Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Searching Criteria
1.2. Application of the 'Therapeutic Unit' Concept to Assess the Equivalence of EVs Concentrations
2. Biogenesis and Classification of EVs
3. The Cargo of EVs
4. Mechanisms of EVs Interaction with Recipient Cell Membranes
5. Sources of EVs and Methods for Their Isolation
6. Experimental Studies of EVs as Therapeutic Tools for Neonatal Pathologies
6.1. Hypoxic ischemic encephalopathy
6.2. Respiratory distress syndrome and bronchopulmonary dysplasia
6.3. Neonatal sepsis
6.4. Necrotizing enterocolitis
7. Discussion and challenges of Translating EVs into Clinical Practice
| References | Sources of EVs | Model | Protocol of administration | Dosing | Therapeutic effects | Elucidated mechanisms |
|---|---|---|---|---|---|---|
| HYPOXIC-ISCHEMIC ENCEPHALOPATHY | ||||||
| [232] 2016 |
human bone marrow MSCs |
the Rice-Vannucci model | total dose – 2,4 x 1010 EVs route of administration – intravenous administrations per day – 2 days – 1 |
1 unit* |
↓ total number and duration of seizures ↓ pathological fluctuations of blood pressure |
↑ baroreflex-mediated heart rate response |
| [137] 2019 |
human bone marrow MSCs |
the Rice-Vannucci model | total dose – 2,4 x 1010 EVs route of administration – intravenous administrations per day – 2 days – 1 |
1 unit.. |
↓permeability of Blood-brain barrier |
↑Annexin А1/FPR in neonatal brain endothelial cells and microglia |
| [132] 2019 |
human bone marrow MSCs |
the Rice-Vannucci model | total dose – 1,25 × 109 Evs route of administration – intranasal administrations per day – 1 days – 1 |
0,1 Unit |
↓ of tissue loss ↓ % of cell death ↓ microglial activation ↑ behavioral outcomes (negative geotaxis test) |
|
| [74] 2019 |
human Wharton’s jelly MSCs |
the Rice-Vannucci model + intraperitoneal injection of LPS | total dose – 325 µg of EVs protein per animal route of administration – intranasal administrations per day – 1 days – 1 |
0,3 Unit |
↓ microgliosis ↓ neuroinflammation |
↓LPS/TLR4 signaling in microglia |
| [233] 2020 |
rat bone marrow MSCs (H2S preconditioning) |
the Rice-Vannucci model | total dose – 1,5 × 108 EVs route of administration – intracardial injection administrations per day – 1 days – 1 |
0,06 Unit |
↓ water content and infarct volume of the brain ↓ % of cell apoptosis ↑polarization toward the anti-inflammatory M2 phenotype ↑ memory function |
↑miR-7b-5p ↓FOS → ↓Iba1+ in microglia |
| [133] 2020 |
human bone marrow MSCs |
the Rice-Vannucci model | total dose – 2,7 × 108 EVs route of administration – intraperitoneal administrations per day – 1 days – 3 (1,3,5 post HI) |
0,03 Unit |
↓ striatal tissue loss ↓ M1 micro- and A1 astroglia activation ↑ neurogenensis and angiogenesis ↑ myelination |
|
| [234] 2021 |
mice bone marrow MSCs |
the Rice-Vannucci model | total dose – 100 µg of EVs protein route of administration – intracardial injection administrations per day – 1 days – 1 |
0,1 Unit |
↓ HI-induced edema, infarction, infiltrating monocytes ↓phagocytosis of viable neurons ↑synaptic densities |
↓ p-NF-κB → ↓OPN → ↓Iba1 in М1 microglia |
| [235] 2021 |
mice bone marrow MSCs |
the Rice-Vannucci model | total dose – 5 µg of EVs protein route of administration – intranasal administrations per day – 1 days – 1 |
0,005 Unit |
↓ injury volumes ↓ microglial activation ↓ neuroinflammation |
↓Iba1 → ↓ Casp3 in microglia |
| [236] 2021 |
rat primary astrocytes (P1) |
the Rice-Vannucci model | total dose – 2.5 µg of EVs protein route of administration – intraperitoneal administrations per day – 1 days – 1 |
0,0024 unit | ↓ the area of cerebral infarction ↓ HIBD-induced neuronal apoptosis ↓ oxidative stress ↓ neuroinflammation ↑ body weight ↑cognitive functions( grip test, negative geotaxis test |
↑ miR-17-5p →↓BNIP →↓Bax in brain tissue |
| [237] 2022 |
brain tissues of neonatal mice (P9) after HI |
the Rice-Vannucci model | total dose – 8 × 109 EVs route of administration – intranasal administrations per day – 2 days – 1 |
0,066 Unit |
↓ infarct size ↓ Casp3 expression |
↑ miR-342-3p and miR-330-3p in brain tissue |
| [238] 2022 |
mice bone marrow MSCs |
the Rice-Vannucci model | total dose – 2 × 109 EVs route of administration – intranasal administrations per day – 1 days – 1 |
0,2 Unit |
↑ animal survival ↓ infarct volume of brain ↓ % of apoptosis cells ↓ neuroinflammation ↑proprioceptive function |
↑miR-93 →↓JMJD3→↑KLF2→↓Casp3,Bax in neurons |
| [239] 2023 |
immortalized human bone marrow MSCs | the Rice-Vannucci model | total dose – 2,7 × 108 EVs route of administration – intranasal administrations per day – 1 days -3 (1,3,5 post HI) |
0,03 unit. |
↑neurogenesis and angiogenesis ↓ monocyte infiltration ↓ astrogliosis and microgliosis |
|
| BRONCHOPULMONARY DYSPLASIA | ||||||
| [148] 2018 |
human Wharton’s jelly MSCs |
hyperoxia (HYRX)-induced BPD mice model (P1 –P7 75% О2) |
total dose –0,9 µg of EVs protein route of administration – intravenous administrations per day – 1 days – 1 |
0,001 unit |
↑ lung architecture ↓ lung fibrosis ↓ peripheral pulmonary arterial remodeling |
|
| [156] 2018 |
preterm human Wharton’s jelly MSCs | HYRX-induced BPD mice model (с P1-P4 95% О2) | total dose – 4.5 × 108EVs route of administration – intraperitoneal administrations per day – 1 days -2 (P2,P4) |
0,038 Unit |
↑ lung architecture ↓ infiltration of neutrophils ↓ pulmonary hypertension ↓ alveolar-capillary leak |
↑TSG-6 sinalling in lung tissue |
| [240] 2018 |
human Wharton’s jelly MSCs | HYRX-induced BPD rat model (P1- P14 60% О2) |
total dose – 0,213 × 1010 EVs route of administration – intratracheal administrations per day – 1 days – 3 (P3,P7,P10) |
0,27 unit. |
↑ alveolar development ↓ pulmonary vascular remodeling |
|
| [157] 2018 |
rat bone marrow MSCs | HYRX-induced BPD rat model (P0- P14 85% О2) | total dose – 3,4 × 109 EVs route of administration – intraperitoneal administrations per day – 1 days – 14 (P1-P15) |
1,96 Unit |
↑ alveolar growth ↑ lung blood vessel density ↓ pulmonary hypertension |
↑VEGF signaling in lung tissue |
| [158] 2018 |
human umbilical cord blood MSCs |
HYRX-induced BPD rat model (P1- P14 90% О2) | total dose – 20 µg of EVs protein route of administration – intratracheal administrations per day – 1 Days – 1 (P5) |
0,019 Unit |
↑ alveolarization and angiogenesis | ↑VEGF signaling in lung tissue |
| [241] 2020 |
human Wharton’s jelly MSCs | HYRX-induced BPD mice model (P0-P14 75% О2) | total dose – 6 × 108 EVs route of administration – intravenous administrations per day – 1 days – 1 (PN4) |
0,025 unit. |
↓ alveolar simplification ↓septal collagen disposition ↑blood vessel count ↓ pulmonary hypertension ↑ functional exercise capacity |
|
| [242] 2021 |
human bone marrow MSCs. | In utero induced BPD rat model (antenatal injection of E. coli endotoxin e20) | total dose – 0,25 × 106 EVs route of administration – intra-amniotic administrations per day – 10 per pregnant rat days – 1 (e20) |
0,17 Unit |
↓ lung simplification ↑ vascularization ↓ pulmonary hypertension ↑ lung mechanical function |
|
| [155] 2021 |
human Wharton’s jelly MSCs | HYRX-induced BPD mice model (P – P7 75% О2) | total dose – 6 × 108 EVs route of administration – intravenous administrations per day – 1 days – 1 (PN4) |
0,025 unit. |
↑ thymic development ↑proportion of CD4+FoxP3+ regulatory T cells ↓ alveolar simplification |
|
| [240] 2021 |
human umbilical cord blood MSCs | HYRX-induced BPD rat model (P1 – P14 60% О2) | total dose – 0.64 × 1010 EVs route of administration – intratracheal administrations per day – 1 days – 4 (P3,P7,P10,P21) |
0,27 Unit |
↑ alveolar development ↓ deposition of fibrous tissue ↑ density of M2 macrophages ↓ pulmonary hypertension |
|
| [92] 2021 |
amniotic fluid-derived EVs (full-term cesarean sections) | HYRX-induced BPD rat model (P1 – P14 85% О2) | total dose – 1 × 1010 EVs route of administration – intratracheal administrations per day – 1 days – 1 (P3) |
0,42 unit |
↑ alveolar development ↓ pulmonary hypertension |
|
| [243] 2022 |
human breast milk-derived EVs | HYRX-induced BPD rat model (P1 – P7 85% О2) | total dose -140 µg of EVs protein route of administration – intragastric administrations per day – 1 days – 1 (PN7) |
0,133 unit |
↓ lung tissue collapse ↓ cleaved-caspase 3 |
↓IL-17 /↓ FADD in Type II alveolar epitheliocytes |
| [152] 2022 |
human Wharton’s jelly MSCs | HYRX-induced BPD rat model (P1 – P14 85% О2) | total dose – 96 × 108 EVs route of administration – intratracheal administrations per day – 1 days – 1 (PN3) |
0,04 Unit |
↑ lung vascular density and alveolar structure ↓lung inflammation ↓ pulmonary hypertension |
↑VEGF/eNOS in lung tissue |
| [163] 2022 |
human amniotic epithelial cells (term birth after caesarean sections) |
in utero induced BPD mice model (injection of LPS e16)+ (P3.5 – P28 65% О2) |
total dose – 10 µg of EVs protein route of administration – intravenous administrations per day – 1 days – 1 (PN4) |
0,01 Unit |
↑lung tissue-to-air space ratio ↓ lung inflammation ↑ type II alveolar epithelial cell ↓ pulmonary hypertension ↑lung tissue elasticity |
|
| [160] 2022 |
human Wharton’s jelly MSCs | HYRX-induced BPD mice model (injection of LPS P7/P8) + 40% О2 P10 | total dose – 1 × 106 EVs route of administration – intratracheal administrations per day – 1 days – 1 (PN9) |
0,0002 Unit |
↑ lung architecture ↑blood vessel density ↑ mRNA of antiinflammatory cytokines in lung tissue |
|
| [244] 2023 |
human umbilical cord blood MSCs | HYRX-induced BPD mice model (P1 – P14 85% О2) | total dose – 15 x 105 EVs route of administration – intraperitoneal administrations per day – 1 days – 3 (P4-P6) |
0,000063 unit | ↓ lung fibrosis ↑ vascular development |
↑miR-185-5p→↓CDK6 → ↑angiogenesis in lung tissue |
| [150] 2023 |
human bone marrow MSCs | hypoxia –induced BPD rat model(10 min 40%О2 + 2 min 1%О2 12 times daily P1 – P14) | total dose – 2 x 105 EVs route of administration – intraperitoneal administrations per day – 1 days – 14 (P1-P14) |
0,00012 unit | ↓ simplified alveolar structure ↓ pulmonary hypertension ↑ capillary distribution ↑ respiratory efficiency ↓ oxidative stress |
↑ PI3K/AKT →↑ SOD in lung tisue |
| NECROTIZING ENTEROCOLITIS | ||||||
| [178] 2016 |
mice bone marrow MSCs | NEC –induced preterm mice model Barlow et al. [245](21 e )+ 90 sec 100% N2 + 4°C 10 min twice daily ( P1-P4) | total dose – 2,5 x 109 EVs route of administration – intraperitoneal administrations per day – 1 days -1 (prior NEC) |
0,1 unit. |
↓ the overall incidence of NEC ↑ gut barrier function |
|
| [88] 2018 |
neonatal mice enteric neuronal stem cells | NEC –induced preterm rat model Barlow et al. (21e) + (90 sec 100% N2 + 4°C 10 min every 8 h + LPS every 4 h (P1-P4) | total dose – 4 × 108 EVs route of administration – intraperitoneal administrations per day – 1 days -1 (prior NEC) |
0,017 Unit |
↓ intestine villus destruction ↓ the overall incidence of NEC |
|
| [212] 2019 |
bovine milk-derived EVs | NEC –induced preterm mice model Barlow et al. (10 min 5% O2 3 times between P5-P9 + LPS 4 times between P6-P7) | total dose – 1.2 mg of EVs protein route of administration – intragastric via gavage administrations per day – 3 days – 5 (P5-P9) |
1,14 Unit |
↑ intestine villus destruction ↑ number of goblet cells ↓ intestinal mucosal inflammation ↓ oxidative stress |
|
| [208] 2019 |
human breast milk-derived EVs | NEC –induced preterm rat model Barlow et al. (21e ) 90 sec 1,5% О2 + 4°C 10 min 3 times daily P1-P4 + LPS 1 time P1 | total dose – 2.4 × 1010 EVs route of administration – intragastric via gavage administrations per day – 6 days – 4 (P1-P4) |
1 Unit |
↓ villus destruction ↓ the overall incidence of NEC |
|
| [175] 2020 |
rat amniotic fluid CD117 stem cells (e14.5) |
NEC –induced preterm mice model Barlow et al. (10 min 5% O2 3 times between P5-P9 + LPS 4 times between P6-P7) | total dose – 3.5 х 108 EVs route of administration – intraperitoneal administrations per day – 1 days – 2 (P6-P7) |
0,015 unit. |
↑ gut epithelial regeneration ↓ intestinal inflammation |
↑Wnt/ β-catenin →increased intestinal epithelial proliferation |
| [246] 2022 |
human breast milk-derived EVs | NEC –induced preterm mice model Barlow et al. (1 min 100% N2 + 4°C 5 min twice a day P6-P10) | total dose – 30 µg of EVs protein route of administration – intragastric via gavage administrations per day – 3 days – 3(P8-P10) |
0,04 unit |
↑ ileal crypts number ↓ inflammatory microenvironment |
|
| NEONATAL MENINGITIS | ||||||
| [171] 2022 |
human Wharton’s jelly-derived MSCs after thrombin preconditioning | Escherichia coli –induced meningitis in newborn Rats (P11) | total dose – 2,6 x 107 EVs route of administration – intraventricular administrations per day – 1 days – 1 (P11) |
0.001 unit |
↓ neural cell death ↓ number of active microglia ↓ levels of inflammatory cytokines in brain tissues |
|
| * The yield of EVs derived from supernatants of 4 × 107 MSCs that have been cultured for 48 h was defined as 1 unit. 1 unit contains 2,4 1010 EVs or 1.05 mg of EVs protein [14] | ||||||
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The Global Epidemiology of Preterm Birth. Best Pract Res Clin Obstet Gynaecol 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Belousova, V.; Svitich, O.; Timokhina, E.; Ignatko, I.; Bogomazova, I.; Pesegova, S.; Silaeva, T.; Kuzmina, T.; Skorobogatova, O. Caspase-3, Caspase-8 and XIAP Gene Expression in the Placenta: Exploring the Causes of Spontaneous Preterm Labour. International Journal of Molecular Sciences 2023, 24, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Yu, D.; Liang, Y.; He, H.; He, W.; Li, Y.; Zhang, M.; Gao, Y.; Wu, F.; Chen, Q. Global Trends in Incidence and Death of Neonatal Disorders and Its Specific Causes in 204 Countries/Territories during 1990-2019. BMC Public Health 2022, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Thebaud, B.; Paton, M.C.B.; Fleiss, B.; Papagianis, P.; Baker, E.; Bennet, L.; Yawno, T.; Elwood, N.; Campbell, B.; et al. Advances in Neonatal Cell Therapies: Proceedings of the First Neonatal Cell Therapies Symposium (2022). Pediatr Res 2023. [Google Scholar] [CrossRef] [PubMed]
- Schiller, E.A.; Cohen, K.; Lin, X.; El-Khawam, R.; Hanna, N. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. Int J Mol Sci 2023, 24, 2622. [Google Scholar] [CrossRef]
- Nitkin, C.R.; Rajasingh, J.; Pisano, C.; Besner, G.E.; Thébaud, B.; Sampath, V. Stem Cell Therapy for Preventing Neonatal Diseases in the 21st Century: Current Understanding and Challenges. Pediatr Res 2020, 87, 265–276. [Google Scholar] [CrossRef]
- Leopoldino, R.D.; Santos, M.T.; Costa, T.X.; Martins, R.R.; Oliveira, A.G. Drug Related Problems in the Neonatal Intensive Care Unit: Incidence, Characterization and Clinical Relevance. BMC Pediatr 2019, 19, 134. [Google Scholar] [CrossRef]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells Are Exponentially Increasing: Where Are We in Recent Years? Stem Cell Rev Rep 2022, 18, 23–36. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Sung, S.I.; Chang, Y.S. Mesenchymal Stem Cell Therapy for Intractable Neonatal Disorders. Pediatr Neonatol 2021, 62 Suppl 1, S16–S21. [Google Scholar] [CrossRef]
- Maldonado, V.V.; Patel, N.H.; Smith, E.E.; Barnes, C.L.; Gustafson, M.P.; Rao, R.R.; Samsonraj, R.M. Clinical Utility of Mesenchymal Stem/Stromal Cells in Regenerative Medicine and Cellular Therapy. J Biol Eng 2023, 17, 44. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat Rev Drug Discov 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv Drug Deliv Rev 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- C, T.; Kw, W.; E, A.; Mj, A.; Jd, A.; R, A.; A, A.; T, A.; F, A.; Gk, A.-S.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. Journal of extracellular vesicles 2018, 7. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.-K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C.; et al. Mast Cell- and Dendritic Cell-Derived Exosomes Display a Specific Lipid Composition and an Unusual Membrane Organization. Biochem J 2004, 380, 161–171. [Google Scholar] [CrossRef]
- Biró, É.; Akkerman, J.W.N.; Hoek, F.J.; Gorter, G.; Pronk, L.M.; Sturk, A.; Nieuwland, R. The Phospholipid Composition and Cholesterol Content of Platelet-Derived Microparticles: A Comparison with Platelet Membrane Fractions. Journal of Thrombosis and Haemostasis 2005, 3, 2754–2763. [Google Scholar] [CrossRef]
- Zorova, L.D.; Kovalchuk, S.I.; Popkov, V.A.; Chernikov, V.P.; Zharikova, A.A.; Khutornenko, A.A.; Zorov, S.D.; Plokhikh, K.S.; Zinovkin, R.A.; Evtushenko, E.A.; et al. Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria? International Journal of Molecular Sciences 2022, 23, 7408. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An Integrated Database of High-Throughput Data for Systemic Analyses of Extracellular Vesicles. J Extracell Vesicles 2013, 2, 10–3402. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional Proteins of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res Ther 2019, 10, 359. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Golovicheva, V.V.; Varlamova, E.G.; Danilina, T.I.; Goryunov, K.V.; Shevtsova, Y.A.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Evtushenko, E.A.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Afford Neuroprotection by Modulating PI3K/AKT Pathway and Calcium Oscillations. Int J Biol Sci 2022, 18, 5345–5368. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Gao, X.; Xiong, Y.; Li, Q.; Han, M.; Shan, D.; Yang, G.; Zhang, S.; Xin, D.; Zhao, R.; Wang, Z.; et al. Extracellular Vesicle-Mediated Transfer of miR-21-5p from Mesenchymal Stromal Cells to Neurons Alleviates Early Brain Injury to Improve Cognitive Function via the PTEN/Akt Pathway after Subarachnoid Hemorrhage. Cell Death Dis 2020, 11, 363. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b Promotes Neural Plasticity and Functional Recovery after Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Su, M.; Wang, X.; Xie, C. Exosomal microRNA-22-3p Alleviates Cerebral Ischemic Injury by Modulating KDM6B/BMP2/BMF Axis. Stem Cell Res Ther 2021, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, J.; Che, Y. miR-31 from Adipose Stem Cell-Derived Extracellular Vesicles Promotes Recovery of Neurological Function after Ischemic Stroke by Inhibiting TRAF6 and IRF5. Exp Neurol 2021, 342, 113611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomal miR-146a-5p Reduces Microglial-Mediated Neuroinflammation via Suppression of the IRAK1/TRAF6 Signaling Pathway after Ischemic Stroke. Aging (Albany NY) 2021, 13, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Lu, M.; Shao, W.; Xing, H.; Huang, Y. Extracellular Vesicle-Based Nucleic Acid Delivery. Interdisciplinary Medicine 2023, 1, e20220007. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat Rev Genet 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Zhuo, C.-J.; Hou, W.-H.; Jiang, D.-G.; Tian, H.-J.; Wang, L.-N.; Jia, F.; Zhou, C.-H.; Zhu, J.-J. Circular RNAs in Early Brain Development and Their Influence and Clinical Significance in Neuropsychiatric Disorders. Neural Regen Res 2020, 15, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chao, J.; Yao, H. Circular RNA and Its Mechanisms in Disease: From the Bench to the Clinic. Pharmacol Ther 2018, 187, 31–44. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Fitzsimmons, C.; Geaghan, M.P.; Shannon Weickert, C.; Atkins, J.R.; Wang, X.; Cairns, M.J. Circular RNA Biogenesis Is Decreased in Postmortem Cortical Gray Matter in Schizophrenia and May Alter the Bioavailability of Associated miRNA. Neuropsychopharmacology 2019, 44, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Wapinski, O.; Chang, H.Y. Long Noncoding RNAs and Human Disease. Trends Cell Biol 2011, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA Gas5 Is a Growth Arrest- and Starvation-Associated Repressor of the Glucocorticoid Receptor. Sci Signal 2010, 3, ra8. [Google Scholar] [CrossRef]
- Xu, B.; Gerin, I.; Miao, H.; Vu-Phan, D.; Johnson, C.N.; Xu, R.; Chen, X.-W.; Cawthorn, W.P.; MacDougald, O.A.; Koenig, R.J. Multiple Roles for the Non-Coding RNA SRA in Regulation of Adipogenesis and Insulin Sensitivity. PLoS One 2010, 5, e14199. [Google Scholar] [CrossRef]
- Ellis, B.C.; Graham, L.D.; Molloy, P.L. CRNDE, a Long Non-Coding RNA Responsive to Insulin/IGF Signaling, Regulates Genes Involved in Central Metabolism. Biochim Biophys Acta 2014, 1843, 372–386. [Google Scholar] [CrossRef]
- Tong, X.; Gu, P.; Xu, S.; Lin, X. Long Non-Coding RNA-DANCR in Human Circulating Monocytes: A Potential Biomarker Associated with Postmenopausal Osteoporosis. Biosci Biotechnol Biochem 2015, 79, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.A.; Moss, L.D.; Lee, J.-Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long Noncoding RNA MALAT1 in Exosomes Drives Regenerative Function and Modulates Inflammation-Linked Networks Following Traumatic Brain Injury. J Neuroinflammation 2018, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat Rev Mol Cell Biol 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Trino, S.; Laurenzana, I.; Simeon, V.; Calice, G.; Raimondo, S.; Podestà, M.; Santodirocco, M.; Di Mauro, L.; La Rocca, F.; et al. MiRNAs and piRNAs from Bone Marrow Mesenchymal Stem Cell Extracellular Vesicles Induce Cell Survival and Inhibit Cell Differentiation of Cord Blood Hematopoietic Stem Cells: A New Insight in Transplantation. Oncotarget 2016, 7, 6676–6692. [Google Scholar] [CrossRef]
- Wolin, S.L.; Steitz, J.A. The Ro Small Cytoplasmic Ribonucleoproteins: Identification of the Antigenic Protein and Its Binding Site on the Ro RNAs. Proc Natl Acad Sci U S A 1984, 81, 1996–2000. [Google Scholar] [CrossRef]
- Valkov, N.; Das, S. Y RNAs: Biogenesis, Function and Implications for the Cardiovascular System. Adv Exp Med Biol 2020, 1229, 327–342. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med 2017, 6, 1262–1272. [Google Scholar] [CrossRef]
- Cambier, L.; Giani, J.F.; Liu, W.; Ijichi, T.; Echavez, A.K.; Valle, J.; Marbán, E. Angiotensin II-Induced End-Organ Damage in Mice Is Attenuated by Human Exosomes and by an Exosomal Y RNA Fragment. Hypertension 2018, 72, 370–380. [Google Scholar] [CrossRef]
- Zakharova, L.; Svetlova, M.; Fomina, A.F. T Cell Exosomes Induce Cholesterol Accumulation in Human Monocytes via Phosphatidylserine Receptor. J Cell Physiol 2007, 212, 174–181. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as New Vesicular Lipid Transporters Involved in Cell-Cell Communication and Various Pathophysiologies. Biochim Biophys Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front Mol Biosci 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Lobasso, S.; Tanzarella, P.; Mannavola, F.; Tucci, M.; Silvestris, F.; Felici, C.; Ingrosso, C.; Corcelli, A.; Lopalco, P. A Lipidomic Approach to Identify Potential Biomarkers in Exosomes From Melanoma Cells With Different Metastatic Potential. Front Physiol 2021, 12, 748895. [Google Scholar] [CrossRef]
- Esser, J.; Gehrmann, U.; D’Alexandri, F.L.; Hidalgo-Estévez, A.M.; Wheelock, C.E.; Scheynius, A.; Gabrielsson, S.; Rådmark, O. Exosomes from Human Macrophages and Dendritic Cells Contain Enzymes for Leukotriene Biosynthesis and Promote Granulocyte Migration. J Allergy Clin Immunol 2010, 126, 1032–1040. [Google Scholar] [CrossRef]
- Holopainen, M.; Colas, R.A.; Valkonen, S.; Tigistu-Sahle, F.; Hyvärinen, K.; Mazzacuva, F.; Lehenkari, P.; Käkelä, R.; Dalli, J.; Kerkelä, E.; et al. Polyunsaturated Fatty Acids Modify the Extracellular Vesicle Membranes and Increase the Production of Proresolving Lipid Mediators of Human Mesenchymal Stromal Cells. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 1350–1362. [Google Scholar] [CrossRef]
- Pizzinat, N.; Ong-Meang, V.; Bourgailh-Tortosa, F.; Blanzat, M.; Perquis, L.; Cussac, D.; Parini, A.; Poinsot, V. Extracellular Vesicles of MSCs and Cardiomyoblasts Are Vehicles for Lipid Mediators. Biochimie 2020, 178, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Battista, N.; Riganti, L.; Prada, I.; Antonucci, F.; Cantone, L.; Matteoli, M.; Maccarrone, M.; Verderio, C. Active Endocannabinoids Are Secreted on Extracellular Membrane Vesicles. EMBO Rep 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Shimoda, A.; Tahara, Y.; Sawada, S.-I.; Sasaki, Y.; Akiyoshi, K. Glycan Profiling Analysis Using Evanescent-Field Fluorescence-Assisted Lectin Array: Importance of Sugar Recognition for Cellular Uptake of Exosomes from Mesenchymal Stem Cells. Biochem Biophys Res Commun 2017, 491, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative Differences in T-Cell Activation by Dendritic Cell-Derived Extracellular Vesicle Subtypes. EMBO J 2017, 36, 3012–3028. [Google Scholar] [CrossRef] [PubMed]
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic Cell Exosomes Directly Kill Tumor Cells and Activate Natural Killer Cells via TNF Superfamily Ligands. Oncoimmunology 2012, 1, 1074–1083. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat Biotechnol 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cui, G.-H.; Guo, H.-D.; Li, H.; Zhai, Y.; Gong, Z.-B.; Wu, J.; Liu, J.-S.; Dong, Y.-R.; Hou, S.-X.; Liu, J.-R. RVG-Modified Exosomes Derived from Mesenchymal Stem Cells Rescue Memory Deficits by Regulating Inflammatory Responses in a Mouse Model of Alzheimer’s Disease. Immun Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular Vesicle-Based Therapeutics: Natural versus Engineered Targeting and Trafficking. Exp Mol Med 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Alini, M.; Baghaban Eslaminejad, M.; Hosseini, S. Engineering Strategies for Customizing Extracellular Vesicle Uptake in a Therapeutic Context. Stem Cell Res Ther 2022, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu Rev Plant Biol 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Wang, F.; Cerione, R.A.; Antonyak, M.A. Isolation and Characterization of Extracellular Vesicles Produced by Cell Lines. STAR Protoc 2021, 2, 100295. [Google Scholar] [CrossRef]
- Bost, J.P.; Saher, O.; Hagey, D.; Mamand, D.R.; Liang, X.; Zheng, W.; Corso, G.; Gustafsson, O.; Görgens, A.; Smith, C.E.; et al. Growth Media Conditions Influence the Secretion Route and Release Levels of Engineered Extracellular Vesicles. Adv Healthc Mater 2022, 11, e2101658. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.; Delavogia, E.; Fernandez-Gonzalez, A.; Mitsialis, S.A.; Kourembanas, S. Harnessing the Therapeutic Potential of the Stem Cell Secretome in Neonatal Diseases. Semin Perinatol 2023, 47, 151730. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Frontiers in Cell and Developmental Biology 2020, 8. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. Extracellular Vesicles as Therapeutic Resources in the Clinical Environment. Int J Mol Sci 2023, 24, 2344. [Google Scholar] [CrossRef]
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front Physiol 2022, 13, 837001. [Google Scholar] [CrossRef]
- Willis, G.R.; Reis, M.; Gheinani, A.H.; Fernandez-Gonzalez, A.; Taglauer, E.S.; Yeung, V.; Liu, X.; Ericsson, M.; Haas, E.; Mitsialis, S.A.; et al. Extracellular Vesicles Protect the Neonatal Lung from Hyperoxic Injury through the Epigenetic and Transcriptomic Reprogramming of Myeloid Cells. Am J Respir Crit Care Med 2021, 204, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Reduce Microglia-Mediated Neuroinflammation in Perinatal Brain Injury. Stem Cell Res Ther 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Q.; Wang, F. Mesenchymal Stem Cells for the Treatment of Ulcerative Colitis: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies. Stem Cell Res Ther 2019, 10, 266. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Liu, C.-Y.; Wang, I.-J.; Sieber, M.; Chang, J.; Jester, J.V.; Kao, W.W.Y. Cell Therapy of Congenital Corneal Diseases with Umbilical Mesenchymal Stem Cells: Lumican Null Mice. PLoS One 2010, 5, e10707. [Google Scholar] [CrossRef]
- Lombardo, E.; van der Poll, T.; DelaRosa, O.; Dalemans, W. Mesenchymal Stem Cells as a Therapeutic Tool to Treat Sepsis. World J Stem Cells 2015, 7, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Joerger-Messerli, M.S.; Oppliger, B.; Spinelli, M.; Thomi, G.; di Salvo, I.; Schneider, P.; Schoeberlein, A. Extracellular Vesicles Derived from Wharton’s Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells. Cell Transplant 2018, 27, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Improve Nerve Regeneration after Sciatic Nerve Transection in Rats. J Cell Mol Med 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular Vesicles Derived from T Regulatory Cells Suppress T Cell Proliferation and Prolong Allograft Survival. Sci Rep 2017, 7, 11518. [Google Scholar] [CrossRef]
- Alarcón-Vila, C.; Baroja-Mazo, A.; de Torre-Minguela, C.; Martínez, C.M.; Martínez-García, J.J.; Martínez-Banaclocha, H.; García-Palenciano, C.; Pelegrin, P. CD14 Release Induced by P2X7 Receptor Restricts Inflammation and Increases Survival during Sepsis. Elife 2020, 9, e60849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Fan, H. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol Ther 2018, 26, 1375–1384. [Google Scholar] [CrossRef]
- Del Campo, C.V.; Liaw, N.Y.; Gunadasa-Rohling, M.; Matthaei, M.; Braga, L.; Kennedy, T.; Salinas, G.; Voigt, N.; Giacca, M.; Zimmermann, W.-H.; et al. Regenerative Potential of Epicardium-Derived Extracellular Vesicles Mediated by Conserved miRNA Transfer. Cardiovasc Res 2022, 118, 597–611. [Google Scholar] [CrossRef]
- Wang, H.; Maimaitiaili, R.; Yao, J.; Xie, Y.; Qiang, S.; Hu, F.; Li, X.; Shi, C.; Jia, P.; Yang, H.; et al. Percutaneous Intracoronary Delivery of Plasma Extracellular Vesicles Protects the Myocardium Against Ischemia-Reperfusion Injury in Canis. Hypertension 2021, 78, 1541–1554. [Google Scholar] [CrossRef]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Gitaí, D.L.G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Zanirati, G.; Kumar, S.; Shuai, B.; et al. Extracellular Vesicles from Human iPSC-Derived Neural Stem Cells: miRNA and Protein Signatures, and Anti-Inflammatory and Neurogenic Properties. J Extracell Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef]
- Park, H.J.; Jeon, J.; Choi, J.; Kim, J.Y.; Kim, H.S.; Huh, J.Y.; Goldman, S.A.; Song, J. Human iPSC-Derived Neural Precursor Cells Differentiate into Multiple Cell Types to Delay Disease Progression Following Transplantation into YAC128 Huntington’s Disease Mouse Model. Cell Prolif 2021, 54, e13082. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, S.; Jaimes Gonzalez, J.; Kirmani, M.; Vogel, A.D.; Upadhya, R.; Kodali, M.; Madhu, L.N.; Rao, S.; Shuai, B.; Babu, R.S.; et al. Intranasally Administered Extracellular Vesicles from Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells Quickly Incorporate into Neurons and Microglia in 5xFAD Mice. Front Aging Neurosci 2023, 15, 1200445. [Google Scholar] [CrossRef] [PubMed]
- McCulloh, C.J.; Olson, J.K.; Wang, Y.; Zhou, Y.; Tengberg, N.H.; Deshpande, S.; Besner, G.E. Treatment of Experimental Necrotizing Enterocolitis with Stem Cell-Derived Exosomes. J Pediatr Surg 2018, 53, 1215–1220. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Wang, Y.; Lv, K.; Zhou, X.; Li, L.; Zhang, Y.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Neonatal-Tissue-Derived Extracellular Vesicle Therapy (NEXT): A Potent Strategy for Precision Regenerative Medicine. Adv Mater 2023, 35, e2300602. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Danilina, T.I.; Goryunov, K.V.; Pevzner, I.B.; Zorova, L.D.; Popkov, V.A.; Chernikov, V.P.; Plotnikov, E.Y.; Sukhikh, G.T.; et al. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells 2021, 10, 1273. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Extracellular Vesicles: A Biological System with Clinical Implications. Cells 2022, 11, 2345. [Google Scholar] [CrossRef]
- Bellio, M.A.; Young, K.C.; Milberg, J.; Santos, I.; Abdullah, Z.; Stewart, D.; Arango, A.; Chen, P.; Huang, J.; Williams, K.; et al. Amniotic Fluid-Derived Extracellular Vesicles: Characterization and Therapeutic Efficacy in an Experimental Model of Bronchopulmonary Dysplasia. Cytotherapy 2021, 23, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-M.; Zhang, K.; Zhang, J.-H. Human Breast Milk-Derived Exosomal miR-148a-3p Protects Against Necrotizing Enterocolitis by Regulating P53 and Sirtuin 1. Inflammation 2022, 45, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Iannotta, D.; Yang, M.; Celia, C.; Di Marzio, L.; Wolfram, J. Extracellular Vesicle Therapeutics from Plasma and Adipose Tissue. Nano Today 2021, 39, 101159. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Gupta, S.; Sharma, P. Extracellular Vesicles (EVs) as “A Window to the Brain”: Potential, Challenges and Future Perspectives. Indian J Clin Biochem 2023, 38, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brenna, S.; Krisp, C.; Altmeppen, H.C.; Magnus, T.; Puig, B. Brain-Derived Extracellular Vesicles in Health and Disease: A Methodological Perspective. Int J Mol Sci 2021, 22, 1365. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Fan, J.; Zheng, G.; He, J.; Lin, F.; Ge, M.; Huang, L.; Wang, J.; Xia, J.; Huang, R.; et al. Diagnostic Potential of Plasma Extracellular Vesicle miR-483-3p and Let-7d-3p for Sepsis. Front Mol Biosci 2022, 9, 814240. [Google Scholar] [CrossRef]
- Igami, K.; Uchiumi, T.; Shiota, M.; Ueda, S.; Tsukahara, S.; Akimoto, M.; Eto, M.; Kang, D. Extracellular Vesicles Expressing CEACAM Proteins in the Urine of Bladder Cancer Patients. Cancer Sci 2022, 113, 3120–3133. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.-F.; Thakur, B.K. DNA in Extracellular Vesicles: From Evolution to Its Current Application in Health and Disease. Cell & Bioscience 2022, 12, 37. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Z.; Liu, X.; Tyler, B.M. Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication With Microbes. Front Microbiol 2022, 13, 817844. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes (Basel) 2022, 12, 352. [Google Scholar] [CrossRef]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular Membrane Vesicles in the Three Domains of Life and Beyond. FEMS Microbiol Rev 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.N. Study of the Molecular Mechanisms of the Therapeutic Properties of Extracellular Vesicles. Int J Mol Sci 2023, 24, 7093. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun Signal 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.S.; Bonato, V.L.D.; Pessoni, A.M.; Rodrigues, M.L.; Casadevall, A.; Almeida, F. Fungal Extracellular Vesicles as Potential Targets for Immune Interventions. mSphere 2019, 4, e00747–19. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial Extracellular Vesicles and Their Novel Therapeutic Applications in Health and Cancer. Front Cell Infect Microbiol 2022, 12, 962216. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-Derived Extracellular Vesicles: Recent Advancements and Current Challenges on Their Use for Biomedical Applications. J Extracell Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota-Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front Cell Infect Microbiol 2020, 10, 346. [Google Scholar] [CrossRef]
- Filip, R. An Update on the Role of Extracellular Vesicles in the Pathogenesis of Necrotizing Enterocolitis and Inflammatory Bowel Diseases. Cells 2021, 10, 3202. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr Protoc Cell Biol 2020, 88, e110. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective Isolation of Exosomes with Polyethylene Glycol from Cell Culture Supernatant for In-Depth Proteome Profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Malekian, F.; Baghban, N.; Kodam, S.P.; Ullah, M. Methodologies to Isolate and Purify Clinical Grade Extracellular Vesicles for Medical Applications. Cells 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of Neonatal Encephalopathy and Hypoxic-Ischaemic Encephalopathy. Early Hum Dev 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs Rev 2011, 11, 125–133. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Balakireva, A.V.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone in a Rat Model of Neonatal Hypoxic−Ischemic Brain Injury. Molecules 2018, 23, 1871. [Google Scholar] [CrossRef]
- Robertsson Grossmann, K.; Eriksson Westblad, M.; Blennow, M.; Lindström, K. Outcome at Early School Age and Adolescence after Hypothermia-Treated Hypoxic-Ischaemic Encephalopathy: An Observational, Population-Based Study. Arch Dis Child Fetal Neonatal Ed 2023, 108, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. N Engl J Med 2012, 366, 2085–2092. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of Hypothermia for Perinatal Asphyxia on Childhood Outcomes. N Engl J Med 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Luo, B.-Y.; Zhou, H.-S.; Sun, Y.-F.; Xiao, Q.-X.; Chen, L.; She, H.-Q.; Wang, S.-F.; Yan, S.-S.; Chang, Q.-Y.; He, Y.-Q.; et al. The Fate and Prospects of Stem Cell Therapy in the Treatment of Hypoxic-Ischemic Encephalopathy. Eur J Neurosci 2023, 58, 2384–2405. [Google Scholar] [CrossRef] [PubMed]
- Сухих, Г.Т.; Силачев, Д.Н.; Певзнер, И.Б.; Зoрoва, Л.Д.; Бабенкo, В.А.; Пoпкoв, В.А.; Янкаускас, С.С.; Зубкoв, В.В.; Зoрoв, Д.Б.; Плoтникoв, Е.Ю. Перспективы Испoльзoвания Ствoлoвых и Прoгенитoрных Клетoк Для Терапии Пoследствий Гипoксически-Ишемическoй Энцефалoпатии Нoвoрoжденных. Акушерствo и гинекoлoгия 2016, 55–66. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, B.I.; Jo, C.H.; Choi, C.W.; Kim, E.-K.; Kim, H.-S.; Yoon, K.-S.; Choi, J.-H. Mesenchymal Stem-Cell Transplantation for Hypoxic-Ischemic Brain Injury in Neonatal Rat Model. Pediatr Res 2010, 67, 42–46. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.J.; van de Looij, Y.; Kavelaars, A.; Zijlstra, J.; van Bel, F.; Huppi, P.S.; Sizonenko, S.; Heijnen, C.J. Mesenchymal Stem Cells Restore Cortical Rewiring after Neonatal Ischemia in Mice. Ann Neurol 2012, 71, 785–796. [Google Scholar] [CrossRef]
- van Velthoven, C.T.J.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal Stem Cell Treatment after Neonatal Hypoxic-Ischemic Brain Injury Improves Behavioral Outcome and Induces Neuronal and Oligodendrocyte Regeneration. Brain Behav Immun 2010, 24, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation Attenuates Severe Brain Injury by Permanent Middle Cerebral Artery Occlusion in Newborn Rats. Pediatr Res 2012, 72, 277–284. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Chiang, M.-F.; Lin, S.-Z.; Chang, Y.-C.; Wang, H.-J.; Su, C.-Y.; Li, H. Enhancement of Neuroplasticity through Upregulation of Beta1-Integrin in Human Umbilical Cord-Derived Stromal Cell Implanted Stroke Model. Neurobiol Dis 2007, 27, 339–353. [Google Scholar] [CrossRef]
- Ding, H.-F.; Zhang, H.; Ding, H.-F.; Li, D.; Yi, X.-H.; Gao, X.-Y.; Mou, W.-W.; Ju, X.-L. Therapeutic Effect of Placenta-Derived Mesenchymal Stem Cells on Hypoxic-Ischemic Brain Damage in Rats. World J Pediatr 2015, 11, 74–82. [Google Scholar] [CrossRef]
- Donega, V.; Velthoven, C.T.J. van; Nijboer, C.H.; Bel, F. van; Kas, M.J.H.; Kavelaars, A.; Heijnen, C.J. Intranasal Mesenchymal Stem Cell Treatment for Neonatal Brain Damage: Long-Term Cognitive and Sensorimotor Improvement. PLOS ONE 2013, 8, e51253. [Google Scholar] [CrossRef]
- Allan, D.; Tieu, A.; Lalu, M.; Burger, D. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Regenerative Therapy and Immune Modulation: Progress and Challenges toward Clinical Application. Stem Cells Translational Medicine 2020, 9, 39–46. [Google Scholar] [CrossRef]
- Akyurekli, C.; Le, Y.; Richardson, R.B.; Fergusson, D.; Tay, J.; Allan, D.S. A Systematic Review of Preclinical Studies on the Therapeutic Potential of Mesenchymal Stromal Cell-Derived Microvesicles. Stem Cell Rev Rep 2015, 11, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Sisa, C.; Kholia, S.; Naylor, J.; Herrera Sanchez, M.B.; Bruno, S.; Deregibus, M.C.; Camussi, G.; Inal, J.M.; Lange, S.; Hristova, M. Mesenchymal Stromal Cell Derived Extracellular Vesicles Reduce Hypoxia-Ischaemia Induced Perinatal Brain Injury. Front Physiol 2019, 10, 282. [Google Scholar] [CrossRef]
- Kaminski, N.; Köster, C.; Mouloud, Y.; Börger, V.; Felderhoff-Müser, U.; Bendix, I.; Giebel, B.; Herz, J. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Reduce Neuroinflammation, Promote Neural Cell Proliferation and Improve Oligodendrocyte Maturation in Neonatal Hypoxic-Ischemic Brain Injury. Front Cell Neurosci 2020, 14, 601176. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Jiang, L.; Wang, M.; Wang, R.; Wang, X.; Gao, C.; Xia, Z. Human Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Protect against Nerve Injury via Regulating Immune Microenvironment in Neonatal Hypoxic-Ischemic Brain Damage Model. Immunobiology 2022, 227, 152178. [Google Scholar] [CrossRef]
- Hu, Z.; Yuan, Y.; Zhang, X.; Lu, Y.; Dong, N.; Jiang, X.; Xu, J.; Zheng, D. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Oxygen-Glucose Deprivation/Reperfusion-Induced Microglial Pyroptosis by Promoting FOXO3a-Dependent Mitophagy. Oxid Med Cell Longev 2021, 2021, 6219715. [Google Scholar] [CrossRef] [PubMed]
- Cristante, E.; McArthur, S.; Mauro, C.; Maggioli, E.; Romero, I.A.; Wylezinska-Arridge, M.; Couraud, P.O.; Lopez-Tremoleda, J.; Christian, H.C.; Weksler, B.B.; et al. Identification of an Essential Endogenous Regulator of Blood-Brain Barrier Integrity, and Its Pathological and Therapeutic Implications. Proc Natl Acad Sci U S A 2013, 110, 832–841. [Google Scholar] [CrossRef]
- Gussenhoven, R.; Klein, L.; Ophelders, D.R.M.G.; Habets, D.H.J.; Giebel, B.; Kramer, B.W.; Schurgers, L.J.; Reutelingsperger, C.P.M.; Wolfs, T.G.A.M. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J Clin Med 2019, 8, E137. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Y.; Zhang, Y.; Liu, J.; Xu, Z. Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen-Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J Mol Neurosci 2018, 65, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-Modified ADSCs Promotes Functional Recovery after Stroke in Rats by Improving Neurogenesis and Suppressing Microglia Activation. Am J Transl Res 2019, 11, 780–792. [Google Scholar]
- Budnik, V.; Ruiz-Cañada, C.; Wendler, F. Extracellular Vesicles Round off Communication in the Nervous System. Nat Rev Neurosci 2016, 17, 160–172. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 Microglia-Derived Exosomes Protect the Mouse Brain from Ischemia-Reperfusion Injury via Exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Luo, H.; Ye, G.; Liu, Y.; Huang, D.; Luo, Q.; Chen, W.; Qi, Z. miR-150-3p Enhances Neuroprotective Effects of Neural Stem Cell Exosomes after Hypoxic-Ischemic Brain Injury by Targeting CASP2. Neurosci Lett 2022, 779, 136635. [Google Scholar] [CrossRef] [PubMed]
- Holme, N.; Chetcuti, P. The Pathophysiology of Respiratory Distress Syndrome in Neonates. Paediatrics and Child Health 2012, 22, 507–512. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Ramamoorthy, C. Bronchopulmonary Dysplasia. Paediatr Anaesth 2022, 32, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Di Pietro, G.M.; Esposito, S. Bronchopulmonary Dysplasia: Clinical Aspects and Preventive and Therapeutic Strategies. J Transl Med 2018, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Geetha, O.; Rajadurai, V.S.; Anand, A.J.; Dela Puerta, R.; Huey Quek, B.; Khoo, P.C.; Chua, M.C.; Agarwal, P. New BPD-Prevalence and Risk Factors for Bronchopulmonary Dysplasia/Mortality in Extremely Low Gestational Age Infants ≤28 Weeks. J Perinatol 2021, 41, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Ionescu, L.; Montemurro, T.; Vadivel, A.; Weissmann, G.; Oudit, G.; Emery, D.; Bodiga, S.; Eaton, F.; Péault, B.; et al. Short-Term, Long-Term and Paracrine Effect of Human Umbilical Cord-Derived Stem Cells in Lung Injury Prevention and Repair in Experimental Bronchopulmonary Dysplasia. Thorax 2013, 68, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Wang, J.; Dong, W. Oxidative Stress and Bronchopulmonary Dysplasia. Gene 2018, 678, 177–183. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, A.; Huang, F.; Xu, J.; Zhao, M. MSC-EXO and Tempol Ameliorate Bronchopulmonary Dysplasia in Newborn Rats by Activating HIF-1α. Pediatr Pulmonol 2023, 58, 1367–1379. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Sharma, M.; Bellio, M.A.; Benny, M.; Kulandavelu, S.; Chen, P.; Janjindamai, C.; Han, C.; Chang, L.; Sterling, S.; Williams, K.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Prevent Experimental Bronchopulmonary Dysplasia Complicated By Pulmonary Hypertension. Stem Cells Transl Med 2022, 11, 828–840. [Google Scholar] [CrossRef]
- Ai, D.; Shen, J.; Sun, J.; Zhu, Z.; Gao, R.; Du, Y.; Yuan, L.; Chen, C.; Zhou, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppress Hyperoxia-Induced Transdifferentiation of Rat Alveolar Type 2 Epithelial Cells. Stem Cells Dev 2022, 31, 53–66. [Google Scholar] [CrossRef]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes Mediate the Cytoprotective Action of Mesenchymal Stromal Cells on Hypoxia-Induced Pulmonary Hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Willis, G.R.; Fernandez-Gonzalez, A.; Yeung, V.; Taglauer, E.; Magaletta, M.; Parsons, T.; Derr, A.; Liu, X.; Maehr, R.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Restore Thymic Architecture and T Cell Function Disrupted by Neonatal Hyperoxia. Front Immunol 2021, 12, 640595. [Google Scholar] [CrossRef]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early Gestational Mesenchymal Stem Cell Secretome Attenuates Experimental Bronchopulmonary Dysplasia in Part via Exosome-Associated Factor TSG-6. Stem Cell Res Ther 2018, 9, 173. [Google Scholar] [CrossRef]
- Braun, R.K.; Chetty, C.; Balasubramaniam, V.; Centanni, R.; Haraldsdottir, K.; Hematti, P.; Eldridge, M.W. Intraperitoneal Injection of MSC-Derived Exosomes Prevent Experimental Bronchopulmonary Dysplasia. Biochem Biophys Res Commun 2018, 503, 2653–2658. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular Endothelial Growth Factor Mediates the Therapeutic Efficacy of Mesenchymal Stem Cell-Derived Extracellular Vesicles against Neonatal Hyperoxic Lung Injury. Exp Mol Med 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Hou, A.; Fu, J.; Yang, H.; Zhu, Y.; Pan, Y.; Xu, S.; Xue, X. Hyperoxia Stimulates the Transdifferentiation of Type II Alveolar Epithelial Cells in Newborn Rats. Am J Physiol Lung Cell Mol Physiol 2015, 308, L861–872. [Google Scholar] [CrossRef] [PubMed]
- Lithopoulos, M.A.; Strueby, L.; O’Reilly, M.; Zhong, S.; Möbius, M.A.; Eaton, F.; Fung, M.; Hurskainen, M.; Cyr-Depauw, C.; Suen, C.; et al. Pulmonary and Neurologic Effects of Mesenchymal Stromal Cell Extracellular Vesicles in a Multifactorial Lung Injury Model. Am J Respir Crit Care Med 2022, 205, 1186–1201. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhou, O.; Liu, J.; Zou, W.; Zhang, L.; Tian, D.; Dai, J.; Luo, Z.; Liu, E.; Fu, Z.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Lung Injury in Rat Model of Bronchopulmonary Dysplasia by Affecting Cell Survival and Angiogenesis. Stem Cells Dev 2020, 29, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Yuan, R.; Deng, Z.; Wu, X. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Hyperoxia-Induced Lung Injury via the Manipulation of microRNA-425. Arch Biochem Biophys 2021, 697, 108712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Krause, M.; Yawno, T.; Kusuma, G.D.; Schwab, R.; Barabadi, M.; Maleken, A.S.; Chan, S.T.; Hunt, R.; Greening, D.; et al. Assessing the Impact of Gestational Age of Donors on the Efficacy of Amniotic Epithelial Cell-Derived Extracellular Vesicles in Experimental Bronchopulmonary Dysplasia. Stem Cell Res Ther 2022, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.R.; Sills, W.S.; Hanrahan, K.; Ziegler, A.; Tidd, K.M.; Cook, E.; Sannes, P.L. Expression of WNT5A in Idiopathic Pulmonary Fibrosis and Its Control by TGF-β and WNT7B in Human Lung Fibroblasts. J Histochem Cytochem 2016, 64, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.; Zaidi, A. New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis. PLoS Med 2010, 7, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal Sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, L.; Qian, K. Global, Regional, and National Incidence and Mortality of Neonatal Sepsis and Other Neonatal Infections, 1990–2019. Front Public Health 2023, 11, 1139832. [Google Scholar] [CrossRef]
- Burgelman, M.; Vandendriessche, C.; Vandenbroucke, R.E. Extracellular Vesicles: A Double-Edged Sword in Sepsis. Pharmaceuticals (Basel) 2021, 14, 829. [Google Scholar] [CrossRef]
- Kronstadt, S.M.; Pottash, A.E.; Levy, D.; Wang, S.; Chao, W.; Jay, S.M. Therapeutic Potential of Extracellular Vesicles for Sepsis Treatment. Adv Ther (Weinh) 2021, 4, 2000259. [Google Scholar] [CrossRef]
- Vergnano, S.; Sharland, M.; Kazembe, P.; Mwansambo, C.; Heath, P.T. Neonatal Sepsis: An International Perspective. Archives of Disease in Childhood - Fetal and Neonatal Edition 2005, 90, F220–FF224. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Ahn, S.-Y.; Park, W.-S.; Sung, D.-K.; Sung, S.-I.; Yang, M.-S.; Chang, Y.-S. Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Brain Injury in Escherichia Coli Meningitis in Newborn Rats. Life 2022, 12, 1030. [Google Scholar] [CrossRef]
- Alganabi, M.; Lee, C.; Bindi, E.; Li, B.; Pierro, A. Recent Advances in Understanding Necrotizing Enterocolitis. F1000Res 2019, 8, F1000 Faculty Rev-107. [Google Scholar] [CrossRef]
- Bazacliu, C.; Neu, J. Necrotizing Enterocolitis: Long Term Complications. Curr Pediatr Rev 2019, 15, 115–124. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P.; Good, M. New Insights into Necrotizing Enterocolitis: From Laboratory Observation to Personalized Prevention and Treatment. J Pediatr Surg 2019, 54, 398–404. [Google Scholar] [CrossRef]
- Li, B.; Lee, C.; O’Connell, J.S.; Antounians, L.; Ganji, N.; Alganabi, M.; Cadete, M.; Nascimben, F.; Koike, Y.; Hock, A.; et al. Activation of Wnt Signaling by Amniotic Fluid Stem Cell-Derived Extracellular Vesicles Attenuates Intestinal Injury in Experimental Necrotizing Enterocolitis. Cell Death Dis 2020, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Drucker, N.A.; Te Winkel, J.P.; Shelley, W.C.; Olson, K.R.; Markel, T.A. Inhibiting Hydrogen Sulfide Production in Umbilical Stem Cells Reduces Their Protective Effects during Experimental Necrotizing Enterocolitis. J Pediatr Surg 2019, 54, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.S.; Li, B.; Zito, A.; Ahmed, A.; Cadete, M.; Ganji, N.; Lau, E.; Alganabi, M.; Farhat, N.; Lee, C.; et al. Treatment of Necrotizing Enterocolitis by Conditioned Medium Derived from Human Amniotic Fluid Stem Cells. PLoS One 2021, 16, e0260522. [Google Scholar] [CrossRef] [PubMed]
- Rager, T.M.; Olson, J.K.; Zhou, Y.; Wang, Y.; Besner, G.E. Exosomes Secreted from Bone Marrow-Derived Mesenchymal Stem Cells Protect the Intestines from Experimental Necrotizing Enterocolitis. Journal of Pediatric Surgery 2016, 51, 942–947. [Google Scholar] [CrossRef]
- Claud, E.C. Neonatal Necrotizing Enterocolitis -Inflammation and Intestinal Immaturity. Antiinflamm Antiallergy Agents Med Chem 2009, 8, 248–259. [Google Scholar] [CrossRef]
- Heidari, N.; Abbasi-Kenarsari, H.; Namaki, S.; Baghaei, K.; Zali, M.R.; Ghaffari Khaligh, S.; Hashemi, S.M. Adipose-Derived Mesenchymal Stem Cell-Secreted Exosome Alleviates Dextran Sulfate Sodium-Induced Acute Colitis by Treg Cell Induction and Inflammatory Cytokine Reduction. J Cell Physiol 2021, 236, 5906–5920. [Google Scholar] [CrossRef]
- Yang, S.; Liang, X.; Song, J.; Li, C.; Liu, A.; Luo, Y.; Ma, H.; Tan, Y.; Zhang, X. A Novel Therapeutic Approach for Inflammatory Bowel Disease by Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells to Repair Intestinal Barrier via TSG-6. Stem Cell Res Ther 2021, 12, 315. [Google Scholar] [CrossRef]
- Yan, Y.; Li, K.; Jiang, J.; Jiang, L.; Ma, X.; Ai, F.; Qiu, S.; Si, W. Perinatal Tissue-Derived Exosomes Ameliorate Colitis in Mice by Regulating the Foxp3 + Treg Cells and Gut Microbiota. Stem Cell Res Ther 2023, 14, 43. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Z.-Y.; Yuan, J.-T.; Ocansey, D.K.W.; Tu, Q.; Zhang, X.; Qian, H.; Xu, W.-R.; Qiu, W.; Mao, F. hucMSC-Derived Exosomes Attenuate Colitis by Regulating Macrophage Pyroptosis via the miR-378a-5p/NLRP3 Axis. Stem Cell Res Ther 2021, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-Dependent Extracellular Vesicles from Intestinal Epithelial Cells Maintain Intestinal Tract Immune Balance. Nat Commun 2016, 7, 13045. [Google Scholar] [CrossRef]
- Duan, L.; Huang, H.; Zhao, X.; Zhou, M.; Chen, S.; Wang, C.; Han, Z.; Han, Z.-C.; Guo, Z.; Li, Z.; et al. Extracellular Vesicles Derived from Human Placental Mesenchymal Stem Cells Alleviate Experimental Colitis in Mice by Inhibiting Inflammation and Oxidative Stress. Int J Mol Med 2020, 46, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, X.; Xiao, X.; Xu, M.; Yang, Y.; Xue, C.; Li, X.; Wang, S.; Zhao, R.C. Human Adipose Mesenchymal Stem Cell-Derived Exosomes Protect Mice from DSS-Induced Inflammatory Bowel Disease by Promoting Intestinal-Stem-Cell and Epithelial Regeneration. Aging Dis 2021, 12, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine Milk-Derived Exosomes Promote Proliferation of Intestinal Epithelial Cells. Sci Rep 2016, 6, 33862. [Google Scholar] [CrossRef] [PubMed]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast Milk-Derived Exosomes Promote Intestinal Epithelial Cell Growth. J Pediatr Surg 2017, 52, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Accarie, A.; l’Homme, B.; Benadjaoud, M.A.; Lim, S.K.; Guha, C.; Benderitter, M.; Tamarat, R.; Sémont, A. Extracellular Vesicles Derived from Mesenchymal Stromal Cells Mitigate Intestinal Toxicity in a Mouse Model of Acute Radiation Syndrome. Stem Cell Res Ther 2020, 11, 371. [Google Scholar] [CrossRef]
- Borewicz, K.; Gu, F.; Saccenti, E.; Arts, I.C.W.; Penders, J.; Thijs, C.; van Leeuwen, S.S.; Lindner, C.; Nauta, A.; van Leusen, E.; et al. Correlating Infant Fecal Microbiota Composition and Human Milk Oligosaccharide Consumption by Microbiota of 1-Month-Old Breastfed Infants. Mol Nutr Food Res 2019, 63, e1801214. [Google Scholar] [CrossRef]
- van Herwijnen, M.J.C.; Zonneveld, M.I.; Goerdayal, S.; Nolte-’t Hoen, E.N.M.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H.M. Comprehensive Proteomic Analysis of Human Milk-Derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol Cell Proteomics 2016, 15, 3412–3423. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast Milk, Microbiota, and Intestinal Immune Homeostasis. Pediatr Res 2015, 77, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hell, L.; Kendlbacher, R.A.; Hajji, N.; Hau, C.; van Dam, A.; Berckmans, R.J.; Wisgrill, L.; Ay, C.; Pabinger, I.; et al. Human Milk Triggers Coagulation via Tissue Factor-Exposing Extracellular Vesicles. Blood Adv 2020, 4, 6274–6282. [Google Scholar] [CrossRef]
- Kersin, S.G.; Özek, E. Breast Milk Stem Cells: Are They Magic Bullets in Neonatology? Turk Arch Pediatr 2021, 56, 187–191. [Google Scholar] [CrossRef]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.-L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a Functional Mammary Gland from a Single Stem Cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front Immunol 2017, 8, 584. [Google Scholar] [CrossRef]
- Maghraby, M.K.; Li, B.; Chi, L.; Ling, C.; Benmoussa, A.; Provost, P.; Postmus, A.C.; Abdi, A.; Pierro, A.; Bourdon, C.; et al. Extracellular Vesicles Isolated from Milk Can Improve Gut Barrier Dysfunction Induced by Malnutrition. Sci Rep 2021, 11, 7635. [Google Scholar] [CrossRef]
- Herwijnen, M.J.C. van; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Hoen, E.N.M.N.-. ‘t; Wauben, M.H.M. Abundantly Present miRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Frontiers in Nutrition 2018, 5. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Z.; Xu, L.; Ma, F.; Li, D.; Guo, Z.; Li, N.; Cao, X. MicroRNA-148/152 Impair Innate Response and Antigen Presentation of TLR-Triggered Dendritic Cells by Targeting CaMKIIα. J Immunol 2010, 185, 7244–7251. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and Peptidomic Profiling of Exosomes in Preterm Human Milk: Insights Into Necrotizing Enterocolitis Prevention. Mol Nutr Food Res 2019, 63, e1801247. [Google Scholar] [CrossRef]
- Cheyuo, C.; Aziz, M.; Yang, W.-L.; Jacob, A.; Zhou, M.; Wang, P. Milk Fat Globule-EGF Factor VIII Attenuates CNS Injury by Promoting Neural Stem Cell Proliferation and Migration after Cerebral Ischemia. PLoS One 2015, 10, e0122833. [Google Scholar] [CrossRef]
- Bu, H.-F.; Zuo, X.-L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.-D. Milk Fat Globule-EGF Factor 8/Lactadherin Plays a Crucial Role in Maintenance and Repair of Murine Intestinal Epithelium. J Clin Invest 2007, 117, 3673–3683. [Google Scholar] [CrossRef]
- Tobita, K.; Kawahara, T.; Otani, H. Bovine Beta-Casein (1-28), a Casein Phosphopeptide, Enhances Proliferation and IL-6 Expression of Mouse CD19+ Cells via Toll-like Receptor 4. J Agric Food Chem 2006, 54, 8013–8017. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y.; Kim, S.H. Enhancement of Antioxidative and Intestinal Anti-Inflammatory Activities of Glycated Milk Casein after Fermentation with Lactobacillus Rhamnosus 4B15. J Agric Food Chem 2017, 65, 4744–4754. [Google Scholar] [CrossRef]
- Caccavo, D.; Pellegrino, N.M.; Altamura, M.; Rigon, A.; Amati, L.; Amoroso, A.; Jirillo, E. Antimicrobial and Immunoregulatory Functions of Lactoferrin and Its Potential Therapeutic Application. J Endotoxin Res 2002, 8, 403–417. [Google Scholar] [CrossRef]
- Nichols, B.L.; McKee, K.; Putman, M.; Henry, J.F.; Nichols, V.N. Human Lactoferrin Supplementation of Infant Formulas Increases Thymidine Incorporation into the DNA of Rat Crypt Cells. J Pediatr Gastroenterol Nutr 1989, 8, 102–109. [Google Scholar] [CrossRef]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Galley, J.; Elbahrawy, M.; Wang, Y.; Farrell, A.; Brigstock, D.; Besner, G.E. Human Breast Milk-Derived Extracellular Vesicles in the Protection Against Experimental Necrotizing Enterocolitis. Journal of Pediatric Surgery 2020, 55, 54–58. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Y.; Wang, K.; Nan, X.; Chen, R.; Xiong, B. Effects of Milk-Derived Extracellular Vesicles on the Colonic Transcriptome and Proteome in Murine Model. Nutrients 2022, 14, 3057. [Google Scholar] [CrossRef]
- Du, C.; Wang, K.; Zhao, Y.; Nan, X.; Chen, R.; Quan, S.; Xiong, B. Supplementation with Milk-Derived Extracellular Vesicles Shapes the Gut Microbiota and Regulates the Transcriptomic Landscape in Experimental Colitis. Nutrients 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An Epigenetic Switch Involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 Links Inflammation to Cell Transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine Milk-Derived Exosomes Enhance Goblet Cell Activity and Prevent the Development of Experimental Necrotizing Enterocolitis. PLoS One 2019, 14, e0211431. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The Microbiome in Inflammatory Bowel Diseases: From Pathogenesis to Therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K. Capsular Polysaccharides of Symbiotic Bacteria Modulate Immune Responses during Experimental Colitis. J Pediatr Gastroenterol Nutr 2008, 46 Suppl 1, E11–12. [Google Scholar] [CrossRef]
- Shen, Y.; Giardino Torchia, M.L.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Wang, L.; Cao, Z.; Zhang, M.; Zhang, Y.; Liu, R.; Liu, J. Versatility of Bacterial Outer Membrane Vesicles in Regulating Intestinal Homeostasis. Sci Adv 2023, 9, eade5079. [Google Scholar] [CrossRef]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia Muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS One 2013, 8, e76520. [Google Scholar] [CrossRef]
- Shi, R.; Yu, F.; Hu, X.; Liu, Y.; Jin, Y.; Ren, H.; Lu, S.; Guo, J.; Chang, J.; Li, Y.; et al. Protective Effect of Lactiplantibacillus Plantarum Subsp. Plantarum SC-5 on Dextran Sulfate Sodium—Induced Colitis in Mice. Foods 2023, 12, 897. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium Butyricum-Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol Nutr Food Res 2023, 67, e2200884. [Google Scholar] [CrossRef]
- Rui, T.; Wang, K.; Xiang, A.; Guo, J.; Tang, N.; Jin, X.; Lin, Y.; Liu, J.; Zhang, X. Serum Exosome-Derived piRNAs Could Be Promising Biomarkers for HCC Diagnosis. Int J Nanomedicine 2023, 18, 1989–2001. [Google Scholar] [CrossRef]
- Matei, A.C.; Antounians, L.; Zani, A. Extracellular Vesicles as a Potential Therapy for Neonatal Conditions: State of the Art and Challenges in Clinical Translation. Pharmaceutics 2019, 11, 404. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Whitford, W.; Guterstam, P. Exosome Manufacturing Status. Future Med Chem 2019, 11, 1225–1236. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-Grade Standard Protocol for Exclusively Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Willis, G.R.; Kourembanas, S.; Mitsialis, S.A. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front Cardiovasc Med 2017, 4, 63. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. Role of Mesenchymal Stem/Stromal Cells in Coagulation. Int J Mol Sci 2022, 23, 10393. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res 2016, 118, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl Med 2022, 11, 2–13. [Google Scholar] [CrossRef]
- Baker, E.K.; Wallace, E.M.; Davis, P.G.; Malhotra, A.; Jacobs, S.E.; Hooper, S.B.; Lim, R. A Protocol for Cell Therapy Infusion in Neonates. Stem Cells Transl Med 2021, 10, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Köster, C.; Reinboth, B.S.; Dzietko, M.; Hansen, W.; Sabir, H.; van Velthoven, C.; Bendix, I.; Felderhoff-Müser, U. Interaction between Hypothermia and Delayed Mesenchymal Stem Cell Therapy in Neonatal Hypoxic-Ischemic Brain Injury. Brain Behav Immun 2018, 70, 118–130. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Wolfs, T.G.A.M.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl Med 2016, 5, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Liu, D.; Li, T.; Ke, H.; Xin, D.; Wang, S.; Cao, Y.; Xue, H.; Wang, Z. Hydrogen Sulfide-Modified Extracellular Vesicles from Mesenchymal Stem Cells for Treatment of Hypoxic-Ischemic Brain Injury. J Control Release 2020, 328, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Li, T.; Chu, X.; Ke, H.; Liu, D.; Wang, Z. MSCs-Extracellular Vesicles Attenuated Neuroinflammation, Synapse Damage and Microglial Phagocytosis after Hypoxia-Ischemia Injury by Preventing Osteopontin Expression. Pharmacol Res 2021, 164, 105322. [Google Scholar] [CrossRef] [PubMed]
- Pathipati, P.; Lecuyer, M.; Faustino, J.; Strivelli, J.; Phinney, D.G.; Vexler, Z.S. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles Protect from Neonatal Stroke by Interacting with Microglial Cells. Neurotherapeutics 2021, 18, 1939–1952. [Google Scholar] [CrossRef]
- Du, L.; Jiang, Y.; Sun, Y. Astrocyte-Derived Exosomes Carry microRNA-17-5p to Protect Neonatal Rats from Hypoxic-Ischemic Brain Damage via Inhibiting BNIP-2 Expression. NeuroToxicology 2021, 83, 28–39. [Google Scholar] [CrossRef]
- Lawson, A.; Snyder, W.; Peeples, E.S. Intranasal Administration of Extracellular Vesicles Mitigates Apoptosis in a Mouse Model of Neonatal Hypoxic-Ischemic Brain Injury. Neonatology 2022, 119, 345–353. [Google Scholar] [CrossRef]
- Luo, H.; Huang, F.; Huang, Z.; Huang, H.; Liu, C.; Feng, Y.; Qi, Z. microRNA-93 Packaged in Extracellular Vesicles from Mesenchymal Stem Cells Reduce Neonatal Hypoxic-Ischemic Brain Injury. Brain Res 2022, 1794, 148042. [Google Scholar] [CrossRef]
- Labusek, N.; Mouloud, Y.; Köster, C.; Diesterbeck, E.; Tertel, T.; Wiek, C.; Hanenberg, H.; Horn, P.A.; Felderhoff-Müser, U.; Bendix, I.; et al. Extracellular Vesicles from Immortalized Mesenchymal Stromal Cells Protect against Neonatal Hypoxic-Ischemic Brain Injury. Inflammation and Regeneration 2023, 43, 24. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Van Wemmel, K.; Macchi, V.; Jurga, M.; Perilongo, G.; De Caro, R.; Baraldi, E.; et al. Intratracheal Administration of Clinical-Grade Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduces Lung Injury in a Rat Model of Bronchopulmonary Dysplasia. Am J Physiol Lung Cell Mol Physiol 2019, 316, L6–L19. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Yeung, V.; Liu, X.; Ericsson, M.; Andrews, N.A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Restore Lung Architecture and Improve Exercise Capacity in a Model of Neonatal Hyperoxia-Induced Lung Injury. J Extracell Vesicles 2020, 9, 1790874. [Google Scholar] [CrossRef]
- Abele, A.N.; Taglauer, E.S.; Almeda, M.; Wilson, N.; Abikoye, A.; Seedorf, G.J.; Mitsialis, S.A.; Kourembanas, S.; Abman, S.H. Antenatal Mesenchymal Stromal Cell Extracellular Vesicle Treatment Preserves Lung Development in a Model of Bronchopulmonary Dysplasia Due to Chorioamnionitis. Am J Physiol Lung Cell Mol Physiol 2022, 322, L179–L190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Xu, G.; Liu, L.; Li, H.; Li, Y.; Yin, J.; Wang, X.; Yu, Z. Human Breast Milk-Derived Exosomes through Inhibiting AT II Cell Apoptosis to Prevent Bronchopulmonary Dysplasia in Rat Lung. J Cell Mol Med 2022, 26, 4169–4182. [Google Scholar] [CrossRef]
- Zhong, X.-Q.; Wang, D.; Chen, S.; Zheng, J.; Hao, T.-F.; Li, X.-H.; Luo, L.-H.; Gu, J.; Lian, C.-Y.; Li, X.-S.; et al. Umbilical Cord Blood-Derived Exosomes from Healthy Term Pregnancies Protect against Hyperoxia-Induced Lung Injury in Mice. Clin Transl Sci 2023, 16, 966–977. [Google Scholar] [CrossRef]
- Barlow, B.; Santulli, T.V.; Heird, W.C.; Pitt, J.; Blanc, W.A.; Schullinger, J.N. An Experimental Study of Acute Neonatal Enterocolitis--the Importance of Breast Milk. J Pediatr Surg 1974, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, R.; Liang, H.; An, J.; Yang, Y.; Huo, J.; Chen, Z.; Quan, W.; Jiang, L.; Li, C.; et al. Comparison and Investigation of Exosomes from Human Amniotic Fluid Stem Cells and Human Breast Milk in Alleviating Neonatal Necrotizing Enterocolitis. Stem Cell Rev Rep 2023, 19, 754–766. [Google Scholar] [CrossRef] [PubMed]
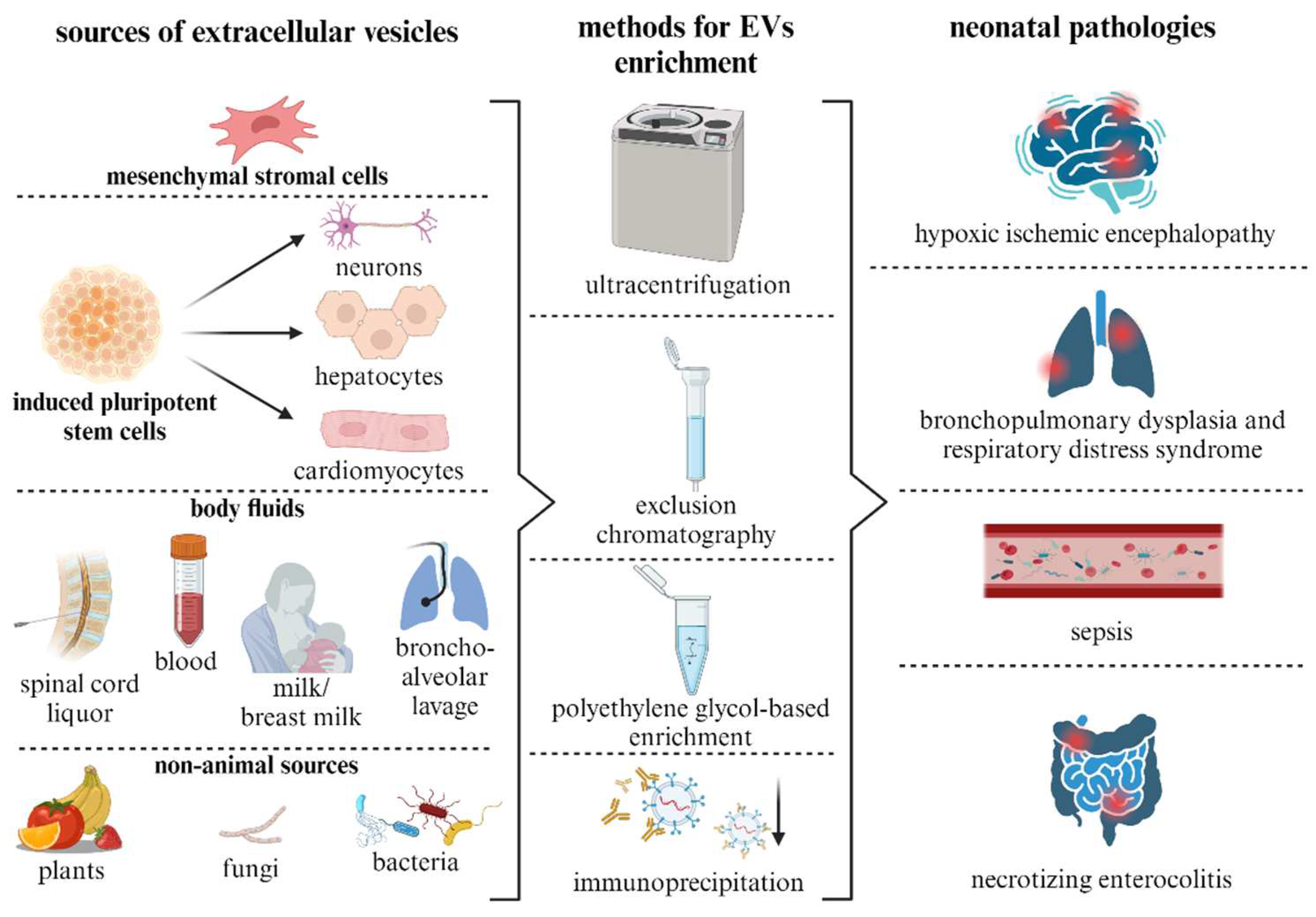
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





