1. Introduction
In recent years, the spotlight has increasingly turned towards the issue of air pollution, paralleled by a growing body of research into colon cancer (CC). CC stands as the second leading cause of cancer-related deaths globally (Tian et al., 2014). The risk factors linked to CC are primarily categorized into modifiable elements (such as smoking, obesity, and diet) and non-modifiable factors (like family history, age, and sex). Recent studies have illuminated that exposure to environmental pollutants, notably heavy metals, could elevate the risk of CC. Among these heavy metal pollutants are commonly cadmium, arsenic, lead, mercury, and chromium (Lin et al., 2018; Rinaldi et al., 2015; Sarkar et al., 2020).
Cadmium (Cd), a hazardous metal prevalent in the environment and commonly used in industrial processes, is classified as a human carcinogen by International Agency for Research on Cancer (IARC) (Briffa et al., 2020; Rahman, 2019). Mounting evidence indicates that Cd present in aquatic environments can induce various adverse health outcomes in humans, including cancer, (Nawrot et al., 2015; Nishijo et al., 2018), cardiovascular diseases (Lin et al., 2021; Tellez-Plaza et al., 2013) and inflammatory conditions (Salama et al., 2021; Taş et al., 2019). Nevertheless, the impact of Cd in the atmosphere on human health, particularly concerning CC, remains elusive. Recent investigations have revealed a notable increase in plasma Cd levels among CC patients compared to healthy individuals (Mahmood et al., 2022). Experimental data further suggests that Cd exerts a mutagenic effect by impeding the deoxyribonucleic acid (DNA) mismatch repair mechanism, thereby exacerbating the progression of CC (Wu et al., 2017). Additionally, studies have demonstrated that Cd can prompt cellular transformation and carcinogenesis in human colon cell line CRL-1807 and xenograft animal models (Lu et al., 2015). Speculations suggest that these cellular responses might be associated with specific molecular mechanisms triggered by Cd exposure, such as the induction of oxidative stress, β-catenin mutation, and upregulation of cyclooxygenase-2 (COX-2), thereby initiating pro-inflammatory reactions. However, further research is needed to comprehensively elucidate the intricate connections between Cd exposure and the molecular pathways involved in CC development.
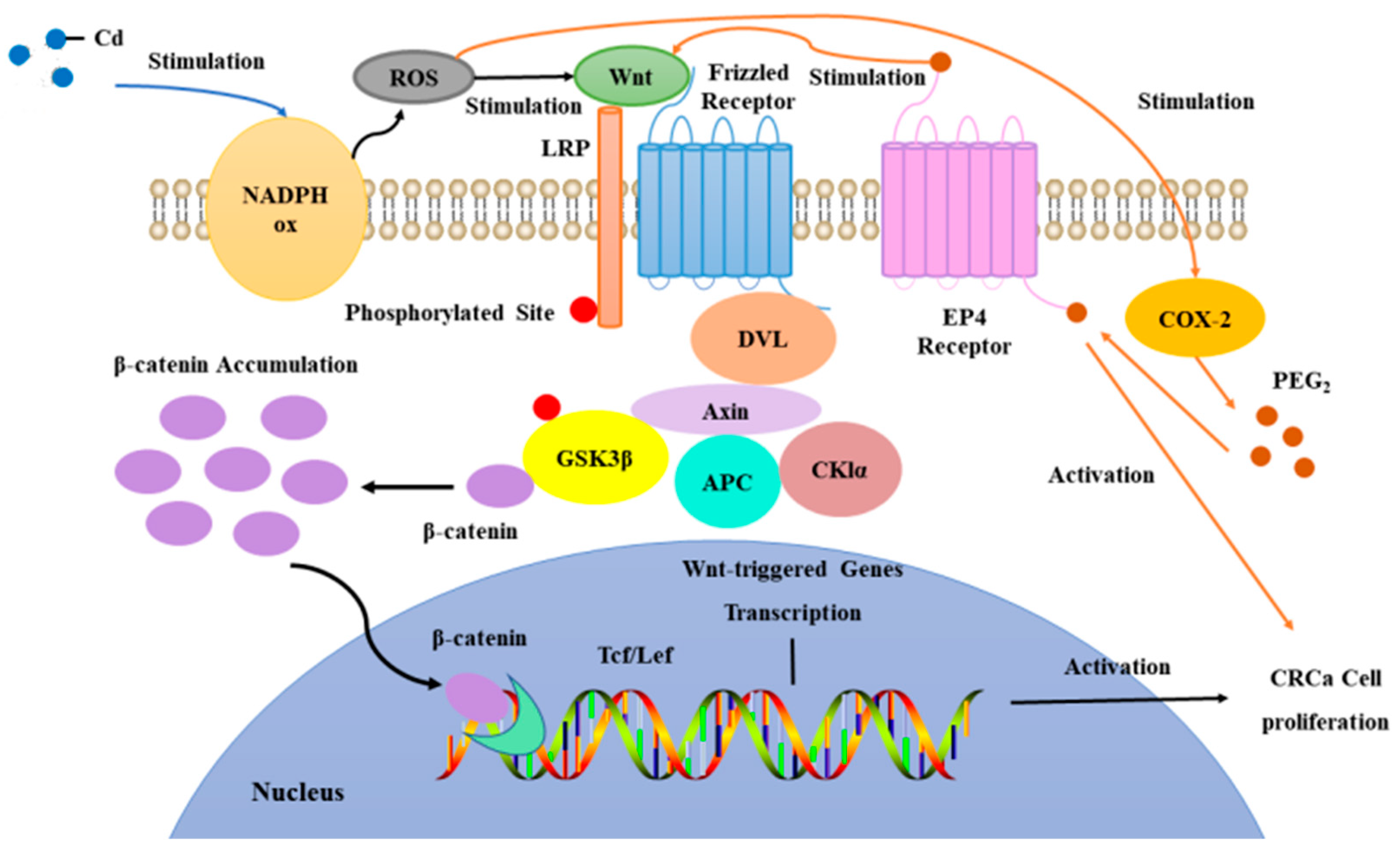
While the molecular intricacies behind Cd-induced carcinogenesis necessitate further investigation, there is a consensus that reactive oxygen species (ROS) assume a pivotal role in instigating cellular damage triggered by Cd, subsequently culminating in cancer development. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) complex stands as a critical physiological system for ROS generation. Studies have revealed that Cd has the capability to activate NOX and disrupt the integrity of the outer mitochondrial membrane, thereby engendering the production of superoxide radicals, hydroxyl radicals, and hydrogen peroxide
(Chou et al., 2004). In the context of the colon, the Wnt/β-catenin pathway, controlled by ROS generated via NOX, regulates the fate of proliferating cells
(Coant et al., 2010a; Ladelfa et al., 2011). This pathway often exhibits differential regulation between normal and cancerous tissues, particularly in cancers such as hepatocellular carcinoma, prostate cancer, and CC
(Jung, 2020). Within healthy cells (
Figure S1), in the absence of Wnt signaling, β-catenin is bound and regulated by a multi-subunit complex consisting of recombinant axis inhibition protein (Axin), adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase 3β (GSK3β). This complex promotes β-catenin phosphorylation, facilitating its interaction with β transducin repeats-containing proteins (β-TRCP) and subsequent ubiquitination and degradation, maintaining low β-catenin expression levels. Conversely, in cancer cells when Wnt signaling is active (
Figure S2), the Wnt signal engages specific receptors like frizzled protein, leading to low density lipoprotein receptor related protein (LRP) phosphorylation. This culminates in the formation of the Wnt-Frizzled-LRP complex, triggering disheveled (DVL) activation and conglomerate aggregation toward the receptor. DVL activation intensifies GSK3β phosphorylation, impeding β-catenin degradation
(Y. Li et al., 2011). Consequently, β-catenin accumulates in the nucleus, interacts with coactivators like T cell factor and lymphoid enhancer factor (TCF/LEF), and stimulates downstream Wnt-target gene transcription, fostering cancer cell proliferation. Concomitantly, the heightened ROS production may incite an inflammatory cascade. The COX-2 pathway emerges as a pivotal inflammatory pathway implicated in CC
(Naji et al., 2019). Substantial data highlights significantly elevated COX-2 gene expression in cancerous colon mucosa compared to healthy tissue
(Mahmoud et al., 2014; Peng et al., 2013; Tatsuguchi et al., 2006). The primary attributor to carcinogenic effect of COX-2 is believed to be its chief metabolite, prostaglandin E2 (PGE2), which exerts its biological function by binding to its target receptor, prostaglandin receptor 4 (EP4)
(Breyer et al., 2001). However, the precise molecular mechanism by which Cd in the atmospheric milieu induces CC remains an area yet to be fully elucidated.
Advancements in medical devices and technologies have led to the gradual adoption of intestinal stents as the primary treatment for colon tract decompression. Evidence-based medicine has validated the short-term safety and efficacy of this approach (Shigeta et al., 2014; Shingu et al., 2013; Yoshida et al., 2013). Nevertheless, significant concerns persist regarding the long-term tumor prognosis in CC patients who undergo bowel stenting as a form of conversion therapy. Studies indicate that preoperative placement of bowel stents in CC patients may disrupt tumor prognosis and potentially trigger tumor cell dissemination during the stent insertion process (Maruthachalam et al., 2007). Furthermore, stent placement has been linked to adverse effects on tumor invasiveness. However, the impact of atmospheric Cd on the invasiveness of stents in cancerous colon remains largely unexplored.
Hence, this study employed CT26WT CC rat models with implanted stents. These models were subjected to atomized air containing Cd via inhalation, mimicking atmospheric exposure, to investigate the influence of Cd in the environment on CC patients with implanted intestinal stents. The hypothesis posited here suggested that Cd in the atmospheric setting might foster the proliferation and dissemination of CC cells through a mechanism reliant on NOX-ROS-COX-2-Wnt/β-catenin pathways. The preliminary verification of this hypothesis was illustrated in
Figure 1.
2. Materials and Methods
Animal and experimental design. The CT26 rat CC cells along with the culture medium were procured from Wuhan Pricella Life Technology Co., Ltd. These CC cells were nurtured in 1640 complete medium containing 10% fetal bovine serum (FBS) and 1% bis-antibodies within a 37°C, 5% carbon dioxide (CO2) incubator. Forty-five 6-week-old female BALB/c rats were acquired from Beijing Sipeifu Biotechnology Co., Ltd. and were housed in accordance with institutional animal care guidelines. To facilitate acclimation, the rats were allowed a week in the new environment before utilization. The rats were accommodated in plastic cages (5 mice/cage) under specific conditions: relative humidity (50±10%), a 12/12 hours light/dark cycle, and a temperature of 23±2°C. For the implantation of CC cell lines, 1×107/mL of cells (200μL/piece) were cultured on the mucosa of the posterior colon wall in rats and observed for tumor formation over a 2-week period utilizing a small animal live imaging device (InVivo Smart-LF, VISQUE, South Korea). The BALB/c rats were then randomly divided into 9 groups based on body weight: healthy groups: Blank Group (H-Blank), Control Group (H-Control), Cd Group (H-Cd); with CC cells but without intestinal stent: Blank Group (C-Blank), Control Group (C-Control), Cd Group (C-Cd); with CC cells and intestinal stent: Blank Group (C-S-Blank), Control Group (C-S-Control), Cd Group (C-S-Cd). The rats in the intestinal stent group were implanted with metal spring coils (length 5 mm, inner diameter 2 mm, outer diameter 3 mm, wire diameter 0.2 mm) through the anus and cultured for 2 weeks. The Cd group was exposed to a simulated environment of oxygen and nitrogen (O2/N2) mixed atomized gas containing Cd for the subsequent 4 weeks. The blank group was exposed to the actual environment without gas introduction, while the control group was exposed to a simulated environment with the introduction of O2/N2 mixed atomized gas. Throughout the experiment, the rats were weighed twice weekly, and any signs of weight loss were closely monitored. At 27 weeks of age, all rats were euthanized using CO2 asphyxiation, and whole colon intestinal tissue was collected. Tissue samples were promptly fixed in 10% formalin or frozen in liquid nitrogen for further analysis.
Establishment of simulation environment. Reference was drawn from the average concentration of Cd in the atmospheric environment spanning from January 2016 to February 2017, specifically under heavy pollution weather conditions (AQI>200) in Heping District, Tianjin. During this period, the average concentration was recorded at 4.33 ng/m
3. As demonstrated in formula (1), the estimated quantity of Cd inhaled by humans in the atmospheric environment amounted to 46.76 ng per day, whereas the estimated intake for BALB/c rats in the atmospheric environment stood at 0.13 ng per day.
was the respiratory exposure to Cd in the atmospheric environment, ng;
was the average concentration of Cd in the atmospheric environment, ng/m
3;
was the respiratory rate, m
3/d. The respiratory rate of BALB/c rats was 19.16 mL/min, which was 0.03m
3/d, and the respiratory rate of humans was 10.8 m
3/d;
was the exposure time, d. The Cd used in the experiment to simulate the polluted atmospheric environment was a solution derived from the Cd inductively coupled plasma (ICP) standard solution obtained from O2si, USA. To align with the inhalation volume for both humans and BALB/c rats, the Cd ICP standard solution was prepared at a concentration of 0.23 μg/mL. Connected the compressed air inlet of the medical nebulizer to the fresh air cylinder (21% O
2/79% N
2) generates dispersion nebulization gas.
Histopathology. All tumor and mucosa specimens underwent hematoxylin-eosin (H&E) staining for histopathological assessment. The samples were fixed with paraformaldehyde and then decalcified in decalcifying solution. Dehydration of the samples was through alcohol, and paraffin removal from the tissue was carried out using xylene solution. The tissue sample underwent embedding by being dipped in melted wax solution and placed into an embedding frame. Once positioned appropriately according to the embedding surface requirements, the sample was cooled on a -20°C freezing platform until the wax solidified. After solidification, the wax block was removed from the embedding frame, trimmed, and placed on a paraffin microtome for slicing. The resulting slices were floated on 40°C warm water in a spreading machine to flatten the tissue. Subsequently, the tissue was picked up with a glass slide and placed in a 60°C oven for baking. Following baking, the slices were dewaxed in xylene twice and dehydrated using absolute ethanol. Hydration of the sample sections was performed using a series of ethanol concentrations (95%, 80%, 70%) and distilled water. Subsequent staining involved hematoxylin staining solution application, followed by differentiation. The samples underwent dehydration, transparency treatment, and sealing. Observation and photography were conducted using a fluorescence microscope (ECLIPSE Ci, Nikon, Japan) to analyze the stained tissue sections.
Immunohistochemistry. To achieve cell permeability and block endogenous peroxidase, sections underwent immersion in blocking permeabilization solution (at room temperature, shielded from light). To uncover the antigen-determining area for antigen retrieval, the slices were submerged in 0.01M sodium citrate buffer (pH=6.0) and heated in a microwave oven until boiling, with regular replenishment of the solution to prevent desiccation. For obstructing the influence of non-specific proteins, the surrounding tissue was outlined using a histochemical pen, followed by the application of 5% sheep serum within the outlined area. The diluted primary antibody was directly added and allowed to incubate overnight at 4°C. Following this, the diluted secondary antibody was applied, and the sections were placed in a constant-temperature oven at 37°C. Subsequent steps involve the addition of streptavidin-perosidase (SP), placement in a 37°C oven, and the introduction of diaminobenzidine (DAB). The staining progression was observed under the fluorescence microscope, controlling the duration based on the color observed. Subsequently, applied the hematoxylin staining solution to stain the nuclear proteins, followed by using phosphate buffered saline (PBS) to restore their blue coloration. The samples underwent dehydration, transparency treatment, and sealing. Observation and photography were conducted using the fluorescence microscope to analyze the stained tissue sections.
Western blot analysis. Western blot analysis was conducted utilizing specific antibodies targeting β-catenin (1:1000, BIOSS) and phospho-GSK3β (1:1000, BIOSS), actin (1:3000, Affinity), SOD1 (1:1000, Affinity), SOD2 (1:1000, Affinity), CAT (1:1000, BIOSS), NOX1 (1:1000, Affinity).
Statistical analysis. Differences among treatment groups were tested using ANOVA. Differences in which p was < 0.05 were considered statistically significant. The analyses were performed using SPSS software (SPSS, Chicago, IL, USA).
3. Results
Colon tumor invasion and metastasis capabilities
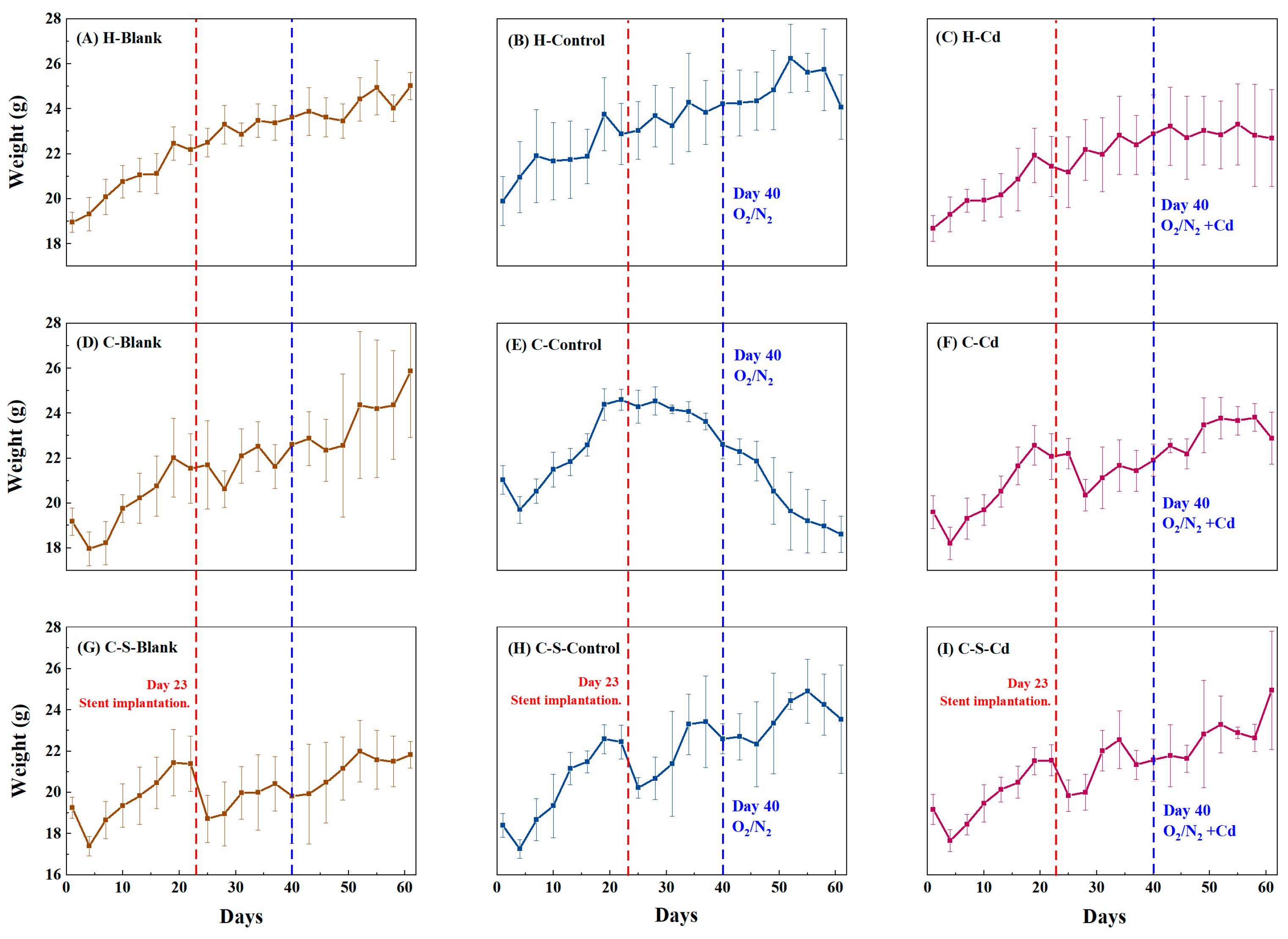
Mice lost weight within 4 days after CC cells were implanted, according to
Figure 2D–I. This weight reduction during the initial stages of CC growth was attributed to the heightened energy demand of proliferating CC cells, consequently depleting the body's resources
(DU, 2016). With tumor progression, a gradual adaptation seemed apparent, marked by a subsequent increase in appetite and weight. However, upon the implantation of intestinal stents on day 23, rats with CC experienced weight loss as indicated in
Figure 2G–I. The presence of these stents induced gastrointestinal discomfort, reducing food intake. Furthermore, their implantation easily triggered inflammation and infection, compelling the body to expend additional energy in managing the immune response
(Choi et al., 2015). Interestingly, the injection of O
2/N
2 atomized gas and Cd-O
2/N
2 atomized gas exhibited no notable impact on the mice's body weight compared to the control group. This discrepancy might be attributed to the overshadowing effect of increased tumor tissue weight during the later phases of the experiment on the rats' weight alterations. Hence, a detailed observation and analysis of in situ colon tumor formation became imperative for comprehensive understanding.
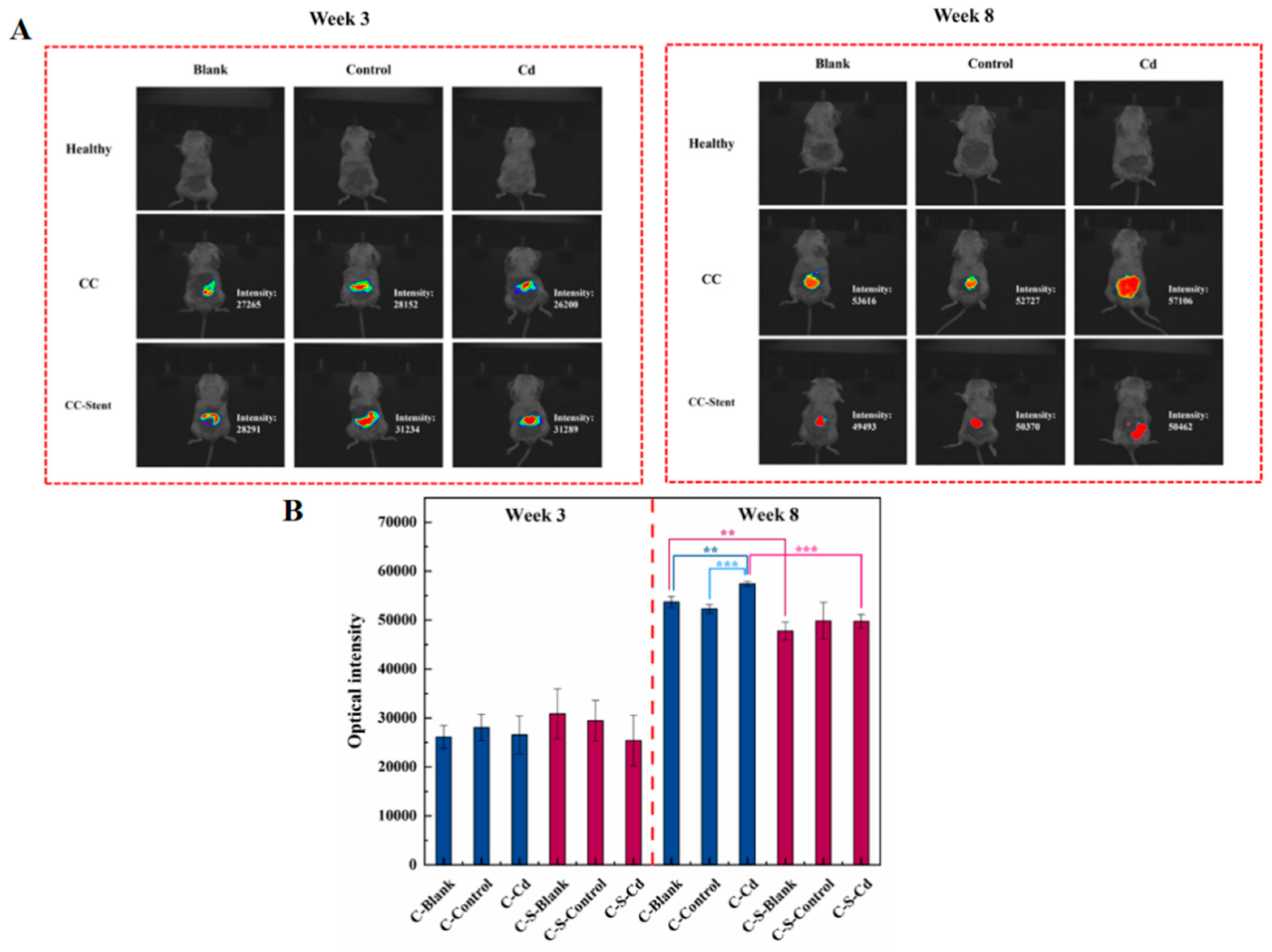
Illustrated in
Figure 3, there was extensive proliferation of CC cells from the 3rd to the 8th week, leading to a heightened fluorescence signal within the colon tumor tissue. Notably, compared to the blank and control groups, the administration of Cd-O
2/N
2 atomized gas further intensified the proliferation of CC cells. The presence of atmospheric Cd activated specific cellular signaling pathways, a phenomenon that warrants detailed exploration in subsequent investigations. However, upon the implantation of intestinal stents, the proliferative capacity of CC cells exhibited attenuation. The introduction of these stents hindered, to a certain extent, the physical growth and dissemination of CC cells.
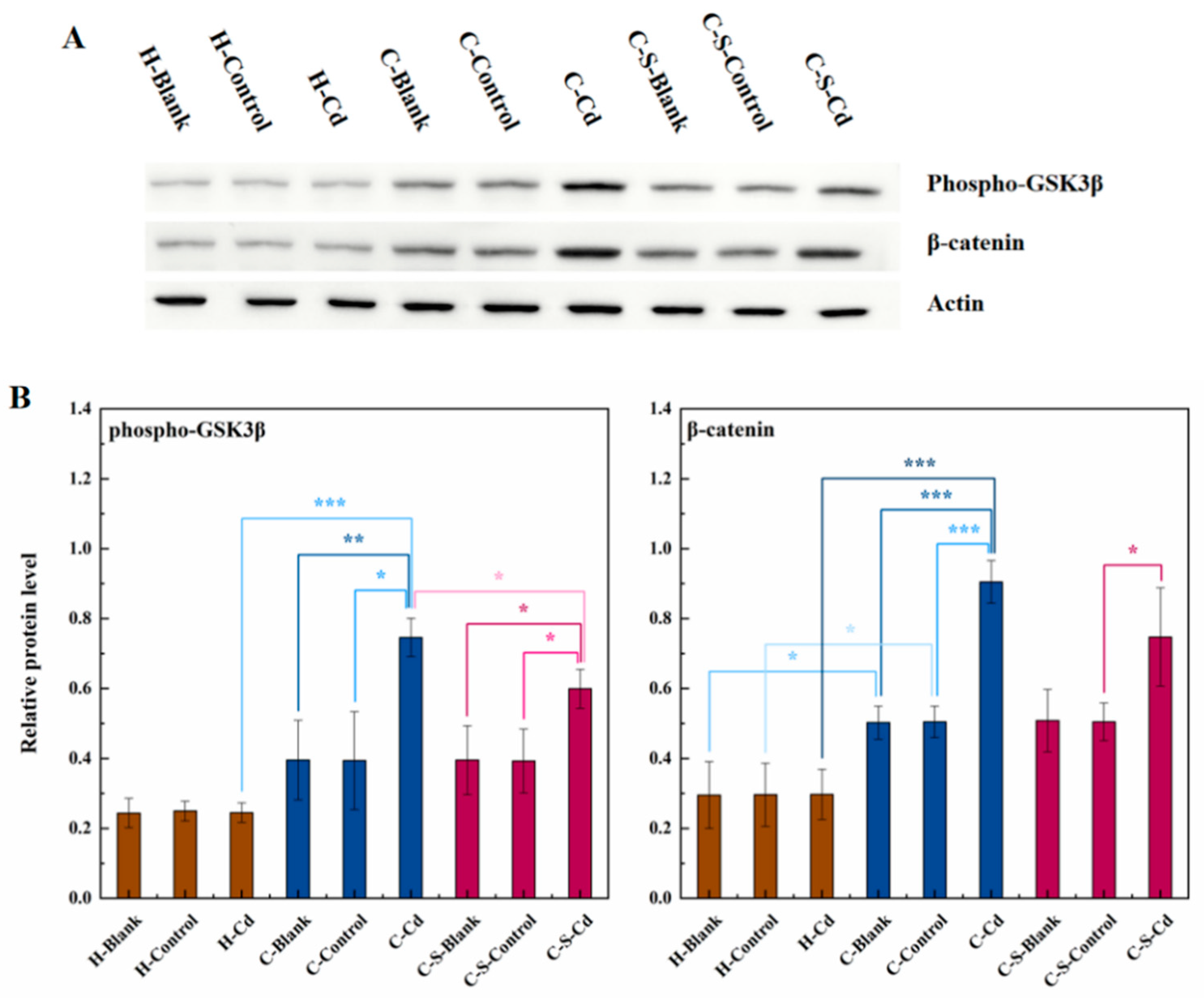
Hence, further investigation was imperative to elucidate the mechanistic influence of atmospheric Cd and intestinal stents on CC cell proliferation. In comparison to healthy rats, the expression levels of β-catenin and phospho-GSK3β were notably elevated within colon tumors of CC rats. Anomalous Wnt protein secretion instigated aberrant activation of the Wnt/β-catenin signaling pathway, prompting GSK3β phosphorylation. Consequently, this inhibition of β-catenin ubiquitination led to its intracellular accumulation, thereby fostering the proliferation of CC cells (Zhang et al., 2023). The administration of Cd-O2/N2 atomized gas further augmented the expression of β-catenin and phospho-GSK3β. Conversely, the implantation of intestinal stents suppressed the expression of phospho-GSK3β. Remarkably, intestinal stents mitigated GSK3β phosphorylation, diminishing the intracellular accumulation of β-catenin and consequently decelerating CC cell proliferation.
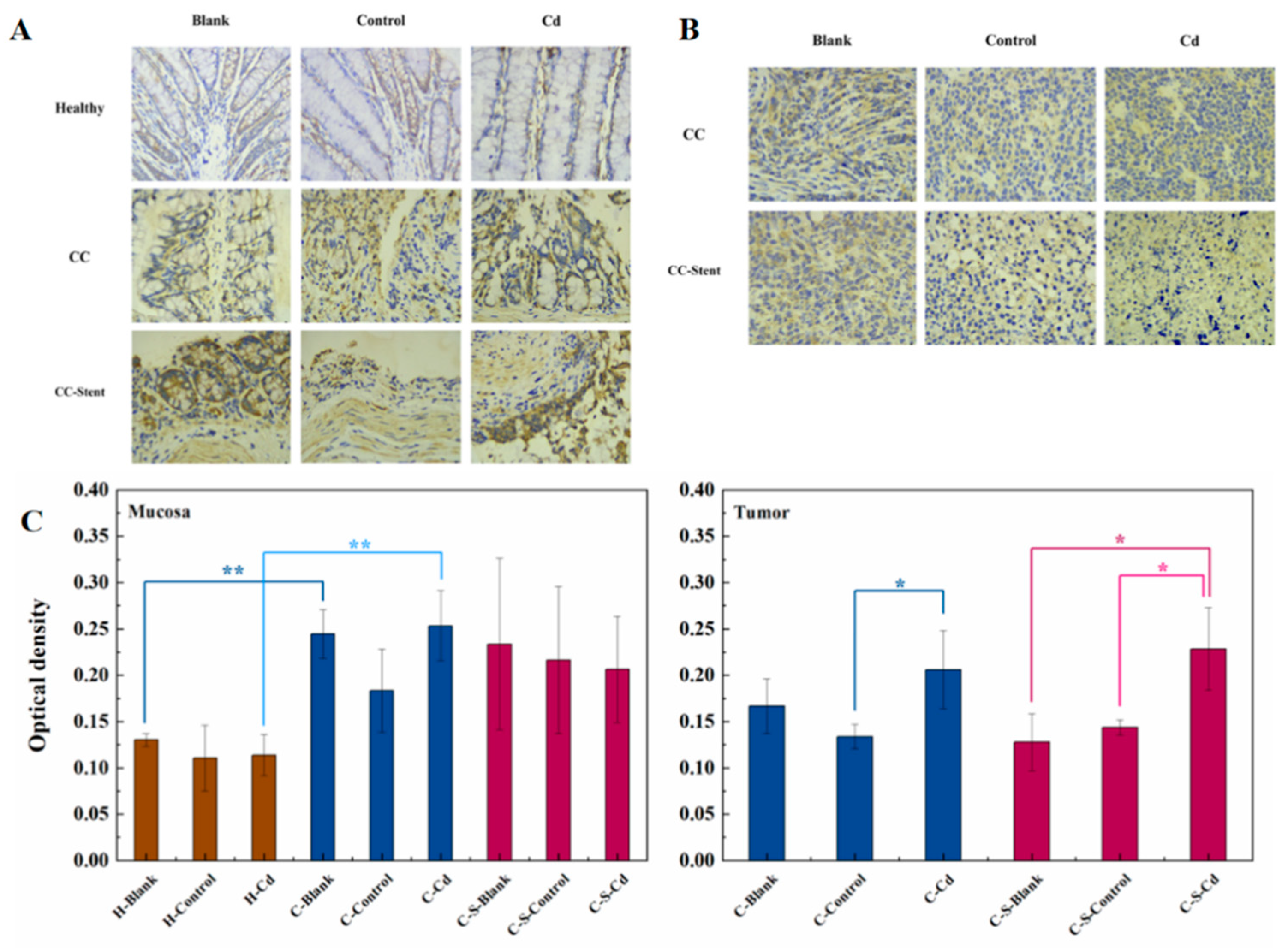
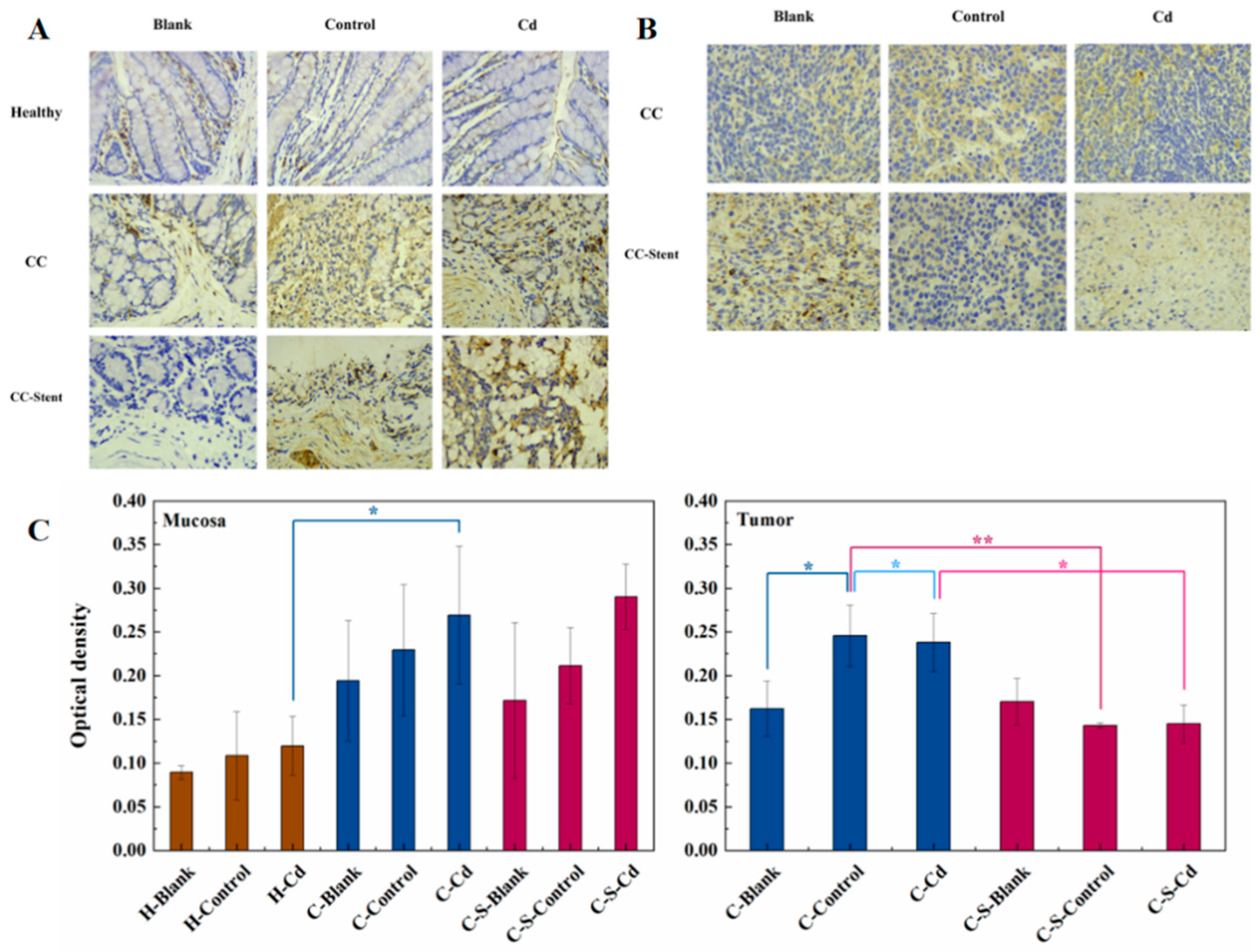
The assessment of colon tumor invasion and metastatic potential involved the examination and analysis of MMP-2 expression. MMP-2 primarily participates in tissue remodeling, cellular migration, and angiogenesis (Santos et al., 2011). In comparison to healthy rats, heightened MMP-2 expression was observed in the colon mucosa of CC rats, as depicted in Figure 4. This escalated MMP-2 expression in the colon mucosa of CC rats corresponded to an increase in tissue protein degradation activity, thereby contributing to the progression and invasiveness of colon tumors. Notably, administration of Cd-O2/N2 atomized gas amplified MMP-2 expression in colon tumors of CC mice relative to the blank and control groups. This Cd-induced elevation in MMP-2 expression facilitated colon tumor restructuring and the migration of tumor cells.
Figure 2.
Changes in body weight of mice (g). Data were means ± SEM.
Figure 2.
Changes in body weight of mice (g). Data were means ± SEM.
Figure 3.
In vivo imaging was performed at the 3rd and 8th weeks after the start of the experiment (before sampling) to observe the (A) in situ tumor formation of the colon and (B) the changes in fluorescence intensity. (B) Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01, ***P<0.001.
Figure 3.
In vivo imaging was performed at the 3rd and 8th weeks after the start of the experiment (before sampling) to observe the (A) in situ tumor formation of the colon and (B) the changes in fluorescence intensity. (B) Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01, ***P<0.001.
Figure 4.
The expression of phospho-GSK3β and β-catenin in colon tumor of mice in each group was detected. (A) Expression of phospho-GSK3β and β-catenin in colon tumor was determined by immunoblotting. Expression of actin served as an internal control. (B) Relative levels of β-catenin and phospho-GSK3β in colon tumor. Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01, ***P<0.001.
Figure 4.
The expression of phospho-GSK3β and β-catenin in colon tumor of mice in each group was detected. (A) Expression of phospho-GSK3β and β-catenin in colon tumor was determined by immunoblotting. Expression of actin served as an internal control. (B) Relative levels of β-catenin and phospho-GSK3β in colon tumor. Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01, ***P<0.001.
Figure 4.
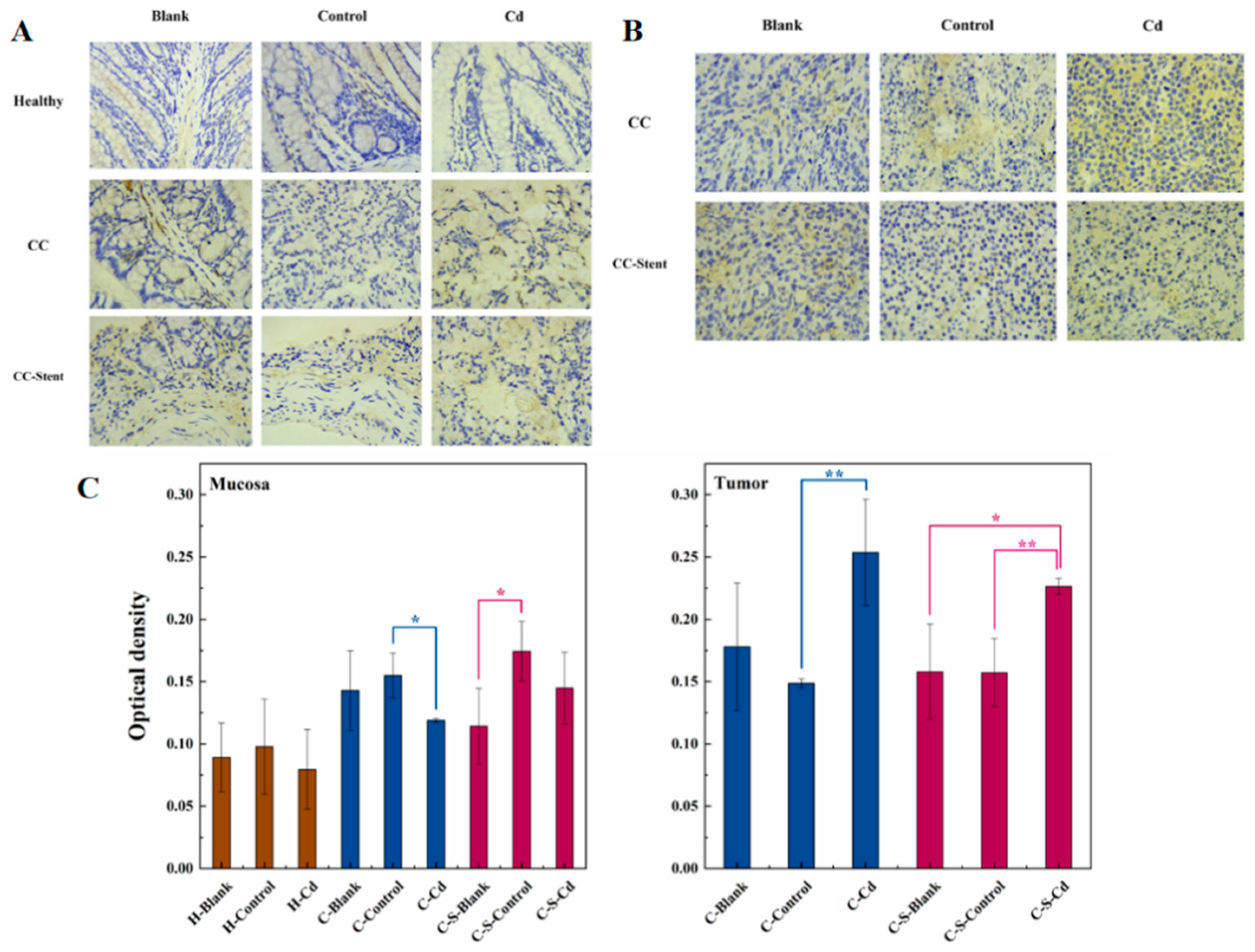
The expression of MMP-2 in colon tumors and mucosa of mice in each group was detected. (A) Immunohistochemical staining of MMP-2 in colonic mucosa in indicated treatments. Magnification was 400×. (B) Immunohistochemical staining of MMP-2 in colonic tumors in indicated treatments. Magnification was 400×. (C) Optical density was used to represent the expression of MMP-2 in colon mucosa and tumors. Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01.
Figure 4.
The expression of MMP-2 in colon tumors and mucosa of mice in each group was detected. (A) Immunohistochemical staining of MMP-2 in colonic mucosa in indicated treatments. Magnification was 400×. (B) Immunohistochemical staining of MMP-2 in colonic tumors in indicated treatments. Magnification was 400×. (C) Optical density was used to represent the expression of MMP-2 in colon mucosa and tumors. Data were means ± SEM of 5–6 mice. *P < 0.05, **P<0.01.
Oxidative stress
Carcinogenic metals were renowned for their capacity to induce ROS
(Wang et al., 2016). As depicted in
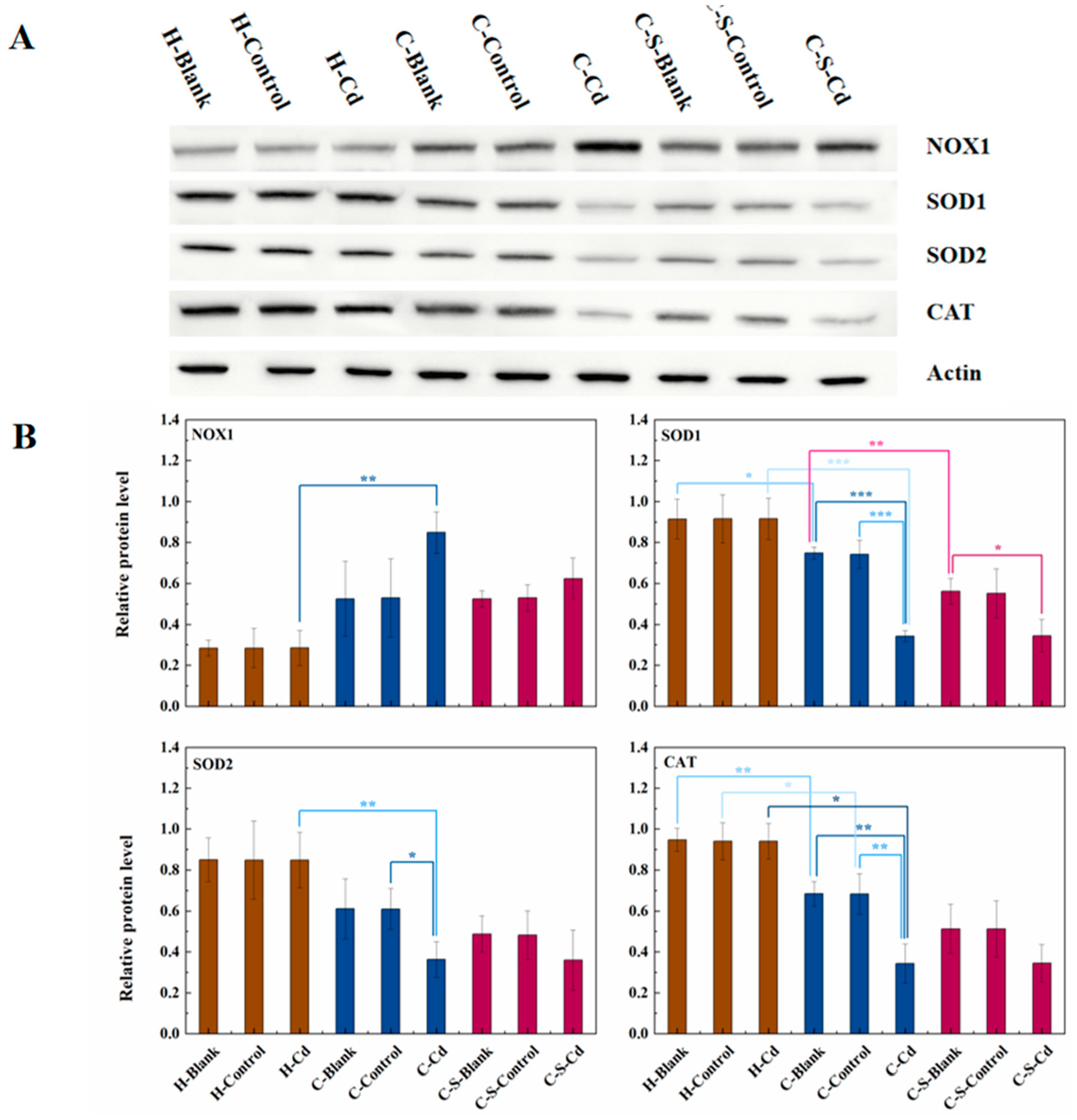
Figure 5, heightened NOX1 expression levels were evident in the colon tumors of CC rats when compared to their healthy counterparts. This elevation signifies increased NOX1 enzyme activity, leading to augmented ROS production. Additionally, the expression levels of SOD1, SOD2, and CAT decreased in these tumors. This decline indicates reduced activity of these enzymes responsible for ROS elimination, consequently fostering the gradual accumulation of ROS within CC cells. Administration of Cd-O
2/N
2 atomized gas further suppressed the expression of SOD1, SOD2, and CAT in colon tumors of CC rats. Conversely, the implantation of intestinal stents inhibited SOD1 expression in colon tumors of CC rats.
ROS generated by NOX played a pivotal role in governing cell proliferation within the colon, exerting control through the regulation of the Wnt/β-catenin signaling pathway (Coant et al., 2010b; Kajla et al., 2012; Wang et al., 2012; Zhang et al., 2011). Notably, alterations in the expression levels of NOX1, β-catenin, and phospho-GSK3β followed a similar pattern. Synthesizing insights from these references, an initial deduction can be drawn: Cadmium amplifies ROS production by enhancing NOX1 activity within CC cells. Subsequently, ROS promoted anomalous Wnt protein secretion, thereby instigating abnormal activation of the Wnt/β-catenin signaling pathway. This sequence of events ultimately fostered the proliferation of CC cells.
8-OHdG served as a commonly utilized biomarker to detect oxidative DNA damage resulting from oxidative stress
(Bláhová et al., 2023). Relative to healthy rats, elevated expression levels of 8-OHdG were observed within the colon mucosa of CC-afflicted mice, as illustrated in
Figure 6. This surge in 8-OHdG points to oxidative DNA damage induced by oxidative stress, a contributing factor to the dissemination of CC cells. The administration of Cd-O
2/N
2 atomized gas further amplified the expression of 8-OHdG in the colon mucosa.
Inflammation
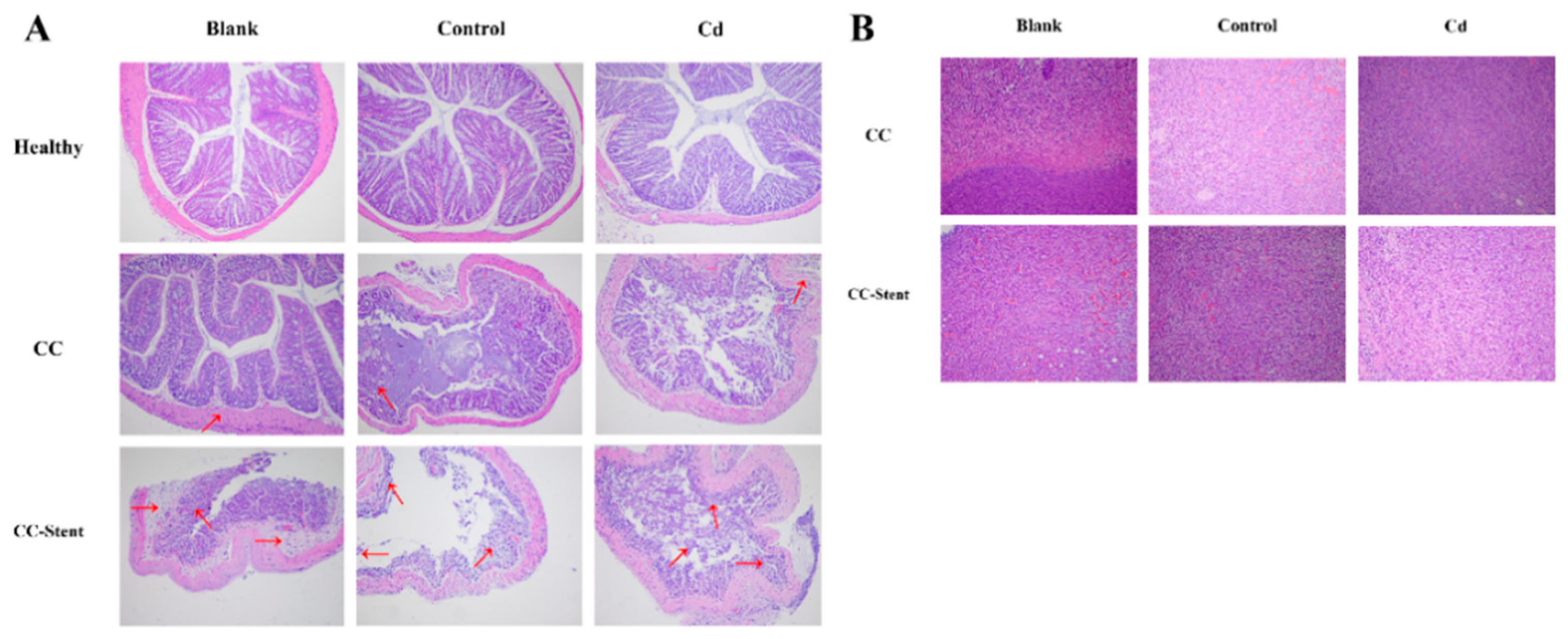
As depicted in
Figure 7, the colon mucosa of healthy rats exhibited a well-maintained structure characterized by neatly arranged glands and an absence of inflammatory cell infiltration. Conversely, the colon mucosa of CC mice displayed evident damage, with disordered glandular arrangement and noticeable infiltration of inflammatory cells. Remarkably, the mucosa of CC rats implanted with intestinal stents exhibited severe damage, featuring disorganized glands and pronounced inflammatory cell infiltration. Notably, tumors observed across all groups of rats exhibited irregular nuclear morphology and variable sizes. Cell arrangement appeared disordered, lacking distinct tissue structure, and showcasing irregular intercellular spaces. However, no significant differences were noted between the groups in this regard.
Inflammation exhibits a close association with the onset and progression of cancer. MMP-9 primarily participates in the activation and migration of inflammatory cells
(Lee et al., 2020). Relative to healthy rats, heightened MMP-9 expression was observed within the colon mucosa of CC mice, as depicted in
Figure 8. This increased MMP-9 expression in the colon mucosa of CC mice correlated with an augmented inflammatory response, contributing to the proliferation and metastasis of tumor cells. The administration of Cd-O
2/N
2 atomized gas suppressed the expression of MMP-9 in colon tumors of CC rats. Cd inhibited the activation and migration of inflammatory cells within colon tumors. Similarly, the implantation of intestinal stents restrained MMP-9 expression in colon tumors of CC rats. Furthermore, the presence of these stents impeded the activation and migration of inflammatory cells within colon tumors. Variances were noted in MMP-9 expression among the colon tumors of CC rats in the blank and control groups. The disparity between the actual and simulated environments might lead to substantial discrepancies in MMP-9 expression within colon tumors.
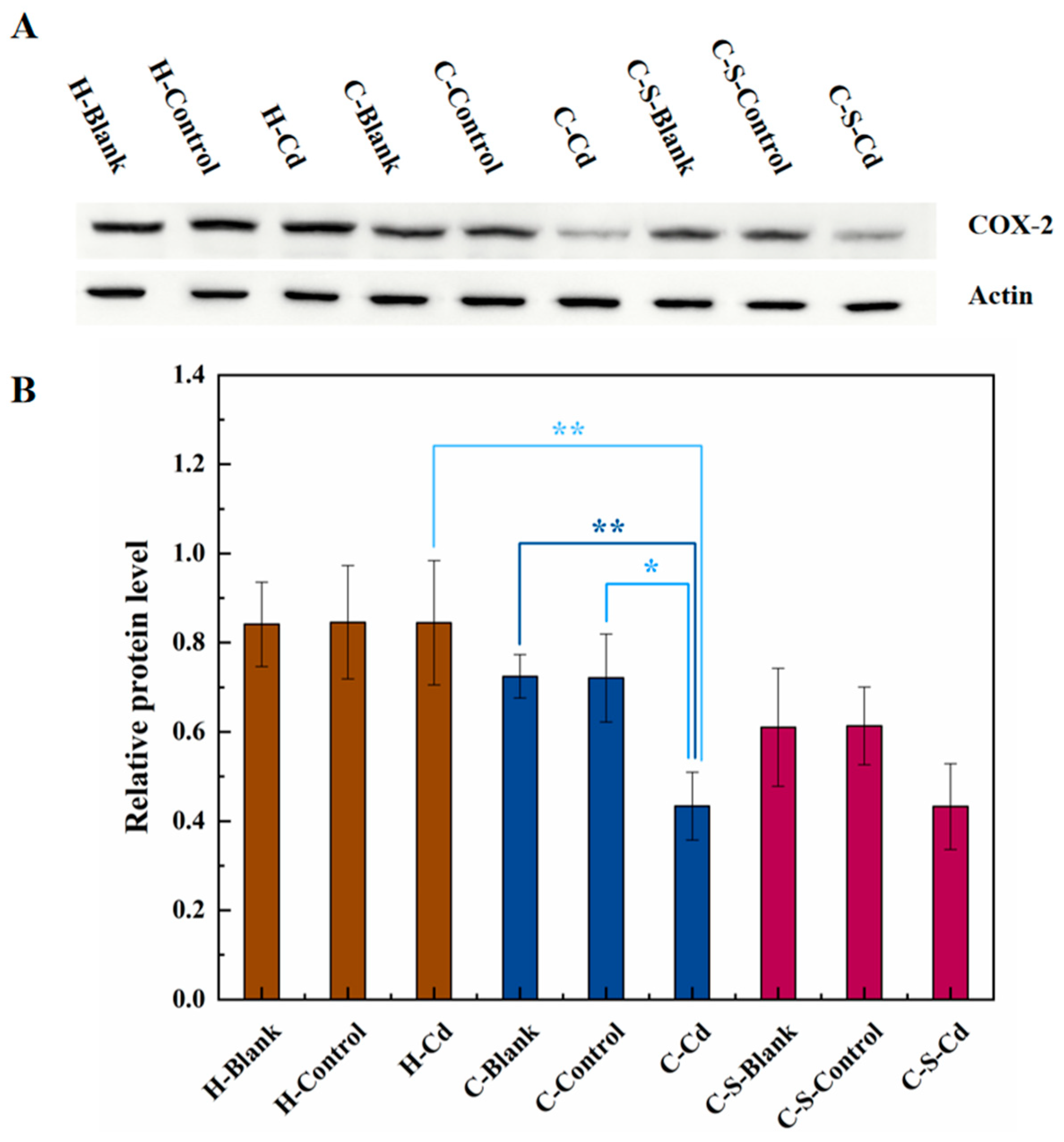
Inflammatory stimuli often trigger an upsurge in intracellular COX-2 expression
(Ramadhan, 2020). Depicted in
Figure 9, the expression of COX-2 was notably suppressed within colon tumors of CC mice compared to healthy counterparts. The administration of Cd-O
2/N
2 atomized gas further inhibited COX-2 expression within colon tumors.
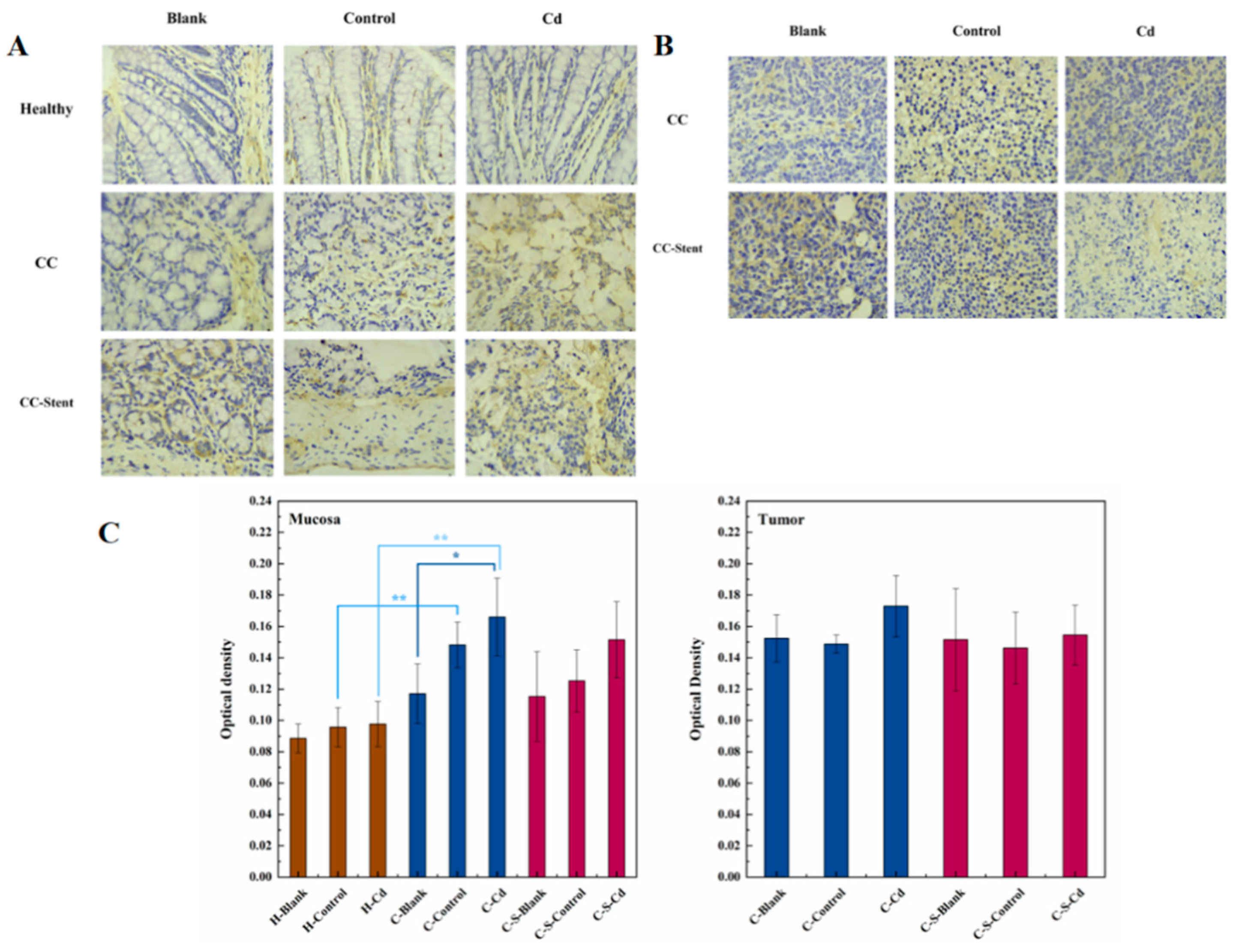
The expression level of Iba1 serves as a reflection of the cellular inflammatory response intensity (Waller et al., 2021). Notably, the administration of Cd-O2/N2 atomized gas led to a reduction in Iba1 expression within the colon mucosa. Cd induced oxidative stress, prompting the generation of oxygen free radicals within colon cells. This oxidative stress negatively impacted immune cells, curbing the production of inflammatory cytokines and subsequently dampening the inflammatory response. Intriguingly, the same administration of Cd-O2/N2 atomized gas prompted an elevation in Iba1 expression within colon tumors. Here, Cd instigated an upsurge in the inflammatory response of colon tumor cells, thereby fostering the proliferation of these cells. Significant variance was observed in the expression of Iba1 within the colonic mucosa of the blank and control groups following stent implantation. This discrepancy suggests that the difference between the actual and simulated environments might engender considerable discrepancies in the expression of Iba1 within the colonic mucosa.
Figure 10.
The expression of Iba1 in colon tumors and mucosa of mice in each group was detected. (A) Immunohistochemical staining of Iba1 in colonic mucosa in indicated treatments. Magnification was 400×. (B) Immunohistochemical staining of Iba1 in colonic tumors in indicated treatments. Magnification was 400×. (C) Optical density was used to represent the expression of Iba1 in colon mucosa and tumor. Data are means ± SEM of 5–6 mice. *P < 0.05, **P<0.01.
Figure 10.
The expression of Iba1 in colon tumors and mucosa of mice in each group was detected. (A) Immunohistochemical staining of Iba1 in colonic mucosa in indicated treatments. Magnification was 400×. (B) Immunohistochemical staining of Iba1 in colonic tumors in indicated treatments. Magnification was 400×. (C) Optical density was used to represent the expression of Iba1 in colon mucosa and tumor. Data are means ± SEM of 5–6 mice. *P < 0.05, **P<0.01.
4. Discussion
In this study, rat models of CC were employed to scrutinize the implications of heavy metal Cd exposure in the atmospheric environment on CC progression and its impact on the invasiveness of intestinal stents within the cancerous colon. An abnormal secretion of Wnt protein within CC cells triggered an unconventional activation of the Wnt/β-catenin signaling pathway, culminating in the phosphorylation of GSK3β. Consequently, this cascade hindered the ubiquitination process of β-catenin, resulting in its intracellular accumulation and consequent promotion of CC cell proliferation. Wnt ligands constitute a diverse and highly conserved group of secreted proteins pivotal for normal cellular development (Yu et al., 2023). Their influence extends across various cellular processes, encompassing cell proliferation, stem cell self-renewal, determination of cell fate, establishment of cell polarity, and orchestration of convergent extension behavior during cell migration (Braunschweig et al., 2015; Qiu et al., 2011; Yadav et al., 2024). Each of these orchestrated consequences steered by Wnt signaling is crucial for maintaining normal intestinal development (Zhang et al., 2023). Upon secretion, Wnt molecules engage specific receptors on the cell surface, initiating a series of phosphorylation and dephosphorylation events in downstream proteins. This cascade ultimately leads to the accumulation of β-catenin. This multifunctional protein interacts with E-cadherin at cellular junctions and actively participates in the formation of adhesive zones. When present in its unbound state, β-catenin can translocate into the cell nucleus, influencing gene expression. However, aberrant expression or activation of β-catenin has been linked to tumorigenesis (Li et al., 2023).
The proliferation demands imposed on CC cells led to increased energy consumption, contributing to an initial decline in body weight among CC mice. Subsequently, in the later stages of the trial, an observable rise in tissue protein degradation within the colon mucosa of CC mice significantly contributed to the invasive nature of colon tumors. Additionally, Cd amplified CC cell proliferation by further stimulating GSK3β phosphorylation and consequent intracellular β-catenin accumulation. This finding aligns with the observations made by Wei et al. (2017), who noted Cd's role in advancing malignant tumors by inhibiting GSK3β activity while concurrently enhancing β-catenin expression. Similarly, Chakraborty et al. (2010) suggested that Cd-induced alterations in cancerous conditions might plausibly occur through Wnt signaling pathways. This enhancement didn't markedly affect the body weight of the CC rats, likely due to the overshadowing effect of increased tumor tissue weight in the later stages of the experiment. Additionally, in the latter phase of the study, Cd notably facilitated colon tumor remodeling and instigated tumor cell migration. This corroborates with Wang et al. (2019) findings, where Cd exposure induced MMP-2 mRNA expression, consequently fostering migration and invasion capabilities in human breast cancer cells. Implantation of intestinal stents relieved GSK3β phosphorylation, reducing intracellular β-catenin accumulation, thereby slowing down CC cell proliferation. The stent takes up part of the intestinal space and prevents colon tumors from expanding. And intestinal stents can affect the blood supply and reduce the blood supply to colon tumors. Colon tumor growth requires large amounts of oxygen and nutrients, and if the blood supply is reduced, the reproductive capacity of CC cells will be inhibited (Feng et al., 2022). This aligns with Matsuda et al. (2019) observations, suggesting that mechanical compression induced by intestinal stents could hinder cancer cell proliferation in cases of malignant large bowel obstruction. However, the implantation of intestinal stents induced gastrointestinal discomfort in CC rats, resulting in reduced food intake and weight loss.
ROS generated by oxidative stress in the colonic mucosa of CC mice induces oxidative DNA damage, significantly contributing to the proliferation and spread of CC cells. This aligns with the conclusions drawn by Nilsson et al., (2020). Additionally, heightened NOX1 activity within CC cells generates increased ROS levels, prompting aberrant activation of the Wnt/β-catenin signaling pathway by disrupting Wnt protein secretion. Consequently, this abnormal activation fosters the uncontrolled proliferation of CC cells. Cd exacerbates ROS production by further amplifying NOX1 activity in CC cells, a phenomenon also observed by Tyagi et al. (2023) and Lian et al. (2015). Implantation of intestinal stents inhibited the expression of SOD1 in colon tumors of CC mice. However, the impact on NOX1, SOD2, and CAT enzyme activities was not evident. SOD, categorized into three groups (SOD1, SOD2, SOD3), plays crucial roles in maintaining intracellular ROS homeostasis (Zelko et al., 2002). Specifically, SOD1 facilitates the dismutation of O2- into H2O2, a stable ROS messenger pivotal for regulating oxidative stress and supporting oncogene-driven cancer cell proliferation (Picazo et al., 2023). SOD2 scavenges superoxide radicals formed in the respiratory electron transport chain, impacting cell cycle signaling and cancer progression (Miar et al., 2015). CAT acts as an enzymatic scavenger, catalyzing the breakdown of H2O2 into oxygen and water, shielding cells from H2O2 toxicity and serving as a key element in the biological defense system (Ighodaro et al., 2018). Consequently, the observed lack of substantial increase in intracellular ROS following intestinal stent implantation suggests a less pronounced impact on the Wnt/β-catenin signaling pathway. This effect may even contribute to slowing down the dysregulation of the Wnt/β-catenin signaling pathway.
CC rats exhibited colon mucosal damage, disorder in glandular arrangement, and infiltration of inflammatory cells. However, upon the implantation of intestinal stents, severe damage to the colonic mucosa and noticeable infiltration of inflammatory cells were observed. Cd exhibited a dual impact on immune response within the colon mucosa of CC rats. It inhibited the expression of Iba1, likely due to its induction of oxidative stress, triggering the generation of oxygen free radicals in colon cells. This unfavorable effect on immune cells resulted in reduced production of inflammatory cytokines, thereby mitigating the inflammatory response (Shah et al., 2024). Conversely, Cd promoted the expression of Iba1 in colon tumors of CC rats. This indicated that Cd heightened the inflammatory response in CC cells, consequently fostering the proliferation of colon tumor cells. It stimulated the activity of certain immune cells, notably macrophages, triggering inflammatory reactions and elevating the expression levels of immune markers such as Iba1. A similar study by Yang et al. also reported increased gliosis, indicated by a rise in the number of Iba1-positive cells, following Cd poisoning. Moreover, both Cd and intestinal stents inhibited the expression of MMP-9 and COX-2 in colon tumors. This inhibition might be linked to Cd's ability to suppress the activity of inflammation-related cells, possibly including those responsible for producing MMP-9 and COX-2 within colon tumors. However, this conclusion necessitates further validation and investigation.
5. Conclusions
The presence of the heavy metal Cd within the atmospheric milieu acts as a catalyst in the progression of tumorigenesis within murine models of CC. The implementation of intestinal stents demonstrates a mitigating effect on tumor incidence within the CC murine model. Significantly, aberrant activation of the ROS-mediated Wnt/β-catenin signaling pathway emerges as a pivotal mechanism contributing to the facilitation of this tumorigenic promotion.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Shuai Zhang: Conceptualization, Validation, Investigation, Resources, Supervision, Project administration. Ruikang Li: Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing-original draft, Writing-review & editing, Visualization, Supervision. Jing Xu: Resources, Project administration. Yan Liu: Resources, Supervision. Yanjie Zhang: Resources, Supervision.
Acknowledgments
This research was supported by Study on the influence of stents on the invasiveness of rectal cancer, Project of Tianjin People's Hospital (2020YJ001). The authors are grateful to the anonymous reviewers for their insightful comments.
Conflicts of Interest
The authors declare there is no financial interests/personal relationships which may be considered as potential competing interests.
References
- Bláhová, L., Janoš, T., Mustieles, V., Rodríguez-Carrillo, A., Fernández, M. F., & Bláha, L. (2023). Rapid extraction and analysis of oxidative stress and DNA damage biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in urine: Application to a study with pregnant women. International Journal of Hygiene and Environmental Health, 250. [CrossRef]
- Braunschweig, L., Meyer, A. K., Wagenführ, L., & Storch, A. (2015). Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Molecular and Cellular Neuroscience, 67, 84–92. [CrossRef]
- Breyer, R. M., Bagdassarian, C. K., Scott, a, & Breyer, M. D. (2001). P ROSTANOID R ECEPTORS : Subtypes. Prostaglandins, 41, 661–690.
- Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 6(9), e04691. [CrossRef]
- Chakraborty, P. K., Scharner, B., Jurasovic, J., Messner, B., Bernhard, D., & Thévenod, F. (2010). Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicology Letters, 198(1), 69–76. [CrossRef]
- Choi, S. J. in, Min, J. W. on, Yun, J. M. in, Ahn, H. S. hin, Han, D. J. ae, Lee, H. J. eong, & Kim, Y. O. k. (2015). Portal vein thrombosis with sepsis caused by inflammation at colonic stent insertion site. The Korean Journal of Gastroenterology = Taehan Sohwagi Hakhoe Chi, 65(5), 316–320. [CrossRef]
- Chou, W. C., Jie, C., Kenedy, A. A., Jones, R. J., Trush, M. A., & Dang, C. V. (2004). Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4578–4583. [CrossRef]
- Coant, N., Ben Mkaddem, S., Pedruzzi, E., Guichard, C., Tréton, X., Ducroc, R., Freund, J.-N., Cazals-Hatem, D., Bouhnik, Y., Woerther, P.-L., Skurnik, D., Grodet, A., Fay, M., Biard, D., Lesuffleur, T., Deffert, C., Moreau, R., Groyer, A., Krause, K.-H., … Ogier-Denis, E. (2010a). NADPH Oxidase 1 Modulates WNT and NOTCH1 Signaling To Control the Fate of Proliferative Progenitor Cells in the Colon. Molecular and Cellular Biology, 30(11), 2636–2650. [CrossRef]
- Coant, N., Ben Mkaddem, S., Pedruzzi, E., Guichard, C., Tréton, X., Ducroc, R., Freund, J.-N., Cazals-Hatem, D., Bouhnik, Y., Woerther, P.-L., Skurnik, D., Grodet, A., Fay, M., Biard, D., Lesuffleur, T., Deffert, C., Moreau, R., Groyer, A., Krause, K.-H., … Ogier-Denis, E. (2010b). NADPH Oxidase 1 Modulates WNT and NOTCH1 Signaling To Control the Fate of Proliferative Progenitor Cells in the Colon. Molecular and Cellular Biology, 30(11), 2636–2650. [CrossRef]
- DU, J. (2016). Association between shortage of energy supply and nuclear gene mutations leading to carcinomatous transformation. Molecular and Clinical Oncology, 4(1), 11–12. [CrossRef]
- Feng, Y., Chen, Y., Chen, Y., He, X., Khan, Y., Hu, H., Lan, P., Li, Y., Wang, X., Li, G., & Kaplan, D. (2022). Intestinal stents: Structure, functionalization and advanced engineering innovation. In Biomaterials Advances (Vol. 137). Elsevier Ltd. [CrossRef]
- Ighodaro, O. M., & Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine, 54(4), 287–293. [CrossRef]
- Jung, Y. S., & Park, J. Il. (2020). Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Experimental and Molecular Medicine, 52(2), 183–191. [CrossRef]
- Kajla, S., Mondol, A. S., Nagasawa, A., Zhang, Y., Kato, M., Matsuno, K., Yabe-Nishimura, C., & Kamata, T. (2012). A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. The FASEB Journal, 26(5), 2049–2059. [CrossRef]
- Ladelfa, M. F., Toledo, M. F., Laiseca, J. E., & Monte, M. (2011). Interaction of p53 with tumor suppressive and oncogenic signaling pathways to control cellular reactive oxygen species production. Antioxidants and Redox Signaling, 15(6), 1749–1761. [CrossRef]
- Lee, T. H., Chen, J. L., Liu, P. S., Tsai, M. M., Tsai, M. M., Wang, S. J., Hsieh, H. L., & Hsieh, H. L. (2020). Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. Journal of Neuroinflammation, 17(1). [CrossRef]
- Li, C., Cheng, B., Yang, X., Tong, G., Wang, F., Li, M., Wang, X., & Wang, S. (2023). SOX8 promotes tumor growth and metastasis through FZD6-dependent Wnt/β-catenin signaling in colorectal carcinoma. Heliyon, 9(12). [CrossRef]
- Li, Y., Bavarva, J. H., Wang, Z., Guo, J., Qian, C., Thibodeau, S. N., Golemis, E. A., & Liu, W. (2011). HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene, 30(23), 2633–2643. [CrossRef]
- Lian, S., Xia, Y., Khoi, P. N., Ung, T. T., Yoon, H. J., Kim, N. H., Kim, K. K., & Jung, Y. Do. (2015). Cadmium induces matrix metalloproteinase-9 expression via ROS-dependent EGFR, NF-κB, and AP-1 pathways in human endothelial cells. Toxicology, 338, 104–116. [CrossRef]
- Lin, H. C., Hao, W. M., & Chu, P. H. (2021). Cadmium and cardiovascular disease: An overview of pathophysiology, epidemiology, therapy, and predictive value. Revista Portuguesa de Cardiologia, 40(8), 611–617. [CrossRef]
- Lin, X., Peng, L., Xu, X., Chen, Y., Zhang, Y., & Huo, X. (2018). Connecting gastrointestinal cancer risk to cadmium and lead exposure in the Chaoshan population of Southeast China. Environmental Science and Pollution Research, 25(18), 17611–17619. [CrossRef]
- Lu, J., Zhou, Z., Zheng, J., Zhang, Z., Lu, R., Liu, H., Shi, H., & Tu, Z. (2015). 2D-DIGE and MALDI TOF/TOF MS analysis reveal that small GTPase signaling pathways may play an important role in cadmium-induced colon cell malignant transformation. Toxicology and Applied Pharmacology, 288(1), 106–113. [CrossRef]
- Mahmood, M. H. R., Qayyum, M. A., Yaseen, F., Farooq, T., Farooq, Z., Yaseen, M., Irfan, A., Muddassir, K., Zafar, M. N., Qamar, M. T., Abbasi, A. M., & Liu, H. Y. (2022). Multivariate Investigation of Toxic and Essential Metals in the Serum from Various Types and Stages of Colorectal Cancer Patients. Biological Trace Element Research, 200(1), 31–48. [CrossRef]
- Mahmoud, A. S., Umair, A., Azzeghaiby, S. N., Hussain, F., Hanouneh, S., & Tarakji, B. (2014). Expression of Cyclooxygenase-2 ( COX-2 ) in Colorectal A d e n o c a rc i n o m a : a n I m m u n o h i s t o c h e m i c a l a n d Histopathological Study. 15, 6787–6790.
- Maruthachalam, K., Lash, G. E., Shenton, B. K., & Horgan, A. F. (2007). Tumour cell dissemination following endoscopic stent insertion. British Journal of Surgery, 94(9), 1151–1154. [CrossRef]
- Matsuda, A., Miyashita, M., Matsumoto, S., Sakurazawa, N., Kawano, Y., Yamahatsu, K., Sekiguchi, K., Yamada, M., Hatori, T., & Yoshida, H. (2019). Colonic stent-induced mechanical compression may suppress cancer cell proliferation in malignant large bowel obstruction. Surgical Endoscopy, 33(4), 1290–1297. [CrossRef]
- Miar, A., Hevia, D., Muñoz-Cimadevilla, H., Astudillo, A., Velasco, J., Sainz, R. M., & Mayo, J. C. (2015). Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radical Biology and Medicine, 85, 45–55. [CrossRef]
- Naji, S., Issa, K., Eid, A., Iratni, R., & Eid, A. H. (2019). Cadmium induces migration of colon cancer cells: Roles of reactive oxygen species, p38 and cyclooxygenase-2. Cellular Physiology and Biochemistry, 52(6), 1517–1534. [CrossRef]
- Nawrot, T. S., Martens, D. S., Hara, A., Plusquin, M., Vangronsveld, J., Roels, H. A., & Staessen, J. A. (2015). Association of total cancer and lung cancer with environmental exposure to cadmium: the meta-analytical evidence. Cancer Causes and Control, 26(9), 1281–1288. [CrossRef]
- Nilsson, R., & Liu, N. A. (2020). Nuclear DNA damages generated by reactive oxygen molecules (ROS) under oxidative stress and their relevance to human cancers, including ionizing radiation-induced neoplasia part I: Physical, chemical and molecular biology aspects. In Radiation Medicine and Protection (Vol. 1, Issue 3, pp. 140–152). KeAi Communications Co. [CrossRef]
- Nishijo, M., Nakagawa, H., Suwazono, Y., Nogawa, K., Sakurai, M., Ishizaki, M., & Kido, T. (2018). Cancer mortality in residents of the cadmium-polluted Jinzu River basin in Toyama, Japan. Toxics, 6(2). [CrossRef]
- Peng, L., Zhou, Y., Wang, Y., Mou, H., & Zhao, Q. (2013). Prognostic Significance of COX-2 Immunohistochemical Expression in Colorectal Cancer: A Meta-Analysis of the Literature. PLoS ONE, 8(3), 1–9. [CrossRef]
- Picazo, C., Padilla, C. A., McDonagh, B., Matallana, E., Bárcena, J. A., & Aranda, A. (2023). Regulation of metabolism, stress response, and sod1 activity by cytosolic thioredoxins in yeast depends on growth phase. Advances in Redox Research, 9, 100081. [CrossRef]
- Qiu, W., Chen, L., & Kassem, M. (2011). Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochemical and Biophysical Research Communications, 413(1), 98–104. [CrossRef]
- Rahman, Z., & Singh, V. P. (2019). The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environmental Monitoring and Assessment, 191(7). [CrossRef]
- Ramadhan, U. (2020). In vitro assessment of anti-inflammatory and COX-2 inhibitory action of some medicinal plants. Journal of Biological Research (Italy), 93(1). [CrossRef]
- Rinaldi, L., Barabino, G., Klein, J. P., Bitounis, D., Pourchez, J., Forest, V., Boudard, D., Leclerc, L., Sarry, G., Roblin, X., Cottier, M., & Phelip, J. M. (2015). Metals distribution in colorectal biopsies: New insight on the elemental fingerprint of tumour tissue. Digestive and Liver Disease, 47(7), 602–607. [CrossRef]
- Salama, S. A., Mohamadin, A. M., & Abdel-Bakky, M. S. (2021). Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sciences, 287(September), 120121. [CrossRef]
- Santos, A., Lopes, C., Frias, C., Amorim, I., Vicente, C., Gärtner, F., & Matos, A. de. (2011). Immunohistochemical evaluation of MMP-2 and TIMP-2 in canine mammary tumours: A survival study. Veterinary Journal, 190(3), 396–402. [CrossRef]
- Sarkar, S., Mukherjee, S. K., Roy, K., & Ghosh, P. (2020). Evaluation of Role of Heavy Metals in Causation of Colorectal Cancer. Journal of Evolution of Medical and Dental Sciences, 9(13), 1036–1039. [CrossRef]
- Shah, R., Ibis, B., Kashyap, M., & Boussiotis, V. A. (2024). The role of ROS in tumor infiltrating immune cells and cancer immunotherapy. Metabolism, 151, 155747. [CrossRef]
- Shigeta, K., Baba, H., Yamafuji, K., Kaneda, H., Katsura, H., & Kubochi, K. (2014). Outcomes for Patients with Obstructing Colorectal Cancers Treated with One-Stage Surgery Using Transanal Drainage Tubes. Journal of Gastrointestinal Surgery, 18(8), 1507–1513. [CrossRef]
- Shingu, Y., Hasegawa, H., Sakamoto, E., Komatsu, S., Kurumiya, Y., Norimizu, S., & Taguchi, Y. (2013). Clinical and oncologic safety of laparoscopic surgery for obstructive left colorectal cancer following transanal endoscopic tube decompression. Surgical Endoscopy, 27(9), 3359–3363. [CrossRef]
- Taş, İ., Zhou, R., Park, S. Y., Yang, Y., Gamage, C. D. B., Son, Y. J., Paik, M. J., & Kim, H. (2019). Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicology in Vitro, 59(May), 300–311. [CrossRef]
- Tatsuguchi, A., Kishida, T., Fujimori, S., Tanaka, S., Gudis, K., Shinji, S., Furukawa, K., Tajiri, T., Sugisaki, Y., Fukuda, Y., & Sakamoto, C. (2006). Differential expression of cyclo-oxygenase-2 and nuclear β-catenin in colorectal cancer tissue. Alimentary Pharmacology and Therapeutics Symposium Series, 2(1), 153–159. [CrossRef]
- Tellez-Plaza, M., Jones, M. R., Dominguez-Lucas, A., Guallar, E., & Navas-Acien, A. (2013). Cadmium exposure and clinical cardiovascular disease: A systematic review topical collection on nutrition. Current Atherosclerosis Reports, 15(10). [CrossRef]
- Tian, R., Chen, J., & Niu, R. (2014). The development of low-molecular weight hydrogels for applications in cancer therapy. Nanoscale, 6(7), 3474–3482. [CrossRef]
- Tyagi, A., Chandrasekaran, B., Navin, A. K., Shukla, V., Baby, B. V., Ankem, M. K., & Damodaran, C. (2023). Molecular interplay between NOX1 and autophagy in cadmium-induced prostate carcinogenesis. Free Radical Biology and Medicine, 199, 44–55. [CrossRef]
- Waller, R., Narramore, R., Simpson, J. E., Heath, P. R., Verma, N., Tinsley, M., Barnes, J. R., Haris, H. T., Henderson, F. E., Matthews, F. E., Richardson, C. D., Brayne, C., Ince, P. G., Kalaria, R. N., & Wharton, S. B. (2021). Heterogeneity of cellular inflammatory responses in ageing white matter and relationship to Alzheimer’s and small vessel disease pathologies. Brain Pathology, 31(3). [CrossRef]
- Wang, L., Wise, J. T. F., Zhang, Z., & Shi, X. (2016). Progress and Prospects of Reactive Oxygen Species in Metal Carcinogenesis. In Current Pharmacology Reports (Vol. 2, Issue 4, pp. 178–186). Springer International Publishing. [CrossRef]
- Wang, X., Mandal, A. K., Saito, H., Pulliam, J. F., Lee, E. Y., Ke, Z. J., Lu, J., Ding, S., Li, L., Shelton, B. J., Tucker, T., Evers, B. M., Zhang, Z., & Shi, X. (2012). Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/β-catenin signaling pathway. Toxicology and Applied Pharmacology, 262(1), 11–21. [CrossRef]
- Wang, Y., Shi, L., Li, J., Li, L., Wang, H., & Yang, H. (2019). Long-term cadmium exposure promoted breast cancer cell migration and invasion by up-regulating TGIF. Ecotoxicology and Environmental Safety, 175, 110–117. [CrossRef]
- Wei, Z., & Shaikh, Z. A. (2017). Cadmium stimulates metastasis-associated phenotype in triple-negative breast cancer cells through integrin and β-catenin signaling. Toxicology and Applied Pharmacology, 328, 70–80. [CrossRef]
- Wu, C. L., Huang, L. Y., & Chang, C. L. (2017). Linking arsenite- and cadmium-generated oxidative stress to microsatellite instability in vitro and in vivo. Free Radical Biology and Medicine, 112, 12–23. [CrossRef]
- Yadav, R. P., & Baranwal, S. (2024). Kindlin-2 regulates colonic cancer stem-like cells survival and self-renewal via Wnt/β-catenin mediated pathway. Cellular Signalling, 113, 110953. [CrossRef]
- Yoshida, S., Watabe, H., Isayama, H., Kogure, H., Nakai, Y., Yamamoto, N., Sasaki, T., Kawakubo, K., Hamada, T., Ito, Y., Yashima, Y., Sasahira, N., Hirano, K., Yamaji, Y., Tada, M., Omata, M., & Koike, K. (2013). Feasibility of a new self-expandable metallic stent for patients with malignant colorectal obstruction. Digestive Endoscopy, 25(2), 160–166. [CrossRef]
- Yu, M., Qin, K., Fan, J., Zhao, G., Zhao, P., Zeng, W., Chen, C., Wang, A., Wang, Y., Zhong, J., Zhu, Y., Wagstaff, W., Haydon, R. C., Luu, H. H., Ho, S., Lee, M. J., Strelzow, J., Reid, R. R., & He, T.-C. (2023). The evolving roles of Wnt signaling in stem cell proliferation and differentiation, the development of human diseases, and therapeutic opportunities. Genes & Diseases. [CrossRef]
- Zelko, I. N., Mariani, T. J., & Folz, R. J. (2002). SUPEROXIDE DISMUTASE MULTIGENE FAMILY: A COMPARISON OF THE CuZn-SOD (SOD1), Mn-SOD (SOD2), AND EC-SOD (SOD3) GENE STRUCTURES, EVOLUTION, AND EXPRESSION.
- Zhang, X., Li, C., Wu, Y., & Cui, P. (2023). The research progress of Wnt/β-catenin signaling pathway in colorectal cancer. In Clinics and Research in Hepatology and Gastroenterology (Vol. 47, Issue 3). Elsevier Masson s.r.l. [CrossRef]
- Zhang, Z., Wang, X., Cheng, S., Sun, L., Son, Y. O., Yao, H., Li, W., Budhraja, A., Li, L., Shelton, B. J., Tucker, T., Arnold, S. M., & Shi, X. (2011). Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/β-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicology and Applied Pharmacology, 256(2), 114–121. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).