Submitted:
10 January 2024
Posted:
11 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
1. Results
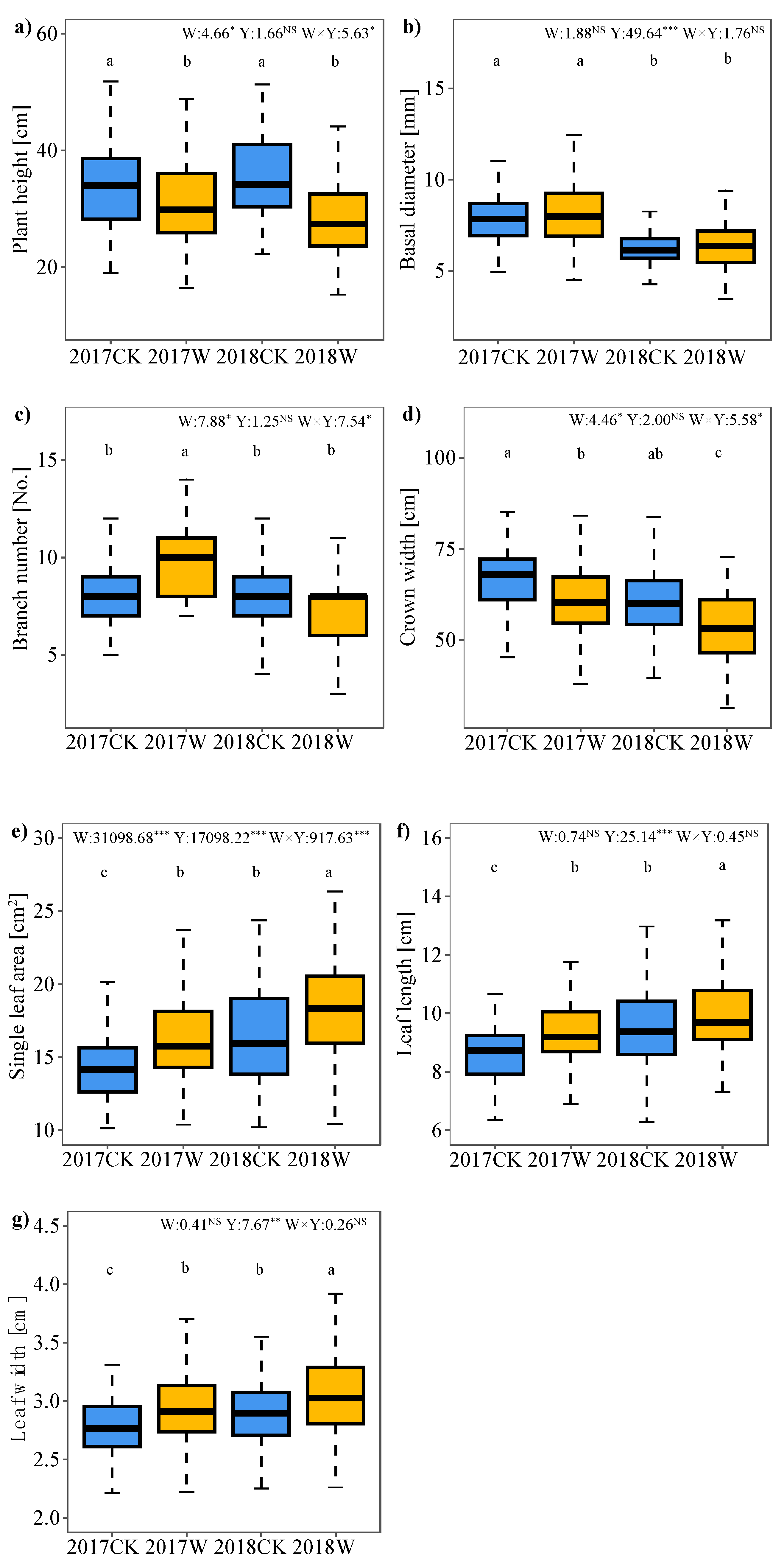
1.1. Plant Vegetative Growth
1.2. Flower Longevity and Flowering Duration
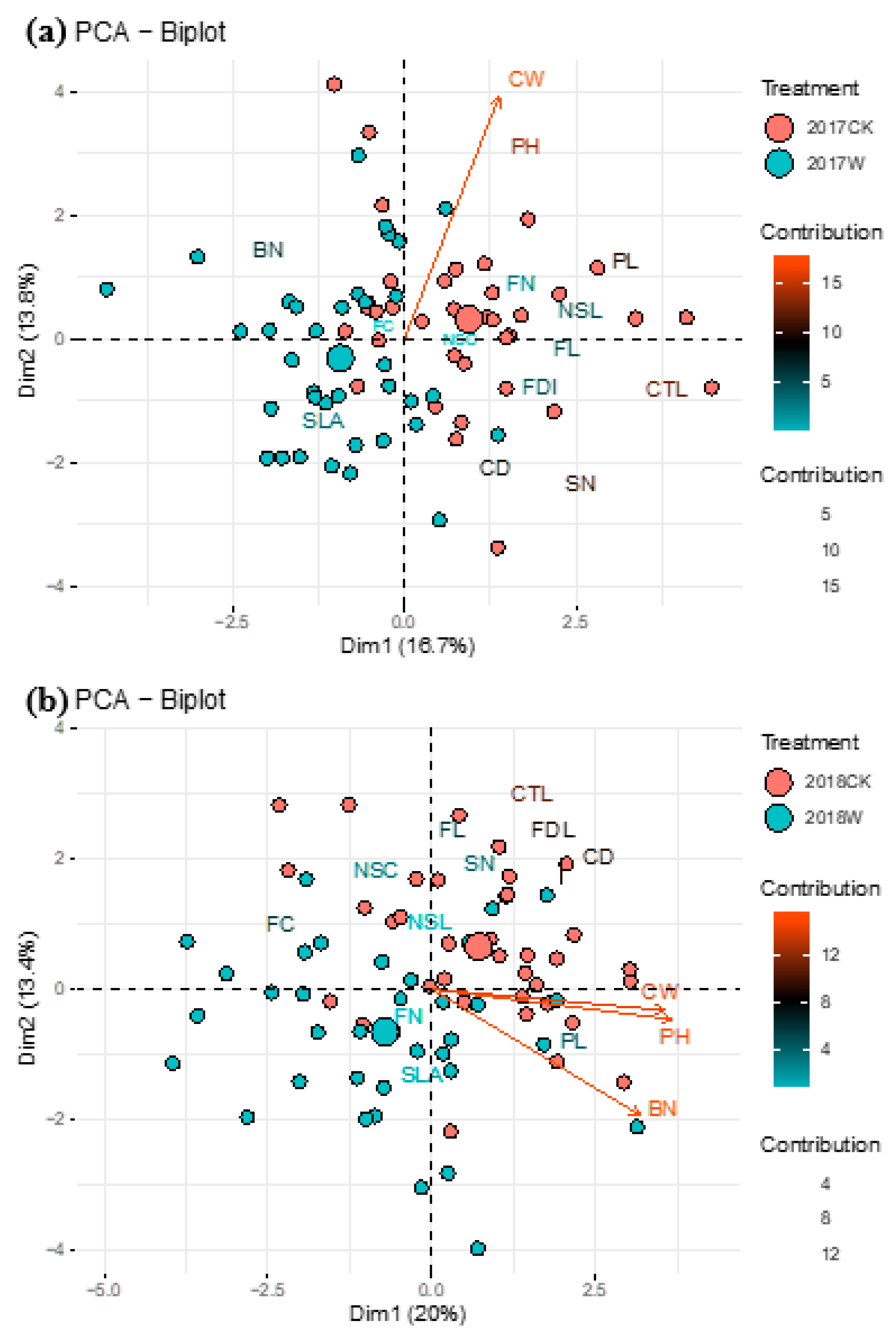
1.3. Floral Traits
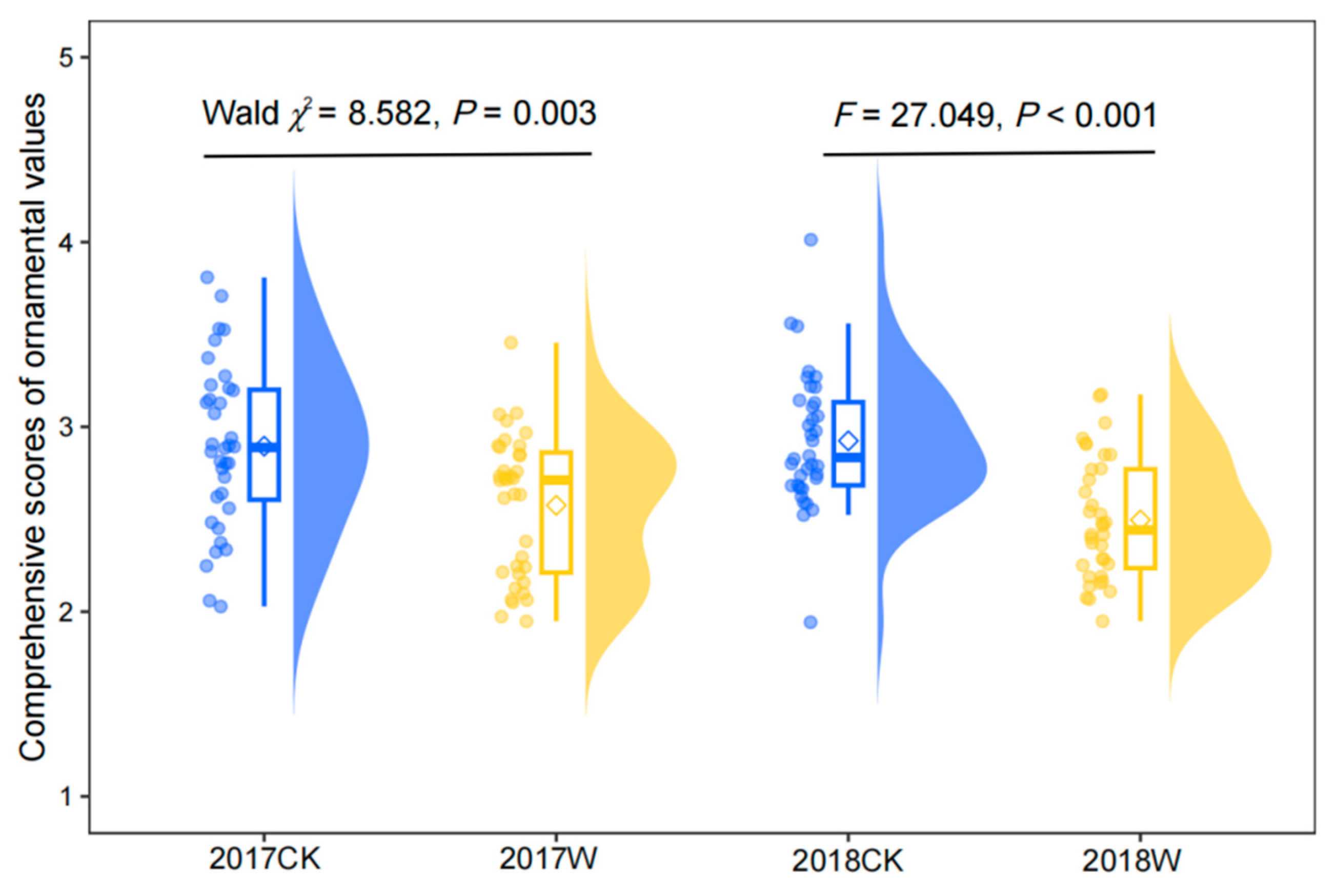
1.4. Comprehensive Ornamental Value
2. Discussion
2.1. Effect of Warming on Plant Vegetative Growth
2.2. Effect of Warming on the Ornamental Time and Flowering Period
2.3. Effect of Warming on Flower Ornamental Characteristics
2.4. Effect of Warming on Comprehensive Ornamental Value
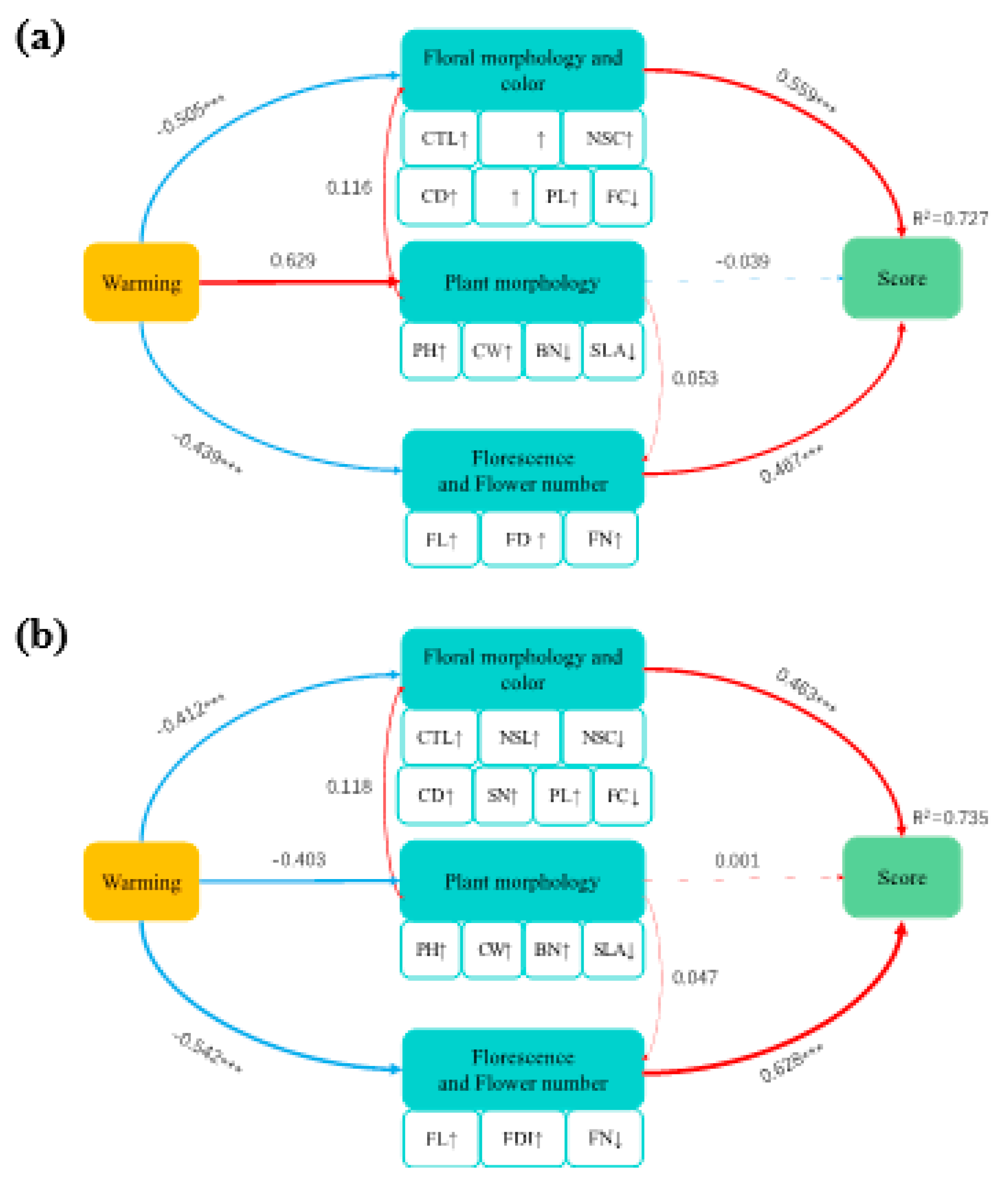
2.5. Study Site and Plant Materials
2.6. Warming Treatment
2.7. Determination of Air Temperature, Humidity and Soil Temperature
2.8. Measurement of Plant Morphology
2.9. Determination of Ornamental Traits of Flower
2.10. Determination of Anthocyanin Content
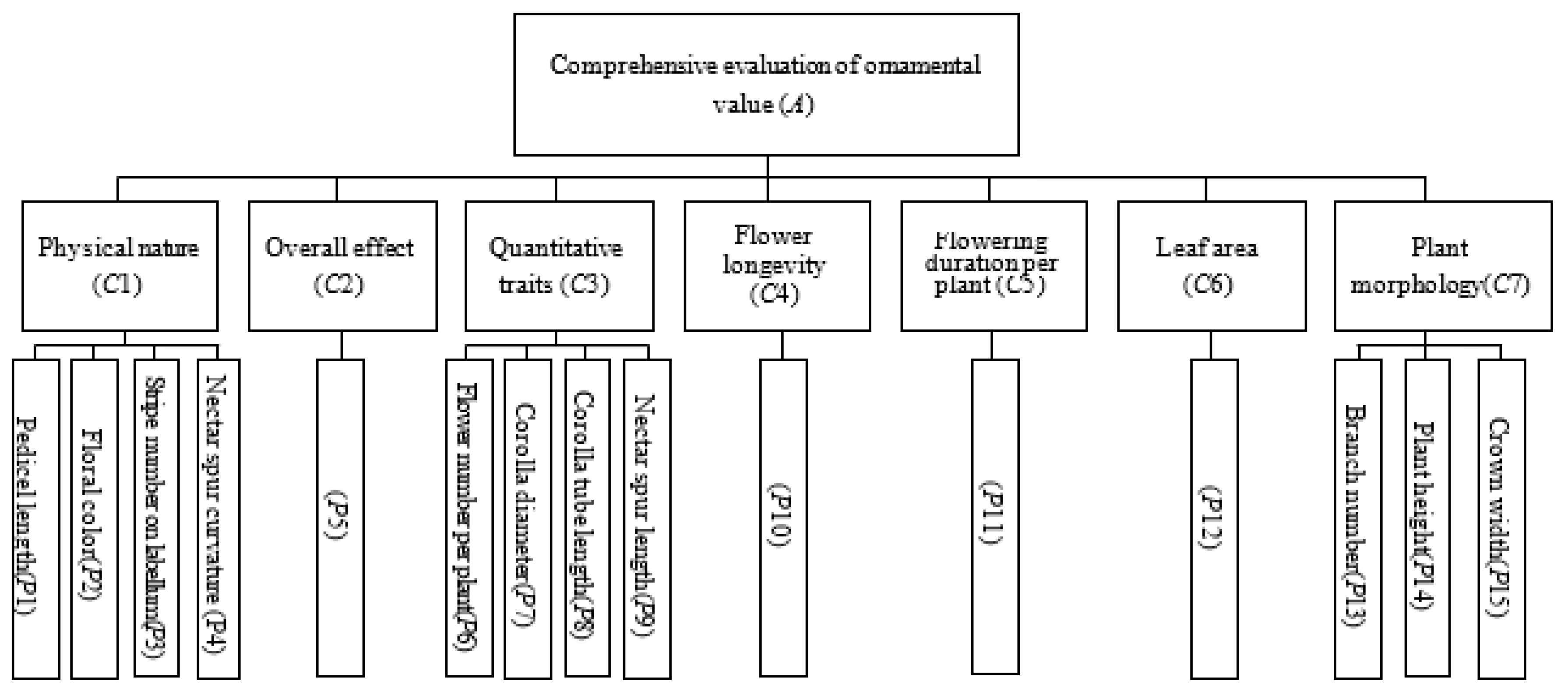
2.11. Evaluation of Ornamental Value
2.12. Treatment
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennisi, E. On the origin of flowering plants. Science, 2009, 324(5923): 28-31. [CrossRef]
- Matthews HD, Wynes S. Current global efforts are insufficient to limit warming to 1.5°C. Science, 2022, 376(6600): 1404-1409. [CrossRef]
- Mckay DA, Staal A, Abrams FJ, et al. Exceeding 1.5°C global warming could trigger multiple climate tipping points. Science, 2022, 377(6611): 1171-1171. [CrossRef]
- Patil RH, Laegdssmand M, Olesen JE, et al. Growth and yield response of winter wheat to soil warming and rainfall patterns. Journal of Agricultural Science, 2010, 148(5): 553-566. [CrossRef]
- Wigge, P.A. Ambient temperature signalling in plants. Current opinion in plant biology, 2013, 16(5): 661-666. [CrossRef]
- Mcclung CR, Lou P, Victor H, et al. The importance of ambient temperature to growth and the induction of flowering. Frontiers in Plant Science, 2016, 7: 1266. [CrossRef]
- Quint M, Delker C, Franklin KA, et al. Molecular and genetic control of plant thermomorphogenesis. Nature Plants, 2016, 2(1): 15190. [CrossRef]
- Panetta AM, Stanton ML, Harte J. Climate warming drives local extinction: evidence from observation and experimentation. Science Advances, 2018, 4(2): eaaq1819. [CrossRef]
- Kirillova IA, Kirillov DV. Impact of weather conditions on seasonal development, population structure and reproductive success on Dactylorhiza traunsteineri (Orchidaceae) in the Komi Republic (Russia). Nature Conservation Research, 2020, 5(Suppl.1): 77-89. [CrossRef]
- Jiang HY, Chen JJ, Liu GY, et al. Screening of early flowering lotus (Nelumbo nucifera Gaertn.) cultivars and effects of different cultivars on flowering period. Plants, 2023, 12(8): 1683-1683. [CrossRef]
- Awasthi R, Bhandari K, Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Frontiers in Environmental Science, 2015, 3:11-11. [CrossRef]
- Harsant J, Pavlovic L, Chiu G, et al. High temperature stress and its effect on pollen development and morphological components of harvest index in the C3 model grass Brachypodium distachyon. Journal of Experimental Botany, 2013, 64(10): 2971-2983. [CrossRef]
- Djanaguiraman M, Prasad PVV, Schapuagh WT. High day-or nighttime temperature alters leaf assimilation, reproductive success and phosphatidic acid of pollen grain in Soybean [Glycine max L. Merr.). Crop Science, 2013, 53(4): 1594-1604.
- Prasad PVV, Djanaguiraman M. Response of floret fertility and individual grain weight of wheat to high temperature stress: sensitive stages and thresholds for temperature and duration. Functional Plant Biology, 2014, 41(12): 1261-1269. [CrossRef]
- Singh V, Nguyen CT, Oosterom VEJ, et al. Sorghum genotypes differ in high temperature responses for seed set. Field Crops Research, 2015, 171: 32-40. [CrossRef]
- Devireddy A R, Tschaplinski T J, Tuskan G A, et al. Role of reactive oxygen species and hormones in plant responses to temperature changes. International Journal of Molecular Sciences, 2021, 22(16):8843-8843. [CrossRef]
- Monder MJ, Bbelewski P, Szperlik J, et al. The adjustment of China endemic Heptacodium miconioides Rehd. to temperate zone of Poland. BMC Plant Biology, 2023, 23(1): 184-184. [CrossRef]
- Meineri E, Skarpaas O, Spindelbock J, et al. Direct and size-dependent effects of climate on flowering performance in alpine and lowland herbaceous species. Journal of Vegetation Science, 2014, 25(1): 275-286. [CrossRef]
- Nicole EMS, Jennifer CG, James D.F, et al. Functional mismatch in a bumble bee pollination mutualism under climate change. Science, 2015, 349(6255): 1541-1544. [CrossRef]
- Haeuser E, Dawson W, Kleunen MV. The effects of climate warming and disturbance on the colonization potential of ornamental alien plant species. Journal of Ecology, 2017, 105(6): 1698-1708. [CrossRef]
- Springate DA, Kover PX. Plant responses to elevated temperatures: a field study on phenological sensitivity and fitness responses to simulated climate warming. Global change biology, 2014, 20(2): 456-465. [CrossRef]
- Hoover SE, Ladley JJ, Shchepetkina AA, et al. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecology Letters, 2012, 15(3): 227-234. [CrossRef]
- de Manincor N, Fisogni A, Rafferty NE. Warming of experimental plant-pollinator communities advances phenologies, alters traits, reduces interactions and depresses reproduction. Ecology Letters, 2023, 26(2): 323-334. [CrossRef]
- Dela G, Or E, Ovadia R, et al. Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high-temperature conditions. Plant Science, 2003, 164(3): 333-340. [CrossRef]
- Dai WK, Ochola AC, Li YQ. Spatio-temporal variations in pollen limitation and floral traits of an alpine lousewort (Pedicularis rhinanthoides) in relation to pollinator availability. Plants, 2022, 12(1): 78. [CrossRef]
- Arroyo MTK, Dudley LS, Jespersen G, et al. Temperature-driven flower longevity in a high-alpine species of Oxalis influences reproductive assurance. New Phytologist, 2013, 200: 1260-1268. [CrossRef]
- Seymour RS, Gibernau M, Pirintsos SA. Thermogenesis of three species of Arum from Crete. Plant, Cell & Environment, 2009, 32(10): 1467-1476. [CrossRef]
- Chen, Y.L. Floral of China. Volume 47 (Second Division). Beijing Science Press, 2001.
- Dan Y, Baxter A, Zhang S, et al. Development of efficient plant regeneration and transformation system for impatiens using agrobacterium tumefaciens and multiple bud cultures as explants. BMC Plant Biology, 2010, 10(1): 165-165. [CrossRef]
- Jin XF, Ding BY. The impatiens of zhejiang wild flower resources and development. Chinese Wild Plant Resources, 2000, 19(4): 27-29.
- Bronson DR, Gower ST, Tanner M, et al. Effect of ecosystem warming on boreal black spruce bud burst and shoot growth. Global Change Biology, 2009, 15: 1534-1543. [CrossRef]
- Wang, Y. Collection and preservation of Impatiens spp. PhD Thesis. Beijing: Beijing Forestry University, 2008.
- Wang, Q. Biological effects of experimental warming on pollination in Impatiens oxyanthera (Balsaminaceae). PhD Thesis. Beijing: The University of Chinese Academy of Sciences, 2013.
- Cui MM, Yang B, Ren GQ, et al. Effects of warming, phosphorous deposition, and both treatments on the growth and physiology of invasive Solidago canadensis and native Artemisia argyi. Plants, 2023, 12(6): 1370-1370. [CrossRef]
- Marlène A, François O. Growth temperature affects inflorescence architecture in Arabidopsis thaliana. Botany, 2013, 91(9): 642-651. [CrossRef]
- Lysenko EA, Kozuleva MA, Klaus AA, et al. Lower air humidity reduced both the plant growth and activities of photosystems I and II under prolonged heat stress. Plant Physiology and Biochemistry, 2023, 194: 246-262. [CrossRef]
- Nagarathn TK, Shadakshari YG, Jagadish KS, et al. Interactions of auxin and cytokinins in regulating axillary bud formation in sunflower (Helianthus annuus L.). Helia, 2010, 33(52): 85-94. [CrossRef]
- Li YB, Hou RX, Tao FL. Wheat morpho-physiological traits and radiation use efficiency under interactive effects of warming and tillage management. Plant, Cell & Environment, 2020, 44(7): 2386-2401. [CrossRef]
- Chen BM, Gao Y, Liao HX, et al. Differential responses of invasive and native plants to warming with simulated changes in diurnal temperature ranges. AoB PLANTS, 2017, 9(4): plx028. [CrossRef]
- Aspi J, Jakalaniemi A, Tuomi J, et al. Multilevel phenotypic selection on morphological characters in a metapopulation of Silene tatarica. Evolution, 2003, 57: 509-517. [CrossRef]
- Zeng Z, Huan HH, Liu G, et al. Effects of elevated temperature and CO2 concentration on growth and leaf quality of Morus alba seedlings. Chinese Journal of Applied Ecology, 2016, 27(8): 2445-2451. [CrossRef]
- Sage TL, Bagha S, Lundsgaard-Nielsen V, et al. The effect of high temperature stress on male and female reproduction in plants. Field Crops Research, 2015, 182: 30-42. [CrossRef]
- Ishii HS, Sakai S. Effects of display size and position on individual floral longevity in racemes of Narthecium asiaticum (Liliaceae). Functional Ecology, 2001, 15(3): 396-405. [CrossRef]
- Itagaki T, Sakai S. Relationship between floral longevity and sex allocation among flowers within inflorescences in Aquilegia buergeriana var.Oxysepala (Ranunculaceae). American Journal of Botany, 2006, 93(9):1320-1327.
- Bock A, Sparks TH, Estrella N, et al. Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Global Change Biology, 2014, 20: 3508-3519. [CrossRef]
- Sood A, Duchin S, Adamov Z, et al. Abscisic acid mediates the reduction of petunia flower size at elevated temperatures due to reduced cell division. Planta, 2022, 255(1): 18. [CrossRef]
- Wang Li, Yang Youqin, Wang Qiong. Photosynthetic physiological response of Impatiens oxyanthera to Simulated Warming. Journal of China West Normal University (Natural Sciences), 2019, 40(4): 339-345.
- Suraweera DD, Groom T, Nicolas ME. Nicolas. Exposure to heat stress during flowering period reduces flower yield and pyrethrins in Pyrethrum (Tanacetum cinerariifolium). Journal of Agronomy and Crop Science, 2020, 206(5): 568-578. [CrossRef]
- Lambrecht, S.C. Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). International Journal of Plant Sciences, 2013, 174: 74-84. [CrossRef]
- Gómez JM, Bosch J, Perfectti F, et al. Association between floral traits and rewards in Erysimum mediohispanicum (Brassicaceae). Annals of Botany, 2008, 101(9): 1413-1420. [CrossRef]
- Ellis AG, Johnson SD, Conner JK. Gender differences in the effects of floral spur length manipulation on fitness in a hermaphrodite orchid. International Journal of Plant Sciences, 2010, 171(9):1010-1019.
- Sletvold N, Ågren J. Nonadditive effects of floral display and spur length on reproductive success in a deceptive orchid. Ecology, 2011, 92(12): 2167-2174. [CrossRef]
- Boberg E, Ågren J. Despite their apparent integration, spur length but not perianth size affects reproductive success in the moth-pollinated orchid Platanthera bifolia. Functional Ecology, 2009, 23: 1022-1028. [CrossRef]
- Jia Y, Zhao JL, Pan YZ, et al. Collection and evaluation of Primula species of western Sichuan in China. Genetic Resources and Crop Evolution, 2014, 61(7): 1245-1262. [CrossRef]
- Shen Gangxu, Wang WeiLung. Circlize package in R and Analytic Hierarchy Process (AHP): Contribution values of ABCDE and AGL6 genes in the context of floral organ development. PloS one, 2022, 17(1): e0261232. [CrossRef]
- Wang YS, Chen LJ, Yang XJ, et al. A comprehensive evaluation of the wild ground cover plants resources in Yunshan, Hunan. Acta Prataculturae Sinica, 2015, 24((07): 30-40. [CrossRef]
- Cicevan R, Sestras AF, Plazas M, et al. Biological traits and genetic relationships amongst cultivars of three species of tagetes (Asteraceae). Plants, 2022, 11(6):760-760. [CrossRef]
- Xing GM, Qu LW, Zhang YQ, et al. Collection and evaluation of wild tulip (Tulipa spp.) resources in China. Genetic Resources and Crop Evolution, 2017, 64(4):641-652. [CrossRef]
- Yang Z, Meng TF, Bi XY, Lei JJ. Investigation and evaluation of wild Iris resources in Liaoning Province, China. Genetic Resources and Crop Evolution, 2017, 64(5): 967-978. [CrossRef]
- Ye A, Chen L, Lan SR, et al. Comprehensive evaluation of the ornamental value of Cymbidium ensifolium cultivars using analytical hierarchy process method. Journal of Fujian Agriculture and Forestry University (Natural Science Edition), 2019, 48(6): 736-741.
- Anjali C, Meenakshi T, Anjali R, et al. Exogenous applications of gibberellic acid modulate the growth, flowering and longevity of calla lily. Heliyon, 2023, 9(5): e16319. [CrossRef]
- Yang K, Wu H, Qin J, et al. Recent climate changes over the Tibetan Plateau and their impacts on energy and water cycle: A review. Global and Planetary Change, 2014, 112:79-91. [CrossRef]
- Liu L, Wu W, Zheng YL, et al. Variations on the chemical components of the volatile oil of Houttuynia cordata Thunb. populations from different valleys and altitudes of Mt. Emei. Acta Ecologica Sinica, 2007, 27(06): 2239-2250.
- Gu HY, Li CH. Biodiversity and flora of the mixed evergreen and deciduous broadleaved forest in Emei. Bulletin of botanical research, 2006, 26(5): 618-624.
- Li ZY and Shi L. Plants of Mount Emei. Beijing Science and Technology Press, 2007.
- Zhao QY, Zhang X, Cao MH, et al. Investigation and evaluation on plant resources of Impatiens in southwest Sichuan. Seed, 2023,42(2): 64-71,82.
- IPCC(2014) Summary for Policymakers. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds C.B. Field, V.R. Barros, D.J. Dokken et al.). World Meteorological Organization, Geneva, Switzerland: 1-190.
- Chen DF, Zhang Y, Fang Z. Study on the content of anthocyanin and related biochemical substances during the petal development in Impatiens hawkeri. Journal of Agricultural University of Hebei, 2008, 31(3): 28-32.
- Bates D, Maechler M, Bolker B, et al. 2019. lme4: linear mixed-effects models using ‘eigen’ and s4. R package version 1.1-21. https://cran.r-project.org/package=lme4/ (28 June 2021).
- Fox J, Weisberg S. An R companion to applied regression. Thousand Oaks, CA: Sage Publications, 2011.
- Lenth, R., Singmann H, Love J, et al. 2020. Emmeans: estimated marginal means, aka least-squares means. R package ver. 1. 5. 1. https://CRAN.Rproject.org/package=emmeans. (28 June 2021).
| Trait | 2017 | 2018 | W | Y | W × Y | |||
|---|---|---|---|---|---|---|---|---|
| Control | Warming | Control | Warming | |||||
| Male phase (d) | 2.417 ± 0.072 a | 2.185 ± 0.068 b | 2.898 ± 0.093 a | 2.583 ± 0.085 a | NS | * | NS | |
| Female phase (d) | 1.065 ± 0.051 a | 1.028 ± 0.051 a | 1.333 ± 0.068 a | 1.213 ± 0.052 a | NS | NS | NS | |
| Flower longevity (d) | 3.481 ± 0.083 b | 3.213 ± 0.076 b | 4.231 ± 0.085 a | 3.796 ± 0.070 ab | NS | ** | NS | |
| Flowering duration per plant (d) | 65.278 ± 1.474 a | 58.944 ± 1.330 b | 65.917 ± 1.899 a | 53.361 ± 1.840 c | * | NS | ** | |
| Flower number per plant (No.) |
80.778 ± 5.155 a | 73.306 ± 4.251 b | 73.472 ± 4.357 b | 65.500 ± 5.361 c | NS | *** | NS | |
| Trait | 2017 | 2018 | W | Y | W × Y | |||
|---|---|---|---|---|---|---|---|---|
| Control | Warming | Control | Warming | |||||
| Vexillum length (mm) | 12.776 ± 0.245 a | 11.840 ± 0.213 b | 12.546 ± 0.101 a | 12.181 ± 0.152 ab | *** | NS | *** | |
| Wing petal length (mm) | 23.058 ± 0.240 b | 22.289 ± 0.258 c | 23.650 ± 0.263 a | 22.807 ± 0.217 bc | NS | ** | NS | |
| Corolla diameter (mm) | 21.916 ± 0.407 b | 21.626 ± 0.360 b | 23.434 ± 0.361 a | 22.465 ± 0.384 ab | NS | ** | NS | |
| Corolla tube length (mm) | 20.202 ± 0.310 a | 19.275 ± 0.220 b | 20.444 ± 0.215 a | 18.637 ± 0.252 b | *** | NS | *** | |
| Stripe number on the labellum (No.) | 11.167 ± 0.232 a | 10.713 ± 0.193 ab | 10.667 ± 0.183 ab | 10.222 ± 0.186 b | NS | NS | NS | |
| Nectar spur length (mm) | 30.079 ± 0.399 a | 27.447 ± 0.536 b | 28.458 ± 0.410 b | 27.820 ± 0.314 b | *** | *** | *** | |
| Nectar spur curvature (°) | 303.333 ± 12.626 a | 300.000 ± 10.992 a | 334.352 ± 10.114 a | 326.389 ± 9.408 a | NS | * | NS | |
| Pedicel length (mm) | 46.285 ± 1.725 a | 38.664 ± 1.292 b | 40.055 ± 1.107 b | 38.960 ± 1.379 b | ** | ** | * | |
| relative anthocyanin content of vexillum (A. g-1 FW) |
5.624 ± 0.136 a | 5.867 ± 0.159 a | 5.563 ± 0.120 a | 5.851 ± 0.114 a | NS | NS | NS | |
| relative anthocyanin content of corolla tube (A. g-1 FW) |
3.659 ± 0.078 a | 3.722 ± 0.095 a | 3.560 ± 0.056 a | 3.723 ± 0.112 a | NS | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





