Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis Methods
2.3. Preparation of Immobilized Lipases
2.4. Enzymatic Transesterification/Esterification Between PSs and Various Fatty Acyl Donors
2.5. Preparation of Partially Transesterified Plant Oils via Lipase-catalyzed Transesterification of Oil TGs and Ethanol
3. Results and Discussion
3.1. Screening of Lipases of Transesterification Activity to Produce PSEs
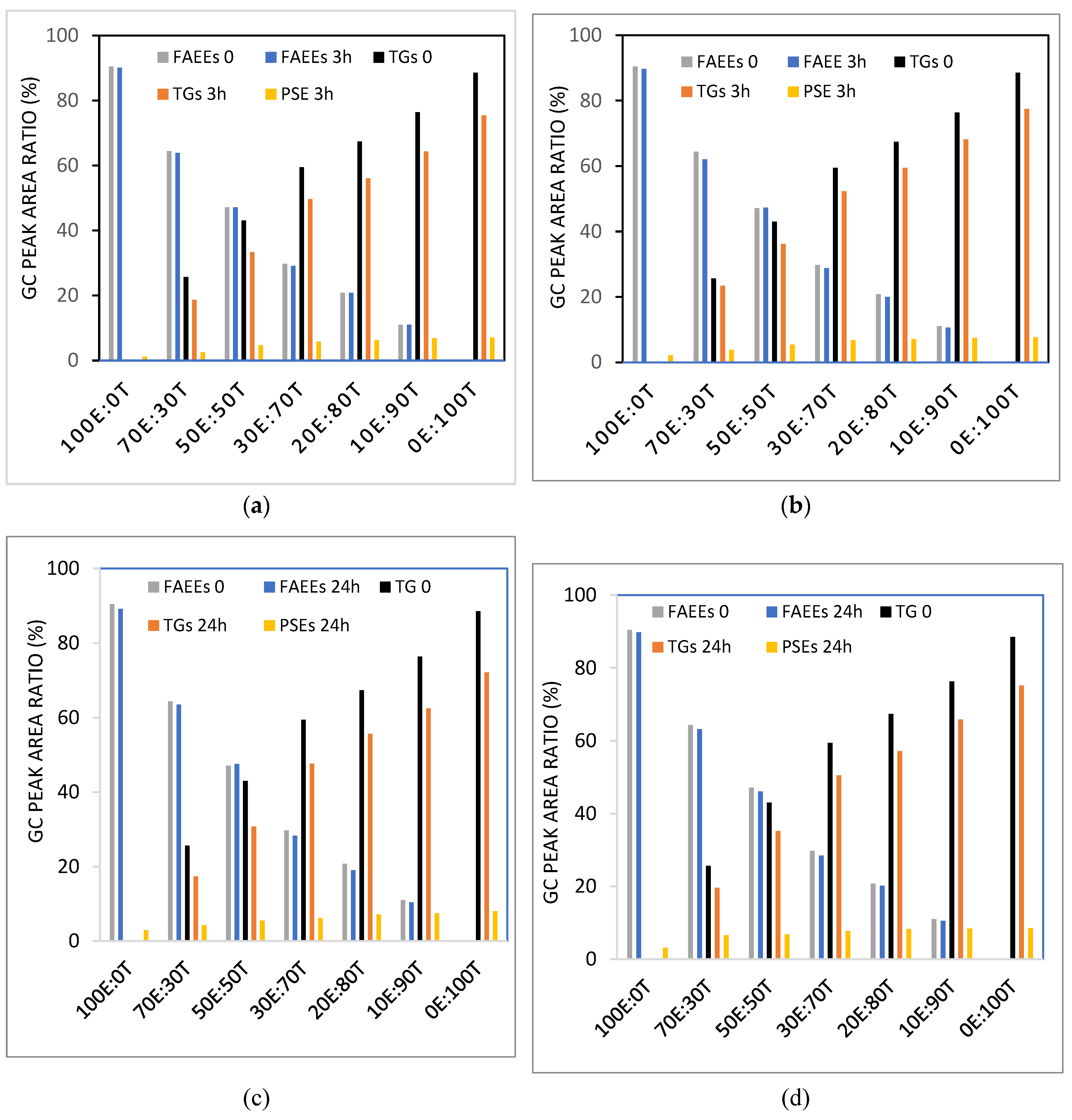
3.2. Enzymatic Transesterification of PSs and Mixtures of Different Weight Ratios of Canola Oil TGs and FAEEs
3.3. Transesterification/Esterification of PSs with Mixtures Comprised of Different Weight Ratios of Canola oil TGs, MGs and FFAs as Fatty acyl donors
3.4. Screening of Lipases of Transesterification Activity to Produce PSEs Using Partially Transesterified Plant Oils with Ethanol as a Reaction Medium
3.5. Enzymatic Transesterification of PSs and Partially Transesterified Oils with Ethanol Containing Low Levels of FAEEs
3.6. Enzymatic Transesterification of PSs with Partially Enzymatically Transesterified Oil Comprised of FAEEs, MGs, and TGs/or with Oil Mixtures Prepared by Mixing Appropriate Weight Ratios of FAEEs, MGs, DGs and TGs
| FAEEs % | MGs % | DGs % | TGs % |
| 67.8 | 12.4 | 9.7 | 9.1 |
| FAEEs % | MGs % | DGs % | TGs % |
| 68.4 | 12.7 | 9.6 | 9.3 |
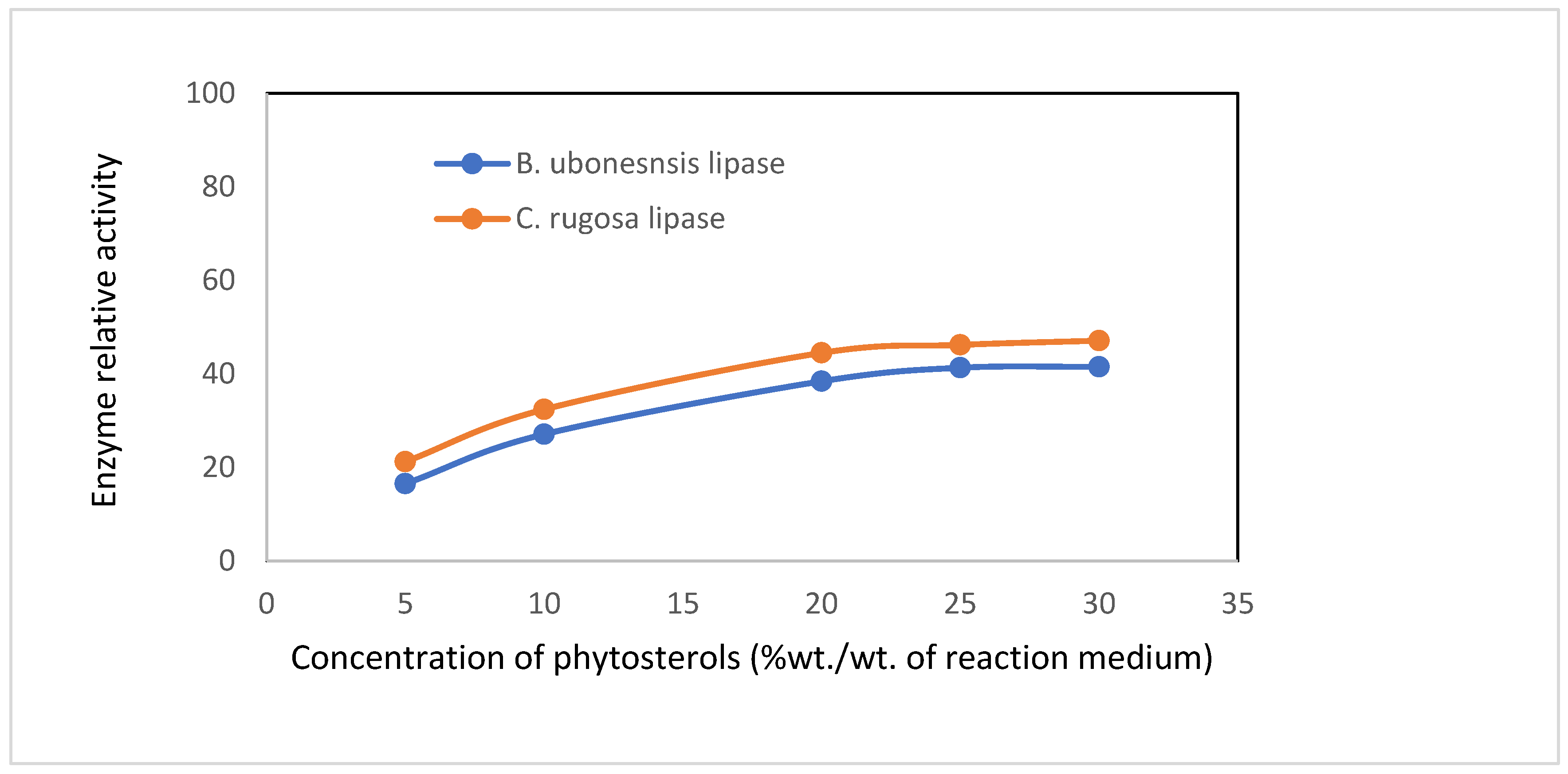
3.7. The Effect of Phytosterol Concentration
4. Conclusions
5. Patents
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, G.; Ma, C.; Chen, X. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain Oil Sci. Technol. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant sterols: chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Ma, L.; Ostlund Jr, R.E. Natural Dietary Phytosterols. J. AOAC Int. 2015, 98, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Abe, T.; Hudaka, I.; Kojima, K.; Yoshino, H.; Aoyama, T.; Okazaki, M.; Kondo, K. Effects of Phytosterol Esters-Enriched Vegetable Oil on Cholesterol and Assessment of Safety in Healthy Men. J. Oleo Sci. 2003, 52, 205–213. [Google Scholar] [CrossRef]
- Weber, N.; Weitkamp, P.; Mukherjee, K.D. Cholesterol-lowering food additives: lipase-catalysed preparation of phytosterol and phytostanol esters. Food Res. Int. 2002, 35(2-3), 177-181. [CrossRef]
- Yu, K.; Zhang, Y.; Ying, J. Phytosterol compositions of enriched products influence their cholesterol-lowering efficacy: a meta-analysis of randomized controlled trials. Eur J. Clin. Nutr. 2019, 73, 1579–1593. [Google Scholar] [CrossRef]
- Nekrasov, P.O.; Berezka, T.O.; Nekrasov, O.P.; Gudz, O.M.; Rudneva, S.I.; Molchenko, S.M. Study of biocatalytic synthesis of phytosterol esters as formulation components of nutritional systems for health purposes. J. Chem. Technol. 2022, 30, 404–409. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Can, A.; Techunenbou-Magaia, F.L. Development and Characterization of Phytosterol-Enriched Oil Microcapsules for Foodstuff Application. Food Bioprocess Technol. 2018, 11, 152–163. [Google Scholar] [CrossRef]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in functional foods and food supplements: Tradition, efficacy, and regulatory aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; MacClements, D.J.; Xu, D.; Chen, S. Production, Characterization, delivery, and Cholesterol-lowering Mechanism of Phytosterols: A Review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef]
- Vaikousi, H.; Lazaridou, A.; Biliaderis, C.G.; Zawistowski, J. Phase transitions, solubility, and crystallization kinetics of phytosterols and phytosterol− oil blends. J. Agric. Food Chem. 2007, 55, 1790–1798. [Google Scholar] [CrossRef]
- Soupas, L.; Juntunen, L.; Lampi, A.M.; Piironen, V. Effects of sterol structure, temperature, and lipid medium on phytosterol oxidation. J. Agric. Food Chem. 2004, 52, 6485–6491. [Google Scholar] [CrossRef]
- Pavani, M.; Singha, P.; Singh, S.K. Development of Phytosterol Enriched Functional Edible oils: Study of Physical, Chemical, Thermal and Structural Properties. J. Sci. Ind. Res. 2022, 81, 549–560 http://opniscprresin/indexphp/JSIR/article/view/59585. [Google Scholar]
- Hellner, G.; Tőke, E.R.; Nagy, V.; Szakács, G.; Poppe, L. Integrated enzymatic production of specific structured lipid and phytosterol ester compositions. Process Biochem., 2010, 45, 1245–1250. [Google Scholar] [CrossRef]
- Zheng, M.M.; Huang, Q.; Huang, F.H.; Guo, P.M.; Xiang, X.; Deng, Q.C.; Li, W.L.; Wan, C.Y.; Zheng, C. Production of novel “functional oil” rich in diglycerides and phytosterol esters with “one-pot” enzymatic transesterification. J. Agric. Food Chem., 2014, 62, 5142–5148. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef]
- Pereira, A.D.S.; De Souza, A.H.; Fraga, J.L.; Villeneuve, P.; Torres, A.G.; Amaral, P.F. Lipases as effective green biocatalysts for phytosterol esters’ production: A review. Catal. 2022, 12, p88. [Google Scholar] [CrossRef]
- Pouilloux, Y.; Courtois, G.; Boisseau, M.; Piccirilli, A.; Barrault, J. Solid base catalysts for the synthesis of phytosterol esters. Green Chem. 2003, 5, 89–91. [Google Scholar] [CrossRef]
- Coelho A.L.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61:1689-1703. [CrossRef]
- Negishi, S.; Hidaka, I.; Takahashi, I.; Kunita, S. Transesterification of phytosterol and edible oil by lipase powder at high temperature. J. Am. Oil Chem. Soc. 2003, 80, 905–907. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Modeling and optimization of lipase-catalyzed synthesis of phytosteryl esters of oleic acid by response surface methodology. Food Chem. 2007, 102, 336–342. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, B.; Wang, X.; Chen, J.; Yang, G. Solvent-free synthesis of phytosterol linoleic acid esters at low temperature. RSC adv. 2021, 11, 10738–10746. [Google Scholar] [CrossRef]
- de Menezes, L.H.S.; do Espírito Santo, E.L.; Dos Santos, M.M.O.; de Carvalho Tavares, I.M.; Mendes, A.A.; Franco, M.; de Oliveira, J.R. Candida rugosa lipase immobilized on hydrophobic support Accurel MP 1000 in the synthesis of emollient esters. Biotechnol. Lett. 2022, 44, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Honcharov, D.S.; Tkachenko, N.A.; Nikolaieva, V.G. Transesterification of a Mixture of Vegetable Fats with the Addition of Phytosterols. European j. agric. food sci. 2021, 3, 45–48. [Google Scholar] [CrossRef]
- Basheer, S.; Watanabe, Y. Enzymatic conversion of acid oils to biodiesel. Lipid Technol. 2016, 28, 16–18. [Google Scholar] [CrossRef]
- Zheng, M.M.; Lu, Y.; Dong, L.; Guo, P.M.; Deng; Q.C.; Li, W.L.; Feng, Y.Q.; Huang, F.H. Immobilization of Candida rugosa lipase on hydrophobic/strong cation-exchange functional silica particles for biocatalytic synthesis of phytosterol esters. Bioresour. Technol. 2012, 115, 141-146. [CrossRef]
| Commercial name | Species of microorganism | Form lipase | Manufacturer |
|---|---|---|---|
| Lipase F-AP | Rhizopus oryzae | Powder |
Amano Enzymes, Japan |
| Lipase G | Penicillium camemberti | Powder | |
| Lipase A | Aspergillus niger | Powder | |
| Lipase PS | Pseudomonas cepacia | Powder | |
| Lipase PF | Pseudomonas fluorescens | Powder | |
| Lipase R | Penicillium roqueforti | Powder | |
| Lipase F | Rhizopus niveus | Powder | |
| Lipase AY | Candida (C.) rugosa | Powder | |
| Lipase CR | C. rugosa | Powder | Enzyme Development Corporation, USA |
| Lipase AN | Aspergillus niger | Powder | |
| Lipase QLM | Burkholderia (B.) ubonensis | Powder |
Meito Sangio, Japan |
| Lipase OF | C. rugosa | Powder | |
| Lipase TL | Pseudomonas stutzeri | Powder | |
| Lipase SL | Pseudomonas (B.) cepacia | Powder | |
| Novocor AD L | C. antarctica A | Liquid |
Novozymes, Denmark |
| Eversa Transform 2.0 | Thermomyces lanuginosus | Liquid | |
| Lipozyme CALB L | C. antarctica B | Liquid | |
| Novozym 435 (Immobilized) |
C. antarctica B | Immobilized | |
| Lipozyme RM IM (Immobilized) |
Rhizomucor miehei | immobilized |
| GC Peak area ratio (%) | |||||||
| Name of lipase | Source of lipase | FFAs | MGs | PSs | DGs | PSEs | TGs |
| Control | - | 1.4 | 0.3 | 9 | 5.1 | 0 | 84.2 |
| Lipase F-AP | Rhizopus oryzae | 4.8 | 3 | 6.8 | 7 | 2.2 | 76.2 |
| Lipase G | Penicillium camemberti | 3.3 | 1.3 | 7 | 2.3 | 2 | 84.1 |
| Lipase A | Aspergillus niger | 2.5 | 1.2 | 7.5 | 3.2 | 1.5 | 84.1 |
| Lipase PS | Pseudomonas cepacia | 7.9 | 3.6 | 4 | 11.9 | 5 | 67.6 |
| Lipase PF | Pseudomonas fluorescens | 6.8 | 2.6 | 3 | 12.5 | 6 | 69.1 |
| Lipase R | Penicillium roqueforti | 8.4 | 3.9 | 7 | 11.4 | 2 | 67.3 |
| Lipase F | Rhizopus niveus | 3.7 | 0.9 | 7.1 | 2.4 | 1.9 | 84 |
| Lipase AY | C. rugosa | 7.4 | 3.7 | 0.3 | 13.4 | 8.7 | 66.5 |
| Lipase CR | C. rugosa | 7.6 | 3.9 | 0.1 | 15 | 8.9 | 64.5 |
| Lipase AN | Aspergillus niger | 8.6 | 3.9 | 2.8 | 9.5 | 6.2 | 69 |
| Lipase QLM | B. ubonensis | 9.5 | 3.7 | 0.1 | 14.2 | 8.7 | 63.8 |
| Lipase OF | C. rugosa | 8.4 | 3.5 | 0.3 | 13.9 | 8.7 | 65.2 |
| Lipase TL | Pseudomonas stutzeri | 8.2 | 4.3 | 0.2 | 12.7 | 8.6 | 66 |
| Lipase SL | B. cepacia | 11.3 | 3.2 | 0.8 | 13.5 | 8.2 | 63 |
| Novocor AD L | C. antarctica A | 17.5 | 6.5 | 6 | 18.4 | 3 | 48.6 |
| Eversa Transform 2.0 | Thermomyces lanuginosus | 21.6 | 9.9 | 7.5 | 16.6 | 1.5 | 42.9 |
| Lipozyme CALB L | C. antarctica B | 3.3 | 2 | 7.5 | 1.7 | 1.5 | 84 |
| Novozym 435 (Immobilized) |
C. antarctica B | 7.5 | 3.8 | 6.7 | 12.2 | 2.3 | 67.5 |
| Lipozyme RM IM (Immobilized) |
Rhizomucor miehei | 9.7 | 3.3 | 7 | 10.9 | 2 | 67.1 |
| Time (h) | Lipase source | GC Peak area ratio (%) | |||||
|---|---|---|---|---|---|---|---|
| FFAs | MGs | PSs | DGs | PSEs | TGs | ||
|
0 |
Control | 24.25 | 38.27 | 25.14 | 11.92 | 0 | 0.42 |
| Control | 14.01 | 22.74 | 20.29 | 10.94 | 0 | 32.02 | |
| Control | 9.36 | 14.83 | 16.75 | 8.73 | 0 | 50.33 | |
| Control | 5.1 | 8.01 | 15.04 | 5.8 | 0 | 66.05 | |
| Control | 4.5 | 4.11 | 13.67 | 4.5 | 0 | 73.22 | |
| Control | 2 | 2.67 | 10.59 | 3.02 | 0 | 81.72 | |
| Control | 0.86 | 0.02 | 10.14 | 2.77 | 0 | 86.21 | |
|
3 |
B. ubonensis | 29.55 | 34.88 | 23.38 | 10.22 | 1.76 | 0.21 |
| B. ubonensis | 20.22 | 22.47 | 17.59 | 22.55 | 2.7 | 14.47 | |
| B. ubonensis | 12.66 | 20.77 | 14.11 | 26.57 | 2.64 | 23.25 | |
| B. ubonensis | 8.55 | 15.46 | 11.41 | 28.66 | 3.63 | 32.29 | |
| B. ubonensis | 7.2 | 11.47 | 9.34 | 27.82 | 4.33 | 39.84 | |
| B. ubonensis | 5.8 | 6.6 | 5.39 | 23.94 | 5.2 | 53.07 | |
| B. ubonensis | 4.6 | 1.45 | 3.74 | 12.12 | 6.4 | 71.69 | |
| C. rugosa | 28.89 | 34.66 | 23.03 | 11.12 | 2.11 | 0.19 | |
| C. rugosa | 19.88 | 17.47 | 16.48 | 18.64 | 3.81 | 23.72 | |
| C. rugosa | 16.25 | 12.36 | 12.42 | 18.51 | 4.33 | 36.13 | |
| C. rugosa | 12.48 | 7.55 | 10.27 | 19.5 | 4.77 | 45.43 | |
| C. rugosa | 9.55 | 6.32 | 8.86 | 19.96 | 4.81 | 50.5 | |
| C. rugosa | 6.3 | 4.11 | 5.5 | 17.02 | 5.09 | 61.98 | |
| C. rugosa | 4.6 | 1.32 | 3.93 | 9.94 | 6.21 | 74 | |
|
24 |
B. ubonensis | 31.22 | 33.54 | 19.19 | 9.99 | 5.95 | 0.11 |
| B. ubonensis | 21.44 | 20.69 | 10.03 | 22.52 | 10.26 | 15.06 | |
| B. ubonensis | 17.1 | 19.39 | 7.42 | 26.04 | 9.33 | 20.72 | |
| B. ubonensis | 13 | 14.72 | 2.44 | 28.17 | 12.6 | 29.07 | |
| B. ubonensis | 11.3 | 11.98 | 4.55 | 26.5 | 9.12 | 36.55 | |
| B. ubonensis | 9.8 | 8.1 | 2.04 | 24.68 | 8.55 | 46.83 | |
| B. ubonensis | 8.5 | 2.74 | 0.59 | 13.3 | 9.55 | 65.32 | |
| C. rugosa | 31.92 | 31.89 | 22.79 | 10.83 | 2.35 | 0.22 | |
| C. rugosa | 21.01 | 18.47 | 14.97 | 18.88 | 5.32 | 21.35 | |
| C. rugosa | 15.77 | 12.65 | 10.81 | 18.27 | 5.94 | 36.56 | |
| C. rugosa | 12.91 | 8.83 | 6.84 | 21.14 | 8.2 | 42.08 | |
| C. rugosa | 12 | 6.76 | 6.62 | 21.39 | 7.05 | 46.18 | |
| C. rugosa | 9.9 | 5.35 | 2.71 | 18.24 | 7.88 | 55.92 | |
| C. rugosa | 5.31 | 1.72 | 1.38 | 11.46 | 8.76 | 71.37 | |
| GC Peak area ratio (%) | ||||||||
| Name of lipase | Immobilized lipase | FFAs | FAEEs | MGs | PSs | DGs | PSEs | TGs |
| Control | - | 1.2 | 56.5 | 13.4 | 10.5 | 10.4 | 0 | 8 |
| Lipase F-AP | Rhizopus oryzae | 8.3 | 56.2 | 6.9 | 8.5 | 10.3 | 2 | 7.8 |
| Lipase G | Penicillium camemberti | 4 | 57.2 | 8 | 9.6 | 12.3 | 0.9 | 8 |
| Lipase A | Aspergillus niger | 6.2 | 56.8 | 7.1 | 9.5 | 11.8 | 1 | 7.6 |
| Lipase PS | Pseudomonas cepacia | 8.6 | 56.8 | 6.2 | 6.3 | 10.1 | 4.2 | 7.8 |
| Lipase PF | Pseudomonas fluorescens | 9.5 | 55.5 | 6.3 | 8.4 | 10.2 | 2.1 | 8 |
| Lipase R | Penicillium roqueforti | 7.3 | 56.3 | 8.1 | 9.6 | 10.1 | 0.9 | 7.7 |
| Lipase F | Rhizopus niveus | 3.6 | 57.5 | 7.8 | 9.7 | 12.8 | 0.8 | 7.8 |
| Lipase AY | C. rugosa | 14 | 55.2 | 5.2 | 0.4 | 8.2 | 10.1 | 6.9 |
| Lipase CR | C. rugosa | 10.47 | 55.18 | 5.64 | 0.7 | 10.51 | 9.8 | 7.7 |
| Lipase AN | Aspergillus niger | 6.57 | 56.2 | 7.74 | 0.4 | 11.19 | 10.1 | 7.8 |
| Lipase QLM | B. ubonensis | 12.8 | 55.6 | 4.8 | 0.5 | 8.4 | 10 | 7.9 |
| Lipase OF | C. rugosa | 13.9 | 53.3 | 5.7 | 0.8 | 8.6 | 9.7 | 8 |
| Lipase TL | Pseudomonas stutzeri | 14.9 | 55.2 | 6.2 | 0.2 | 6.5 | 10.3 | 6.7 |
| Lipase SL | Pseudomonas (B.) cepacia | 12.7 | 56 | 5.6 | 0.4 | 7.4 | 10.1 | 7.8 |
| Novocor AD L | C. antarctica A | 9.7 | 55.8 | 5.8 | 9.4 | 10.6 | 1.1 | 7.6 |
| Eversa Transform 2.0 | Thermomyces lanuginosus | 10.68 | 55.32 | 7.4 | 9.6 | 8.1 | 0.9 | 8 |
| Lipozyme CALB L | C. antarctica B | 9.9 | 56 | 7.4 | 9.6 | 8.4 | 0.9 | 7.8 |
| Novozym 435 (Immobilized) |
C. antarctica B | 7.1 | 55.2 | 8.3 | 9.6 | 11 | 0.9 | 7.9 |
| Lipozyme RM IM (Immobilized) |
Rhizomucor miehei | 9.2 | 56.1 | 6.9 | 9.5 | 10.5 | 1 | 6.8 |
| GC Peak area ratio (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Immobilized lipase | Water (wt./wt.%) of oil | FAEEs | FFAs | MGs | PSs | DGs | PSEs | TGs |
| 0 | Control | 0 | 20.8 | 1.2 | 27.3 | 14.0 | 20.8 | 0.0 | 15.9 |
|
3 |
B. ubonensis | 0 | 20.2 | 2.3 | 17.2 | 10.6 | 19.5 | 5.3 | 24.8 |
| B. ubonensis | 0.5 | 21.1 | 6.5 | 16.2 | 11.2 | 20.8 | 4.9 | 19.3 | |
| C. rugosa | 0 | 20.2 | 4.0 | 20.4 | 8.6 | 23.1 | 8.7 | 15.0 | |
| C. rugosa | 0.5 | 22.0 | 7.3 | 12.5 | 3.5 | 19.2 | 16.9 | 18.6 | |
|
6 |
B. ubonensis | 0 | 20.5 | 1.5 | 14.5 | 8.1 | 25.0 | 9.0 | 21.3 |
| B. ubonensis | 0.5 | 20.8 | 6.8 | 14.5 | 9.3 | 19.6 | 7.9 | 21.1 | |
| C. rugosa | 0 | 20.7 | 3.2 | 18.9 | 9.1 | 23.7 | 8.9 | 15.5 | |
| C. rugosa | 0.5 | 21.0 | 7.7 | 10.8 | 3.1 | 20.3 | 17.4 | 19.7 | |
|
24 |
B. ubonensis | 0 | 20.1 | 1.6 | 11.9 | 4.2 | 25.0 | 15.7 | 21.5 |
| B. ubonensis | 0.5 | 20.3 | 6.8 | 12.2 | 4.9 | 21.4 | 14.4 | 19.9 | |
| C. rugosa | 0 | 21.0 | 3.5 | 18.8 | 9.1 | 22.9 | 8.8 | 15.9 | |
| C. rugosa | 0.5 | 21.3 | 8.3 | 11.0 | 3.0 | 18.5 | 17.1 | 20.7 | |
| GC Peak area ratio (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Reaction mixture | Immobilized lipase | FAEEs | FFAs | MGs | PSs | DGs | PSEs | TGs |
|
0 |
Mixture A | T=0 | 62 | 1.4 | 11 | 10.6 | 7.9 | 0 | 7.1 |
| Mixture B | T=0 | 61.1 | 1.7 | 11.2 | 10.6 | 7.8 | 0 | 7.6 | |
|
3 |
Mixture A | B. ubonensis | 60.9 | 5.1 | 8.9 | 4.7 | 7.7 | 5.9 | 6.8 |
| Mixture B | B. ubonensis | 59.8 | 5.5 | 9.5 | 5.6 | 7.5 | 5 | 7.1 | |
| Mixture A | C. rugosa | 58.9 | 7.1 | 9.9 | 1.8 | 7.5 | 8.8 | 6 | |
| Mixture B | C. rugosa | 59.2 | 7.9 | 8.7 | 3.5 | 6.8 | 7.1 | 6.8 | |
|
6 |
Mixture A | B. ubonensis | 58.8 | 5.3 | 10.5 | 2.8 | 7.8 | 7.8 | 7 |
| Mixture B | B. ubonensis | 58.4 | 6.8 | 10.1 | 4.4 | 7.5 | 6.2 | 6.6 | |
| Mixture A | C. rugosa | 59 | 7.1 | 9.7 | 0.6 | 7.2 | 10 | 6.4 | |
| Mixture B | C. rugosa | 58.3 | 7.7 | 8.7 | 1.8 | 7.5 | 8.8 | 7.2 | |
|
24 |
Mixture A | B. ubonensis | 59 | 6.6 | 9.8 | 1.1 | 7.6 | 9 | 6.9 |
| Mixture B | B. ubonensis | 59.3 | 6.2 | 10.5 | 2.2 | 7 | 8.4 | 6.4 | |
| Mixture A | C. rugosa | 58.2 | 6.8 | 9.5 | 0.1 | 7.8 | 10.5 | 7.1 | |
| Mixture B | C. rugosa | 59.2 | 7.9 | 9.2 | 1.6 | 7.6 | 9.5 | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




