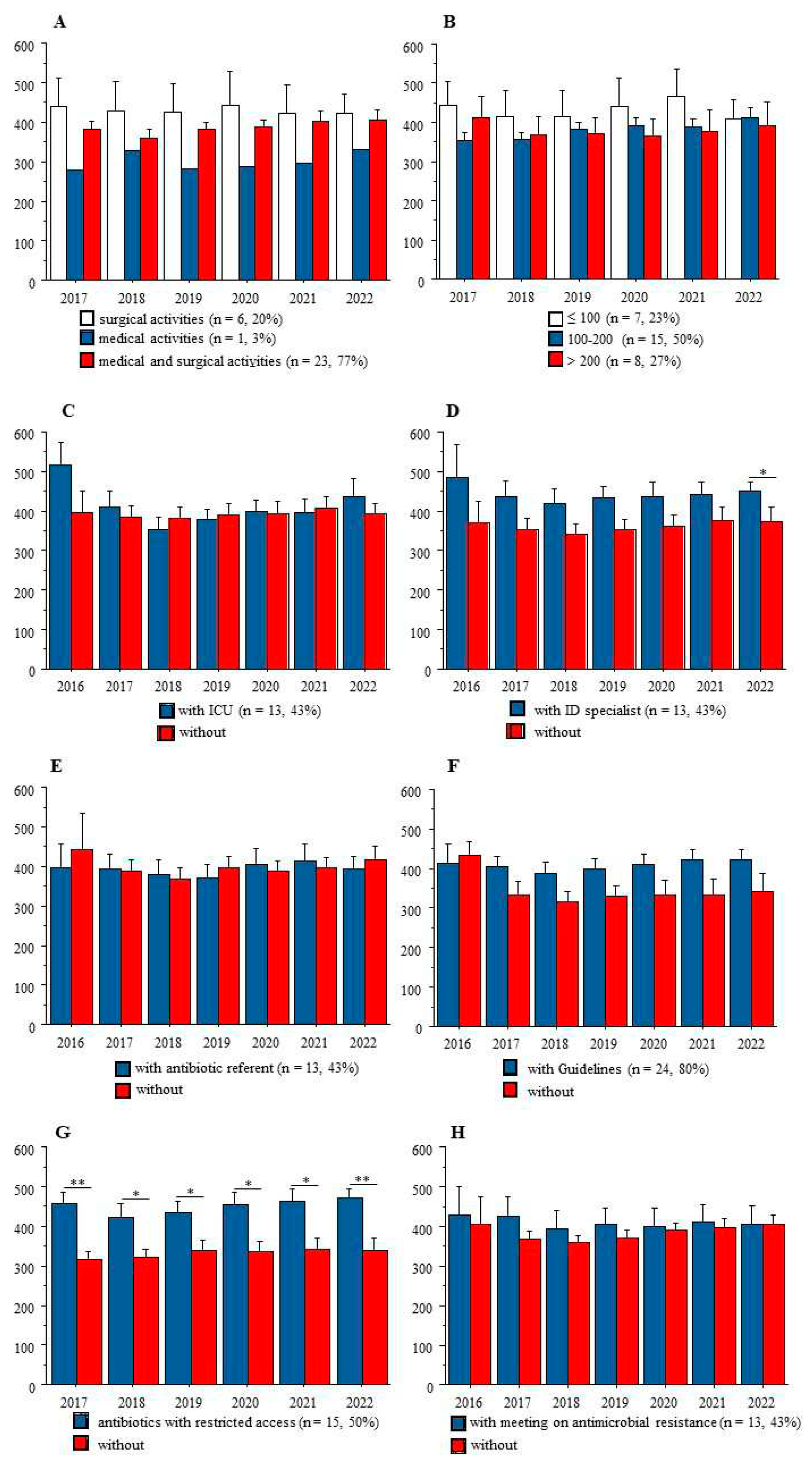
Figure 2 shows that at any time-point from 2017 to 2022, AC was not related to the medical and/or surgical activities of these institutions, nor to the number of beds. Also, among the 5 AMS tools, the list of antibiotics with restricted access was associated with significantly higher AC at the institution’ levels.
Covid-19 pandemic and antimicrobial consumption
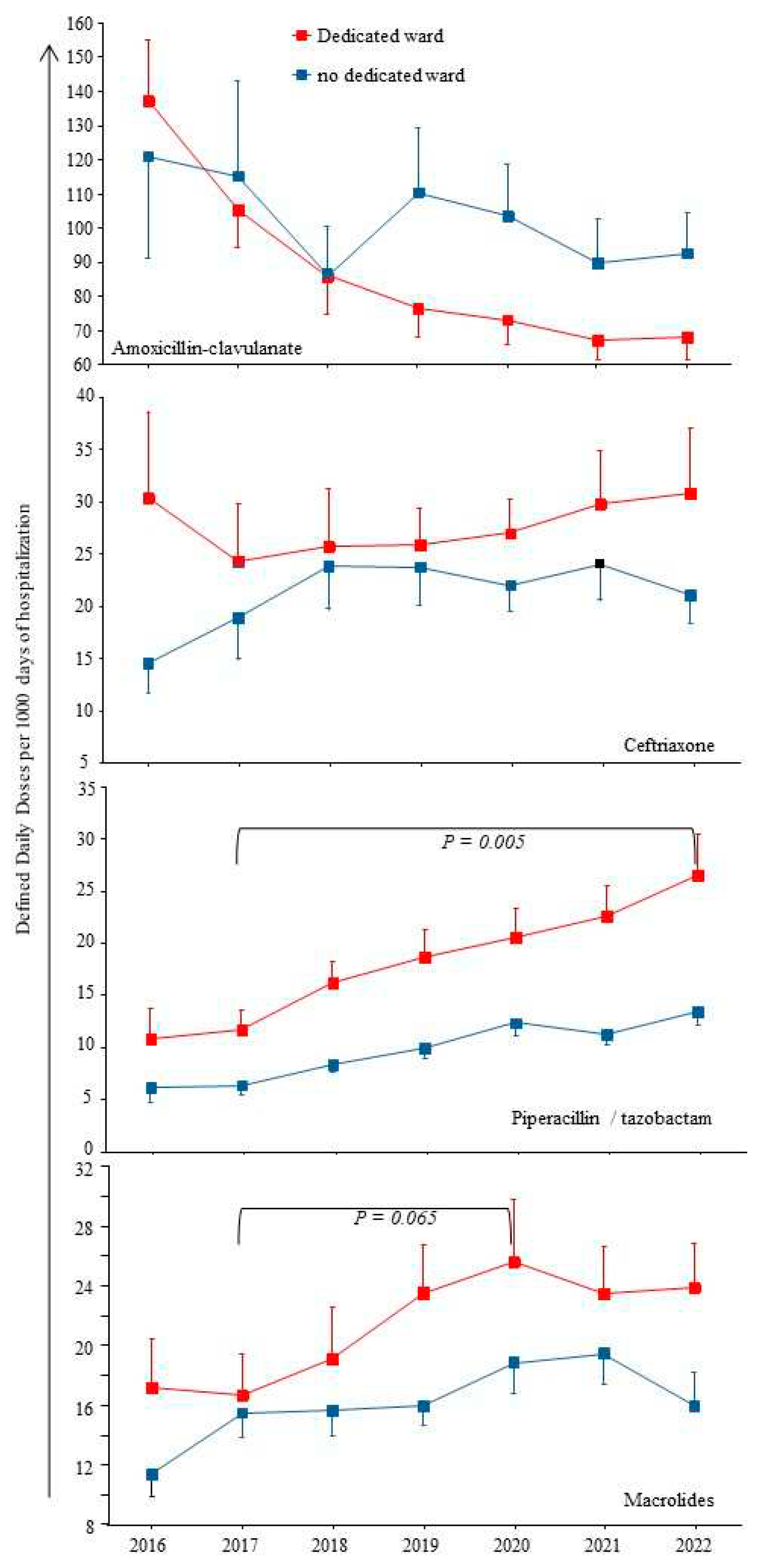
The COVID-19 pandemic might lead to an increase of antibiotic prescriptions for respiratory infections, i.e. AMC or ceftriaxone and/or macrolides, or even Pip/taz in case of healthcare-associated pneumonia. Ten institutions (33%) had a dedicated unit for COVID-19 patients and 7 (23%) had a dedicated intensive care unit.
Figure 3 shows that most antibiotics prescribed for respiratory infections (AMC or ceftriaxone) were not more used in institutions with dedicated units for COVID-19 patients compared to others. Regarding macrolides, a trend toward a higher consumption was observed from 2017 to 2020, from 15 to 25, p = 0.065. Of note, azithromycin emerged in the top ten molecules during the COVID-19 waves in two institutions with dedicated units.
Also, the constant augmentation of Pip/taz use over the study period was significantly accelerated from 2020 to 2022 in institutions with dedicated units (p ≤ 0.003).
Lastly, consumption of other antibiotic compounds such as amoxicillin, carbapenems and aminoglycosides did not vary significantly from 2017 to 2022 whatever the comparison: number of AMS tools or COVID-19 pandemic (data not shown).
Discussion
Our study shows that AC over 6 years in participating hospitals were inversely correlated to the number of tools implemented for antimicrobial stewardship policy. Also, care to COVID-19 patients was associated with an increase of macrolide use. Finally, use of piperacillin/tazobactam increased significantly from 2017 to 2022 without relationship with AMS tools, but with an accelerated consumption during COVID-19 waves.
It is worth remembering that private hospitals in France provide health care for at least 30% of the population, following AMS national recommendations; they participate to both medical education and clinical research.
Our study has several limitations. First, 30 out of 120 institutions among the same group gave their data and we do not know if our sample was representative. Second, we do not know all significant changes at work in these institutions during the study period. For example, one institution had to close its emergency ward, and another had to close a medical ward, due to the lack of human resources in both cases. Also, we had observed a high turn-over of physicians in one hospital, leading to a brutal increase of AC (+90% in one year) [
4]. Additionally, physicians’ reinforcements in overcrowded hospitals during COVID-19 pandemic have been associated with inadequate knowledge of internal guidelines and heterogeneous practices [
12]. Considering these limitations, we did not perform multivariate analyses.
In the current literature, AC are provided by the national healthcare system in France and/or surveillance reports from European or American institutes [
8]. Few reports indicated AC in a lower scale,
i.e. several institutions, trying to link AC to AMS tools. One French online survey described the AMS tools in 97 hospitals in 2020, showing that their implementations were common (between 84 to 95% of the participating institutions), but there was no data on AC, preventing any assessment of their effectiveness [
2].
Our first result showing higher AC in institutions with ≥ 3 AMS tools is counterintuitive. It might suggest that these institutions with high AC knew their need for implementation of most AMS tools, but the latter were still insufficient to fight antibiotic misuse. A large study performed in 2007 in 977 acute French hospitals showed a relationship between
information technology support for prescription and lower antibiotic consumption [
13]
. Of note, there was no difference between public and private hospitals. However, in our work all institutions used the same system of electronic patient records for several years, allowing permanent link between
pharmacy, laboratory and wards. Thus, the benefits of such established technology might decrease over time. In a more recent study performed in 2013 an “intensity score” of antimicrobial stewardship was assessed in 44 academic centers in US, but only the strategy component of the score was partly related to the amount of antimicrobial used [
14]
. Of note, ten years ago, we showed the absence of relationship between AMS tools and the quality of the antibiotic treatments at bedside in four public hospitals in France [
3].
Another counterintuitive result from our study is that the presence of an ID specialist in the institution was not associated with lower AC. This result could be explained at least by the paucity of the salary support to the AMS team: in the French survey cited above, most members received no salary support for their time spent on AMS activities, implying that ID specialists must develop their own activities [
2]. Together these data suggest the need for new tools in those modern hospitals with high AC, because in the latter most rules of antimicrobial stewardship policy are already in place.
Our second result is that COVID-19 pandemic was associated with little changes in AC. The measurable impact of the pandemic was a brief increase of macrolide consumption in 2020 compared to 2017. This result is in accordance with the putative efficacy of azithromycin against SARS-CoV-2 used during the first phase of the pandemic, and which was finally infirmed in 2021 [
15]. The weak impact of the pandemic on global AC has been reported, but always with some specific changes among beta-lactams or fluoroquinolones. As an example, in Northern Ireland, consumption of third generation cephalosporins as well as levofloxacin increased in the hospital setting [
16]. We observed a significant increase in Pip/taz consumption each year from 2017 to 2022 (see
Figure 3). Our result is in accordance with the latest report on the 2022-point prevalence survey on healthcare-associated infections and antimicrobial use in French healthcare facilities, showing that Pip/taz was the third drug to be prescribed, behind AMC and ceftriaxone: its prevalence was increased from 0.99% in 2017 to 1.64% in 2022 [
17]. The accelerated Pip/taz consumption during the COVID-19 waves could be related to the increase rate of healthcare-associated infections in overcrowded intensive care units [
18]. However, we also observed in clinical practices that Pip/taz can replaced AMC in community-acquired infections. This result suggested introducing specific measure to control its use.
Lastly, considering that AC is linked to antimicrobial resistance, one may ask for its potential increase during the COVID-19 pandemic. A meta-analysis including 28 studies, mainly from Unites States, Italy and Brazil, reported that antimicrobial resistance did not increase significantly during the pandemic [
19]. Moreover, infection prevention and control measures and antimicrobial stewardship program were not significantly associated with the rates of antimicrobial resistance [
19].
Materials and Methods
Antibiotic consumption
We used the national software Consores
to determine the AC in Defined Daily Doses (DDD) / 1000 days of hospitalization from 2017 to 2022 in voluntary-based private institutions working in a network in France [
20]. Consores
is a web tool recommended since 2015 by the French health authorities, allowing the analysis of antibiotic consumption in every hospital ward of a healthcare institution. The balance sheets of AC produced by the software were available in the first quarter of each year and included data from the previous year for comparison.
We extracted systematically the total amount of AC and more specifically the following drugs: amoxicillin, amoxicillin / clavulanate (AMC), piperacillin / tazobactam (Pip/taz), carbapenems, ceftriaxone and total consumptions of third generation cephalosporins (TGC), fluoroquinolones (FQ, and in details ciprofloxacin, ofloxacin, levofloxacin), macrolides and aminoglycosides.
Antimicrobial stewardship
Core elements of AMS program have been published and are now largely all over the world in the hospital setting [
1,
20]. For our study, pharmacists and/or ID specialists of the participating institutions described these main AMS tools in place at the beginning of the study’s period: 1/ existence of internal guidelines 2/ list of antibiotic with restricted access (i.e. those with a significant ecological impact (e.g. carbapenem), those with a non-negligible financial cost and those that we feel should be protected (e.g. fluoroquinolones) 3/ presence of an antibiotic referent (a pharmacist or microbiologist, or any other physician amenable to participate in antimicrobial stewardship) and/or 4/ access to an Infectious Diseases specialist advice 5/ proof of bi-annual meeting between the AMS team and the physicians to discuss about antimicrobial resistance and antibiotic consumption.
In accordance with our second goal, the institutions which had a dedicated medical and/or intensive care units for COVID-19 patients were specified. Of note, four successive major waves were observed between March 2020 to October 2022 in mainland France [
11].
Statistical Analysis
The data were analyzed with StatView software version 5.0, and statistical significance was established at α = .05. Continuous variables were compared with the Student t test, the Mann-Whitney non-parametric test or the Kruskal-Wallis test when appropriate. Proportions were compared with the χ2 or Fisher exact test when appropriate. The ratio of antibiotic consumption from one year to another was calculated as follows: (Qx−Qy)/ Qy. In all figures, results are presented as means ± standard errors. Only significant P values or trends (P < 0.2) are shown; P values in horizontal brackets indicate comparisons over a period, while vertical arrows indicate comparisons between groups at a precise time-point (see below).
Conclusions
The current AMS tools were partially inefficient to curb the antimicrobial consumption at hospital’ level, despite the limited impact of the COVID-19 pandemic on antibiotic use. Antimicrobial stewardship policies would need to be renewed for better results in the mid-terms.
Author Contributions
All authors contributed significantly to the study, and all have read and consent to this submission. E.D. and P-M.R. contributed to the study design and to the statistical analysis; E.D., A.A., J.R., P.Q., S.U., A.C., O.B., V.J. and P-M. R. contributed to the writing of the article.
Funding
none: the study was done as part of routine work.
Institutional Review Board Statement
not required.
Informed Consent Statement
not required.
Data Availability Statement
The data used during the current study is available from the corresponding author on reasonable request.
Acknowledgments
We thank professionnals who took part to the study: Nathalie Chalut, Hygiéniste, and Véronique Anduz-Acher, Pharmacie, Clinique St Roch, Cabestany; Eve Montera, Pharmacie, Clinique St Michel, Prades; Agnès Simand, Pharmacie, Clinique Centre République, Clermont Ferrand; Valérie Sohn, Clinique Médicale et Cardiologique d’Aressy, Pau; Catherine Sarret, Pharmacie, Clinique Montagard, Avignon; Sylvie Comparot, Pharmacie, Clinique Fonvert, Sorgues; Christine Hauchart, Pharmacie, Hôpital Privé St Claude, Saint-Quentin; Michel Vitris, Intensive care unit, Clinique du Pont de Chaume, Montauban; Nathalie Troadec, Pharmacie, Clinique St Augustin, Bordeaux ; Laura Bestman, Service Qualité , Clinique St Louis, Poissy; Isabelle Lallemand, Pharmacie, Clinique du Cap d’Or, La Seyne-sur-Mer; Soizic Morin, Pharmacie, Clinique Les Fleurs, Ollioules; Olivier Pantaloni, Pharmacie, Clinique St Pierre, Perpignan; Sophie Anjuere, Pharmacie, Clinique d’Orange, Orange; Romain Reboul, Pharmacie, Clinique Urbain V, Avignon; Florence Bouzigues, Pharmacie, Clinique Rhône-Durance, Avignon; Gaelle Borredon, Pharmacie, Clinique Ormeau, Tarbes; Véronique Dautezac et Marc-Antoine Hennet, Pharmacie, Clinique du Sidobre, Castres. All institutions in France.
Conflicts of Interest
all authors declare that they have no competing interest. The authors have no relevant financial or non-financial interests to disclose.
References
- Core Elements of Hospital Antibiotic Stewardship Programs | Antibiotic Use | CDC. Accessed in January 2020.
- Binda, F.; Tebano, G.; Kallen, M.C.; Ten Oever, J.; Hulscher, M.E.; Schouten, J.A.; Pulcini, C. Nationwide survey of hospital antibiotic stewardship programs in France. Med. Mal. Infect. 2020, 50, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Etienne, P.; Roger, P-M.; Brofferio, P.; Labate, C.; Blanc, V.; Tiger F.; Négrin, N.; Léotard, S. Antimicrobial stewardship program and quality of antibiotic prescriptions. Med. Mal. Infect. 2011, 41, 608–612. [CrossRef]
- Roger, P-M.; Espinet, A.; Ravily, D.; Meyer, MJ.; Moll, F.; Montera, E.; Rancezot, A.; Dautezac, V.; Pantaloni, O. Simplified therapeutic guidelines: the main tool of antimicrobial stewardship programs associated with optimal antibiotic therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 375–383. [CrossRef] [PubMed]
- Tamma, PD.; Avdic, E.; Keenan, JF.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, SE. What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Schuts, EC.; Hulscher, MEJL.; Mouton, JW.; Verduin, CM.; Stuart, JWTC.; Overdiek, HWPM.; van der Linden, PD.; Natsch, S.; Hertogh, CMPM.; Wolfs, TFW.; Schouten, JA.; Kullberg, BJ.; Prins, JM. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016, 16, 847–856. [CrossRef]
- Zhou, J.; Ma, X. A survey on antimicrobial stewardship in 116 tertiary hospitals in China. Clin Microbiol Infect. 2019, 25, 759.e9–759.e14. [Google Scholar] [CrossRef]
- Antimicrobial consumption in the EU/EEA (ESAC-Net) - Annual Epidemiological Report for 2021 (europa.eu) accessed 24 September 2023.
- Mendelson, M.; Morris, AM.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Halder, Tobler R.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 pandemic on inpatient antibiotic consumption in Switzerland. Antibiotics 2022, 11, 792. [CrossRef]
- Khan, S.; Hasan, SS.; Bond, SE.; Conway, BR.; Aldeyab, MA. Antimicrobial consumption in patients with COVID-19: a systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Markowicz, S.; Ait-Medjber, L.; Ouissa, R.; Delta, D.; Portecop, P.; Foucan, T.; Roger, PM. Dedicated team to ambulatory care for patients with COVID-19 requiring oxygen: low rate of hospital readmission. Int. J. Infect. Dis. 2022, 123, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, B.; Dumartin, C.; Parneix, P.; Fourrier-Réglat, A.; Rogues, AM. Relationship between antibiotic consumption and antibiotic policy: an adjusted analysis in the French healthcare system. J. Antimicrob. Chemother. 2011, 66, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Pakyz, AL.; Moczygemba, LR.; Wang, H.; Stevens, MP.; Edmond, MB. An evaluation of the association between an antimicrobial stewardship score and antimicrobial usage. J. Antimicrob. Chemother. 2015, 70, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Kamel, AM.; Monem, MSA.; Sharaf, NA.; Magdy, N.; Farid SF. Efficacy and safety of azithromycin in Covid-19 patients: a systematic review and meta-analysis of randomized clinical trials. Rev. Med. Virol. 2022, 32, e2258. [CrossRef] [PubMed]
- Aldeyab, MA.; Crowe, W.; Karasneh, RA.; Patterson, L.; Sartaj, M.; Ewing, J.; Lattyak, WJ.; Al-Azzam, S.; Araydah, M.; Darwish Elhajji, F.; Kabbaha, S.; Conway, BR.; Conlon-Bingham, G.; Farren, D.; Scott, M. The impact of the COVID-19 pandemic on antibiotic consumption and prevalence of pathogens in primary and secondary healthcare settings in Northern Ireland. Br J Clin Pharmacol. 2023, 89, 2851–66. [Google Scholar] [CrossRef] [PubMed]
- enquete prevalence IAS 2022.pdf. Accessed 4 August 2023.
- Abubakar, U.; Awaisu, A.; Khan, AH.; Alam, K. Impact of COVID-19 pandemic on healthcare-associated infections: a systematic review and meta-analysis. Antibiotics 2023, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Langford, BJ.; Soucy, JR.; Leung, V.; So, M.; Kwan, ATH.; Portnoff, JS.; Bertagnolio, S.; Raybardhan, S.; MacFadden, DR.; Daneman, N. Antibiotic resistance associated with the COVID-19 pandemic: a systematic review and meta-analysis. Clin Microbiol Infect. 2023, 29, 302-9. [CrossRef] [PubMed]
- Boussat, S.; Demoré, B.; Lozniewski, A.; Aissa, N.; Rabaud, C. How to improve the collection and analysis of hospital antibiotic consumption: preliminary results of the ConsoRes software experimental implementation. Med. Mal. Infect. 2012, 42, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Santé publique France Enquêtes Flash : évaluation de la circulation des variants du SARS-CoV-2 en France. Saint-Maurice: Santé publique France. https://www.santepubliquefrance.fr/etudes-et-enquetes/enquetes-flash-evaluation-de-la-circulation-des-variants-du-sars-cov-2-en-france#block-33727. Accessed 25 September 2023. 25 September.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).