Submitted:
30 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. PTB/PTL risk prediction
2.1. Physical testing methods
2.2. Chemical testing method
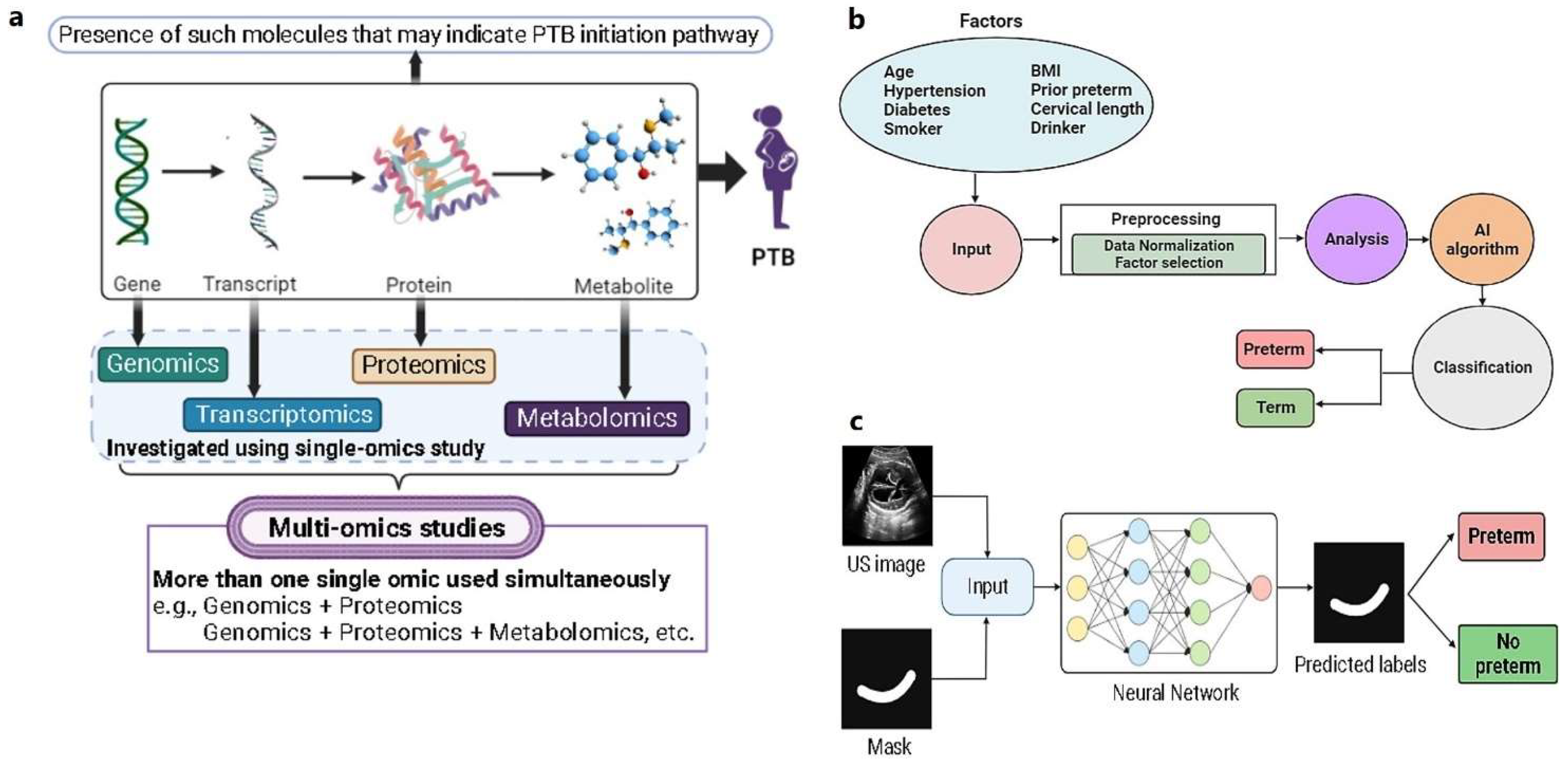
2.3. Multi-omic biomarker studies
2.3.1. Genomic biomarkers:
2.3.2. Transcriptomic biomarkers:
2.3.3. Proteomic biomarkers:
| Sr.no | Markers | Sample | Period (weeks) | Detection limit | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Ref |
| I. Physical Method | |||||||||
| 1. | Cervical length | NA | 22–24 | < 25 mm | 47 | 89 | 37 | 93 | [84] |
| 2. | UCA | NA | 18-36 | ≥111° | 65.1 | 43.6 | 29.8 | 77.3 | [30] |
| 3. | Ferning test | NA | 34-37 | NA | 84.5 | 78.2 | 79.5 | 83.5 | [85] |
| II. Chemical Method | |||||||||
| 1. | Nitrazine Test | Amniotic fluid | 28-36 | NA | 87.3 | 80.9 | 82.1 | 86.4 | [85] |
| III. Biomarker-based method | |||||||||
| Specific Biomarkers | |||||||||
| 1. | fFN | CVF | 23-34 | ≥ 50 µg/mL | 66.7 | 87.9 | 36.4 | 96.2 | [86] |
| 2. | PAMG-1 | CVF | 24-34 | ≥ 4 pg/ml | 90.0 | 93.8 | 78.3 | 97.4 | [87] |
| 66.7 | 98.6 | 75 | 97.9 | [88] | |||||
| 3. | IGFBP-1 | CVF | 20-35 | ≥ 30 µg/ml | 89.5 | 94.1 | 94.4 | 88.9 | [89] |
| 83.3 | 84.4 | 41.7 | 97.4 | [90] | |||||
| 70 | 74 | 48 | 88 | [91] | |||||
| Nonspecific biomarkers | |||||||||
| 1. | Ferritin | Serum | ≥37.5 ng/ml | 78.7 | 68.7 | 71.5 | 76.3 | [68] | |
| 2. | CRP | Serum | ≤20 | ≥5.27 mg/l | 75 | 86.1 | 37.5 | 96.87 | [74] |
| 3. | Prolactin | CVF | 24-36 | >7 ng/mL | 78 | 80 | 88.64 | 64.52 | [72] |
| 20-40 | 9.5 ng/L | 87.03 | 75 | 75.80 | 86.53 | [71] | |||
| 28-36 | 30 ng/L | 95 | 78 | 93 | 84 | [92] | |||
| 4. | Urocortin-1 | Amniotic fluid | 13-28 | ≥57.88 pg/mL | 81.8 | 40.0 | 40 | 82 | [93] |
| 5. | CRH | Serum | 24-36 | 10.45 pg/ml | 80 | 100 | 100 | 55.56 | [76] |
| 6. | ACTH | Serum | 24-36 | 14.65 pg/ml | 80 | 100 | 100 | 55.56 | [76] |
| 7. | MMP-8 | Amniotic fluid | 20 to 36 | >30 ng/mL | 82.4 | 78.0 | 36.0 | 97.7 | [94] |
2.3.4. Metabolomic biomarkers:
2.3.5. Multi-omic biomarkers:
| Sr. No. | Identified biomarkers | Phenotype | Ref. |
| Genomic biomarkers | |||
| 1 | ABCA13 | PTB | [44] |
| 2 | microRNAs (miRNA) and miR | PTB | [45-47] |
| 3 | TIMP2 | Inflammation and infection | [34,35] |
| 4 | COL4A3 | Inflammation and infection | [35,36] |
| 5 | TNF | Inflammation and infection | [37-40] |
| 6 | TNF1 and TNF2 | PTB | [40], [49], [50] |
| 7 | TNFRSF6 | PPROM | [39], [36] |
| 8 | Toll-like receptor | PPROM | [41] |
| Transcriptomic biomarkers | |||
| 9 | miR-21, miR-142, miR-30e, miR-148b, miR-29b and miR-223 | ↓ Gestational period | [47] |
| 10 | MIR4266, MIR1251, MIR601 and MIR3612 | ↑ sPTB risk | [55] |
| 11 | LINC00870 and LINC00094 | ↑ PTB risk | [55] |
| 12 | TLR4 | ↑ PTB risk | [56], [57] |
| 13 | IL-6R | [58] | |
| Proteomic biomarkers | |||
| 14 | lipocalin-type prostaglandin D2 synthase | ↑ PTB risk | [102] |
| 15 | ILs | ↑ PTB and PPROM risk | [60], [61] |
| Metabolomics biomarkers | |||
| 16 | ↑ Glutamate, dulcitol, urocanic acid, N-acetyl glutamine, 1-methyladenine, salicylamide, oleic acid, diglyceride | ↑ PTB risk | [36,96,99] |
| ↓ Glutamine, pyruvate, inositol, alanine, pyroglutamic acid, glutamine, galactose, hexose cluster 5 and 3, inositol, urea, phosphatidylcholines, phosphatidylinositol, ceramides | ↑ PTB risk | [36,96,99] | |
| Multi-omics studies | |||
| 1 | metabolomic (e.g., arabitol, xylitol, etc.), proteomic (e.g., VEGF 121, activin-A, MMPs, etc.), and immunome (e.g., CD56, INF-α, etc.) markers | combine metabolome, proteome, and immunome | [100] |
| 2 | IL-6 polymorphisms and MMP-9 | combine genomics and proteomics | [101] |
| 3 | TLR4 and TNF-α genes with TLR4 mRNA level | combine transcriptomics and genetics | [57] |
| PTB: preterm birth; sPTB: spontaneous preterm birth; PPROM: preterm premature rupture of membrane; TNF: tissue necrosis factor; TLR-4: toll-like receptor 4; INF-α: Interferon α; VEGF 121: Vascular endothelial growth factor; | |||
2.4. AI/ML Methods
3. Principles of biomarker detection
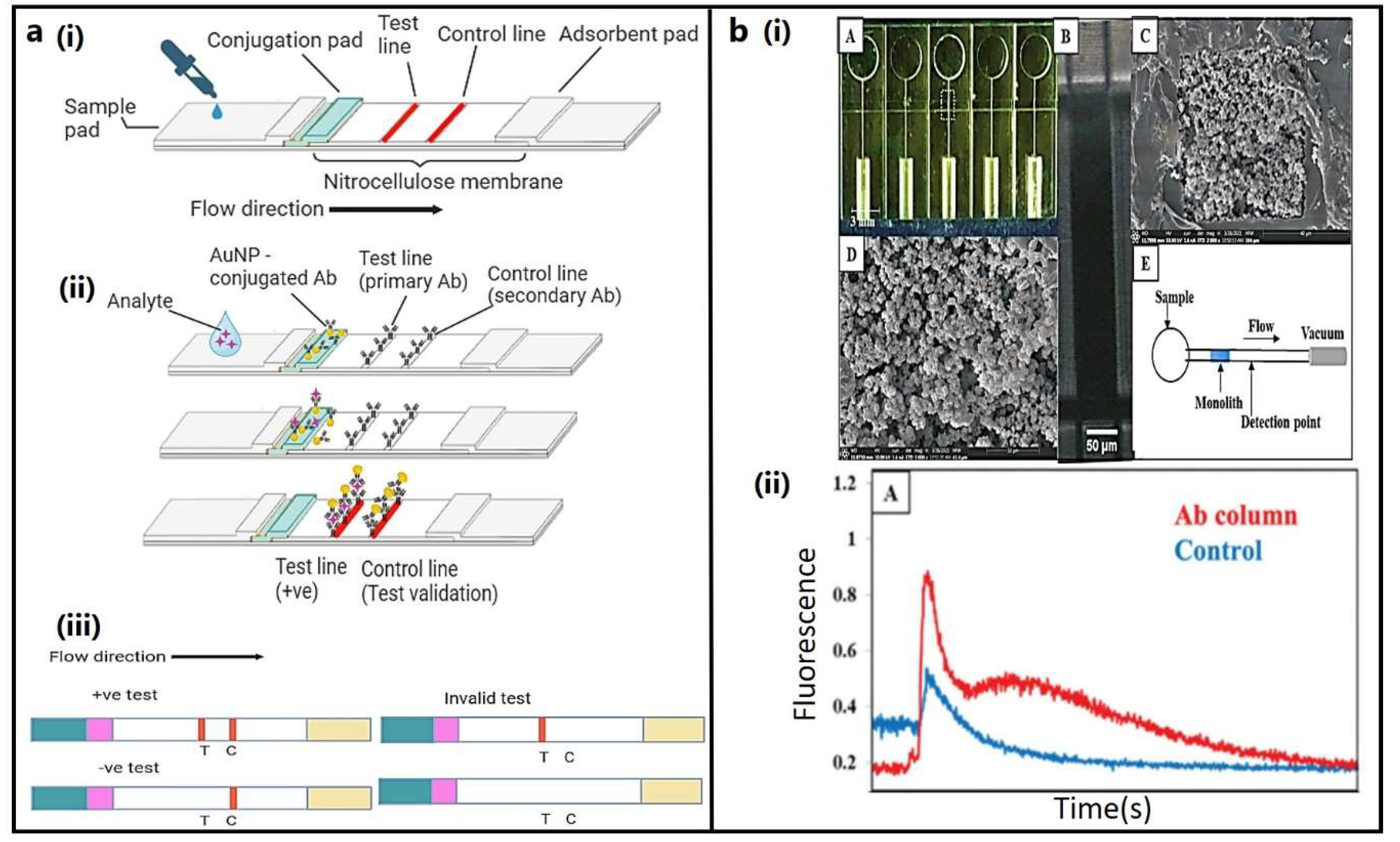
3.1. Lateral flow immunoassay (LFIA):
3.2. Microfluidic devices:
4. Point-of-care testing (POCT) devices
4.1. PartoSure® test
4.2. QuikCheck™ fFN
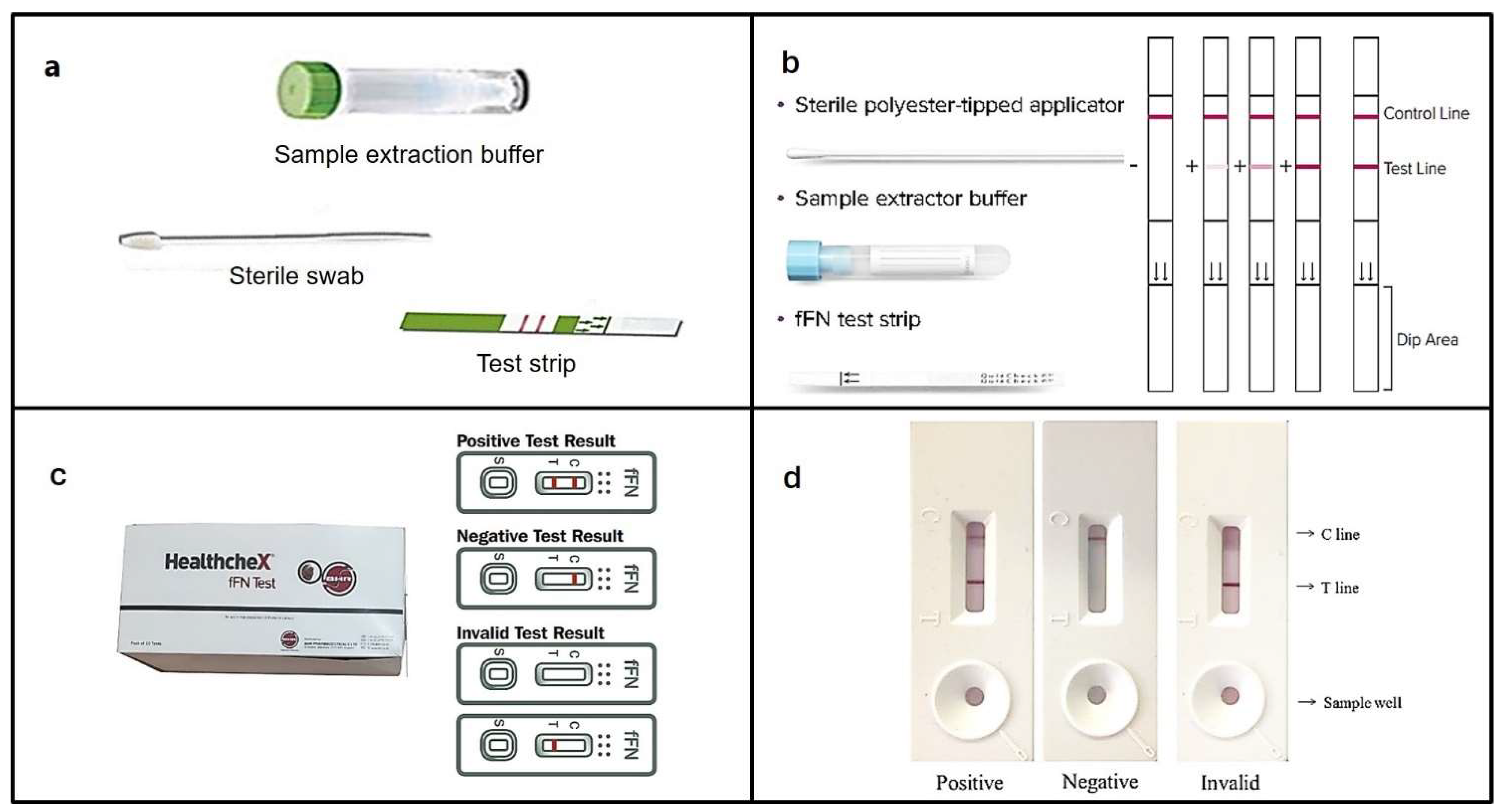
4.3. HealthcheX® Foetal Fibronectin (fFN) Test
4.4. Human Fetal Fibronectin XpressCard
4.5. Actim® Partus
4.6. Premaquick©
| Sr. no. | Device | Biomarker | Sample | LOD (ng/mL) |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | Ref. |
| 1. | PartoSure® test | PAMG-1 | CVS | 1.0 | 80 (<7 d) |
95 | 96 | 76 | - | [124] |
| 63 (<14 d) |
96 | 89 | 91 | - | ||||||
| 2. | Quikcheck fFN test | fFN | CVS | ≥ 50 | 94.5 | 89.1 | 89.7 | 94.2 | 91.8 | [85] |
| 3. | healthcheX fFN test | fFN | CVS | >50 | 98.1 | 98.7 | - | - | 98.4 | [126] |
| 4. | Antagen fFN XpressCard | fFN | Urine | 10 | - | - | - | - | - | [127] |
| 5. | Actim® Partus | ph IGFBP-1 | CVS | 10 | 60 | 67.7 | 23 | 91.3 | 66 | [130] |
| 95 | 92 | 86 | 97 | - | [132] | |||||
| 80 | 94 | 57 | 98 | - | [133] | |||||
| 6. | Premaquick© | IL-6/ phIGFBP-1/ IGFBP-1 | CVS | - | 95.1 | 97.5 | 97.5 | 95.2 | 96.3 | [131] |
5. Challenges
5. Treatment and preventive measures
6. Summary and outlook
Acknowledgment
Conflict of Interest
References
- Behrman, R.E.; Butler, A.S. Preterm birth: causes, consequences, and prevention. 2007.
- (WHO), W.H.O. Preterm birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 10 May 2023).
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet Child & Adolescent Health 2022, 6, 106–115. [Google Scholar]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet global health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Born too soon: decade of action on preterm birth; World Health Organization: 2023.
- Griggs, K.M.; Hrelic, D.A.; Williams, N.; McEwen-Campbell, M.; Cypher, R. Preterm labor and birth: a clinical review. MCN: The American Journal of Maternal/Child Nursing 2020, 45, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, H.C.; Subramaniam, A.; Andrews, W.W. Infection and preterm birth. In Proceedings of the Seminars in Fetal and Neonatal Medicine; 2016; pp. 100–105. [Google Scholar]
- Couceiro, J.; Matos, I.; Mendes, J.J.; Baptista, P.V.; Fernandes, A.R.; Quintas, A. Inflammatory factors, genetic variants, and predisposition for preterm birth. Clinical Genetics 2021, 100, 357–367. [Google Scholar] [CrossRef]
- Sharami, S.H.; Darkhaneh, R.F.; Zahiri, Z.; Milani, F.; Asgharnia, M.; Shakiba, M.; Didar, Z. The relationship between vaginal bleeding in the first and second trimester of pregnancy and preterm labor. Iranian journal of reproductive medicine 2013, 11, 385. [Google Scholar] [PubMed]
- Waldorf, K.M.A.; Singh, N.; Mohan, A.R.; Young, R.C.; Ngo, L.; Das, A.; Tsai, J.; Bansal, A.; Paolella, L.; Herbert, B.R. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. American journal of obstetrics and gynecology 2015, 213, 830. e831–830. e819. [Google Scholar] [CrossRef] [PubMed]
- Hackney, D.N.; Glantz, J.C. Vaginal bleeding in early pregnancy and preterm birth: systemic review and analysis of heterogeneity. The Journal of Maternal-Fetal & Neonatal Medicine 2011, 24, 778–786. [Google Scholar]
- Many, A.; Hill, L.M.; Lazebnik, N.; Martin, J.G. The association between polyhydramnios and preterm delivery. Obstetrics & Gynecology 1995, 86, 389–391. [Google Scholar]
- Lilliecreutz, C.; Larén, J.; Sydsjö, G.; Josefsson, A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC pregnancy and childbirth 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, S.; Kimura, T.; Kakigano, A.; Sato, T.; Iso, H.; Group, J.E.C.s.S.; Saito, H.; Kishi, R.; Yaegashi, N.; Hashimoto, K. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: the Japan Environment and Children's Study. BJOG: An International Journal of Obstetrics & Gynaecology 2019, 126, 1448–1454. [Google Scholar]
- Mercer, B.M.; Goldenberg, R.L.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.F.; Caritis, S.N.; Miodovnik, M.; Menard, M.K.; Thurnau, G.R. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. American journal of obstetrics and gynecology 1999, 181, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Cobo, T.; Kacerovsky, M.; Jacobsson, B. Risk factors for spontaneous preterm delivery. International Journal of Gynecology & Obstetrics 2020, 150, 17–23. [Google Scholar]
- Voltolini, C.; Torricelli, M.; Conti, N.; Vellucci, F.L.; Severi, F.M.; Petraglia, F. Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions. Reproductive sciences 2013, 20, 1274–1292. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. Preterm birth and mortality in adulthood: a systematic review. Journal of Perinatology 2020, 40, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Chersich, M.F.; Pham, M.D.; Areal, A.; Haghighi, M.M.; Manyuchi, A.; Swift, C.P.; Wernecke, B.; Robinson, M.; Hetem, R.; Boeckmann, M. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. bmj 2020, 371. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. The lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Neonatal Network, G.C.f.L.R.; Consortium, P.I.a.t.b.o.l. Preterm birth and sustained inflammation: consequences for the neonate. In Proceedings of the Seminars in immunopathology; 2020; pp. 451–468. [Google Scholar]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus. In Proceedings of the Seminars in Immunopathology; 2020; pp. 413–429. [Google Scholar]
- Garg, A.; Jaiswal, A. Evaluation and Management of Premature Rupture of Membranes: A Review Article. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Yild, C.; Tanir, H.; Sener, T. Comparison of conventional methods (nitrazine test, ferning test) and placental alphamicroglobulin-1 (PAMG-1) in cervicovaginal discharge for the diagnosis of rupture of membranes. Int J Gynaecol Obstet 2009, 107, S530. [Google Scholar]
- Medley, N.; Poljak, B.; Mammarella, S.; Alfirevic, Z. Clinical guidelines for prevention and management of preterm birth: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology 2018, 125, 1361–1369. [Google Scholar]
- da Fonseca, E.B.; Damião, R.; Moreira, D.A. Preterm birth prevention. Best Practice & Research Clinical Obstetrics & Gynaecology 2020, 69, 40–49. [Google Scholar]
- Dochez, V.; Ducarme, G.; Gueudry, P.; Joueidi, Y.; Boivin, M.; Boussamet, L.; Pelerin, H.; Le Thuaut, A.; Lamoureux, Z.; Riche, V.-P. Methods of detection and prevention of preterm labour and the PAMG-1 detection test: a review. Journal of Perinatal Medicine 2021, 49, 119–126. [Google Scholar] [CrossRef]
- Obstetricians, A.C.o.; Gynecologists. Prelabor rupture of membranes: ACOG practice bulletin, number 217. Obstet Gynecol 2020, 135, e80–e97. [Google Scholar]
- Daskalakis, G.; Theodora, M.; Antsaklis, P.; Sindos, M.; Grigoriadis, T.; Antsaklis, A.; Papantoniou, N.; Loutradis, D.; Pergialiotis, V. Assessment of uterocervical angle width as a predictive factor of preterm birth: a systematic review of the literature. BioMed research international 2018, 2018. [Google Scholar] [CrossRef]
- Luechathananon, S.; Songthamwat, M.; Chaiyarach, S. Uterocervical angle and cervical length as a tool to predict preterm birth in threatened preterm labor. International Journal of Women's Health 2021, 153–159. [Google Scholar] [CrossRef]
- Olarinoye, A.O.; Olaomo, N.O.; Adesina, K.T.; Ezeoke, G.G.; Aboyeji, A.P. Comparative diagnosis of premature rupture of membrane by nitrazine test, urea, and creatinine estimation. International Journal of Health Sciences 2021, 15, 16. [Google Scholar]
- Dai, X.; Shen, L. Advances and trends in omics technology development. Frontiers in Medicine 2022, 9, 911861. [Google Scholar] [CrossRef]
- Gupta, J.K.; Alfirevic, A. Systematic review of preterm birth multi-omic biomarker studies. Expert Reviews in Molecular Medicine 2022, 24, e18. [Google Scholar] [CrossRef]
- Frey, H.A.; Stout, M.J.; Pearson, L.N.; Tuuli, M.G.; Cahill, A.G.; Strauss III, J.F.; Gomez, L.M.; Parry, S.; Allsworth, J.E.; Macones, G.A. Genetic variation associated with preterm birth in African-American women. American journal of obstetrics and gynecology 2016, 215, 235. e231–235. e238. [Google Scholar] [CrossRef]
- Romero, R.; Edwards, D.R.V.; Kusanovic, J.P.; Hassan, S.S.; Mazaki-Tovi, S.; Vaisbuch, E.; Kim, C.J.; Chaiworapongsa, T.; Pearce, B.D.; Friel, L.A. Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes. American journal of obstetrics and gynecology 2010, 202, 431. e431–431. e434. [Google Scholar] [CrossRef]
- Romero, R.; Friel, L.A.; Edwards, D.R.V.; Kusanovic, J.P.; Hassan, S.S.; Mazaki-Tovi, S.; Vaisbuch, E.; Kim, C.J.; Erez, O.; Chaiworapongsa, T. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). American journal of obstetrics and gynecology 2010, 203, 361. e361–361. e330. [Google Scholar] [CrossRef]
- Gebhardt, S.; Bruiners, N.; Hillermann, R. A novel exonic variant (221delT) in the LGALS13 gene encoding placental protein 13 (PP13) is associated with preterm labour in a low risk population. Journal of reproductive immunology 2009, 82, 166–173. [Google Scholar] [CrossRef]
- Annells, M.F.; Hart, P.H.; Mullighan, C.G.; Heatley, S.L.; Robinson, J.S.; Bardy, P.; McDonald, H.M. Interleukins-1,-4,-6,-10, tumor necrosis factor, transforming growth factor-β, FAS, and mannose-binding protein C gene polymorphisms in Australian women: risk of preterm birth. American journal of obstetrics and gynecology 2004, 191, 2056–2067. [Google Scholar] [CrossRef]
- Ramos, B.R.d.A.; Mendes, N.D.; Tanikawa, A.A.; Amador, M.A.T.; Santos, N.P.C.d.; Santos, S.E.B.d.; Castelli, E.C.; Witkin, S.S.; Silva, M.G.d. Ancestry informative markers and selected single nucleotide polymorphisms in immunoregulatory genes on preterm labor and preterm premature rupture of membranes: a case control study. BMC Pregnancy and childBirth 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Fortunato, S.J.; Menon, R.; Velez, D.R.; Thorsen, P.; Williams, S.M. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. American journal of obstetrics and gynecology 2008, 198, 666. e661–666. e610. [Google Scholar] [CrossRef]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.-P.; Russell, L. Genetic associations with gestational duration and spontaneous preterm birth. New England Journal of Medicine 2017, 377, 1156–1167. [Google Scholar] [CrossRef]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nature communications 2018, 9, 260. [Google Scholar] [CrossRef]
- Rappoport, N.; Toung, J.; Hadley, D.; Wong, R.J.; Fujioka, K.; Reuter, J.; Abbott, C.W.; Oh, S.; Hu, D.; Eng, C. A genome-wide association study identifies only two ancestry specific variants associated with spontaneous preterm birth. Scientific reports 2018, 8, 226. [Google Scholar] [CrossRef]
- Heng, Y.J.; Pennell, C.E.; McDonald, S.W.; Vinturache, A.E.; Xu, J.; Lee, M.W.; Briollais, L.; Lyon, A.W.; Slater, D.M.; Bocking, A.D. Maternal whole blood gene expression at 18 and 28 weeks of gestation associated with spontaneous preterm birth in asymptomatic women. PloS one 2016, 11, e0155191. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Anton, L.; Bastek, J.; Brown, A.G. Can microRNA profiling in maternal blood identify women at risk for preterm birth? American journal of obstetrics and gynecology 2015, 212, 782–e781. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Brown, A.G.; Anton, L.; Gilstrop, M.; Heiser, L.; Bastek, J. Distinct cervical microRNA profiles are present in women destined to have a preterm birth. American journal of obstetrics and gynecology 2014, 210, 221–e221. [Google Scholar] [CrossRef]
- Sanders, A.P.; Burris, H.H.; Just, A.C.; Motta, V.; Svensson, K.; Mercado-Garcia, A.; Pantic, I.; Schwartz, J.; Tellez-Rojo, M.M.; Wright, R.O. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics 2015, 10, 221–228. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews genetics 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Menon, R.; Velez, D.R.; Morgan, N.; Lombardi, S.J.; Fortunato, S.J.; Williams, S.M. Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Human genetics 2008, 124, 243–253. [Google Scholar] [CrossRef]
- Plunkett, J.; Muglia, L.J. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Annals of medicine 2008, 40, 167–179. [Google Scholar] [CrossRef]
- Kalish, R.B.; Nguyen, D.P.; Vardhana, S.; Gupta, M.; Perni, S.C.; Witkin, S.S. A single nucleotide A> G polymorphism at position− 670 in the Fas gene promoter: relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. American journal of obstetrics and gynecology 2005, 192, 208–212. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS computational biology 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Gray, C.; McCowan, L.M.; Patel, R.; Taylor, R.S.; Vickers, M.H. Maternal plasma miRNAs as biomarkers during mid-pregnancy to predict later spontaneous preterm birth: a pilot study. Scientific reports 2017, 7, 815. [Google Scholar] [CrossRef]
- Cook, J.; Bennett, P.R.; Kim, S.H.; Teoh, T.G.; Sykes, L.; Kindinger, L.M.; Garrett, A.; Binkhamis, R.; MacIntyre, D.A.; Terzidou, V. First trimester circulating microRNA biomarkers predictive of subsequent preterm delivery and cervical shortening. Scientific reports 2019, 9, 5861. [Google Scholar] [CrossRef]
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. The Journal of Maternal-Fetal & Neonatal Medicine 2022, 35, 1239–1247. [Google Scholar]
- Zhou, G.; Holzman, C.; Chen, B.; Wang, P.; Heng, Y.J.; Kibschull, M.; Lye, S.J.; Kasten, E.P. EBF1-correlated long non-coding RNA transcript levels in 3rd trimester maternal blood and risk of spontaneous preterm birth. Reproductive Sciences 2021, 28, 541–549. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, M. Association of TLR4 and TNF-α gene polymorphisms and TLR4 mRNA levels in preterm birth in a northern Indian population. Indian Pediatrics 2019, 56, 202–204. [Google Scholar] [CrossRef]
- Lee, S.Y.; Buhimschi, I.A.; Dulay, A.T.; Ali, U.A.; Zhao, G.; Abdel-Razeq, S.S.; Bahtiyar, M.O.; Thung, S.F.; Funai, E.F.; Buhimschi, C.S. IL-6 trans-signaling system in intra-amniotic inflammation, preterm birth, and preterm premature rupture of the membranes. The Journal of Immunology 2011, 186, 3226–3236. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World Journal of Biological Chemistry 2021, 12, 57. [Google Scholar] [CrossRef]
- Gunko, V.; Pogorelova, T.; Linde, V. Proteomic profiling of the blood serum for prediction of premature delivery. Bulletin of Experimental Biology and Medicine 2016, 161, 829–832. [Google Scholar] [CrossRef]
- Liong, S.; Di Quinzio, M.K.; Heng, Y.J.; Fleming, G.; Permezel, M.; Rice, G.E.; Georgiou, H.M. Proteomic analysis of human cervicovaginal fluid collected before preterm premature rupture of the fetal membranes. Reproduction 2013, 145, 137–147. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Nowicki, B.J.; Izban, M.G.; Pratap, S.; Sashti, N.A.; Sanderson, M.; Nowicki, S. Spontaneous preterm labor is associated with an increase in the proinflammatory signal transducer TLR4 receptor on maternal blood monocytes. BMC pregnancy and childbirth 2010, 10, 1–9. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Goepfert, A.R.; Ramsey, P.S. Biochemical markers for the prediction of preterm birth. American journal of obstetrics and gynecology 2005, 192, S36–S46. [Google Scholar] [CrossRef]
- Kolev, N.; Atanasova, T. COMBINED APPLICATION OF PLACENTAL ALPHA MICROGLOBULIN-1 (PAMG-1) AND INSULIN-LIKE GROWTH FACTOR IN THE DIAGNOSIS OF PRETERM BIRTH-OUR RESULTS. KNOWLEDGE-International Journal 2022, 52, 497–500. [Google Scholar]
- Leitich, H.; Kaider, A. Fetal fibronectin—how useful is it in the prediction of preterm birth? BJOG: An International Journal of Obstetrics & Gynaecology 2003, 110, 66–70. [Google Scholar]
- Chen, M.X.; Dansereau, J.; Hoag, G.N. Comparison of Fetal Fibronectin and Phosphorylated Insulin-Like Growth Factor Binding Protein-1 Testing to Predict Preterm Delivery in Symptomatic Women: A 10-Year Retrospective Study. Journal of Obstetrics and Gynaecology Canada 2020, 42, 971–976. [Google Scholar] [CrossRef]
- Khambalia, A.Z.; Collins, C.E.; Roberts, C.L.; Morris, J.M.; Powell, K.L.; Tasevski, V.; Nassar, N. High maternal serum ferritin in early pregnancy and risk of spontaneous preterm birth. British Journal of Nutrition 2015, 114, 455–461. [Google Scholar] [CrossRef]
- Jahedbozorgan, T.; Yaghmaei, M.; Naserieh, M. Comparison of serum ferritin levels in pregnant women with preterm and term deliveries. Immunopathologia Persa 2020, 6, e25–e25. [Google Scholar] [CrossRef]
- Shah, K.H.; Anjum, A.; Nair, P.; Bhat, P.; Bhat, R.G.; Bhat, S. Pregnancy associated plasma protein A: An indicator of adverse obstetric outcomes in a South India population. Turkish journal of obstetrics and gynecology 2020, 17, 40. [Google Scholar] [CrossRef]
- Vitale, S.G.; Laganà, A.S.; Rapisarda, A.M.C.; Scarale, M.G.; Corrado, F.; Cignini, P.; Butticè, S.; Rossetti, D. Role of urocortin in pregnancy: an update and future perspectives. World journal of clinical cases 2016, 4, 165. [Google Scholar] [CrossRef]
- Kariman, N.; Hedayati, M.; Majd, S.A. The role of vaginal prolactin in diagnosis of premature rupture of membranes. Iranian Red Crescent Medical Journal 2012, 14, 352. [Google Scholar]
- Mehrotra, S.; Solanki, V.; Natu, S.; Chauhan, S.; Sharma, R. Study of Prolactin in Cervicovaginal Secretion in Women with Preterm Labor and Normal Pregnancy. Journal of South Asian Federation of Obstetrics and Gynaecology 2020, 12, 34–37. [Google Scholar]
- Vadillo-Ortega, F.; Estrada-Gutiérrez, G. Role of matrix metalloproteinases in preterm labour. BJOG: An International Journal of Obstetrics & Gynaecology 2005, 112, 19–22. [Google Scholar]
- Nikbakht, R.; Moghadam, E.K.; Nasirkhani, Z. Maternal serum levels of C-reactive protein at early pregnancy to predict fetal growth restriction and preterm delivery: A prospective cohort study. International journal of reproductive biomedicine 2020, 18, 157. [Google Scholar] [CrossRef]
- Moghaddam Banaem, L.; Mohamadi, B.; Asghari Jaafarabadi, M.; Aliyan Moghadam, N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. Journal of Obstetrics and Gynaecology Research 2012, 38, 780–786. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Semmler, M.; Briese, V.; Eckerle, H.; Minas, V.; Mylonas, I.; Friese, K.; Jeschke, U. Maternal serum corticotropin-releasing hormone and ACTH levels as predictive markers of premature labor. International Journal of Gynecology & Obstetrics 2007, 97, 115–119. [Google Scholar]
- Beutler, B.; Greenwald, D.; Hulmes, J.; Chang, M.; Pan, Y.-C.; Mathison, J.; Ulevitch, R.; Cerami, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985, 316, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Lombardi, S.J.; Fortunato, S.J. TNF-α promotes caspase activation and apoptosis in human fetal membranes. Journal of assisted reproduction and genetics 2002, 19, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zeng, W.-Y. Relationship among TNF-alpha gene promoter-308 site polymorphism, the levels of maternal serum TNF-alpha, and the mRNA expression placental TNF-alpha in preterm labor. Sichuan da xue xue bao. Yi xue ban= Journal of Sichuan University. Medical Science Edition 2009, 40, 77–80. [Google Scholar] [PubMed]
- Wang, X.-J.; Li, L.; Cui, S.-H. Role of collagen III, CTGF and TNF-alpha in premature rupture of human fetal membranes. Sichuan da xue xue bao. Yi xue ban= Journal of Sichuan University. Medical Science Edition 2009, 40, 658–661, 675. [Google Scholar] [PubMed]
- Puchner, K.; Iavazzo, C.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Kouskouni, E.; Economou, E.; Malamitsi-Puchner, A.; Creatsas, G. The implication of second-trimester amniotic fluid TNF-alpha, cytochrome C and cell death nucleosomes in the prediction of preterm labor and/or premature rupture of membranes. Archives of gynecology and obstetrics 2012, 285, 37–43. [Google Scholar] [CrossRef] [PubMed]
- McNamara, H.; Mallaiah, S. Managing coagulopathy following PPH. Best Practice & Research Clinical Obstetrics & Gynaecology 2019, 61, 106–120. [Google Scholar]
- Elovitz, M.A.; Baron, J.; Phillippe, M. The role of thrombin in preterm parturition. American journal of obstetrics and gynecology 2001, 185, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Wildschut, H.I.; Weiner, C.P.; Peters, T.J. When to screen in obstetrics and gynecology; Elsevier Health Sciences: 2006.
- Abdelazim, I.A. Fetal fibronectin (Quick Check fFN test®) for detection of premature rupture of fetal membranes. Archives of gynecology and obstetrics 2013, 287, 205–210. [Google Scholar] [CrossRef]
- Liong, S.; Di Quinzio, M.; Fleming, G.; Permezel, M.; Rice, G.; Georgiou, H. New biomarkers for the prediction of spontaneous preterm labour in symptomatic pregnant women: a comparison with fetal fibronectin. BJOG: An International Journal of Obstetrics & Gynaecology 2015, 122, 370–379. [Google Scholar]
- Nikolova, T.; Bayev, O.; Nikolova, N.; Di Renzo, G.C. Evaluation of a novel placental alpha microglobulin-1 (PAMG-1) test to predict spontaneous preterm delivery. Journal of perinatal medicine 2014, 42, 473–477. [Google Scholar] [CrossRef]
- Lotfi, G.; Faraz, S.; Nasir, R.; Somini, S.; Abdeldayem, R.M.; Koratkar, R.; Alsawalhi, N.; Ammar, A. Comparison of the effectiveness of a PAMG-1 test and standard clinical assessment in the prediction of preterm birth and reduction of unnecessary hospital admissions. The Journal of Maternal-Fetal & Neonatal Medicine 2019, 32, 793–797. [Google Scholar]
- Lembet, A.; Eroglu, D.; Ergin, T.; Kuscu, E.; Zeyneloglu, H.; Batioglu, S.; Haberal, A. New rapid bed-side test to predict preterm delivery: phosphorylated insulin-like growth factor binding protein-1 in cervical secretions. Acta obstetricia et gynecologica Scandinavica 2002, 81, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, D.; Yanık, F.; Oktem, M.; Zeyneloglu, H.B.; Kuscu, E. Prediction of preterm delivery among women with threatened preterm labor. Gynecologic and obstetric investigation 2007, 64, 109–116. [Google Scholar] [CrossRef]
- Tanir, H.M.; Sener, T.; Yildiz, Z. Cervical phosphorylated insulin-like growth factor binding proteın-1 for the prediction of preterm delivery in symptomatic cases with intact membranes. Journal of Obstetrics and Gynaecology Research 2009, 35, 66–72. [Google Scholar] [CrossRef]
- Buyukbayrak, E.; Turan, C.; Unal, O.; Dansuk, R.; Cengizoğlu, B. Diagnostic power of the vaginal washing-fluid prolactin assay as an alternative method for the diagnosis of premature rupture of membranes. The Journal of Maternal-Fetal & Neonatal Medicine 2004, 15, 120–125. [Google Scholar]
- Karaer, A.; Celik, E.; Celik, O.; Simsek, O.Y.; Ozerol, İ.H.; Yılmaz, E.; Turkcuoglu, I.; Duz, S.A. Amniotic fluid urocortin-1 concentrations for the prediction of preterm delivery. Journal of Obstetrics and Gynaecology Research 2013, 39, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Maymon, E.; Romero, R.; Chaiworapongsa, T.; Berman, S.; Conoscenti, G.; Gomez, R.; Edwin, S. Amniotic fluid matrix metalloproteinase–8 in preterm labor with intact membranes. American journal of obstetrics and gynecology 2001, 185, 1149–1155. [Google Scholar] [CrossRef]
- Everett, J. NMR-based pharmacometabonomics: A new approach to personalised medicine. NMR in Pharmaceutical Science 2015, 359. [Google Scholar]
- Virgiliou, C.; Gika, H.G.; Witting, M.; Bletsou, A.A.; Athanasiadis, A.; Zafrakas, M.; Thomaidis, N.S.; Raikos, N.; Makrydimas, G.; Theodoridis, G.A. Amniotic fluid and maternal serum metabolic signatures in the second trimester associated with preterm delivery. Journal of proteome research 2017, 16, 898–910. [Google Scholar] [CrossRef]
- Considine, E.C.; Khashan, A.S.; Kenny, L.C. Screening for preterm birth: potential for a metabolomics biomarker panel. Metabolites 2019, 9, 90. [Google Scholar] [CrossRef]
- Eick, S.M.; Ferguson, K.K.; Milne, G.L.; Rios-McConnell, R.; Vélez-Vega, C.; Rosario, Z.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Repeated measures of urinary oxidative stress biomarkers and preterm birth in Puerto Rico. Free Radical Biology and Medicine 2020, 146, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Morillon, A.-C.; Yakkundi, S.; Thomas, G.; Gethings, L.A.; Langridge, J.I.; Baker, P.N.; Kenny, L.C.; English, J.A.; McCarthy, F.P. Association between phospholipid metabolism in plasma and spontaneous preterm birth: a discovery lipidomic analysis in the cork pregnancy cohort. Metabolomics 2020, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, I.A.; Ghaemi, M.S.; Han, X.; Ando, K.; Hédou, J.J.; Feyaerts, D.; Peterson, L.S.; Rumer, K.K.; Tsai, E.S.; Ganio, E.A. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Science translational medicine 2021, 13, eabd9898. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Awasthi, S.; Baranwal, S. IL-6: an endogenous activator of MMP-9 in preterm birth. Journal of Reproductive Immunology 2020, 141, 103147. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Palaia, T.; Hall, C.E.; Ragolia, L. Role of Lipocalin-type prostaglandin D2 synthase (L-PGDS) and its metabolite, prostaglandin D2, in preterm birth. Prostaglandins & other lipid mediators 2015, 118, 28–33. [Google Scholar]
- AlSaad, R.; Malluhi, Q.; Boughorbel, S. PredictPTB: an interpretable preterm birth prediction model using attention-based recurrent neural networks. BioData Mining 2022, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Prema, N.; Pushpalatha, M. Machine learning approach for preterm birth prediction based on maternal chronic conditions. In Proceedings of the Emerging Research in Electronics, Computer Science and Technology: Proceedings of International Conference, ICERECT 2018, 2019; pp. 581–588.
- Raja, R.; Mukherjee, I.; Sarkar, B.K. A machine learning-based prediction model for preterm birth in rural India. Journal of Healthcare Engineering 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Zhu, L.; Cai, Z. Evaluation measures of the classification performance of imbalanced data sets. In Proceedings of the Computational Intelligence and Intelligent Systems: 4th International Symposium, ISICA 2009, Huangshi, China, 2009. Proceedings 4, October 23-25, 2009; pp. 461–471.
- Mercer, B.; Goldenberg, R.; Das, A.; Moawad, A.; Iams, J.; Meis, P.; Copper, R.; Johnson, F.; Thom, E.; McNellis, D. The preterm prediction study: a clinical risk assessment system. American journal of obstetrics and gynecology 1996, 174, 1885–1895. [Google Scholar] [CrossRef]
- Goodwin, L.K.; Iannacchione, M.A.; Hammond, W.E.; Crockett, P.; Maher, S.; Schlitz, K. Data mining methods find demographic predictors of preterm birth. Nursing research 2001, 50, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Osmundson, S.; Edwards, D.R.V.; Jackson, G.P.; Malin, B.A.; Chen, Y. Deep learning predicts extreme preterm birth from electronic health records. Journal of biomedical informatics 2019, 100, 103334. [Google Scholar] [CrossRef]
- Weber, A.; Darmstadt, G.L.; Gruber, S.; Foeller, M.E.; Carmichael, S.L.; Stevenson, D.K.; Shaw, G.M. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Annals of epidemiology 2018, 28, 783–789. e781. [Google Scholar] [CrossRef] [PubMed]
- Mailath-Pokorny, M.; Polterauer, S.; Kohl, M.; Kueronyai, V.; Worda, K.; Heinze, G.; Langer, M. Individualized assessment of preterm birth risk using two modified prediction models. European Journal of Obstetrics & Gynecology and Reproductive Biology 2015, 186, 42–48. [Google Scholar]
- Son, M.; Miller, E.S. Predicting preterm birth: cervical length and fetal fibronectin. In Proceedings of the Seminars in perinatology; 2017; pp. 445–451. [Google Scholar]
- Elaveyini, U.; Devi, S.P.; Rao, K.S. Neural networks prediction of preterm delivery with first trimester bleeding. Archives of gynecology and obstetrics 2011, 283, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, T.; Płotka, S.; Rokita, P.; Sochacki-Wójcicka, N.; Wójcicki, J.; Lipa, M.; Trzciński, T. Spontaneous preterm birth prediction using convolutional neural networks. In Proceedings of the Medical Ultrasound, and Preterm, Perinatal and Paediatric Image Analysis: First International Workshop, ASMUS 2020, and 5th International Workshop, PIPPI 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, October 4-8 2020, Proceedings 1, 2020; pp. 274–283.
- Włodarczyk, T.; Płotka, S.; Szczepański, T.; Rokita, P.; Sochacki-Wojcicka, N.; Wojcicki, J.; Lipa, M.; Trzciński, T. Machine learning methods for preterm birth prediction: a review. Electronics 2021, 10, 586. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays in biochemistry 2016, 60, 111–120. [Google Scholar] [PubMed]
- Wong, R.; Tse, H. Lateral flow immunoassay; Springer Science & Business Media: 2008.
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Analytical and Bioanalytical Chemistry 2022, 414, 167–180. [Google Scholar] [CrossRef]
- Esplin, M.S.; Merrell, K.; Goldenberg, R.; Lai, Y.; Iams, J.D.; Mercer, B.; Spong, C.Y.; Miodovnik, M.; Simhan, H.N.; Van Dorsten, P. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. American journal of obstetrics and gynecology 2011, 204, 391. e391–391. e398. [Google Scholar] [CrossRef]
- Sahore, V.; Kumar, S.; Rogers, C.; Jensen, J.; Sonker, M.; Woolley, A. Pressure-actuated microfluidic devices for electrophoretic separation of pre-term birth biomarkers. Analytical and bioanalytical chemistry 2016, 408, 599–607. [Google Scholar] [CrossRef]
- Almughamsi, H.M.; Howell, M.K.; Parry, S.R.; Esene, J.E.; Nielsen, J.B.; Nordin, G.P.; Woolley, A.T. Immunoaffinity monoliths for multiplexed extraction of preterm birth biomarkers from human blood serum in 3D printed microfluidic devices. Analyst 2022, 147, 734–743. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Nielsen, A.V.; Carson, R.H.; Lin, H.J.L.; Hanson, R.L.; Sonker, M.; Mortensen, D.N.; Price, J.C.; Woolley, A.T. Analysis of thrombin-antithrombin complex formation using microchip electrophoresis and mass spectrometry. Electrophoresis 2019, 40, 2853–2859. [Google Scholar] [CrossRef]
- Rouholamin, S.; Razavi, M.; Rezaeinejad, M.; Sepidarkish, M. A diagnostic profile on the PartoSure test. Expert review of molecular diagnostics 2020, 20, 1163–1170. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Abdelrazak, K.M.; Al-Kadi, M.; Yehia, A.H.; Abdulkareem, A.F. Fetal fibronectin (Quick Check fFN test) versus placental alpha microglobulin-1 (AmniSure test) for detection of premature rupture of fetal membranes. Archives of gynecology and obstetrics 2014, 290, 457–464. [Google Scholar] [CrossRef] [PubMed]
- BHR Pharmaceuticals Ltd. healthchex fFN test instructions. (accessed on). Available online: https://professional.bhr.co.uk/pub/media/wysiwyg/ffn-userguide.pdf.
- Antagen Pharmaceuticals, I. Human Fetal Fibronectin XpressCard. (accessed on). Available online: https://www.antagen.net/wp-content/uploads/2015/05/fFN-XpressCard-Antagen.pdf.
- Lembet, A.; Eroglu, D.; Ergin, T.; Kuscu, E.; Zeyneloglu, H.; Batioglu, S.; Haberal, A. New rapid bed-side test to predict preterm delivery: phosphorylated insulin-like growth factor binding protein-1 in cervical secretions. Acta obstetricia et gynecologica Scandinavica 2002, 81, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Varley-Campbell, J.; Mújica-Mota, R.; Coelho, H.; Ocean, N.; Barnish, M.; Packman, D.; Dodman, S.; Cooper, C.; Snowsill, T.; Kay, T. Biomarker tests to help diagnose preterm labour in women with intact membranes. 2018.
- Tenoudji-Cohen Couka, L.; Donato, X.-C.; Glowaczower, E.; Squercioni-Aumont, A.; Katsogiannou, M.; Desbriere, R. Does Assessment of Cervical Phosphorylated Insulin-like Growth Factor Binding Protein-1 by Bedside Vaginal Swab Test Really Predict Preterm Birth? Reproductive Sciences 2021, 28, 2006–2011. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Amer, O.O.; Shikanova, S.; Karimova, B. Diagnostic accuracy of PremaQuick in detection of preterm labor in symptomatic women. Ginekologia Polska 2022, 93, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Tyagi, S.; Mala, Y.M.; Singh, N.; Pandey, N.B.; Yadav, P. Comparison of rapid bedside tests for phosphorylated insulin-like growth factor-binding protein 1 and fetal fibronectin to predict preterm birth. International Journal of Gynecology & Obstetrics 2016, 135, 47–50. [Google Scholar]
- Azlin, M.N.; Bang, H.K.; An, L.J.; Mohamad, S.; Mansor, N.; Yee, B.S.; Zulkifli, N.; Tamil, A.M. Role of phIGFBP-1 and ultrasound cervical length in predicting pre-term labour. Journal of Obstetrics and Gynaecology 2010, 30, 456–459. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN. PartoSure test. (accessed on). Available online: https://herqiagen.com/partosure/.
- Hologic, I. QuikCheck fFN Test Kit Instructions. (accessed on). Available online: https://www.hologic.com/sites/default/files/2018-05/AW-05842-002_004_02.pdf.
- Bruijn, M.M.; Kamphuis, E.I.; Hoesli, I.M.; de Tejada, B.M.; Loccufier, A.R.; Kühnert, M.; Helmer, H.; Franz, M.; Porath, M.M.; Oudijk, M.A. The predictive value of quantitative fibronectin testing in combination with cervical length measurement in symptomatic women. American journal of obstetrics and gynecology 2016, 215, 793. e791–793. e798. [Google Scholar] [CrossRef] [PubMed]
- Faron, G.; Balepa, L.; Parra, J.; Fils, J.-F.; Gucciardo, L. The fetal fibronectin test: 25 years after its development, what is the evidence regarding its clinical utility? A systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine 2020, 33, 493–523. [Google Scholar]
- Conde-Agudelo, A.; Romero, R. Cervical phosphorylated insulin-like growth factor binding protein-1 test for the prediction of preterm birth: a systematic review and metaanalysis. American journal of obstetrics and gynecology 2016, 214, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Hubinont, C.; Debiève, F. Prevention of preterm labour: 2011 update on tocolysis. Journal of pregnancy 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.D.; Reitman, E.; Gallos, G. Tocolysis: Present and future treatment options. In Proceedings of the Seminars in perinatology; 2017; pp. 493–504. [Google Scholar]
- Garfield, L.; Chin, E. Pharmacology for preterm labor. The Journal of Perinatal & Neonatal Nursing 2020, 34, 155–161. [Google Scholar]
- Yamaji, N.; Suzuki, H.; Saito, K.; Swa, T.; Namba, F.; Vogel, J.P.; Ramson, J.A.; Cao, J.; Tina, L.; Ota, E. Tocolytic Therapy Inhibiting Preterm Birth in High-Risk Populations: A Systematic Review and Meta-Analysis. Children 2023, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Ludmir, J. Drugs for the treatment and prevention of preterm labor. Clinics in perinatology 2019, 46, 159–172. [Google Scholar] [CrossRef]
- Organization, W.H. WHO recommendations on antenatal corticosteroids for improving preterm birth outcomes. In WHO recommendations on antenatal corticosteroids for improving preterm birth outcomes; 2022; pp. 40-40.
- Hassan, S.; Romero, R.; Vidyadhari, D.; Fusey, S.; Baxter, J.; Khandelwal, M.; Vijayaraghavan, J.; Trivedi, Y.; Soma-Pillay, P.; Sambarey, P. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound in Obstetrics & Gynecology 2011, 38, 18–31. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).