Submitted:
04 January 2024
Posted:
04 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Preparation and General Characterization
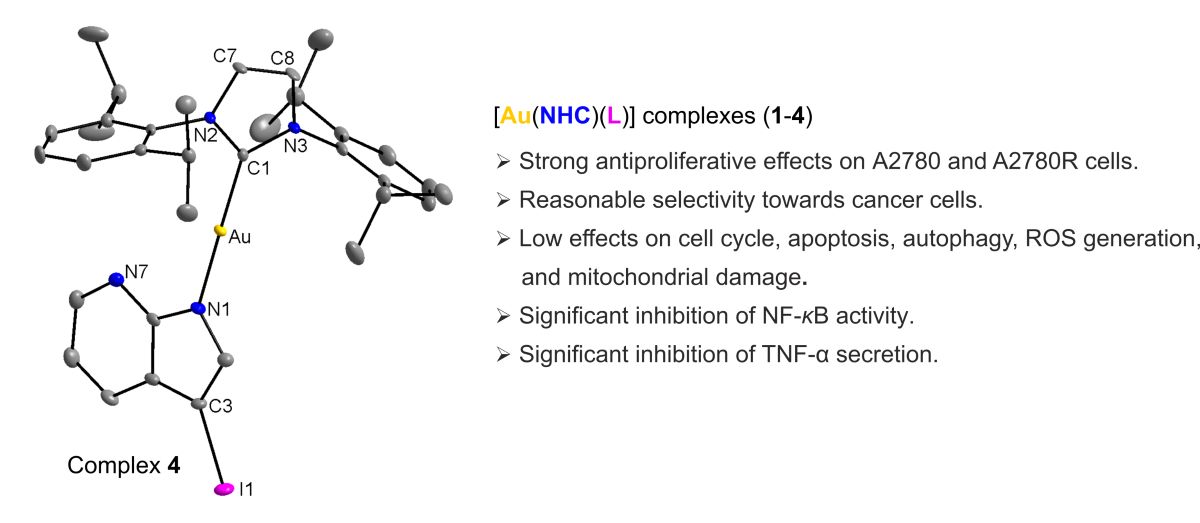
2.2. Single Crystal X-ray Analysis
2.3. In Vitro Cytotoxicity Studies
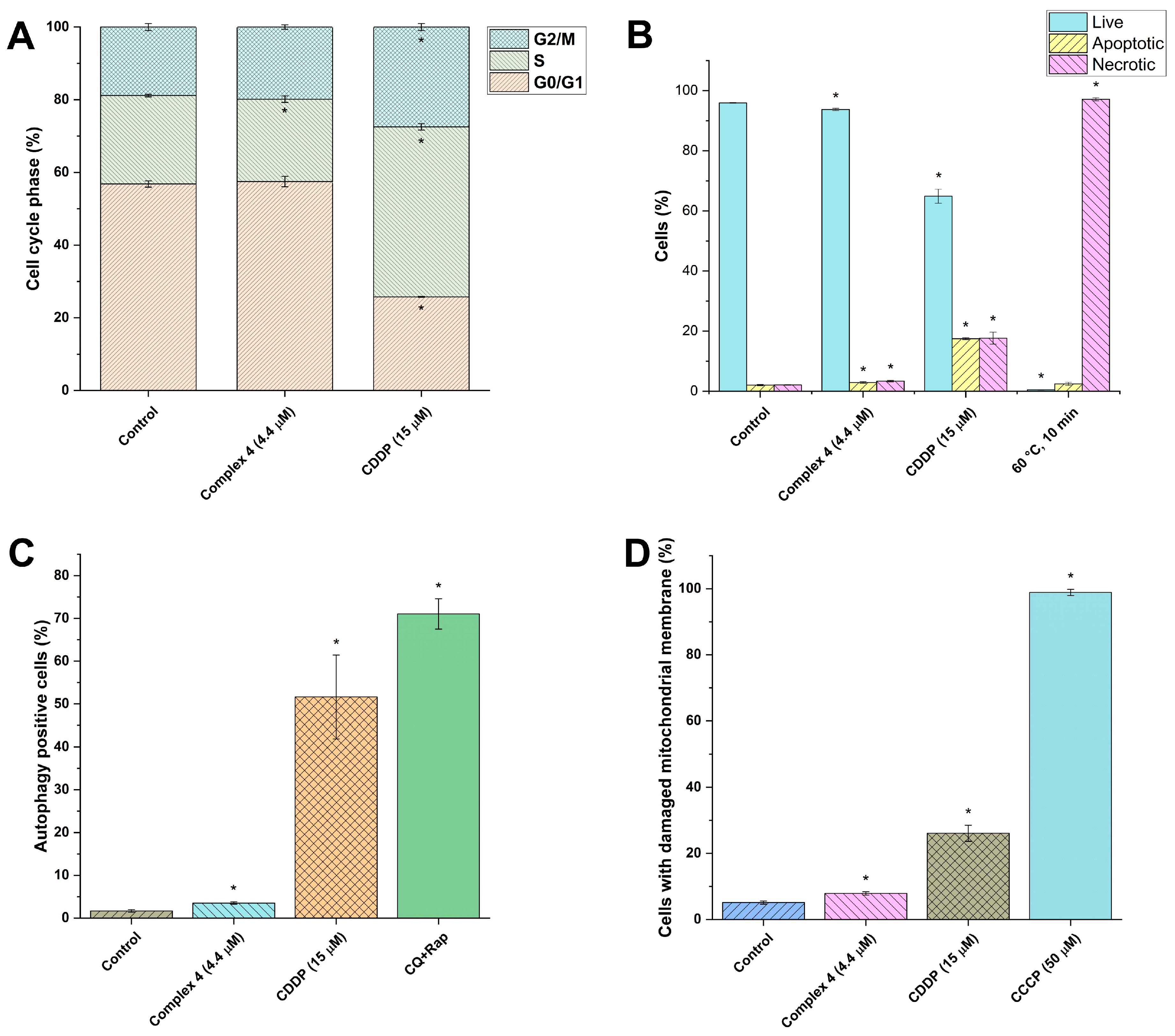
2.4. Cellular Effects of Complex 4 in A2780 Cells
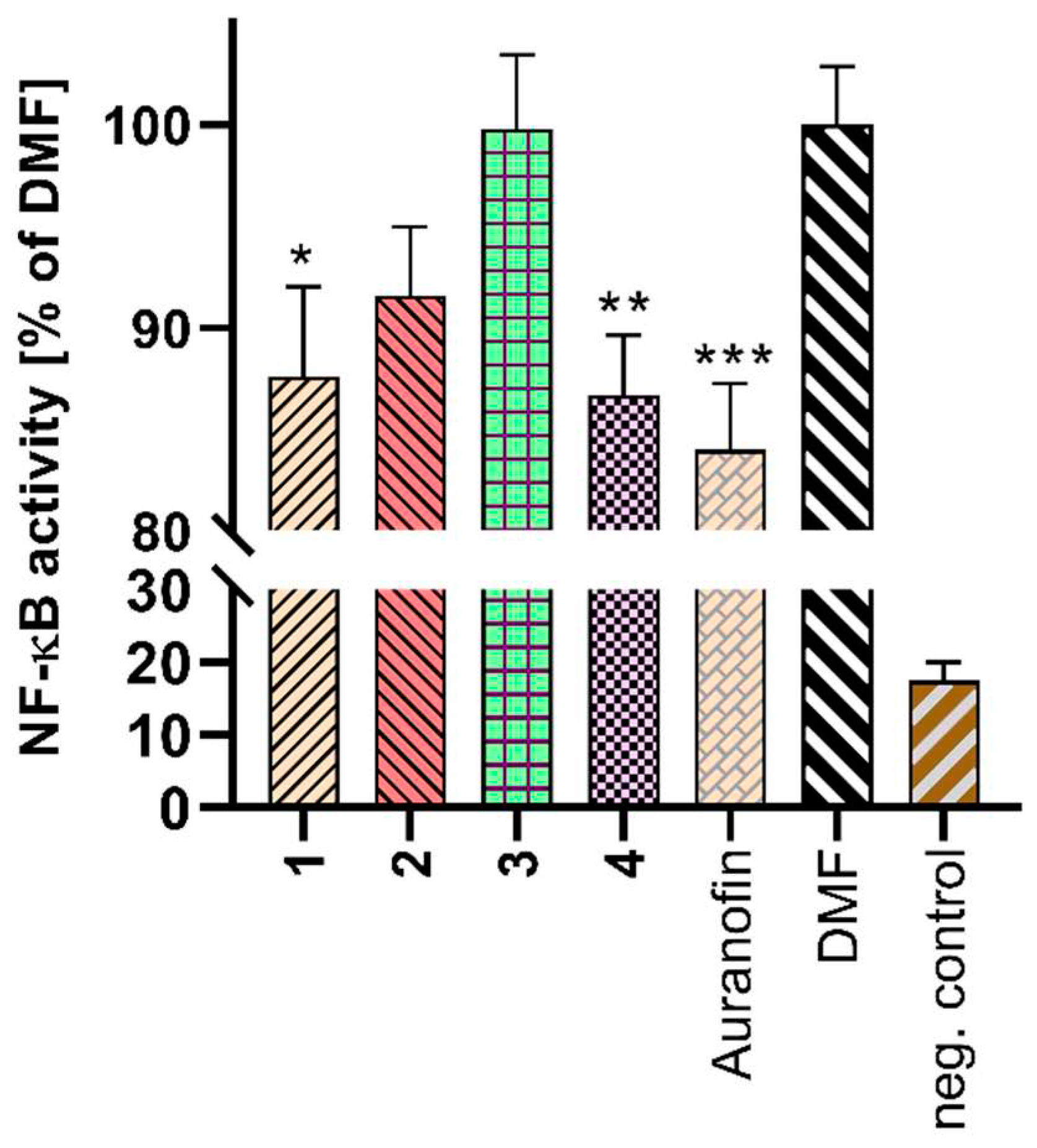
2.5. Cell Viability and NF-κB Activity Determination
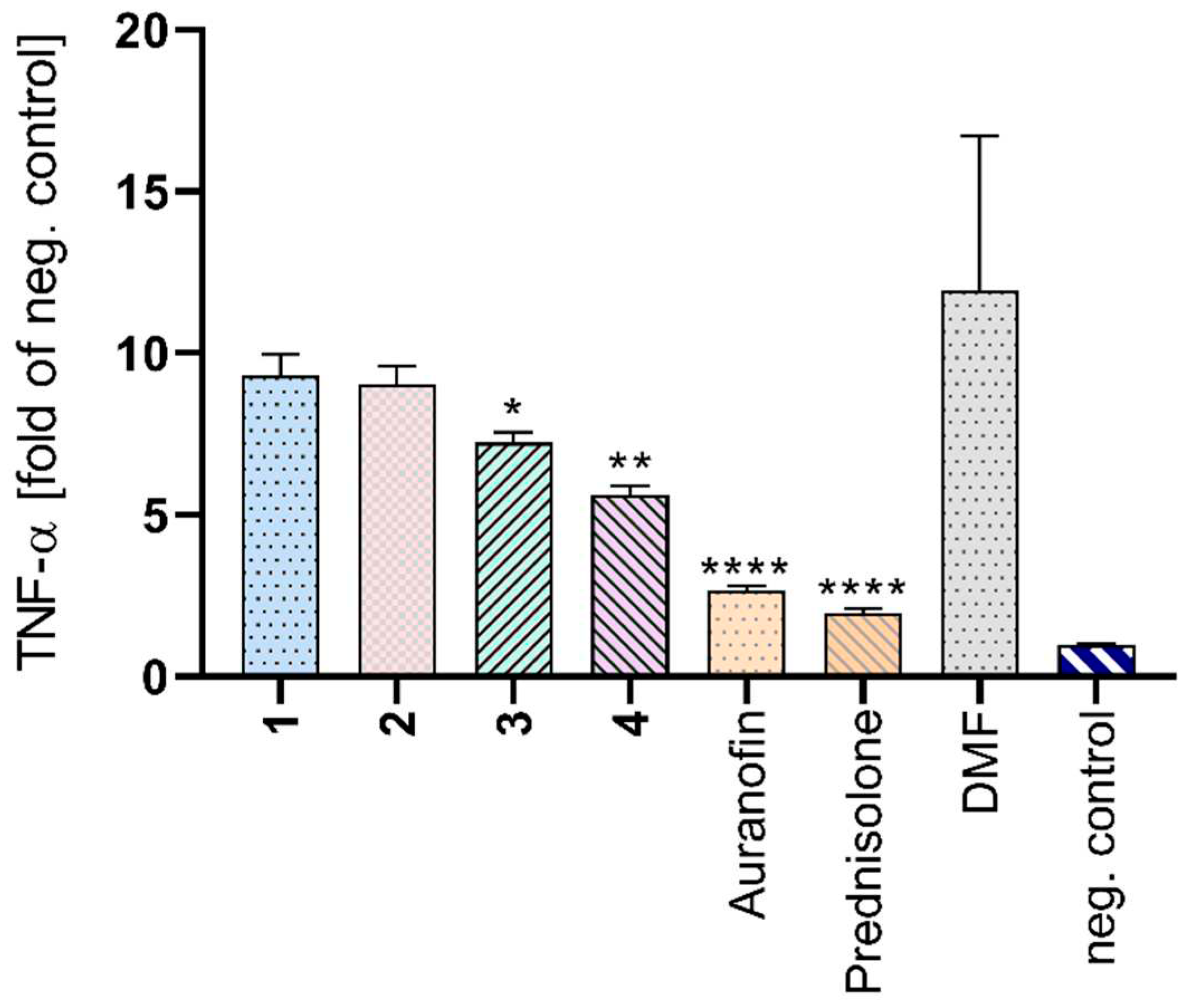
2.6. TNF-α Detection
3. Materials and Methods
3.1. General Methods Used for Characterization
3.2. Preparation and Characterization of the Complexes (1-4)
- Complex (1): Anal. Calcd. for C34H41N4FAu (Mr = 721,7): C, 56.6; H, 5.7; N, 7.8. Found: C, 56.5; H, 5.5; N, 7.6 %. ESI+MS (methanol, m/z): 1201.60 (calcd. 1201.53) [(Au(iPr))2(CH3O)]+, 639.29 (calcd. 639.26) {[Au(iPr)(CH3O)]+Na}+, 1255.56 (calcd. 1255.53) {[(Au(iPr)(CH3O))2]+Na}+, 1305.61 (calcd. 1305.54) [(Au(iPr))2(µ-L1)]+. ESI-MS (methanol, m/z): 719.34 (calcd. 719.29) [Au(iPr)(L1)-H]-, 615.27 (calcd. 615.27) {[Au(iPr)(CH3O)]-H}-. IR (ATR; cm-1): 417 (w), 453 (m), 472 (m), 500 (m), 551 (w), 619 (w), 634 (w), 702 (vs), 739 (s), 762 (s), 799 (s), 806 (s), 870 (m), 947 (w), 977 (m), 1059 (m), 1127 (s), 1181 (s), 1213 (w), 1261 (m), 1301 (m), 1332 (m), 1360 (w), 1385 (s), 1419 (m), 1467 (s), 1552 (w), 1572 (w), 1592 (w), 2868 (w), 2925 (w), 2955 (m), 2964 (m), 3129 (w), 3157 (w).
- Complex (2): Anal. Calcd. for C34H41N4BrAu (Mr = 782,6): C, 52.2; H, 5.3; N, 7.2. Found: C, 52.3; H, 5.1 N, 7.1 %. ESI+MS (methanol, m/z): 1201.60 (calcd. 1201.53) [(Au(iPr))2(CH3O)]+, 639.29 (calcd. 639.26) {[Au(iPr)(CH3O)]+Na}+, 1255.57 (calcd. 1255.53) {[(Au(iPr)(CH3O))2]+Na}+, 1367.52 (calcd. 1365.46 for the first isotopic peak) [(Au(iPr))2(µ-L2)]+, 803.25 (calcd. 803.20) {[Au(iPr)(L2)]+Na}+, 783.28 (calcd. 781.22 for the first isotopic peak) {[Au(iPr)(L2)]}+. IR (ATR; cm-1): 426 (w), 454 (w), 474 (m), 550 (w), 601 (w), 613 (w), 641 (w), 681 (m), 706 (m), 730 (s), 757 (s), 776 (m), 802 (s), 877 (m), 912 (w), 936 (w), 953 (m), 1060 (m), 1103 (w), 1117 (w), 1169 (s), 1186 (w), 1214 (w), 1246 (w), 1291 (m), 1334 (m), 1362 (m), 1386 (s), 1419 (w), 1452 (s), 1466 (s), 1554 (w), 1577 (w), 1594 (w), 2867 (w), 2936 (m), 2960 (m), 3085 (w), 3121 (w), 3149 (w).
- Complex (3): Anal. Calcd. for C34H41N4ClAu (Mr = 7381): C, 49.2; H, 5.0; N, 6.8. Found: C, 56.5; H, 5.5; N, 7.6 %. ESI+MS (methanol, m/z): 1201.61 (calcd. 1201.53) [(Au(iPr))2(CH3O)]+, 639.30 (calcd. 639.26) {[Au(iPr)(CH3O)]+Na}+, 1255.56 (calcd. 1255.53) {[(Au(iPr)(CH3O))2]+Na}+, 1321.57 (calcd. 1321.52) [(Au(iPr))2(µ-L3)]+, 759.32 (calcd. 759.25) {[Au(iPr)(L3)]+Na}+, 737.34 (calcd. 737.27) {[Au(iPr)(L3)]}+. IR (ATR; cm-1): 418 (w), 454 (w), 538 (m), 550 (m), 567 (w), 591 (w), 642 (w), 707 (m), 760 (vs), 773 (s), 787 (s), 803 (s), 924 (w), 935 (w), 947 (w), 1010 (s), 1034 (w), 1115 (w), 1159 (w), 1205 (w), 1271 (m), 1300 (m), 1326 (m), 1344 (w), 1362 (w), 1384 (m), 1401 (s), 1419 (w), 1469 (s), 1548 (w), 1593 (m), 2868 (w), 2929 (w), 2961 (m), 3032 (w), 3079 (w), 3121 (w), 3149 (w).
- Complex (4): Anal. Calcd. for C34H41N4IAu (Mr = 829,6): C, 55.3; H, 5.6; N, 7.6. Found: C, 55.6; H, 5.5; N, 7.5 %. ESI+MS (methanol, m/z): 1201.60 (calcd. 1201.53) [(Au(iPr))2(CH3O)]+, 639.30 (calcd. 639.26) {[Au(iPr)(CH3O)]+Na}+, 1255.56 (calcd. 1255.53) {[(Au(iPr)(CH3O))2]+Na}+, 1413.49 (calcd. 1413.45) [(Au(iPr))2(µ-L4)]+, 829.25 (calcd. 829.20) {[Au(iPr)(L4)]}+, 851.23 (calcd. 851.19) {[Au(iPr)(L4)]+Na}+. IR (ATR; cm-1): 417 (w), 430 (w), 455 (w), 472 (w), 517 (m), 551 (w), 574 (w), 588 (m), 670 (w), 707 (m), 766 (s), 786 (s), 804 (m), 854 (m), 919 (w), 983 (w), 1035 (w), 1060 (w), 1110 (w), 1159 (w), 1184 (w), 1201 (w), 1237 (w), 1267 (w), 1292 (m), 1318 (m), 1364 (w), 1392 (s), 1416 (m), 1466 (s), 1488 (w), 1542 (w), 1552 (w), 1589 (m), 2866 (w), 2925 (w), 2955 (m), 2962 (w), 3029 (w), 3080 (w), 3115 (w), 3145 (w).
3.3. Single-Crystal X-ray Diffraction Analysis
3.4. In Vitro Cytotoxicity against Human Ovarian Cancerous (A2780 and A2780R) and Normal (MRC-5) Cell Lines
3.5. Cell Cycle Analysis in A2780
3.6. Cell Death Induction in A2780
3.7. Autophagy Induction in A2780
3.8. ROS and Superoxide Detection
3.9. Mitochondrial Membrane Potential Analysis
3.10. Descriptive Statistics Evaluation
3.11. Cell Maintenance and Viability Determination
3.12. NF-κB Activity Determination
3.13. TNF-α Release Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in gold catalysis. Chem. Soc. Rev 2008, 37, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.C.; Cadge, J.A.; Bower, J.F.; Russell, C.A. A Hemilabile NHC-Gold Complex and its Application to the Redox Neutral 1,2-Oxyarylation of Feedstock Alkenesl. Angew. Chem. Int. Ed. 2023, 62, e202301526. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Goetzfried, S.K.; Kapitza, P.; Gallati, C.M.; Nindl, A.; Cziferszky, M.; Hermann, M.; Kircher, B.; Gust, R. Investigations of the reactivity, stability and biological activity of halido (NHC)gold(I) complexes. Dalton Trans 2022, 51, 1395–1406. [Google Scholar] [CrossRef]
- Bertrand, B.; Casini, A. A golden future in medicinal inorganic chemistry: the promise of anticancer gold organometallic compounds. Dalton Trans 2014, 43, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J. Organomet. Chem 2019, 882, 96e111. [Google Scholar] [CrossRef]
- Augello, G.; Azzolina, A.; Rossi, F.; Prencipe, F.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Cervello, M.; Tesauro, D. New Insights into the Behaviour of NHC-Gold Complexes in Cancer Cells. Pharmaceutics 2023, 15, 466. [Google Scholar] [CrossRef]
- König, P.; Zhulenko, R.; Suparman, E.; Hoffmeister, H.; Bückreiß; Ott, I.; Bandas, G. A biscarbene gold(I)-NHC-complex overcomes cisplatin-resistance in A2780 and W1 ovarian cancer cells highlighting pERK as regulator of apoptosis. Cancer Chemotherapy and Pharmacology 2023, 92, 57–69. [Google Scholar] [CrossRef]
- Massai, L.R.; Messori, L.; Carpentieri, A.; Amoresano, A.; Melchiorre, Ch.; Fiaschi, T.; Modesti, A.; Gamberi, T.; Magherini, F. The effects of two gold-N-heterocyclic carbene (NHC) complexes in ovarian cancer cells: a redox proteomic study. Cancer Chemotherapy and Pharmacology. 2022, 89, 809–823. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Zolotarev, A.A.; Ott, I. A novel aminotriazole-based NHC complex for the design of gold(i) anti-cancer agents: synthesis and biological evaluation. Med. Chem. Commun 2015, 6, 1186–1189. [Google Scholar] [CrossRef]
- Scattolin, T.; Lippmann, P.; Beliš, M.; van Hecke, K.; Ott, I.; Nolan, S.P. A simple synthetic entryway into (N-heterocyclic carbene)gold-steroidyl complexes and their anticancer activity. Appl. Organomet. Chem. 2022, e6624. [Google Scholar] [CrossRef]
- Filho, M.S.; Scatollin, T.; Dao, P.; Tzouras, N.V.; Benhida, R.; Saab, M.; van Hecke, K.; Lippman, P.; et al. Straightforward synthetic route to gold(i)-thiolato glycoconjugate complexes bearing NHC ligands (NHC = N-heterocyclic carbene) and their promising anticancer activity. New J. Chem 2021, 45, 9995–10001. [Google Scholar] [CrossRef]
- Ott, I. Chapter Four - Metal N-heterocyclic carbene complexes in medicinal chemistry. Advances in Inorganic Chemistry 2020, 75, 121–148. [Google Scholar] [CrossRef]
- Shen, S.; Shen, J.; Wang, F.; Min, J. Molecular mechanisms and clinical implications of the gold drug auranofin. Coord. Chem. Rev. 2023, 493, 215323. [Google Scholar] [CrossRef]
- Hatem, E.; Banna, N. E.; Heneman-Masurel, A.; Baile, D.; Vernis, E.; Riquier, S.; Colinelli-Cohen, M.; Guittet, O.; et al. Cancers 2022, 14(19), 4864. [CrossRef]
- Liu, Y.; Lu, Y.; Xu, Z.; Ma, X.; Chen, X.; Liu, W. Repurposing of the gold drug auranofin and a review of its derivatives as antibacterial therapeutics. Drug Discovery Today 2022, 27, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Vančo, J.; Čajan, M.; Belza, J.; Popa, I.; Hošek, J.; Lenobel, R.; Dvořák, Z. Gold(I) N-heterocyclic carbene (NHC) complexes containing 6-mercaptopurine derivatives and their in vitro anticancer and anti-inflammatory effects. Appl. Organomet. Chem 2023. under review. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Crlíková, Z.; Vančo, J.; Kašpárková, J.; Dvořák, Z. Half-Sandwich Ir(III) Complex of N1-Pyridyl-7-azaindole Exceeds Cytotoxicity of Cisplatin at Various Human Cancer Cells and 3D Multicellular Tumor Spheroids. Organometallics. 2018, 37, 2749–2759. [Google Scholar] [CrossRef]
- Štarha, P.; Hošek, J.; Dvořák, Z.; Suchý, P.; Popa, I.; Pražanová, G.; Trávníček, Z. Pharmacological and Molecular Effects of Platinum(II) Complexes Involving 7-Azaindole Derivatives. PLoS ONE 2014, 9, e90341. [Google Scholar] [CrossRef] [PubMed]
- Z. Trávníček, J. Vančo, Z. Dvořák, N-heterocyclic carbene complexes of gold with bicyclic N-donor ligands and using these complexes to prepare antitumor therapy medicaments, Czech Patent no. 307954, granted 31.07.2019. Czech Patent. Available online: https://isdv.upv.cz/webapp/resdb.print_detail.det?pspis=PT/2018-87&plang=CS.
- Pouchert, C.J. The Aldrich Library of Infrared Spectra, 3rd ed., Aldrich Chemical Company Press: Milwaukee, USA, 1981; pp. 1-1850.
- Lemasters, J.J.; Qian, T.; Bradham, C.A.; Brenner, D.A.; Cascio, W.E.; Trost, L.C.; Nishimura, Y.; Niemine, A.-L.; Herman, B. Mitochondrial Dysfunction in the Pathogenesis of Necrotic and Apoptotic Cell Death. J Bioenerg Biomembr., 1999, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef]
- Gamberi, T.; Chiappetta, G.; Fiaschi, T.; Modesti, A.; Sorbi, F.; Magherini, F. Upgrade of an old drug: Auranofin in innovative cancer therapies to overcome drug resistance and to in-crease drug effectiveness. Med Res Rev 2022, 42, 1111–1146. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12(1), 49–62. [Google Scholar] [CrossRef] [PubMed]
- APEX3 Software Suite, © 2016 Bruker AXS Inc., 5465 East Cheryl Parkway, Madison, WI 53711.
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Diamond - Crystal and Molecular Structure Visualization, 2021, Crystal Impact - Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany.
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0 - new features for the visualization and investigation of crystal structures. J. Appl. Crystallography 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]

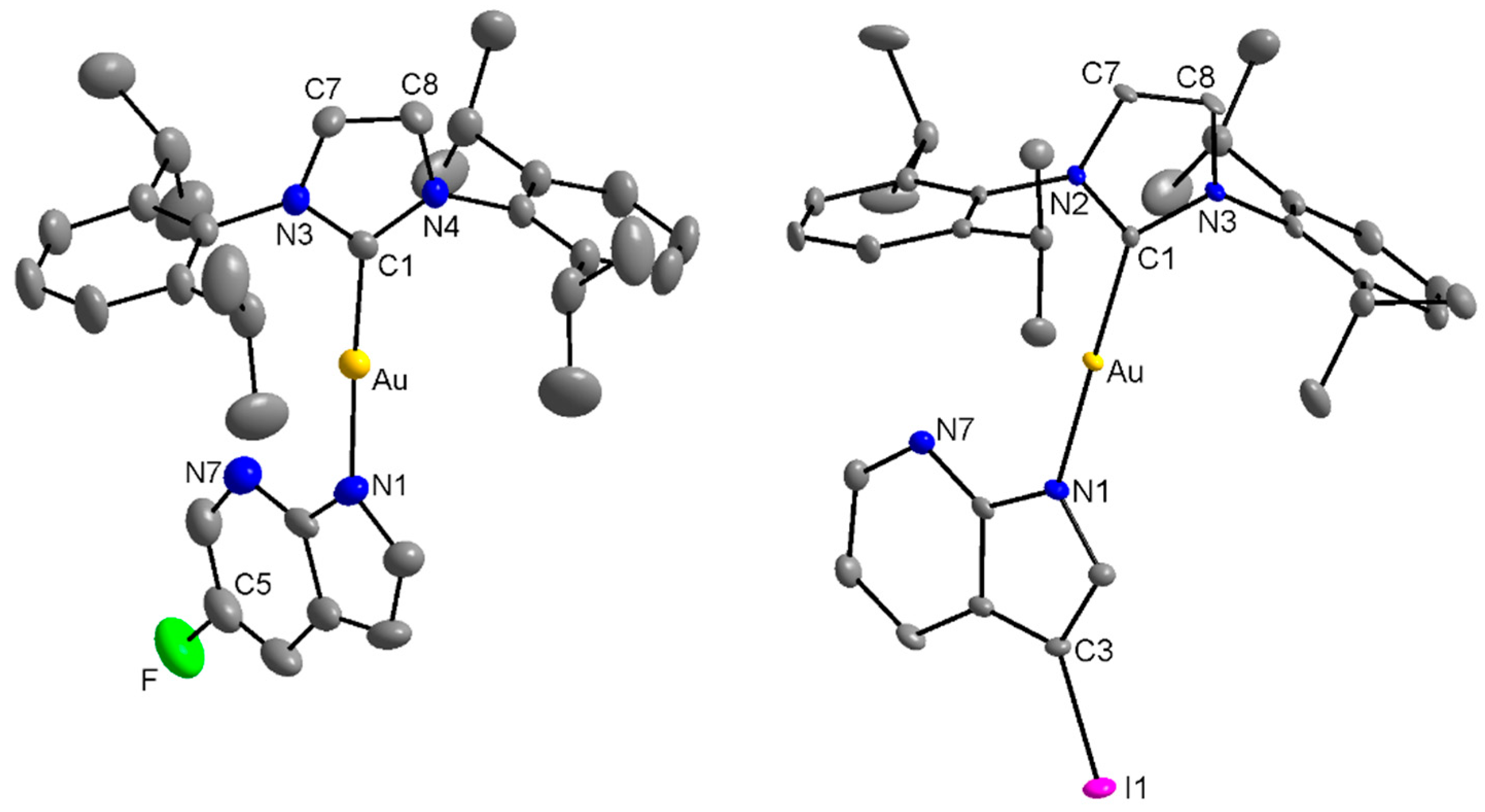
| Compound | [Au(iPr)(L1)] 1 | [Au(iPr)(L4)] 4 | [Au(iPr)(Pur1)]* | [Au(iPr)(Pur2)]** | |
|---|---|---|---|---|---|
| Selected bond lengths [Å] | Au-N | 2.026(4) | 2.024(2) | 2.018(3) | 2.033(6) |
| Au-C | 1.963(5) | 1.976(3) | 1.974(4) | 1.978(7) | |
| Selected angle [°] | N-Au-C | 175.54(18) | 179.29(12) | 173.58(16) | 175.7(3) |
| Compound | Human cell lines | RF* | SI** | ||
|---|---|---|---|---|---|
| A2780 | A2780R | MRC5 | |||
| [Au(iPr)(L1)] (1) | 7.9±2.4 | 5.0±1.5 | 19.8±3.0 | 0.6 | 2.5 |
| [Au(iPr)(L2)] (2) | 9.0±1.4 | >25 | >25 | >2.8 | >2.8 |
| [Au(iPr)(L3)] (3) | 4.4±0.5 | 7.4±2.2 | 13.6±1.4 | 1.7 | 3.1 |
| [Au(iPr)(L4)] (4) | 5.0±1.8 | 7.9±2.4 | 18.7±1.4 | 1.6 | 3.7 |
| [Au(iPr)Cl] | 33.6±0.9 | >50 | >50 | >1.5 | >1.5 |
| Auranofin | 1.5±0.5 | 3.1±0.2 | 4.7±0.8 | 2.1 | 3.1 |
| Cisplatin (CDDP) | 16.8 ± 0.3 | >50 | >50 | >3.0 | >3.0 |
| Compound | 24 h | 48 h | 72 h |
|---|---|---|---|
| [Au(iPr)(L1)] (1) | 7.9±2.4 | 3.8±0.5 | 3.4±0.2 |
| [Au(iPr)(L2)] (2) | 9.0±1.4 | 6.6±2.5 | 5.1±0.7 |
| [Au(iPr)(L3)] (3) | 4.4±0.5 | 3.8±1.1 | 3.0±0.2 |
| [Au(iPr)(L4)] (4) | 5.0±1.8 | 4.1±0.8 | 3.8±0.5 |
| [Au(iPr)Cl] | 33.6±0.9 | 25.7±0.6 | 26.2±1.5 |
| Auranofin | 1.5±0.5 | 1.0±0.5 | 0.9±0.4 |
| Cisplatin (CDDP) | 16.8 ± 0.3 | 11.6 ± 3.2 | 4.4 ± 0.4 |
| Empirical formula Formula weight Temperature Wavelength Crystal system Space group Unit cell dimensions Volume Z Density (calculated) Absorption coefficient F(000) Crystal size θ range for data collection Index ranges Reflections collected Independent reflections Completeness to θ = 25.0° Absorption correction Refinement method Data / restraints / parameters Goodness-of-fit on F2 Final R indices [I>2σ(I)] R indices (all data) |
C34 H41 N4 F Au 720.66 293(2) K 0.71073 Å Triclinic P-1 a = 10.812(4) Å, α = 87.641(15)° b = 11.617(5) Å, β = 77.852(13)° c = 14.396(5) Å, γ = 66.83(2)° 1623.4(11) Å3 2 1.474 g/cm3 4.564 mm-1 720 0.260 x 0.240 x 0.200 mm 1.909 to 24.999°. -12 ≤ h ≤ 12, -13 ≤ k ≤ 13, -17 ≤ l ≤ 17 29392 5713 [R(int) = 0.0666] 100.0 % Semi-empirical from equivalents Full-matrix least-squares on F2 5713 / 0 / 369 1.086 R1 = 0.0312, wR2 = 0.0680 R1 = 0.0465, wR2 = 0.0715 |
C34 H41 N4 I Au 828.56 150(2) K 0.71073 Å Triclinic P-1 a = 9.563(4) Å, α = 81.364(16)° b = 11.986(5) Å, β = 87.163(14)° c = 16.130(7) Å, γ = 77.766(18)° 1786.0(12) Å3 2 1.541 g/cm3 5.008 mm-1 808 0.160 x 0.120 x 0.100 mm 2.180 to 25.000°. -11 ≤ h ≤ 11, -14 ≤ k ≤ 14, -19 ≤ l ≤ 19 37929 6293 [R(int) = 0.0481] 99.9 % Semi-empirical from equivalents Full-matrix least-squares on F2 6293 / 0 / 369 1.053 R1 = 0.0195, wR2 = 0.0393 R1 = 0.0263, wR2 = 0.0406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





