1. Introduction
Preterm birth (PTB) complicates over 10% of pregnancies in the USA [
1] and is a leading cause of neonatal morbidity and mortality [
2]. A potential strategy to reduce the rate of PTB is to identify patients at increased risk and to target specific interventions to those patients. As examples, vaginal progesterone reduces early PTB in patients with mid-trimester sonographic short cervix [
3,
4], and low-dose aspirin reduces preterm preeclampsia in patients with preeclampsia risk factors [
5] and reduces spontaneous PTB in patients with prior PTB [
6]. These interventions have been in widespread use for a decade, but the overall rate of PTB has not decreased, in part because only a small percentage of patients are identified as candidates for treatment.
A newer method of identifying patients at risk for PTB is the PreTRM™ test (Sera Prognostics Inc., Salt Lake City, UT, USA), developed through analysis of the maternal serum proteome. This test designates a patient at increased risk if a second-trimester blood sample has an elevated ratio of insulin-like growth factor binding protein-4 (IGFBP4) to sex hormone binding globulin (SHBG) [
7]. The test is a significant predictor of both indicated and spontaneous PTB <32 weeks, neonatal morbidity, and neonatal length of stay (LOS) [
8,
9], and has been suggested to be both cost-effective and cost-saving [
10,
11].
Prediction of PTB is of clinical value only if it leads to interventions that reduce the risk of PTB or its complications. For patients identified by the PreTRM test as having increased PTB risk, Branch et al. [
12] hypothesized that PTB could be reduced by a suite of interventions including progestogen treatment, low-dose aspirin, and a care management protocol comprising increased outreach, patient education and specialist care. To test this, they conducted the PREVENT-PTB trial, in which patients without traditional PTB risk factors were randomly allocated to be screened with the PreTRM test versus no screening. Screened patients who were identified as high-risk by the test were offered the interventions. The results showed no significant between-group difference in the median gestational age at birth (GA
birth) or in the proportion with PTB <37 wks. However, early termination of the trial due to funding restrictions left it underpowered for these outcomes. Interestingly, newborns in the screened group had shorter LOS in the neonatal intensive care unit (NICU) following PTB and a trend toward improved neonatal morbidity. The authors speculated that these improvements may have resulted from a lower rate of PTB <35 weeks in the screened group but did not evaluate this further.
The primary analysis of the PREVENT-PTB trial appropriately followed a prespecified statistical analysis plan (SAP). However, there is substantial loss of information when a continuous outcome such as GAbirth is dichotomized using arbitrary cut-points such as PTB <37 weeks. Dichotomization might mask clinically relevant differences of several days or even weeks in early PTBs. Further, the median GAbirth in the PREVENT-PTB trial (39.1 weeks in both groups) was largely driven by the majority of patients who delivered at term. We hypothesized that analysis of GAbirth as a continuous variable with a focus on the decile of patients with earliest births might reveal a significant difference that would explain the observed shorter LOS and trend toward reduced morbidity among those randomized to screening. The present study was designed to test this hypothesis. In addition, we also sought to explore which, if any, of the interventions was associated with increased GAbirth.
2. Materials and Methods
Synopsis of the PREVENT-PTB Trial
The PREVENT-PTB trial has been previously described in detail [
12]. Briefly, patients without current or historical PTB risk factors were randomly allocated to have the PreTRM test between 19
5/7 and 20
6/7 weeks of gestation (screened group, N = 595) versus standard obstetric care without the PreTRM test (control group, N = 596). In the screened group, a PreTRM test result indicating ≥14% risk of PTB was considered screen-positive and occurred in 33% (196 of 595). Screen-positive patients were offered prophylactic progestogen (either 17-hydroxyprogesterone caproate [17OHPC] 250 mg weekly or vaginal progesterone 200 mg each night), low-dose aspirin (81 mg daily), sonographic cervical length measurement, and care management (visits to a high-risk clinic, weekly phone contact, a smartphone app for symptom review, and access to 24-hour support). The trial was prospectively registered on clinicaltrials.gov (NCT 03530332), approved by Intermountain Healthcare’s institutional review board, and conducted with informed consent of all participants.
The primary outcome was the rate of spontaneous PTB <37 weeks. Using an adaptive study design, a planned sample size of 3,000 to 10,000 patients was targeted to power the trial to detect a reduction in spontaneous PTB from 6.4% in controls to 4.7% in screened patients. The trial was terminated at <40% of the planned sample size due to limited funding.
The primary outcome occurred in 2.7% of the screened group and in 3.5% of controls (P = 0.41). Rates of PTB <35 weeks (0.2% vs 0.8%, respectively) and PTB <32 weeks (0.2% vs 0.3%, respectively) were similar in the two groups. Median neonatal LOS was similar (1.9 days in both groups) overall, but NICU LOS was significantly shorter for preterm newborns in the screened group (7.6 days vs 36.7 days, P = 0.028). Fewer preterm newborns in the screened group had high scores on a composite morbidity and mortality index (16% vs 31%, P = 0.24) [
13].
Secondary Analysis
For the secondary analysis, the clinician investigators (CAC, JAFZ) met and agreed upon an SAP without having access to the primary data from the PREVENT-PTB trial. The trial sponsor, Sera Prognostics, Inc., released the requested de-identified primary data to the statistician investigators (MW, JS), who suggested minor modifications to the SAP after a preliminary review.
Our primary outcome of interest was GA
birth as a continuous outcome, comparing the screened group vs controls. To avoid diluting the outcome by the majority who delivered at term, we restricted the analyses to the subgroup defined by the earliest decile of each group, i.e., the 10% of subjects with the lowest GA
birth. Although it might have seemed more intuitive to restrict the analysis to those with PTB <37 weeks, we knew from the trial publication that the number of such births was too small to support our planned analyses. We generated Kaplan-Meier survival plots of GA
birth in each subgroup and calculated hazard ratios using Cox proportional hazards models with and without adjustment for maternal age (dichotomized as <40 years vs ≥40 years [
14]) and parity (dichotomized as nulliparous vs parous [
15,
16]). We repeated all these analyses after excluding the subgroup of patients who were treated with 17OHPC because the U. S. Food and Drug Administration has withdrawn approval of this medication [
17], and we wished to determine whether absence of 17OHPC treatment would influence the result.
Analysis of NICU LOS was similarly restricted to the earliest decile of each group. This analysis was also restricted to those who were admitted to NICU because NICU LOS is technically not defined for patients not admitted to NICU. Cox models for NICU LOS included hazard ratios with and without adjustment for maternal age, parity, and GAbirth.
Neonatal intensive respiratory support was defined as ventilator use (with intubation), high flow nasal cannula (≥2 L/min), continuous positive airway pressure , or nasal intermittent mechanical ventilation. Analysis of respiratory support was similarly restricted to the earliest decile and those who received any support. Cox models for respiratory support included hazard ratios with and without adjustment for maternal age, parity, and GAbirth.
To evaluate which, if any, of the interventions contributed to pregnancy prolongation, we evaluated the GAbirth outcome in the screen-positive patients in the screened group, comparing those who declined all the offered interventions to those who accepted some or all of the interventions, in various combinations. The specific interventions analyzed were progestogens, low-dose aspirin, and care management. We generated Kaplan-Meier plots of GAbirth comparing intervention vs declined-intervention subgroups and calculated hazard ratios using Cox proportional hazards models with and without adjustment for maternal age and parity.
Analyses were performed using R software version 4.2.2 [
18]. Between-group differences in hazard ratios were evaluated using log-rank test, with 2-tailed P-values <0.05 considered significant. No correction for multiple comparisons was made.
3. Results
From the total trial pool of 1191 randomized subjects, the earliest decile of GAbirth comprised 123 subjects (63 in the screened group, 60 in controls).
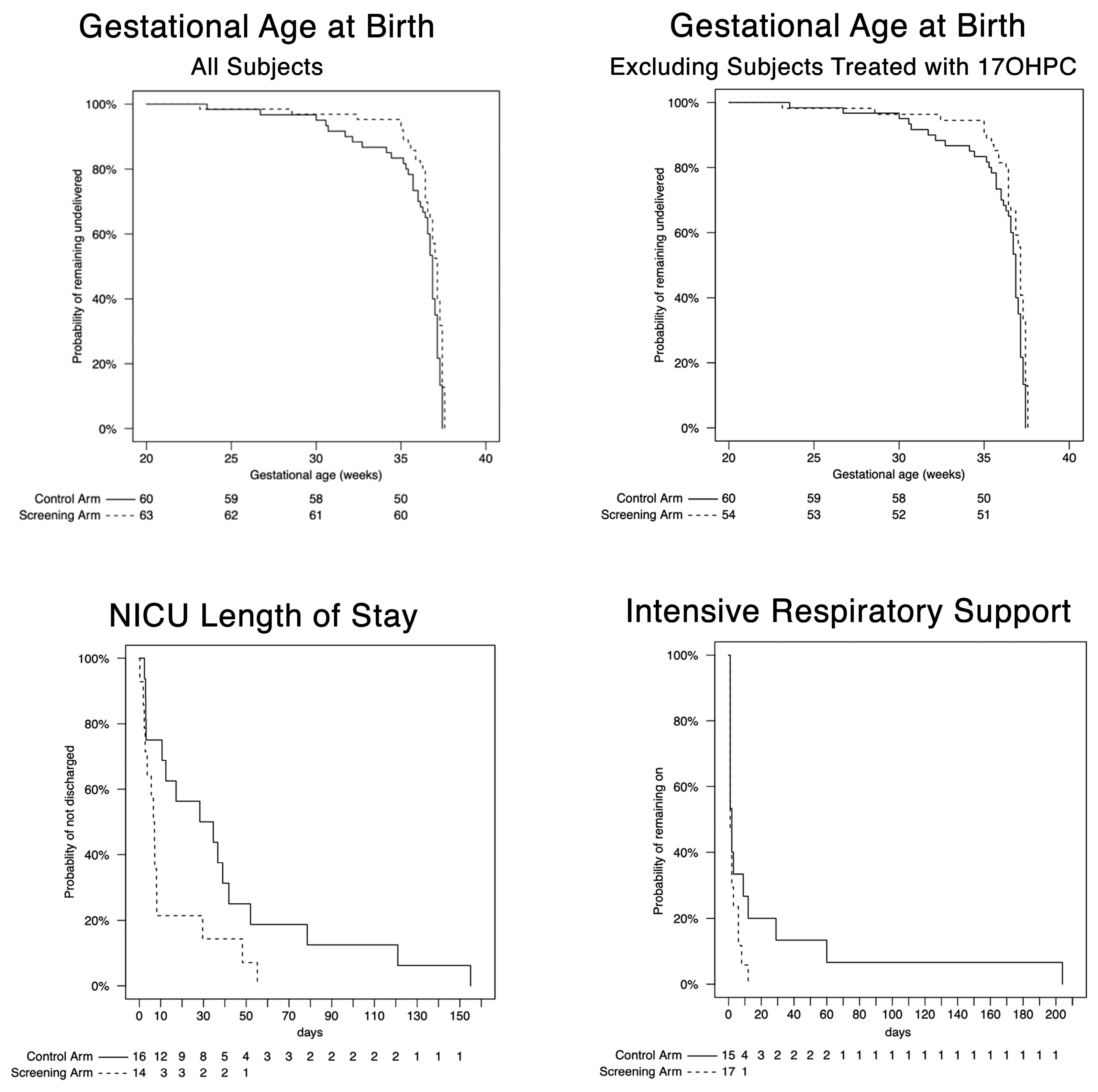
The survival plots of GA
birth, NICU LOS, and days of respiratory support in screened versus control subjects in the earliest decile are shown in
Figure 1. For GA
birth, there was a distinct separation between the curves between 32 weeks and 37 weeks reflecting fewer births in the screened group at these gestational ages, as shown in the upper left panel of
Figure 1. This separation persisted after excluding the 9 patients who were treated with 17OHPC in the earliest decile, as shown in the upper right panel. The adjusted hazard ratio 0.53 (95% CI, 0.36-0.78) was significant (P < 0.01,
Table 1), indicating a prolongation of pregnancy in the screened group. This effect persisted after excluding those treated with 17OHPC. However, despite this prolongation, the median GA
birth was similar in the screened subjects (37.1 weeks; interquartile range [IQR],], 36.4-37.4 weeks) compared to control subjects (36.9 weeks; IQR, 35.7-37.1). The prolongation of pregnancy in the screened group was associated with shorter NICU LOS, as shown in the middle panel of
Figure 1 and reflected in the adjusted hazard ratio 2.84 (95% CI, 1.12-6.70). Although duration of respiratory support was not significantly different between the 2 groups, the lower right panel of
Figure 1 is suggestive of a trend toward shorter duration in the screened group. After adjustment for GA
birth, there was no longer a significant difference in NICU LOS or any trend toward shorter duration of respiratory support.
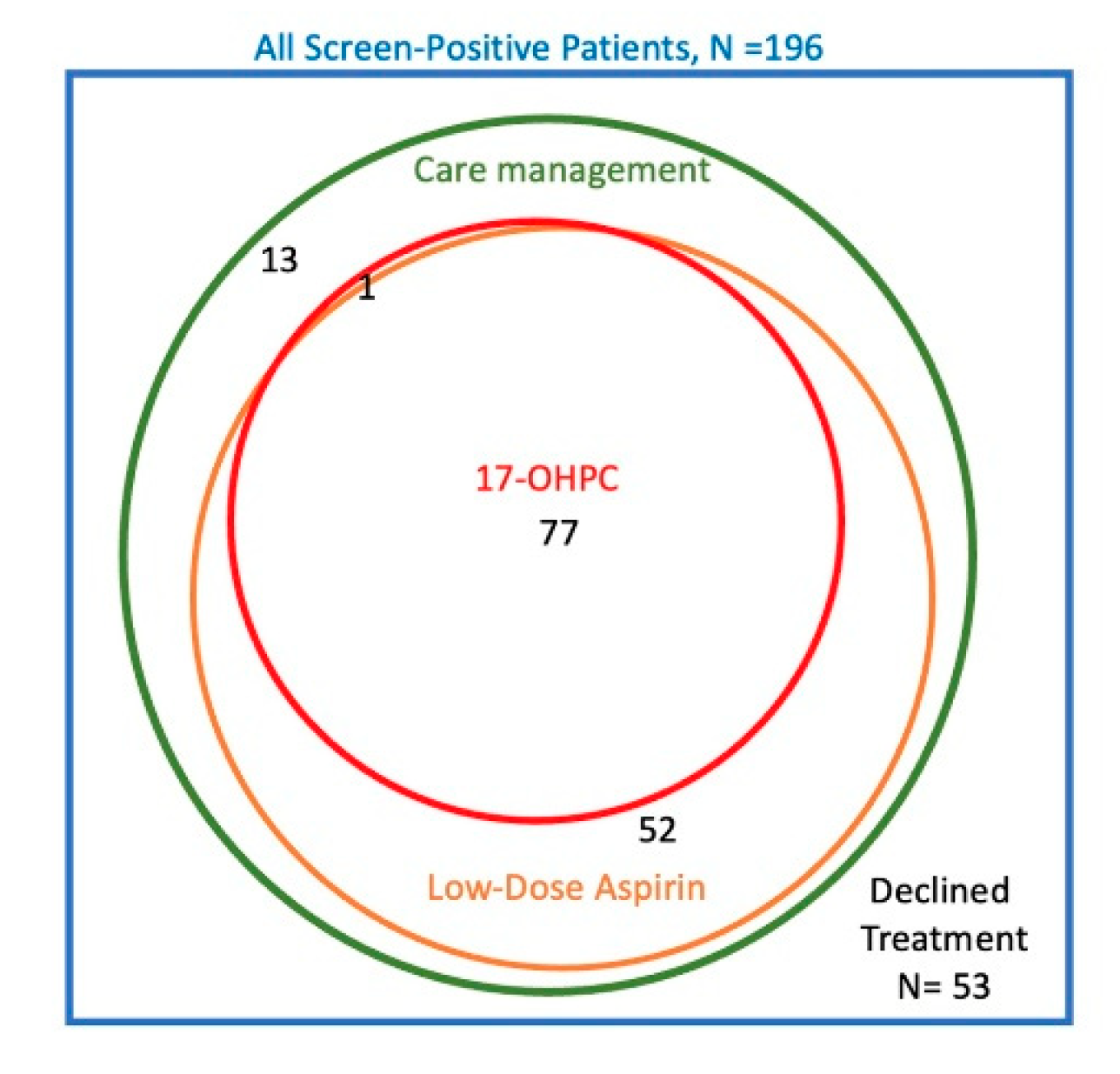
The Venn diagram in
Figure 2 summarizes the treatments chosen by the 196 subjects in the screened group who were screen positive. In total, 53 subjects (27%) declined all the offered interventions, 143 (73%) total elected care management, 129 (66%) elected low-dose aspirin, 78 (40%) elected prophylactic 17OHPC, and none used prophylactic vaginal progesterone. There were large overlaps in the treatments chosen. All patients who used either aspirin or 17OHPC were also enrolled in care management; only 13 subjects had care management alone without either medication. Similarly, 77 of 78 subjects (99%) who elected 17OHPC also took aspirin. On the other hand, 52 of 129 subjects (40%) who elected aspirin did not use 17OHPC. Because of these overlaps, it was not possible to evaluate the independent association of each treatment with GA
birth. Instead, we analyzed GA
birth among those who declined all treatment versus 3 comparison groups: those who accepted any-or-all treatments; those who had care management with-or-without aspirin but without 17OHPC; and those who had care management alone.
Table 2 summarizes the Cox regression statistics for these comparisons. There was no overall difference in GA
birth comparing those who had any treatment versus those who declined all treatments. Those who had care management with-or-without aspirin but who did not have 17OHPC vs those who declined treatment had significant prolongation of pregnancy (adjusted hazard ratio 0.66, 95% CI 0.46-0.97, P = 0.03). A similar prolongation was noted among those with care management alone compared to those who declined treatment (adjusted hazard ratio 0.48, 95% CI 0.24-0.94, P = 0.03).
4. Discussion
The principal finding of this secondary analysis of the PREVENT-PTB trial is that screening with the PreTRM test was associated with a significant prolongation of pregnancy compared to no screening. The prolongation is detectable as a separation of the survival curves between 32 and 37 weeks of gestation with GAbirth analyzed as a continuous variable. The prolongation was not detected in the primary analysis of the trial, in which GAbirth was dichotomized (i.e., < 37 vs ≥ 37 weeks of gestation, term vs preterm). The prolongation also was not reflected in an increase in median GAbirth. These observations underscore the importance of analyzing continuous variables as continuous variables and avoiding the data loss that occurs when collapsing them into dichotomous or categorical variables or expressing them as a synopsis measure of central tendency, such as median or mean.
We suggest that the pregnancy prolongation in the group screened with the PreTRM test, while modest, is clinically relevant. Newborns of subjects who had screening with the PreTRM test had shorter NICU LOS. There was also a trend toward less respiratory morbidity in the screened group, though statistical power was limited by the small number of subjects. Adjustment for GA
birth attenuated these effects (rightmost columns of
Table 1), suggesting that these benefits of screening and treatment are likely entirely attributable to reduction in early PTB in the screened group.
Because of the overlap in treatments chosen, we are unable to make definitive statements about whether the prolongation of pregnancy seen in the screened group was due to the use of care management, aspirin, or both in combination. It is reassuring that the benefits persisted after exclusion of subjects treated with 17OHPC, now that this drug is no longer approved for use in the United States.
Care management alone may reduce PTB in patients at increased risk, as suggested by a recent review [
19]. Our results support this suggestion, though the number of subjects in the PREVENT-PTB trial who had only care management was small.
Prophylactic low-dose aspirin has been associated with reduction of recurrent PTB in patients with prior PTB [
6] and our results are suggestive of a potential benefit for patients selected by a positive PreTRM test. However, at this time, the American College of Obstetricians and Gynecologists does not endorse use of aspirin for prevention of PTB in patients who lack preeclampsia risk factors [
20].
Strengths of the study include the prospective randomized design of the parent trial. A strength of the secondary analysis is the use of statistical methods that assess GAbirth as a continuous outcome rather than a dichotomous variable.
There are also several limitations. First, the number of PTB cases was small because of early termination of the trial. Second, this is a post-hoc, secondary analysis conducted after the primary analysis was reported. Third, we did not have data on the use of antenatal corticosteroids, which can impact preterm neonatal respiratory morbidity. Fourth, the specific treatments chosen in response to the PreTRM test were based on patient preferences, not randomization, and thus the analysis of treatments reveals only associations and not necessarily a causal link between treatments and outcomes. Finally, we did not employ any statistical corrections to adjust for the large number of hypotheses tested; thus, the probability of Type 1 statistical error is larger than the nominal 5%.
Given the limitations, we consider our results to be exploratory only, suggesting potential topics for future trials. We suggest that there is need for large, prospective trials to evaluate the efficacy of specific interventions such as care management and low-dose aspirin in patients identified at increased risk for PTB by the PreTRM test. Ongoing trials may provide additional insights [21-22].
5. Conclusions
We found that screening with the PreTRM test in the PREVENT-PTB trial was associated with significant prolongation of pregnancy and this prolongation was accompanied by improvement in neonatal outcome as reflected by shorter NICU LOS. Future research is needed to evaluate the individual contributions of care management, low-dose aspirin, and perhaps other interventions in achieving these apparent benefits.
Author Contributions
Conceptualization, CAC and JAFZ.; methodology, all authors; formal analysis, MW and JS.; data curation, MW and JS; writing—original draft preparation, CAC.; writing—review and editing, all authors; visualization, JS. All authors have read and agreed to the published version of the manuscript.
Funding
Sera Prognostics, Inc., funded the PREVENT-PTB trial data. For this secondary analysis, Sera Prognostics paid consulting fees to the statistical consultants (MW, ZS) and has agreed to pay article processing charges for open access publishing.
Institutional Review Board Statement
The PREVENT-PTB trial was approved by Intermountain Healthcare’s institutional review board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We requested a relevant subset of the PREVENT-PTB trial data from Sera Prognostics, Inc., the trial sponsor, upon presentation of our statistical analysis plan. We are not authorized to share this proprietary, privately held data. Investigators with reasonable requests are encouraged to seek trial data directly from the sponsor.
Acknowledgments
We thank Drs. D. Ware Branch and Sean Esplin for consenting to our use of the data from the PREVENT-PTB trial. We are grateful for the contributions of all the PREVENT-PTB trial investigators. We thank Jennifer Logan for assistance with copy-editing of the draft manuscript. We acknowledge with gratitude the altruism of the individuals who volunteered for the trial.
Conflicts of Interest
CAC declares no conflicts of interest. Prior to the current project, JAFZ received fees from Sera Prognostics, Inc., for methodological consulting on a different project. MW and JS are paid consultants to Sera Prognostics. Sera Prognostics was the PREVENT-PTB trial sponsor and is the manufacturer of the PreTRM test. Sera Prognostics was not involved in planning the statistical analysis, in the writing of the article, or in the decision to publish.
References
- Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2021. Nat Vital Stat Rep 2023, 72, 1–53. [Google Scholar] [CrossRef]
- Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016, 215, 103.e1-14. [Google Scholar] [CrossRef]
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol 2016, 48, 308–317. [Google Scholar] [CrossRef] [PubMed]
- EPPPIC Group. Evaluating progestogens for preventing preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet 2021, 397, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality. Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021, 326, 1192–1206. [Google Scholar] [CrossRef] [PubMed]
- Kupka E, Hesselman S, Hastie R, Lomartire R, Wikstrom AK, Bergman L. Low-dose aspirin use in pregnancy and the risk of preterm birth: a Swedish register-based cohort study. Am J Obstet Gynecol 2023, 228, 336.e1-9. [Google Scholar] [CrossRef]
- Saade GR, Boggess KA, Sullivan SA, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol 2016, 214, 633.e1-24. [Google Scholar] [CrossRef]
- Markenson GR, Saade GR, Laurent LC, et al. Performance of a proteomic preterm delivery predictor in a large independent cohort. Am J Obstet Gynecol 2020, 2, 100140. [Google Scholar] [CrossRef]
- Burchard J, Polpitiya AS, Fox AC, et al. Clinical validation of a proteomic biomarker threshold for increased risk of spontaneous preterm birth and associated clinical outcomes: a replication study. J Clin Med 2021, 10, 5088. [CrossRef] [PubMed]
- Grabner M, Burchard J, Nguyen et al. Cost-effectiveness of a proteomic test for preterm birth prediction. ClinicoEconom Outcomes Res 2021, 13, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Burchard J, Markenson GR, Saade GR, et al. Clinical and economic evaluation of a proteomic biomarker preterm birth risk predictor: cost-effectiveness modeling of prenatal interventions applied to predicted higher-risk pregnancies within a large and diverse cohort. J Med Econ 2022, 25, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Branch DW, VanBuren JM, Porter TF, et al. Prediction and prevention of preterm birth: a prospective, randomized intervention trial. Am J Perinatol 2021. [CrossRef]
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2011, 38, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Ferre C, Callaghan W, Olson C, Sharma A, Barfield W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates—United States, 2007 and 2014. Morb Mortal Weekly Rep 2016, 65, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Shah PS, Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2010, 89, 862–875. [Google Scholar] [CrossRef]
- Koullali B, van Zijl MD, Kazemier BM, et al. The association between parity and spontaneous preterm birth: a population based study. BMC Pregnancy Childbirth 2020, 20, 233. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Commissioner and Chief Scientist announce decision to withdraw approval of Makena. Available online: https://www.fda.gov/news-events/press-announcements/fda-commissioner-and-chief-scientist-announce-decision-withdraw-approval-makena (accessed on 8 June 2023).
- R Foundation. The R Project for statistical computing. Available online: https://www.r-project.org (accessed on 8 June 2023).
- Garite TJ, Manuck TA. Should case management be considered a component of obstetrical interventions for pregnancies at risk of preterm birth? Am J Obstet Gynecol 2023. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice, Society for Maternal Fetal Medicine. Low-dose aspirin use during pregnancy. ACOG Committee Opinion 743. Obstet Gynecol 2018, 132, e44–e51. [Google Scholar] [CrossRef] [PubMed]
- Hoffman M, Serum assessment of preterm birth outcomes compared to historical controls: AVERT-PRETERM trial. NCT03151330. Available online: https://clinicaltrials.gov/ct2/show/NCT03151330?term=PreTRM&cond=Preterm+Birth&draw=2&rank=2 (accessed on 8 June 2023).
- Iriye B, Gyamfi-Bannerman C, Son M, et al. Prematurity risk assessment combined with clinical interventions for improving neonatal outcomes (PRIME). NCT 04301518. Available online: https://clinicaltrials.gov/ct2/show/NCT04301518?term=PreTRM&cond=Preterm+Birth&draw=2&rank=4 (accessed on 8 June 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).