Submitted:
03 October 2025
Posted:
08 October 2025
You are already at the latest version
Abstract
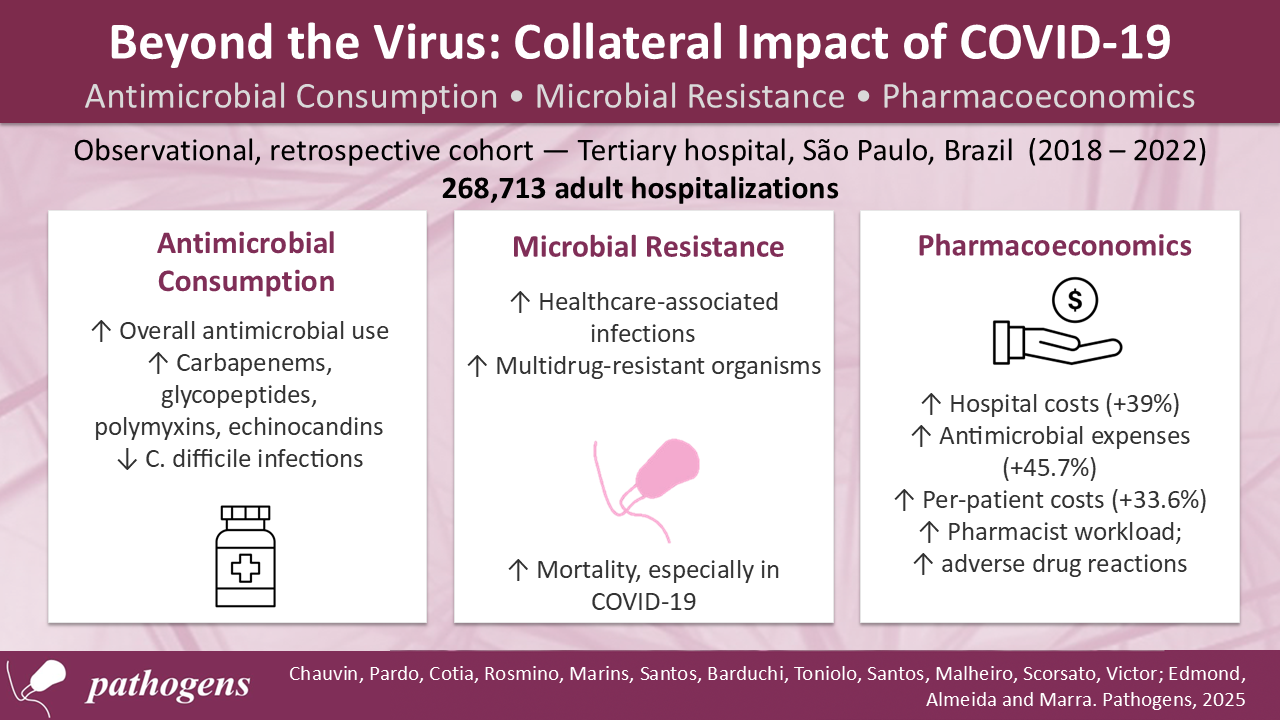
Background: The COVID-19 pandemic had major global repercussions for hospitalized patients, affecting multiple aspects of hospital care. Understanding these effects is important for improving healthcare management and infection control practices. This study aimed to analyze and compare the pandemic’s impact on antimicrobial use in hospitalized patients, with emphasis on therapeutic, microbiological, and pharmacoeconomic aspects. Methods: A retrospective observational study was conducted at a Brazilian tertiary hospital (2018–2022). Adult patients receiving antimicrobials were included. Variables analyzed were antimicrobial consumption, incidence of healthcare-associated infections, resistance profiles, hospital costs, adverse drug reactions, and pharmacy activities. Data were obtained from anonymized institutional records and analyzed using descriptive statistics, time series, and linear regression. Results: Among 268,713 hospitalizations, raw counts of hospitalizations and antimicrobial use were higher during the pandemic, though monthly averages showed no significant increase. Higher consumption of carbapenems, glycopeptides, polymyxins, and echinocandins was linked to more healthcare-associated infections by multidrug-resistant organisms. Clostridioides difficile infections declined. Mortality rose significantly, especially among COVID-19 patients. Costs increased by 39%, with antimicrobial-related expenses up 45.7%. Conclusions: The pandemic intensified antimicrobial use, resistance, and costs. Strengthening antimicrobial stewardship and infection control is essential to reduce future risks.
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Hospitalizations and Mortality
3.2. Antimicrobial Use
3.2.1. Defined Daily Dose (DDD)
3.2.2. Days of Therapy (DOT)
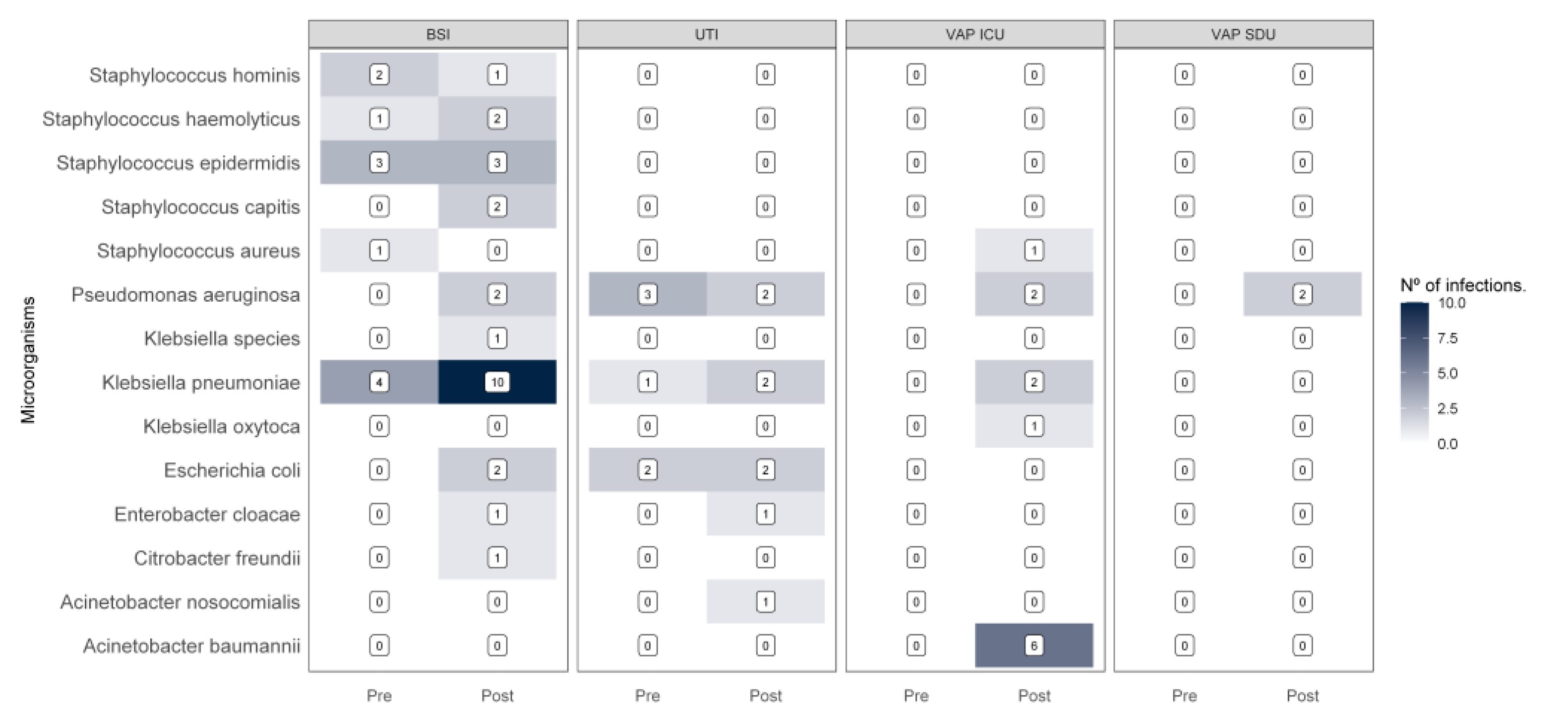
3.3. Healthcare-Associated Infections
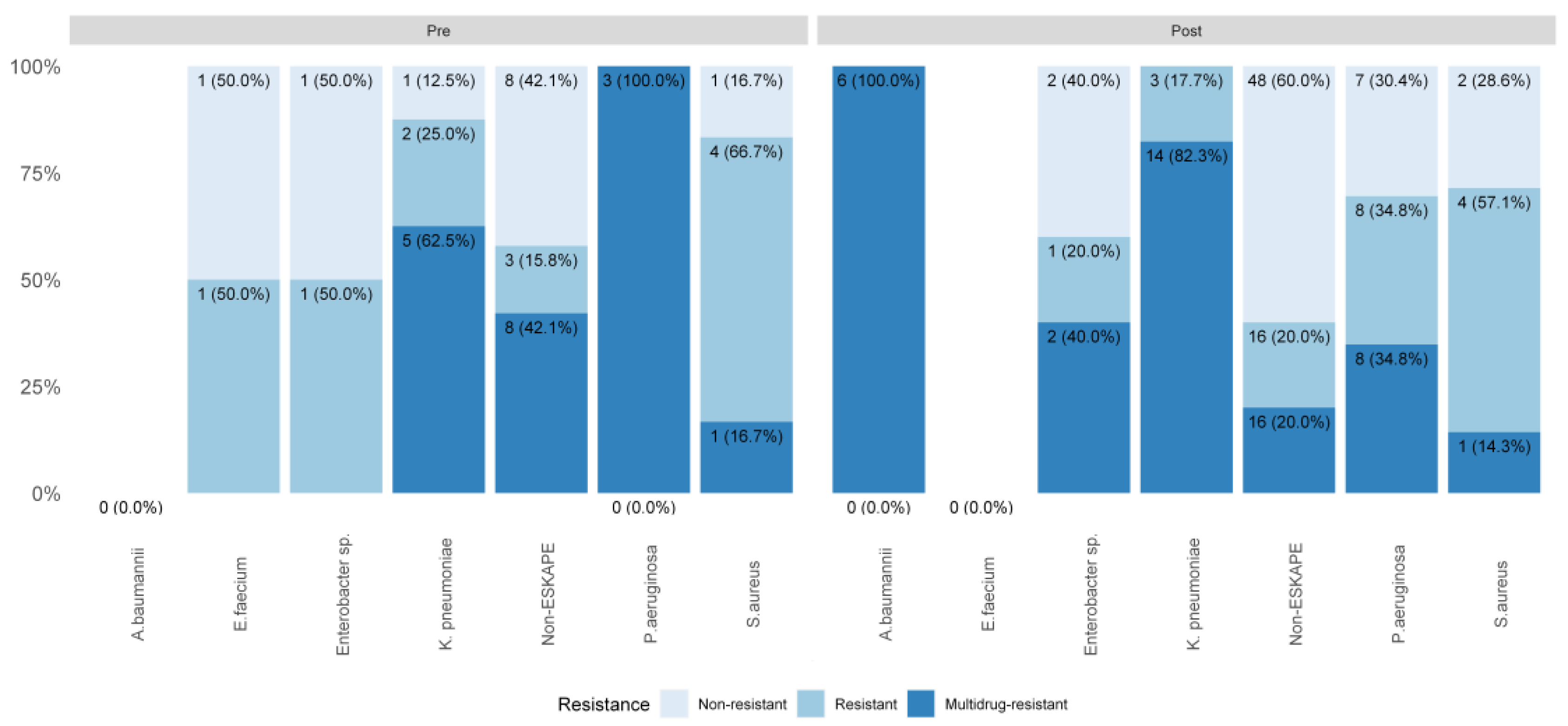
3.3.1. Microbiological Profile of Healthcare-Associated Infections
3.4. Financial Impact
3.5. Pharmacist Activities
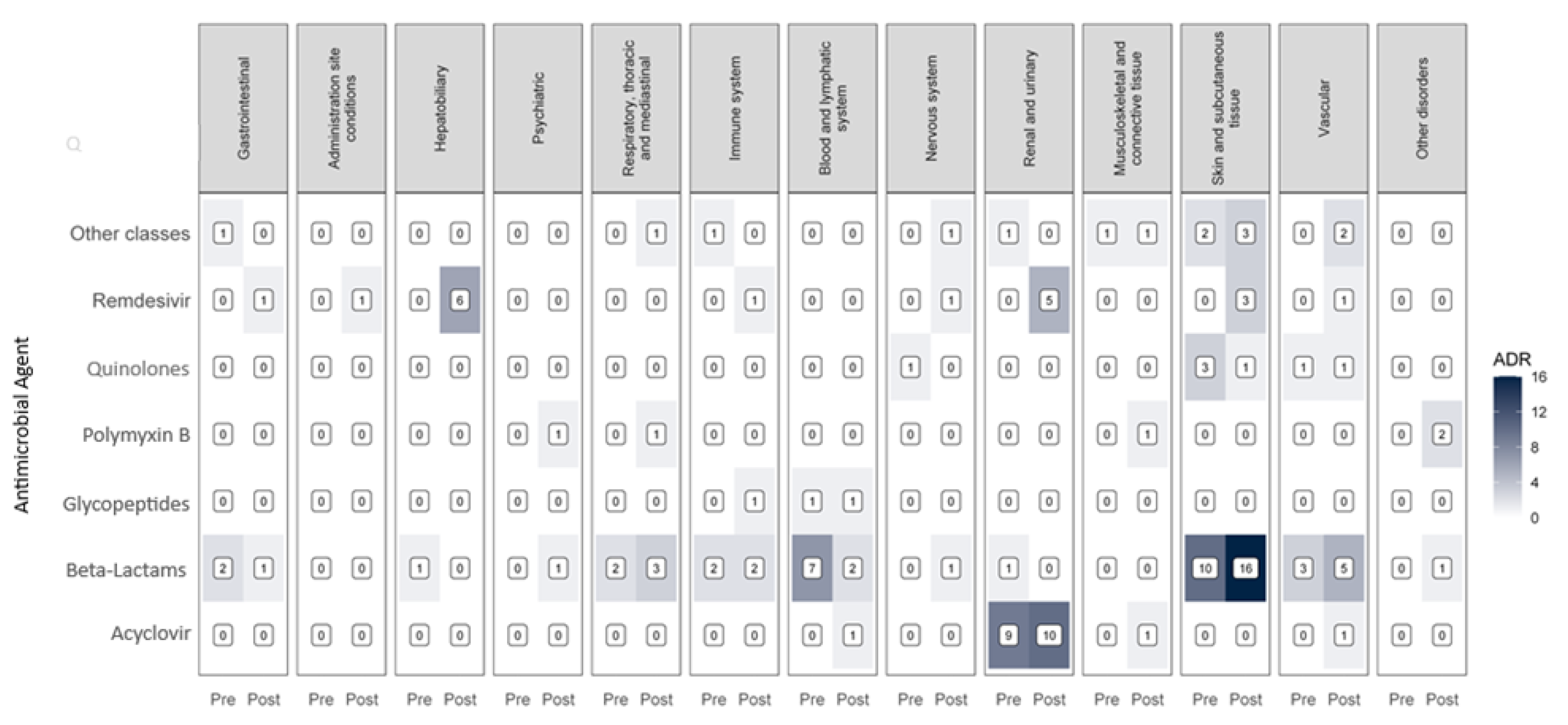
3.6. Adverse Drug Reactions
3.7. Linear Regression Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witt, L.S.; Howard-Anderson, J.R.; Jacob, J.T.; Gottlieb, L.B. The Impact of COVID-19 on Multidrug-Resistant Organisms Causing Healthcare-Associated Infections: A Narrative Review. JAC Antimicrob Resist 2022, 5. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial Resistance (AMR) in COVID-19 Patients: A Systematic Review and Meta-Analysis (November 2019–June 2021). Antimicrob Resist Infect Control 2022, 11, 45. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629. [Google Scholar] [CrossRef]
- Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit. JAC Antimicrob Resist 2019, 1, dlz072. [CrossRef]
- Pierce, J.; Stevens, M.P. COVID-19 and Antimicrobial Stewardship: Lessons Learned, Best Practices, and Future Implications. International Journal of Infectious Diseases 2021, 113, 103. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, R.A.; Umaru, N.; Alharbi, A.B.; Aslanpour, Z. Antimicrobial Stewardship Implementation before and during the COVID-19 Pandemic in the Acute Care Settings: A Systematic Review. BMC Public Health 2023, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Garrouste-Orgeas, M.; Ibn Essaied, W.; Schwebel, C.; Darmon, M.; Mourvillier, B.; Ruckly, S.; Dumenil, A.S.; Kallel, H.; Argaud, L.; et al. Attributable Mortality of ICU-Acquired Bloodstream Infections: Impact of the Source, Causative Micro-Organism, Resistance Profile and Antimicrobial Therapy. Journal of Infection 2017, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Anvisa Nota Técnica GVIMS-GGTES No 05-2017. 2017.
- ATCDDD - ATC/DDD Index Available online:. Available online: https://atcddd.fhi.no/atc_ddd_index_and_guidelines/atc_ddd_index/ (accessed on 18 August 2025).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clinical Microbiology and Infection 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Sills, J.M. World Health Organization Adverse Reaction Terminology Dictionary. Drug Inf J 1989, 23, 211–216. [Google Scholar] [CrossRef]
- Siegel, S.; Para Baixar, D. Estatística Não-Paramétrica Para Ciências Do Comportamento PDF.
- KOSAMBI, D.D. An Extension of the Least-Squares Method for Statistical Estimation. Ann Eugen 1947, 13, 257–261. [Google Scholar] [CrossRef]
- Charnet R, F.Ca.C.E.B.H. Análise de Modelos de Regressão Linear Com Aplicações. 2008.
- Royston, J.P. An Extension of Shapiro and Wilk’s W Test for Normality to Large Samples. Appl Stat 1982, 31, 115. [Google Scholar] [CrossRef]
- Pfaff, B. Analysis of Integrated and Cointegrated Time Series with R. Analysis of Integrated and Cointegrated Time Series with R 2008. [Google Scholar] [CrossRef]
- Menezes-Filho, N.; Komatsu, B.K.; Villares, L. The Impacts of COVID-19 Hospitalizations on Non-COVID-19 Deaths and Hospitalizations: A Panel Data Analysis Using Brazilian Municipalities. PLoS One 2023, 18, e0295572. [Google Scholar] [CrossRef]
- Kadri, S.S.; Sun, J.; Lawandi, A.; Strich, J.R.; Busch, L.M.; Keller, M.; Babiker, A.; Yek, C.; Malik, S.; Krack, J.; et al. Association between Caseload Surge and Covid-19 Survival in 558 u.s. Hospitals, March to August 2020. Ann Intern Med 2021, 174, 1240–1251. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the Characteristics, Morbidity, and Mortality of COVID-19 and Seasonal Influenza: A Nationwide, Population-Based Retrospective Cohort Study. Lancet Respir Med 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clinical Microbiology and Infection 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clinical Infectious Diseases 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Allel, K.; Peters, A.; Conejeros, J.; Martínez, J.R.W.; Spencer-Sandino, M.; Riquelme-Neira, R.; Rivas, L.; Rojas, P.; Orellana Chea, C.; García, P.; et al. Antibiotic Consumption During the Coronavirus Disease 2019 Pandemic and Emergence of Carbapenemase-Producing Klebsiella Pneumoniae Lineages Among Inpatients in a Chilean Hospital: A Time-Series Study and Phylogenomic Analysis. Clinical Infectious Diseases 2023, 77, S20–S28. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial Consumption in Patients with COVID-19: A Systematic Review and Meta-Analysis. Expert Rev Anti Infect Ther 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics 2021, Vol. 10, Page 132 2021, 10, 132. [Google Scholar] [CrossRef]
- Pinte, L.; Ceasovschih, A.; Niculae, C.M.; Stoichitoiu, L.E.; Ionescu, R.A.; Balea, M.I.; Cernat, R.C.; Vlad, N.; Padureanu, V.; Purcarea, A.; et al. Antibiotic Prescription and In-Hospital Mortality in COVID-19: A Prospective Multicentre Cohort Study. J Pers Med 2022, 12, 877. [Google Scholar] [CrossRef]
- Ul Mustafa, Z.; Salman, M.; Aldeyab, M.; Kow, C.S.; Hasan, S.S. Antimicrobial Consumption among Hospitalized Patients with COVID-19 in Pakistan. SN Compr Clin Med 2021, 3, 1691–1695. [Google Scholar] [CrossRef]
- Golli, A.L.; Zlatian, O.M.; Cara, M.L.; Olteanu, M. Pre- and Post-COVID-19 Antimicrobial Resistance Pattern of Pathogens in an Intensive Care Unit. Pharmaceuticals 2024, 17. [Google Scholar] [CrossRef]
- Golli, A.L.; Popa, S.G.; Ghenea, A.E.; Turcu, F.L. The Impact of the COVID-19 Pandemic on the Antibiotic Resistance of Gram-Negative Pathogens Causing Bloodstream Infections in an Intensive Care Unit. Biomedicines 2025, 13, 379. [Google Scholar] [CrossRef]
- Nie, Z.; Sun, T.; Zhao, F. Safety and Efficacy of Antiviral Drugs for the Treatment of COVID-19: A Systematic Review. Infect Drug Resist 2022, 15, 4457. [Google Scholar] [CrossRef]
- AlBahrani, S.; Almogbel, F.; Alanazi, W.; Almutairi, S.H.; Alanazi, M.; Maximos, S.; Azaiez, F.; Osman, A.; Almuthen, S.; Jebakumar, A.Z.; et al. Carbapenem Use Correlates with Percentage of Patients with COVID-19 in Intensive Care Units. Infection 2023, 51, 331–336. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 Pandemic on Antibiotic Consumption: A Systematic Review Comparing 2019 and 2020 Data. Front Public Health 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The Impact of the COVID-19 Pandemic on Healthcare-Associated Infections in Intensive Care Unit Patients: A Retrospective Cohort Study. Antimicrob Resist Infect Control 2021, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Baktash, A.; Goeman, J.J.; Harmanus, C.; Notermans, D.W.; de Greeff, S.C.; Kuijper, E.J. Comparison of Trends in Clostridioides Difficile Infections in Hospitalised Patients during the First and Second Waves of the COVID-19 Pandemic: A Retrospective Sentinel Surveillance Study. The Lancet Regional Health - Europe 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Hilvers, E.; Matizanadzo, J.; McClure, V.; Butterick, P.; Morgan, M. Clostridioides Difficile Infection Following COVID-19: A Nationwide Analysis Using Routine Surveillance Data in Wales. Journal of Hospital Infection 2024, 0. [Google Scholar] [CrossRef]
- Pantasri, T. Expanded Roles of Community Pharmacists in COVID-19: A Scoping Literature Review. Journal of the American Pharmacists Association 2021, 62, 649. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, V.; Cadogan, C.; Fialová, D.; Henman, M.C.; Hazen, A.; Okuyan, B.; Lutters, M.; Stewart, D. Provision of Clinical Pharmacy Services during the COVID-19 Pandemic: Experiences of Pharmacists from 16 European Countries. Research in Social & Administrative Pharmacy 2020, 17, 1507. [Google Scholar] [CrossRef]
- Parreiras Martins, M.A.; Fonseca de Medeiros, A.; Dias Carneiro de Almeida, C.; Moreira Reis, A.M. Preparedness of Pharmacists to Respond to the Emergency of the COVID-19 Pandemic in Brazil: A Comprehensive Overview. Drugs & Therapy Perspectives 2020, 36, 455. [Google Scholar] [CrossRef]
- Liu, C.; Patel, K.; Cernero, B.; Baratt, Y.; Dandan, N.; Marshall, O.; Li, H.; Efird, L. Expansion of Pharmacy Services During COVID-19: Pharmacists and Pharmacy Extenders Filling the Gaps Through Telehealth Services. Hosp Pharm 2021, 57, 349. [Google Scholar] [CrossRef]
- Kapinos, K.A.; Peters, R.M.; Murphy, R.E.; Hohmann, S.F.; Podichetty, A.; Greenberg, R.S. Inpatient Costs of Treating Patients With COVID-19. JAMA Netw Open 2024, 7, e2350145–e2350145. [Google Scholar] [CrossRef]
- Kanerva, M.; Rautava, K.; Kurvinen, T.; Marttila, H.; Finnilä, T.; Rantakokko-Jalava, K.; Pietilä, M.; Mustonen, P.; Kortelainen, M. Economic Impact and Disease Burden of COVID-19 in a Tertiary Care Hospital: A Three-Year Analysis. PLoS One 2025, 20, e0323200. [Google Scholar] [CrossRef]
- Marins, T.A.; Marra, A.R.; Edmond, M.B.; Colombo, L.R.P.; Vieira, S.F.; De Oliveira Xavier, F.; Chauvin, A.G.; Pinho, J.R.R.; De Almeida, S.M.; Junior, M.S.D. Adverse Drug Reactions and Drug Interactions in the Treatment of Hospitalized Patients with Coronavirus Disease 2019 (COVID-19). Antimicrobial Stewardship and Healthcare Epidemiology 2021, 1. [Google Scholar] [CrossRef]
- Sun, J.; Deng, X.; Chen, X.; Huang, J.; Huang, S.; Li, Y.; Feng, J.; Liu, J.; He, G. Incidence of Adverse Drug Reactions in COVID-19 Patients in China: An Active Monitoring Study by Hospital Pharmacovigilance System. Clin Pharmacol Ther 2020, 108, 791. [Google Scholar] [CrossRef] [PubMed]
| Before COVID-19 | During COVID-19 | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Hospitalized patients | |||
| Total | 116,591 (43.4%) | 152,122 (56.6%) | - |
| Monthly mean (± SD) | 4,484 (± 286) | 4,474 (± 874) | 0.586 |
| Hospitalized patients using antimicrobials | |||
| Total | 60,502 (42.8%) | 80,922 (57.2%) | - |
| Monthly mean (± SD) | 2,327 (± 149.1) | 2,380 (± 464.4) | 0.399 |
| Patient/days | |||
| Total | 415,252 (43.3%) | 543,500 (56.7%) | - |
| Monthly mean (± SD) | 15,971 (± 1,915.9) | 15,985 (± 2,325.2) | 0.706 |
| Hospitalized patients with COVID-19 using antimicrobials | - | 6,936 | - |
| Deaths | |||
| Total | 861 (36.6%) | 1494 (63.4%) | - |
| Monthly mean (± SD | 33.12 (± 7.7) | 43.94 (± 13.0) | <0.001 |
| Deaths in COVID-19 patients | - | 449 (30.1%) | - |
| Antimicrobial | Before COVID-19 | After COVID-19 | p-value |
|---|---|---|---|
| 1st and 2nd generation cephalosporin (Cefazolin, Cephalothin, Cefuroxime) | |||
| Mean ± SD | 57.2 ± 5.2 | 52.1 ± 10.4 | |
| Median [IQR] | 57.8 [55.3 – 59.9] | 54.8 [48.3 – 59.3] | 0.061 |
| 3rd, 4th and 5th cephalosporin (Ceftriaxone, Cefotaxime, Ceftazidime, Cefepime, Ceftaroline) | |||
| Mean ± SD | 39.7 ± 2.7 | 44.2 ± 7.6 | |
| Median [IQR] | 39.7 [37.4 – 41.5] | 42.1 [38.1 – 48.60-] | 0.028 |
| Cephalosporin + β-lactamase inhibitors | |||
| Mean ± SD | 2.4 ± 2.8 | 13.2 ± 4.6 | |
| Median [IQR] | 1.1 [0 – 4.2] | 13.3 [11.1 – 15.8] | <0.001 |
| Macrolides | |||
| Mean ± SD | 20.1 ± 3.3 | 28.1 ± 15.8 | |
| Median [IQR] | 19.3 [18.0 – 22.7] | 24.3 [16.5 – 32.5] | 0.128 |
| Carbapenems | |||
| Mean ± SD | 26.8 ± 4.0 | 31.4 ± 4.4 | |
| Median [IQR] | 26.9 [23.6 – 29.7] | 30.8 [28.4 – 34.0] | <0.001 |
| Glycopeptides | |||
| Mean ± SD | 39.2 ± 4.8 | 52.5 ± 11.4 | |
| Median [IQR] | 39.2 [35.2 – 43.0] | 49.1 [45.5 – 56.0] | <0.001 |
| Polymyxin B | |||
| Mean ± SD | 5.1 ± 2.9 | 19.1 ± 14.7 | |
| Median [IQR] | 4.6 [3.6 – 6.3] | 14.4 [8.5 – 24.6] | <0.001 |
| Echinocandins | |||
| Mean ± SD | 17.0 ± 5.1 | 28.4 ± 9.6 | |
| Median [IQR] | 17.6 [14.3 – 20.7] | 26.5 [22.5 – 31.3] | <0.001 |
| Triazole Antifungals | |||
| Mean ± SD | 15.5± 3.3 | 17.0 ± 4.6 | 0.166 |
| Median [IQR] | 15.7 [13.2 – 18.0] | 16.2 [14.0 – 19.8] | |
| Antivirals | |||
| Mean ± SD | 8.8 ± 10.9 | 4.8 ± 6.1 | |
| Median [IQR] | 4.1 [3.3 – 5.9] | 3.0 [1.6 – 6.2] | 0.020 |
| Total Before COVID-19 | Before COVID-19 |
Total During COVID-19 | During COVID-19 | p-value | |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Healthcare-associated infections | 40 (22.5%) | 138 (77.5%) | |||
| Healthcare-associated infections incidence rate per 10,000 patient-days | - | 1.12 | - | 2.30 | <0.001 |
| Bloodstream infections incidence rate per 1000 catheter-days | - | - | |||
| Mean ± SD | 0.28 ± 0.29 | 0.40 ± 0.32 | |||
| Median [IQR] | 0.25 [0.00 – 0.49] | 0.34 [0.16 – 0.61] | 0.216 | ||
| Urinary tract infections incidence rate per 1,000 catheter-days | - | - | |||
| Mean ± SD | 0.35 ± 0.61 | 0.40 ± 0.49 | |||
| Median [IQR] | 0.00 [0.00 – 0.83] | 0.00 [0.00 – 0.73] | 0.453 | ||
| Ventilator-associated infection incidence rate per 1,000 ventilator-days | - | - | |||
| Mean ± SD | 0.72 ± 2.16 | 1.08 ± 1.30 | |||
| Median [IQR] | 0.0 [0.00 – 0.00] | 0.35 [0,00 – 1.97] | 0.016 | ||
| Group ESKAPE infections | 21 (26.6%) | 58 (73.4%) | |||
| Group ESKAPE infections incidence rate per 10,000 patient-days | - | 0.59 | - | 0.97 | 0.076 |
| Before COVID-19 |
During COVID-19 |
Change (%) | p-value | |
|---|---|---|---|---|
| Total Costs (millions of US$) | 844.52 | 1,682.93 | - | |
| Median monthly [IQR] | 32.42 [31.22-34.47] | 53.20 [43.82-56.96] | 39.0% | <0.001 |
| Range | 25.82 – 38.16 | 20.49 - 61.71 | ||
| Monthly antimicrobial costs (millions of US$) | ||||
| Median [IQR] | 4.03 [3.76-4.34] | 7.44 [6.11- 8.22] | 45.7% | <0.001 |
| Range | 3.04 – 5.60 | 3.37 – 9.46 | ||
| Cost per patient (US$) | ||||
| Median [IQR] | 7,194 [6,898-7,502] | 10,840 [10,317 – 11,365] | 33.6% | <0.001 |
| Range | 6,037 – 8,635 | 8,311 – 16,750 | ||
| Total costs of COVID-19 patients (millions of US$) | ||||
| Median [IQR] | - | 8.66 [5.56-12.03] | - | |
| Range | - | 0.21-22.77 | - | |
| Cost per patient with COVID-19 (US$) | ||||
| Median [IQR] | - | 49,660 [33,336 – 76,132] | - | |
| Range | - | 5,302 – 135,866 | - |
| Before COVID-19 | During COVID-19 | p-value | |
|---|---|---|---|
| Monthly pharmaceutical activities | |||
| Median [IQR] | 9,488 [8,703.25 – 10,102.5] | 11,589.5 [8,061.75 – 12,504.25] | 0.042 |
| Range | 7,072 – 11,324 | 6,596 – 14,519 | |
| Monthly pharmaceutical activities per pharmacist | |||
| Median [IQR] | 214.66 [197.86 – 230.95] | 250.92 [164.07 – 262.25] | 0.131 |
| Range | 164.5 – 248.6 | 140.3 – 312.4 | |
| N of pharmaceutics / day | |||
| Median [IQR] | 44 [43 - 44] | 47.5 [45 - 49] | <0.001 |
| Range | 42 - 47 | 43 - 51 | |
| Hospitalized patients / month | |||
| Median [IQR] | 4,524.5 [4,265.5 – 4,706.25] | 4,582.5 [3,897.25 – 5,272.5] | 0.586 |
| Range | 4,002 – 4,948 | 1,979 – 5,565 |
| System | Total ADRs | Before COVID-19 |
During COVID-19 |
|---|---|---|---|
| Skin tissue | 38 | 15 (30.61%) | 23 (28.4%) |
| Renal and urinary | 26 | 11 (22.45%) | 15 (18.52%) |
| Lymphatic and blood* | 12 | 8 (16.33%) | 4 (4.94%) |
| Vascular* | 14 | 4 (8.16%) | 10 (12.35%) |
| Immune | 7 | 3 (6.12%) | 4 (4.94%) |
| Hepatobiliary | 7 | 1 (2.04%) | 6 (7.41%) |
| Gastrointestinal | 5 | 3 (6.12%) | 2 (2.47%) |
| Respiratory | 7 | 2 (4.08%) | 5 (6.17%) |
| Nervous | 4 | 1 (2.04%) | 3 (3.7%) |
| Connective tissue and musculoskeletal | 4 | 1 (2.04%) | 3 (3.7%) |
| Psychiatric | 2 | 0 (0%) | 2 (2.47%) |
| Others | 4 | 0 (0%) | 4 (4,93%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





