Submitted:
28 December 2023
Posted:
29 December 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
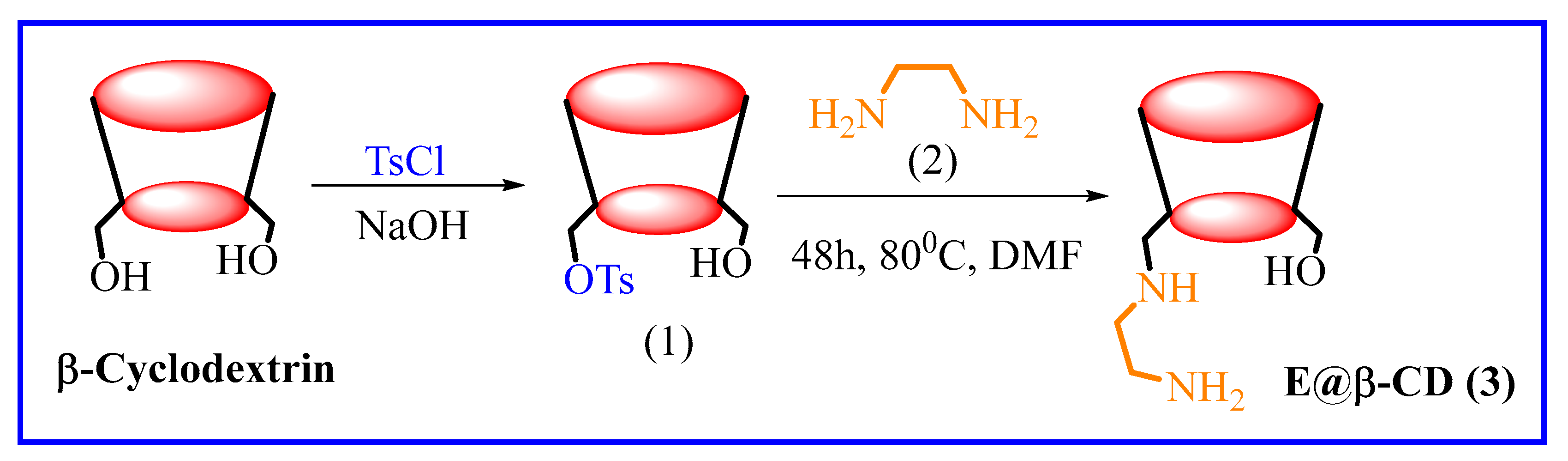
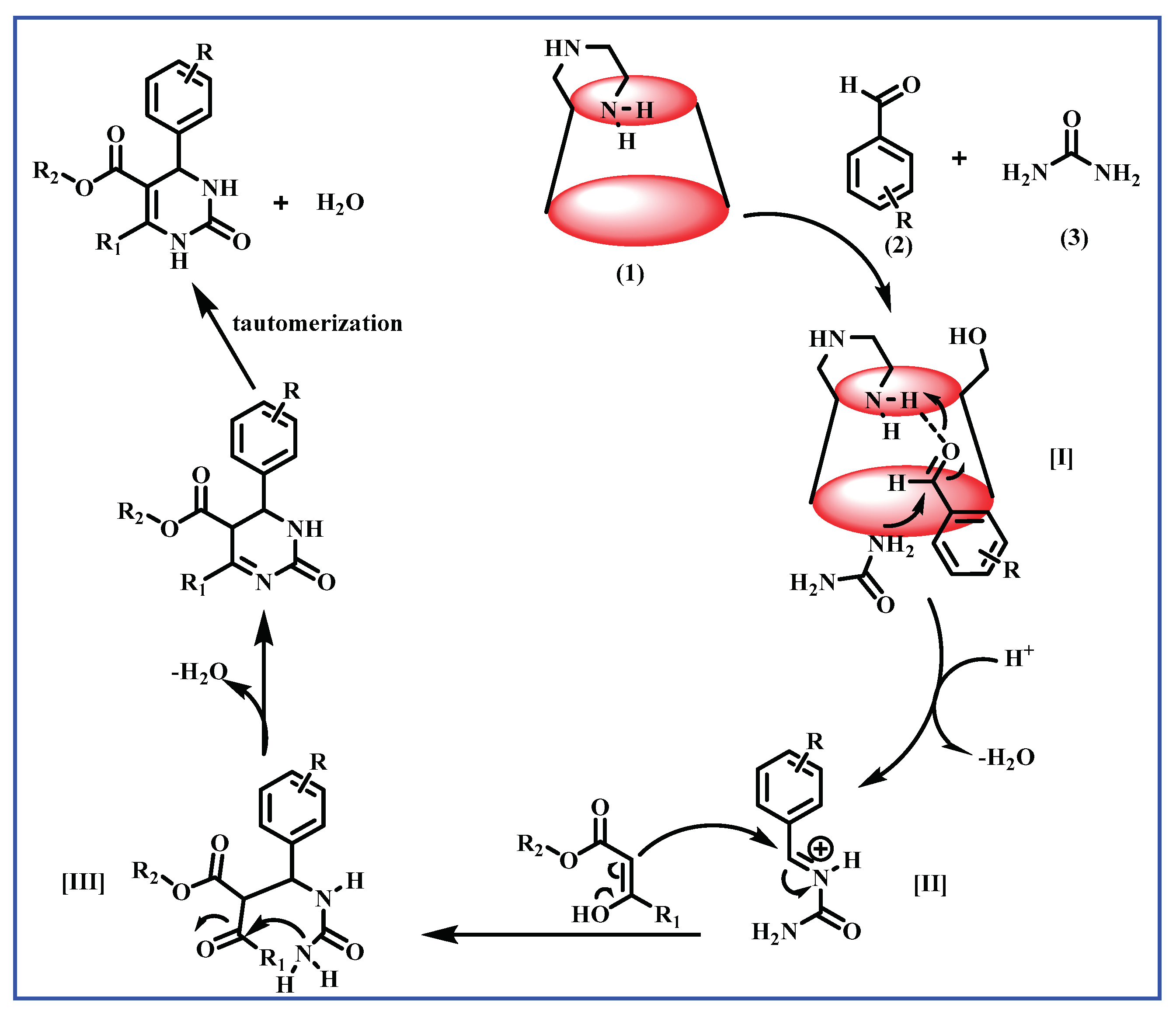
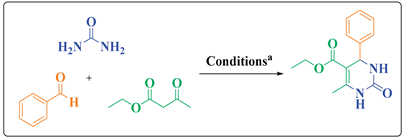
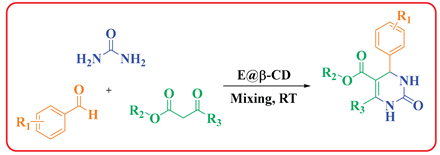
2.1. General Procedure for the Synthesis of 3,4-dihydropyrimidin-2(1H)-ones DHPMs (4 a-r)
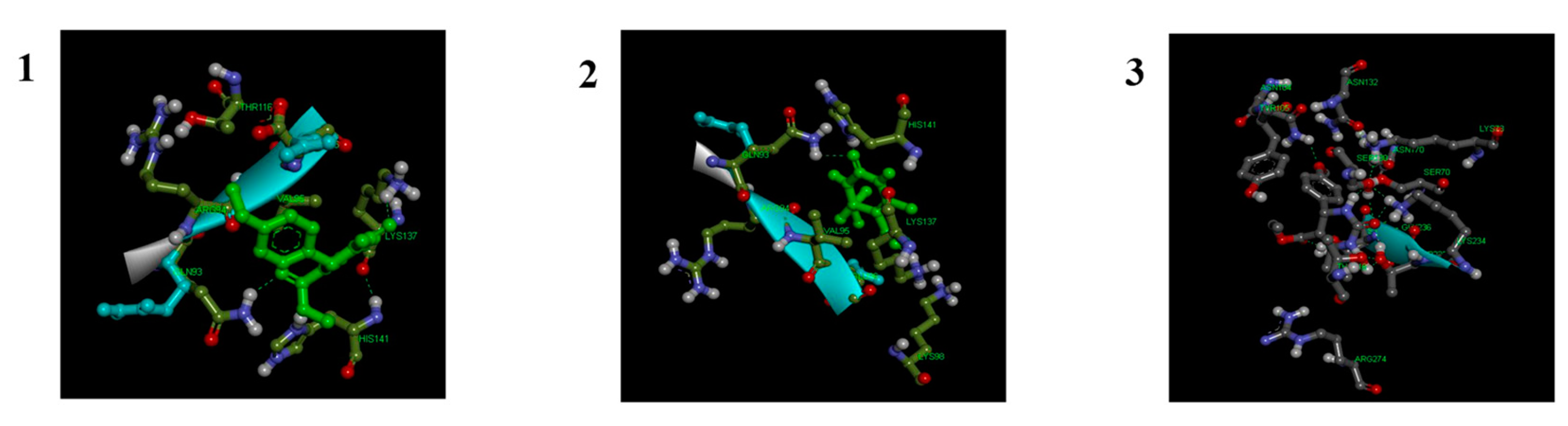
2.2. Molecular Docking Studies
3. Result and Discussion
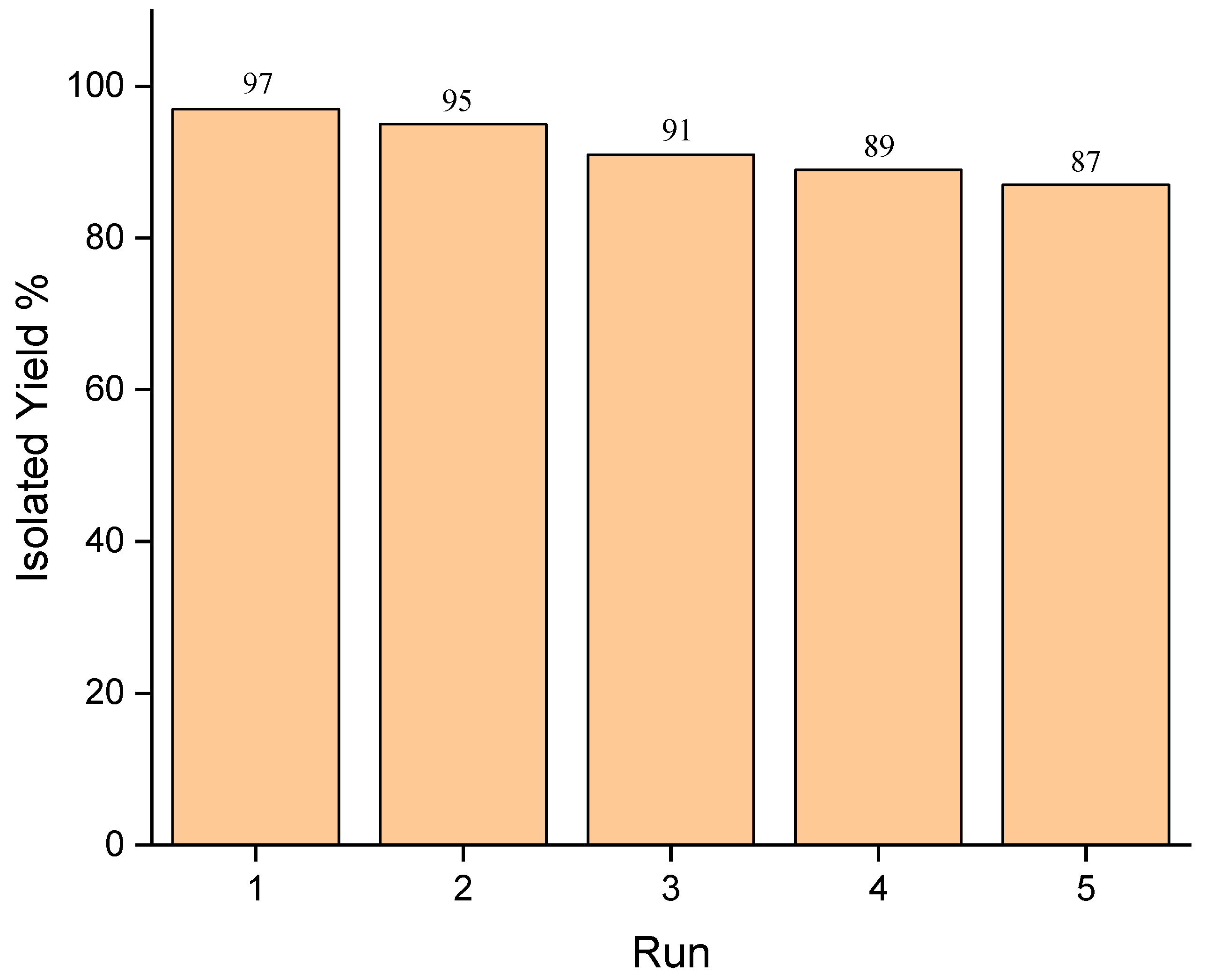
3.1. E@β-CD Catalyzed for Multi-Component Reactions
3.2. In-Silico Analysis

| Entry | vdW + H bond + electrostatic + dissolving energy (1) (kcal/mol) | Final total internal Energy (2) (kcal/mol) | Torsional free energy (3) (kcal/ mol) | Unbound system's energy (4) (kcal/mol) | Estimated free energy of binding [1 + 2 + 3–4] (kcal/mol) |
|---|---|---|---|---|---|
| 4a | -7.53 | -2.83 | 1.49 | -2.83 | -6.04 |
| 4d | -7.21 | -2.28 | 1.49 | -2.28 | -5.72 |
| 4n | -6.21 | -2.88 | 1.69 | -2.25 | -4.96 |
4. Conclusions
Supplementary Information
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregorio, C. Review : A History of Cyclodextrins Gre g. Chemical Reviews 2014. [CrossRef] [PubMed]
- Mitra, B.; Chandra Pariyar, G.; Ghosh, P. β-Cyclodextrin: a supramolecular catalyst for metal-free approach towards the synthesis of 2-amino-4,6-diphenylnicotinonitriles and 2,3-dihydroquinazolin-4(1 H )-one. RSC Advances 2021, 11, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Jie, K.; Zhou, Y.; Yao, Y.; Huang, F. Macrocyclic amphiphiles. Chemical Society Reviews 2015, 44, 3568–3587. [Google Scholar] [CrossRef]
- Hapiot, F.; Monflier, E. Unconventional Approaches Involving Cyclodextrin-Based, Self-Assembly-Driven Processes for the Conversion of Organic Substrates in Aqueous Biphasic Catalysis. Catalysts 2017, 7, 173. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chemical Reviews 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin based novel drug delivery systems. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Shaabani, S.; Shaabani, A.; Ng, S.W. One-pot synthesis of coumarin-3-carboxamides containing a triazole ring via an isocyanide-based six-component reaction. ACS Combinatorial Science 2014, 16, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Saha, A. Free-ZnO nanoparticles: A mild, efficient and reusable catalyst for the one-pot multicomponent synthesis of tetrahydrobenzo[b]pyran and dihydropyrimidone derivatives. New Journal of Chemistry 2013, 37, 4170–4175. [Google Scholar] [CrossRef]
- Xin, J.; Chang, L.; Hou, Z.; Shang, D.; Liu, X.; Feng, X. An enantioselective Biginelli reaction catalyzed by a simple chiral secondary amine and achiral Brønsted acid by a dual-activation route. Chemistry - A European Journal 2008, 14, 3177–3181. [Google Scholar] [CrossRef]
- Shiri, L.; Ghorbani-Choghamarani, A.; Kazemi, M. Synthesis and characterization of DETA/Cu(NO3)2 supported on magnetic nanoparticles: a highly active and recyclable catalyst for the solvent-free synthesis of polyhydroquinolines. Monatshefte Für Chemie - Chemical Monthly 2017, 148, 1131–1139. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahendiran, D.; Bhat, A.R.; Rahiman, A.K. Theoretical, in Vitro Antiproliferative, and in Silico Molecular Docking and Pharmacokinetics Studies of Heteroleptic Nickel (II) and Copper (II) Complexes of Thiosemicarbazone-Based Ligands and Pefloxacin. Chemistry & Biodiversity 2023, 20, e202300702. [Google Scholar]
- Ahmed, S.; Jayathuna, M.A.; Mahendiran, D.; Bharathi, S.; Kalilur Rahiman, A. Heteroleptic silver (I), nickel (II), and copper (II) complexes of N4-substituted thiosemicarbazones and ciprofloxacin: Theoretical, in vitro anti-proliferative, and in silico molecular modeling and pharmacokinetics studies. Applied Organometallic Chemistry 2022, 36, e6782. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Hülsey, M.J.; Yan, N. One-Step Synthesis of N-Heterocyclic Compounds from Carbohydrates over Tungsten-Based Catalysts. ACS Sustainable Chemistry and Engineering 2017, 5, 11096–11104. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lee, J.K.-W.; Ko, C.-C.; Zhu, N. Photochromic Diarylethene-Containing Ionic Liquids and N-Heterocyclic Carbenes. Journal of the American Chemical Society 2009, 131, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Ruamps, M.; Lugan, N.; César, V. A Cationic N-Heterocyclic Carbene Containing an Ammonium Moiety. Organometallics 2017, 36, 1049–1055. [Google Scholar] [CrossRef]
- Nayak, N.; Ramprasad, J.; Dalimba, U. New INH-pyrazole analogs: Design, synthesis and evaluation of antitubercular and antibacterial activity. Bioorganic and Medicinal Chemistry Letters 2015, 25, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Vaja, D.V.; Jadeja, K.A.; Joshi, S.B.; Khedkar, V.M. Synthesis, Biological Evaluation and Molecular Docking Study of Pyrazole, Pyrazoline Clubbed Pyridine as Potential Antimicrobial Agents. Anti-Infective Agents 2019, 18, 306–314. [Google Scholar]
- Amnerkar, N.D.; Bhusari, K.P. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. European Journal of Medicinal Chemistry 2010, 45, 149–159. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, A.; Mahmoud, Z.; Hamed Salem, H.; Abdou, A.M.; Soliman, M.M.H.; Hassan, R.A. Design, ecofriendly synthesis, anticancer and antimicrobial screening of innovative Biginelli dihydropyrimidines using β-aroylpyruvates as synthons. Green Chemistry Letters and Reviews 2021, 14, 221–233. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Baraka, M.M.; Ibrahim, S.M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Rashad, A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. European Journal of Medicinal Chemistry 2009, 44, 3746–3753. [Google Scholar] [CrossRef]
- Chen, W.Y.; Qin, S.D.; Jin, J.R. Efficient biginelli reaction catalyzed by sulfamic acid or silica sulfuric acid under solvent-free conditions. Synthetic Communications 2007, 37, 47–52. [Google Scholar] [CrossRef]
- Slimi, H.; Moussaoui, Y.; ben Salem, R. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction promoted by bismuth(III)nitrate or PPh3 without solvent. Arabian Journal of Chemistry 2016, 9, S510–S514. [Google Scholar] [CrossRef]
- Zahiri, S.; Mokhtary, M. Bi(NO 3 ) 3 ·5H 2 O: An efficient catalyst for one-pot synthesis of 3-((aryl)(diethylamino)methyl)-4-hydroxy-2H-chromen-2-ones and biscoumarin derivatives. Journal of Taibah University for Science 2015, 9, 89–94. [Google Scholar] [CrossRef]
- Ahmed, N.; Siddiqui, Z.N. Sulphated silica tungstic acid as a highly efficient and recyclable solid acid catalyst for the synthesis of tetrahydropyrimidines and dihydropyrimidines. Journal of Molecular Catalysis A: Chemical 2014, 387, 45–56. [Google Scholar] [CrossRef]
- Salim, S.D.; Akamanchi, K.G. Sulfated tungstate: An alternative, eco-friendly catalyst for Biginelli reaction. Catalysis Communications 2011, 12, 1153–1156. [Google Scholar] [CrossRef]
- Davoodnia, A.; Allameh, S.; Fazli, S.; Tavakoli-Hoseini, N. One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo[b] pyrans catalysed by silica gel-supported polyphosphoric acid (PPA-SiO 2) as an efficient and reusable catalyst. Chemical Papers 2011, 65, 714–720. [Google Scholar] [CrossRef]
- Salehi, P.; Dabiri, M.; Zolfigol, M.A.; Bodaghi Fard, M.A. Silica sulfuric acid: an efficient and reusable catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Letters 2003, 44, 2889–2891. [Google Scholar] [CrossRef]
- Thirupathi Reddy, Y.; Rajitha, B.; Narsimha Reddy, P.; Sunil Kumar, B.; Rao, V.P. Bismuth Subnitrate Catalyzed Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones: An Improved Protocol for the Biginelli Reaction. Synthetic Communications 2004, 34, 3821–3825. [Google Scholar] [CrossRef]
- Aswin, K.; Mansoor, S.S.; Logaiya, K.; Sudhan, S.P.N. Triphenylphosphine: An efficient catalyst for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione under thermal conditions. Journal of King Saud University – Science 2014, 26, 141–148. [Google Scholar] [CrossRef]
- Khatri, C.K.; Rekunge, D.S.; Chaturbhuj, G.U. Sulfated polyborate: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction. New Journal of Chemistry 2016, 40, 10412–10417. [Google Scholar] [CrossRef]
- Sharghi, H.; Jokar, M. ChemInform Abstract: Al 2 O 3 /MeSO 3 H: A Novel and Recyclable Catalyst for One-Pot Synthesis of 3,4-Dihydropyrimidinones or Their Sulfur Derivatives in Biginelli Condensation. ChemInform 2009, 40, 958–979. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Hosseinian, A.; Ghashang, M. Reaction in dry media: Silica gel supported ferric chloride catalyzed synthesis of 1,8-dioxo-octahydroxanthene derivatives, Phosphorus. Sulfur and Silicon and the Related Elements 2008, 183, 3136–3144. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.; Taei, M. Aluminatesulfonic acid nanoparticles: Synthesis, characterization and application as a new and recyclable nanocatalyst for the Biginelli and Biginelli-like condensations. RSC Advances 2015, 5, 44978–44989. [Google Scholar] [CrossRef]
- Angeles-Beltrán, D.; Lomas-Romero, L.; Lara-Corona, V.H.; González-Zamora, E.; Negrón-Silva, G. Sulfated zirconia-catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) under solventless conditions: Competitive multicomponent Biginelli vs. Hantzsch reactions. Molecules 2006, 11, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Dilmaghani, K.A.; Zeynizadeh, B.; Parasajam, H. The efficient synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and their sulfur derivatives with H2SO4 immobilized on activated charcoal. Phosphorus, Sulfur and Silicon and the Related Elements 2012, 187, 544–553. [Google Scholar] [CrossRef]
- Su, W.; Li, J.; Zheng, Z.; Shen, Y. One-pot synthesis of dihydropyrimidiones catalyzed by strontium(II) triflate under solvent-free conditions. Tetrahedron Letters 2005, 46, 6037–6040. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Tavazo, M.; Mahdavinia, G.H. Ultrasound promoted one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using dendrimer-attached phosphotungstic acid nanoparticles immobilized on nanosilica. Ultrasonics Sonochemistry 2018, 40, 230–237. [Google Scholar] [CrossRef]
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Huang, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Ultrafast fabrication of fluorescent organic nanoparticles with aggregation-induced emission feature through the microwave-assisted Biginelli reaction. Dyes and Pigments 2019, 165, 90–96. [Google Scholar] [CrossRef]
- Valizadeh, H.; Shockravi, A. Imidazolium-based phosphinite ionic liquid as reusable catalyst and solvent for one-pot synthesis of 3,4-dihydropyrimidin-2(1 H )- (thio)ones. Heteroatom Chemistry 2009, 20, 284–288. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Titanium dioxide supported on MWCNTs as an eco-friendly catalyst in the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones accelerated under microwave irradiation. New Journal of Chemistry 2014, 38, 3514–3521. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Sharma, M.; Sharma, P.; Tomar, R. Synthesis and surfactant modification of clinoptilolite and montmorillonite for the removal of nitrate and preparation of slow release nitrogen fertilizer. Journal of Hazardous Materials 2012, 227, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Ould M’hamed, M.; Alshammari, A.; Lemine, O. Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions. Applied Sciences 2016, 6, 431. [Google Scholar] [CrossRef]
- Dilmaghani, K.A.; Zeynizadeh, B.; Amirpoor, M. Ultrasound-mediated synthesis of 3,4-dihydropyrimidin-2-(1H)-ones (or Thiones) with NaHSO4·H2O, Phosphorus. Sulfur and Silicon and the Related Elements 2013, 188, 1634–1642. [Google Scholar] [CrossRef]
- Suresh, P.; Pitchumani, K. Per-6-amino-β-cyclodextrin catalyzed asymmetric Michael addition of nitromethane and thiols to chalcones in water. Tetrahedron Asymmetry 2008, 19, 2037–2044. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. The aminocyclodextrin/Pd(OAc) 2 complex as an efficient catalyst for the Mizoroki-Heck cross-coupling reaction. Chemistry - A European Journal 2013, 19, 14425–14431. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Pitchumani, K. Per-6-amino-β-cyclodextrin as an Efficient Supramolecular Ligand and Host for Cu(I)-Catalyzed N -Arylation of Imidazole with Aryl Bromides. The Journal of Organic Chemistry 2008, 73, 9121–9124. [Google Scholar] [CrossRef] [PubMed]
- Azath, I.A.; Suresh, P.; Pitchumani, K. Per-6-amino-β-cyclodextrin/CuI catalysed cyanation of aryl halides with K4[Fe(CN)6]. New Journal of Chemistry 2012, 36, 2334. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Suresh, P.; Pitchumani, K. Per-6-amino-β-cyclodextrin as a Reusable Promoter and Chiral Host for Enantioselective Henry Reaction. Organic Letters 2010, 12, 4070–4073. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. Solvent-free multicomponent synthesis of pyranopyrazoles: per-6-amino-β-cyclodextrin as a remarkable catalyst and host. Tetrahedron Letters 2010, 51, 3312–3316. [Google Scholar] [CrossRef]
- Azath, I.A.; Puthiaraj, P.; Pitchumani, K. One-pot multicomponent solvent-free synthesis of 2-amino-4H-benzo[b]pyrans catalyzed by per-6-amino-β-cyclodextrin. ACS Sustainable Chemistry and Engineering 2013, 1, 174–179. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. A pyridinium modified β-cyclodextrin: An ionic supramolecular ligand for palladium acetate in C-C coupling reactions in water. Green Chemistry 2016, 18, 5518–5528. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Lu, M. β-cyclodextrin-capped palladium nanoparticle-catalyzed ligand-free Suzuki and Heck couplings in low-melting β-cyclodextrin/NMU mixtures. Applied Organometallic Chemistry 2014, 28, 635–640. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; He, F.; Li, Z.; Guo, S.; Xie, Y.; Luo, D.; Wu, J. Facile construction of spiroindoline derivatives as potential anti-viral agent via three-component reaction in aqueous with β-cyclodextrin-SO3H as an efficient catalyst. Green Chemistry Letters and Reviews 2022, 15, 139–152. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, A.; Zhu, L.; Yang, X.; Fang, G.; Tang, B. Cyclodextrin-based ocular drug delivery systems: A comprehensive review. Coordination Chemistry Reviews 2023, 476, 214919. [Google Scholar] [CrossRef]
- Yamazaki, S. Efficient Synthesis of Heterocycles Using Highly Electrophilic Ethenetricarboxylates. HETEROCYCLES 2016, 92, 1561. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Ma, J.; Zhang, Y.; Shi, Q.; Luo, L.; Song, B.; Yang, S.; Hu, D. Preparation of 2,3-dihydroquinazolin-4(1H)-one derivatives in aqueous media with β-cyclodextrin-SO 3 H as a recyclable catalyst. Green Chem 2014, 16, 3210–3217. [Google Scholar] [CrossRef]
- Quintas, P.C.; Al-Salami, H.; Pfaff, A.; Li, D.; Koks, S. β-cyclodextrin based nano gene delivery using pharmaceutical applications to treat Wolfram syndrome. Therapeutic Delivery 2022, 13, 449–462. [Google Scholar] [CrossRef]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. European Journal of Medicinal Chemistry 2017, 132, 108–134. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, V.; Mahesh Kumar, P.; Rambabu, D.; Kapavarapu, R.; Shobha Rani, S.; Misra, P.; Pal, M. Novel alkynyl substituted 3,4-dihydropyrimidin-2(1H)-one derivatives as potential inhibitors of chorismate mutase. Bioorganic Chemistry 2013, 51, 48–53. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Composite of cross-linked chitosan beads and a cyclodextrin nanosponge: A metal-free catalyst for promoting ultrasonic-assisted chemical transformations in aqueous media. Journal of Physics and Chemistry of Solids 2021, 156, 110157. [Google Scholar] [CrossRef]
- Bigi, F.; Carloni, S.; Frullanti, B.; Maggi, R.; Sartori, G. A revision of the biginelli reaction under solid acid catalysis. Solvent-free synthesis of dihydropyrimidines over montmorillonite KSF. Tetrahedron Letters 1999, 40, 3465–3468. [Google Scholar] [CrossRef]
- Wang, L.; Qian, C.; Tian, H.; Ma, Y. Lanthanide triflate catalyzed one-pot synthesis of dihydropyrimidin-2(1H)-thiones by a three-component of 1,3-dicarbonyl compounds, aldehydes, and thiourea using a solvent-free Biginelli condensation. Synthetic Communications 2003, 33, 1459–1468. [Google Scholar] [CrossRef]
- Lal, J.; Sharma, M.; Gupta, S.; Parashar, P.; Sahu, P.; Agarwal, D.D. Hydrotalcite: A novel and reusable solid catalyst for one-pot synthesis of 3,4-dihydropyrimidinones and mechanistic study under solvent free conditions. Journal of Molecular Catalysis A: Chemical 2012, 352, 31–37. [Google Scholar] [CrossRef]
- Aswin, K.; Mansoor, S.S.; Logaiya, K.; Sudhan, P.N.; Ahmed, R.N. Facile synthesis of 3,4-dihydropyrimidin-2(1 H )-ones and -thiones and indeno[1,2- d ]pyrimidines catalyzed by p -dodecylbenzenesulfonic acid. Journal of Taibah University for Science 2014, 8, 236–247. [Google Scholar] [CrossRef]
- Liberto, N.A.; De Paiva Silva, S.; De Fátima, Â.; Fernandes, S.A. β-Cyclodextrin-assisted synthesis of Biginelli adducts under solvent-free conditions. Tetrahedron 2013, 69, 8245–8249. [Google Scholar] [CrossRef]
- Gong, K.; Wang, H.; Wang, S.; Ren, X. β-Cyclodextrin-propyl sulfonic acid: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidones via Biginelli reaction. Tetrahedron 2015, 71, 4830–4834. [Google Scholar] [CrossRef]
- Liu, Z.; Larock, R.C. Facile N -Arylation of Amines and Sulfonamides and O -Arylation of Phenols and Arenecarboxylic Acids commonly found in a variety of biologically active and natural. The Journal of Organic Chemistry 2006, 71, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Kolvari, E.; Koukabi, N.; Armandpour, O. A simple and efficient synthesis of 3,4-dihydropyrimidin-2-(1H)-ones via Biginelli reaction catalyzed by nanomagnetic-supported sulfonic acid. Tetrahedron 2014, 70, 1383–1386. [Google Scholar] [CrossRef]
- Quan, Z.J.; Da, Y.X.; Zhang, Z.; Wang, X.C. PS-PEG-SO3H as an efficient catalyst for 3,4-dihydropyrimidones via Biginelli reaction. Catalysis Communications 2009, 10, 1146–1148. [Google Scholar] [CrossRef]
- Zamani, F.; Izadi, E. Synthesis and characterization of sulfonated-phenylacetic acid coated Fe3O4 nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catalysis Communications 2013, 42, 104–108. [Google Scholar] [CrossRef]
- Kalbasi, R.J.; Massah, A.R.; Daneshvarnejad, B. Preparation and characterization of bentonite/PS-SO3H nanocomposites as an efficient acid catalyst for the Biginelli reaction. Applied Clay Science 2012, 55, 1–9. [Google Scholar] [CrossRef]
- Suresh Saini, A.; Kumar, D.; Sandhu, J.S. Multicomponent eco-friendly synthesis of 3,4-dihydropyrimidine-2-(1H)-ones using an organocatalyst Lactic acid. Green Chemistry Letters and Reviews 2009, 2, 29–33. [Google Scholar] [CrossRef]
- de Vasconcelos, A.; Oliveira, P.S.; Ritter, M.; Freitag, R.A.; Romano, R.L.; Quina, F.H.; Pizzuti, L.; Pereira, C.M.P.; Stefanello, F.M.; Barschak, A.G. Antioxidant capacity and environmentally friendly synthesis of dihydropyrimidin-(2H)-ones promoted by naturally occurring organic acids. Journal of Biochemical and Molecular Toxicology 2012, 26, 155–161. [Google Scholar] [CrossRef]
- Amarante, G.W.; Coelho, F. Catalytic Synthesis of 3,4-dihydropyrimidin-2(1H)-ones under Green Conditions and by Keggin type Heteropolyacid catalyst H7[PMo8V4O40]. Química Nova 2009, 32, 469–481. [Google Scholar] [CrossRef]
- Sehout, I.; Boulcina, R.; Boumoud, B.; Boumoud, T.; Debache, A. Solvent-free synthesis of polyhydroquinoline and 1,8-dioxodecahydroacridine derivatives through the Hantzsch reaction catalyzed by a natural organic acid: A green method. Synthetic Communications 2017, 47, 1185–1191. [Google Scholar] [CrossRef]
- Kargar, M.; Hekmatshoar, R.; Mostashari, A.; Hashemi, Z. Efficient and green synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones using imidazol-1-yl-acetic acid as a novel, reusable and water-soluble organocatalyst. Catalysis Communications 2011, 15, 123–126. [Google Scholar] [CrossRef]
- Rajack, A.; Yuvaraju, K.; Praveen, C.; Murthy, Y.L.N. A facile synthesis of 3,4-dihydropyrimidinones/thiones and novel N-dihydro pyrimidinone-decahydroacridine-1,8-diones catalyzed by cellulose sulfuric acid. Journal of Molecular Catalysis A: Chemical 2013, 370, 197–204. [Google Scholar] [CrossRef]
- Ganwir, P.; Gavali, K.; Chaturbhuj, G.U. N -(Phenylsulfonyl)Benzenesulfonamide: A New Organocatalyst for One-Pot, Solvent-Free Synthesis of Biginelli’s 3,4-Dihydropyrimidine-2(1 H )-Thiones. Polycyclic Aromatic Compounds 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Maryam, L.; Khan, A.U. Structural insight into mode of binding of Meropenem to CTX-M-15 type β-lactamase. International Journal of Biological Macromolecules 2017, 96, 78–86. [Google Scholar] [CrossRef] [PubMed]
| Mode of Inclusion | ΔEa (Kcal.M-1) | |
|---|---|---|
| Di-amine group outside the pyr:β-CD cavity | (Mode A) | -64.8800 |
| Di-amine group inside the pyr:β-CD cavity. | (Mode B) | -50.8492 |
 | ||||
| Entry | Catalyst | Medium | Time (h) | Yield (%)b |
| 1 | β-CD | Water | 24 | 28 |
| 2 | Ethylene-di-amine (2) | - | 8 | 26 |
| 3 | Methanol | - | 8 | 28 |
| 4 | Ethanol | - | 8 | 32 |
| 5 | Methylamine | - | 8 | 35 |
| 6 | Diethylamine | - | 8 | 33 |
| 7 | Triethylamine | - | 8 | 34 |
| 8 | Pyridine | - | 8 | 36 |
| 9 | E@β-CD (3) | DMF | 24 | 58 |
| 10 | E@β-CD (3) | DMSO | 24 | 60 |
| 11 | E@β-CD (3) | - | 5 min | 96 |
| c12 | E@β-CD (3) | - | 5 min | 96b |
| d13 | E@β-CD (3) | - | 5 min | 96c |
 | ||||
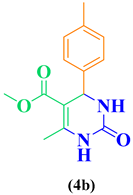
| Entry | R1 in aldehydes | R2 & R3 in esters | Product | Yield (%) |
| 1 | C6H5-CHO | 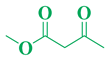 |
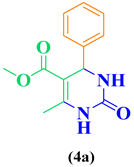 |
86 |
| 2 | CH3-C6H5-CHO |  |
 |
88 |
| 3 | CH3O-C6H5-CHO |  |
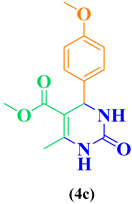 |
88 |
| 4 | Br-C6H5-CHO |  |
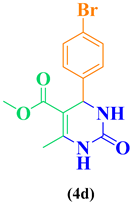 |
93 |
| 5 | CH3O-C6H5-CHO |  |
 |
85 |
| 6 | p-Cl-C6H5-CHO | 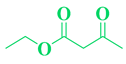 |
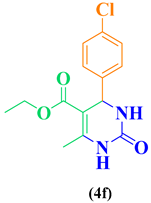 |
92 |
| 7 | CH3-C6H5-CHO | 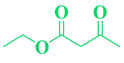 |
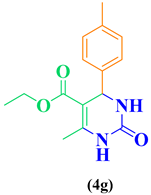 |
88 |
| 8 | p-NO2-C6H5-CHO | 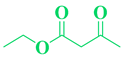 |
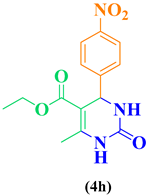 |
94 |
| 9 | m-Cl-C6H5-CHO | 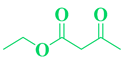 |
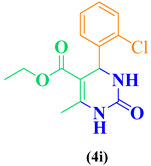 |
98 |
| 10 | p-F-C6H5- CHO |  |
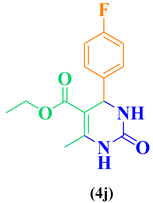 |
97 |
| 11 | m,p-OCH3-C6H5- CHO | 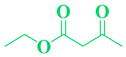 |
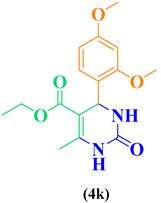 |
83 |
| 12 | p-Br-C6H5- CHO | 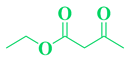 |
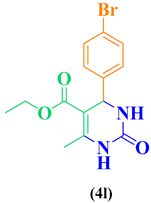 |
93 |
| 13 | o-Cl-C6H5- CHO | 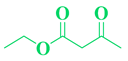 |
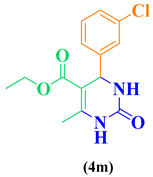 |
98 |
| 14 | p-OCH3-C6H5- CHO | 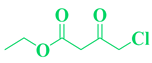 |
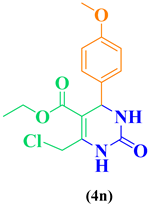 |
89 |
| 15 | o-NO2-C6H5-CHO |  |
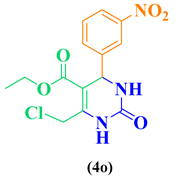 |
94 |
| 16 | p-Cl-C6H5-CHO |  |
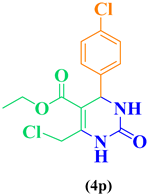 |
96 |
| 17 | C10H9-CHO | 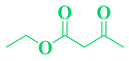 |
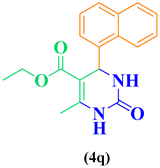 |
93 |
| 18 | C4H3S-CHO | 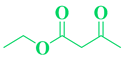 |
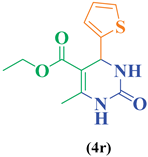 |
95 |
| Entry | Catalyst | Reaction condition | Time | Yield (%) | Ref |
| 1. | E@β-CD / SF | Rt | 30 min | 97 | This work |
| 2. | KSF/SF | 130 0C | 48 h | 74–88 | [61] |
| 3. | Lanthanide triflate/ SF | 100 0C | 1–1.5 min | 81–91 | [62] |
| 4. | strontium(II) triflate/ SF | 70 0C | 4 h | 85–97 | [36] |
| 5. | Mg-Al-CO3 hydrotalcite/SF | 80 0C | 30–60 min | 71–74 | [63] |
| 6. | DBSA (5 mol %) / SF | 80 0C | 2.5–3h | 81–94 | [64] |
| 7. | β-CD SF | 100 0C | 3 h | 85 | [65] |
| 8 | β-CD-SO3H SF | 100 0C | 2 h | 83 | [66] |
| 9 | β-CD-HCl | EtOH/ reflux | 8 h | 92 | [67] |
| 10 | nano-γ-Fe2O3-SO3H SF | 60 ℃ | 3 h | 90 | [68] |
| 11 | PS-PEG-SO3H | Dioxane/80℃ | 10 h | 86 | [69] |
| 12 | Fe3O4/PAA-SO3H SF | RT | 120 min | 90 | [70] |
| 13 | Bentonite/PS-SO3H SF | 120 ℃ | 30 min | 89 | [71] |
| 14 | Tartaric acid | EtOH/Reflux | 4 h | 92 | [72] |
| 15 | Citric acid | EtOH/Reflux | 4 h | 96 | [73] |
| 16 | Lactic acids | EtOH/Reflux | 2.5 h | 92 | [74] |
| 17 | Ascorbic acid | Solvent free | 6 h | 85 | [75] |
| 18 | Imidazole-1yl-acetic acid | Water/reflux | 30 min | 94 | [76] |
| 19 | Sulfanilic acid | Water | 3 h | 98 | [77] |
| 20 | Phenyl Phosphonic acid | ACN/reflux | 4 h | 97 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





