Introduction
Subacute sclerosing panencephalitis (Dawson disease) is a progressive brain inflammation caused by the persistence of the measles virus within the body. This complication usually affects young children and should be suspected in all children with acute cognitive decline, myoclonus, and new onset epileptic symptoms [
1]. The clinical presentation varies ranging from personality changes to coma and death. The diagnosis is challenging but it should be kept as differential in elderly with refractory seizures [
2]. The clinical features, EEG, and CSF analysis help diagnose, while the brain biopsy confirms the disease [
3]. Despite much research, no cure for SSPE has been discovered. Only symptomatic treatment is given to stop disease progression. Thus, to prevent measles and its fatal complications like SSPE, immunisation is a must worldwide.
Case Presentation
A 22-year-old Indian male presented to Medical OPD with the chief complaint of altered behaviour in the form of reduced consciousness for the last 4 days, which was associated with occasional involuntary movement of right > left upper and lower limbs. The patient also complained of fever with chills and rigors for the last 1 day. During the hospital stay, he had 2-3 episodes of febrile seizures following which the patient had respiratory distress.
Negative history of cough, loose stools, abdominal pain, nausea, and vomiting.
Past History
The patient is diagnosed with chronic meningitis and has a ventriculoperitoneal shunt placed for obstructive hydrocephalus.
He has no history of hypertension, diabetes mellitus, and tuberculosis.
Physical Examination
On examination, the patient's general condition was good. He was drowsy, irritable, and not oriented to time, place and person. A nervous system examination revealed that the Glass Glow Coma Scale (GCS) score was E3 M5 V2. His pupils were bilaterally equal in size and diameter and reactive to light. Extraocular Movements (EOM) were normal. Muscle tone is increased in all four limbs (spastic) and strength of the limbs was level 5. Deep Tendon reflexes were present in all the limbs with +3/+3 and ankle clonus was present in both the lower limbs. Babinski sign was present in both lower limbs. The patient had no other biological pathological signs.
Lab Examination
Table 1.
Blood examination.
Table 1.
Blood examination.
| Test |
Observed value |
Reference Range |
| Haemoglobin |
10.7 g/dl |
(12-18) |
| WBC |
6.02 kU/L |
(5.2-12.4) |
| RBC |
3.67*106/ul |
(4.5-5.5) |
| Haematocrit |
33.3% |
(40-50) |
| Platelet counts |
370 kU/L |
(130-400) |
| Neutrophile |
68% |
(49-74) |
| Lymphocyte |
22% |
(26-46) |
| Monocyte |
09% |
(2-12) |
| Eosinophil |
01% |
(0-5) |
| Basophil |
00% |
(0-2) |
Table 2.
Renal Function Test.
Table 2.
Renal Function Test.
| Test |
Observed value |
Reference Range |
| Blood Urea |
9.5 mg/dl |
(15-45) |
| Creatinine serum |
0.37 mg/dl |
(0.5-1.1) |
| Sodium serum |
130 mmol/L |
(132-146) |
| Potassium serum |
4.39 mmol/L |
(3.5-5.5) |
| Chlorine serum |
98 mmol/L |
(99-109) |
Table 3.
CSF examination.
Table 3.
CSF examination.
| Test |
Observed value |
| Quantity |
0.5 ml |
| Color |
Colorless |
| Appearance |
Clear |
| Blood |
Absent |
| Cobweb |
Absent |
| Total count |
12 |
| Polymorphs |
10% |
| Lymphocytes |
90% |
| RBC |
Occasional |
| CSF sugar |
64 mg/dl |
| CSF protein |
210 mg/dl |
Table 4.
Other tests.
| Test |
Observed value |
| CSF culture |
No growth |
| CSF fungal culture |
No growth |
| CSF measles IgG |
Positive |
Radiological Imaging (MRI Brain Plain and Contrast)
Observation:
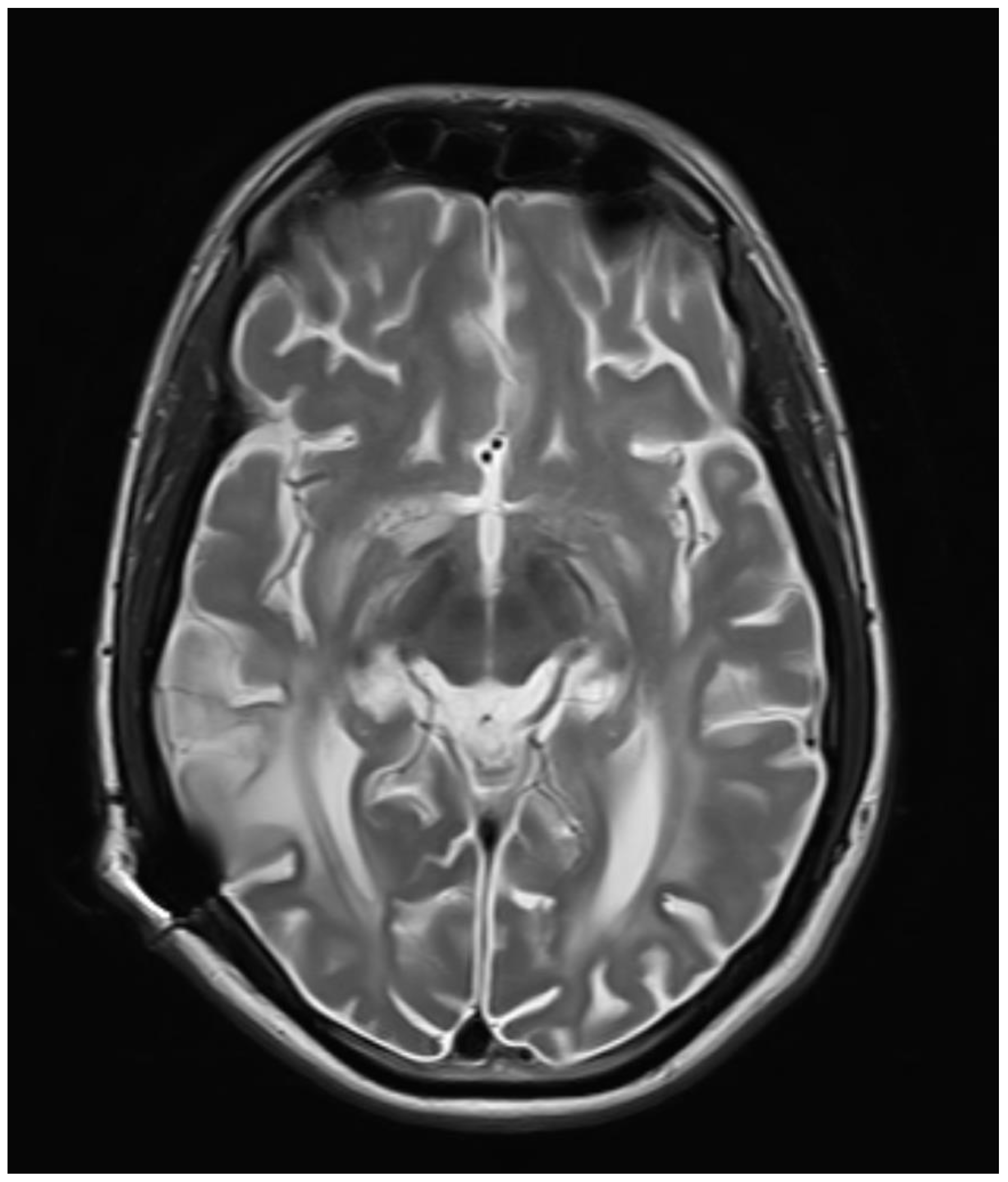
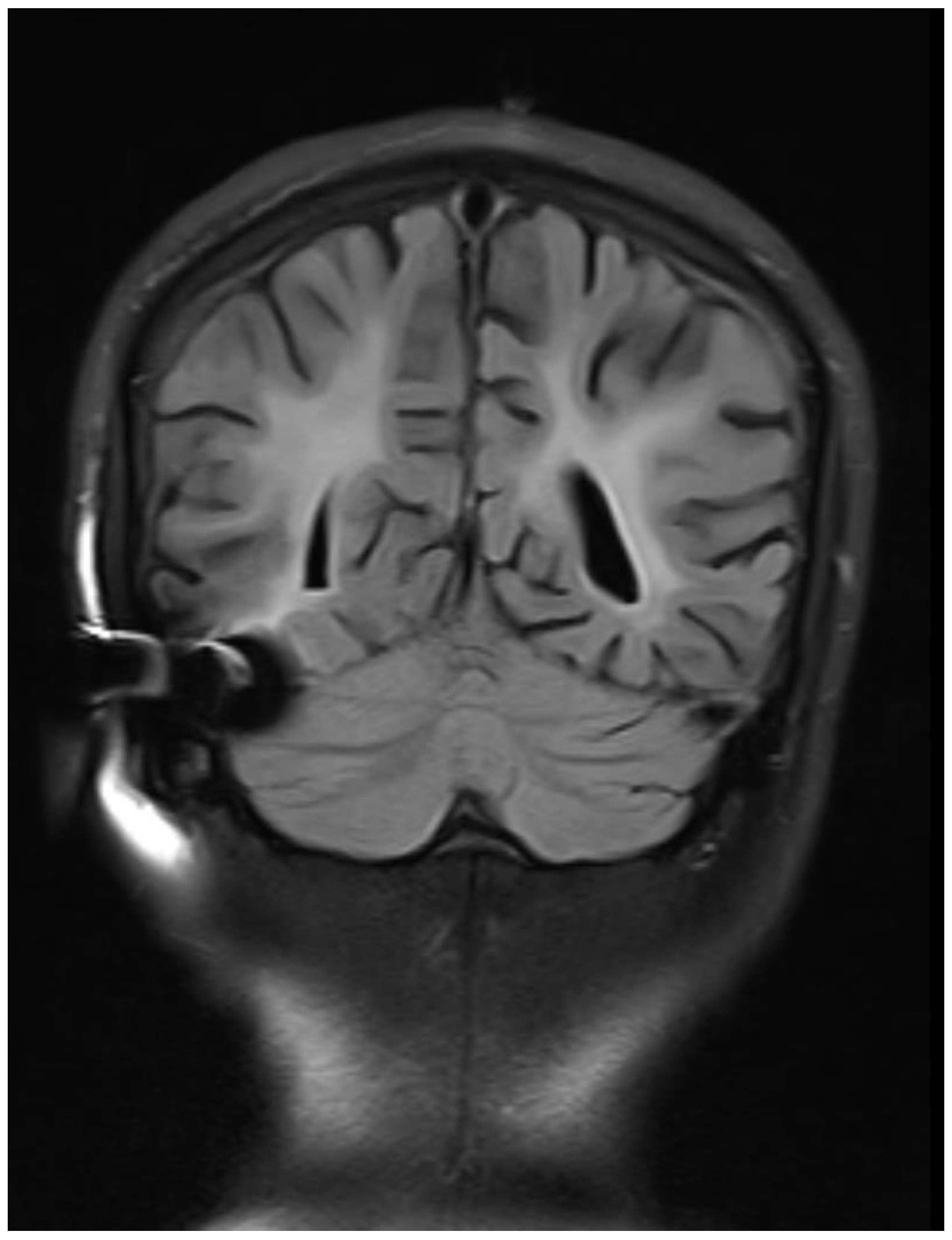
Bilateral symmetrical diffuse confluent T2WI /FLAIR hyperintensities with mild white matter volume loss in the periventricular region extending up to the subcortical region of bilateral cerebral hemispheres, the possibility of demyelinating disease - subacute sclerosing panencephalitis-SSPE likely.
Diffuse pachymeningeal enhancement along bilateral cerebral hemispheres.
Figure 1.
Axial T2WI images showing bilateral symmetrical hyperintensity involving periventricular and subcortical white matter with sparing of subcortical U fibers.
Figure 1.
Axial T2WI images showing bilateral symmetrical hyperintensity involving periventricular and subcortical white matter with sparing of subcortical U fibers.
Figure 2.
Coronal FLAIR images showing bilateral symmetrical white matter hyperintensity with sparing of subcortical U fibers.
Figure 2.
Coronal FLAIR images showing bilateral symmetrical white matter hyperintensity with sparing of subcortical U fibers.
Treatment
The patient was given symptomatic treatment for his condition. Tablet cefixime 200 mg, tablet levetiracetam 500 mg, tablet lacosamide 200 mg, tablet valproic acid 500 mg, tablet clobazam 10 mg, and tablet apixaban 2.5 mg were all given twice a day for 7 days. He was also given supportive therapy like Pantoprazole 40mg, Folic acid and Vitamin B complex, Vitamin D3 and Calcium tablets once a day for 28 days were also given. Due to respiratory distress, the patient was intubated and mechanically ventilated. Ryle’s tube and Foley’s catheterization were also done. After 7 days of intubation, tracheostomy was done. The patient was discharged once his condition was stabilised and symptoms improved.
Follow Up Case
The patient was followed up for around 6 months and there has been no recurrence or aggravation.
Diagnosis
The patient was given appropriate antibiotics and symptomatic treatment for 7 days. Based on the clinical features, lab and imaging findings, previous history, and response to treatment, the patient is diagnosed with subacute sclerosing panencephalitis.
Discussion and Literature
Subacute sclerosing panencephalitis (SSPE), also known as Dawson disease, is a rare neurological complication of measles infection. It commonly affects children and adolescents several years after the initial infection with measles. Measles infection is endemic in countries with poor vaccination access and overcrowding like Yemen, India, Kazakhstan, Ethiopia, etc.[
4]. The cases of measles and its complications have decreased over the years due to vaccination. However, recently due to vaccination refusal, there has been a surge in measles cases. This also increases the possibility of SSPE in future years due to reduced herd immunity [
5].
Measles, also called Rubeola, is caused by the single-stranded RNA measles virus of the Paramyxoviridae family. It is a highly contagious illness transmitted via airborne route. The virus has tropism for upper respiratory tract lymphocytes and lower respiratory tract epithelial cells. There occurs cellular fusion known as Warthin-Finkeldey cells [
6]. The infection spreads to various organ systems via lymphatics. The initial infection causes severe immunosuppression resulting in various secondary infections. SSPE occurs due to poor cellular response.
Risk factors for SSPE include poverty, overcrowding, multiple siblings, and higher birth rank order. It is impossible to get SSPE after measles vaccination. This is because it occurs due to the presence of specific host defense proteins which are found only in wild strains of measles virus [
7]. The actual pathogenesis of this complication is unknown but it has been studied that various mutations occur within the wild measles strain, particularly in the M gene, to develop a persistent vegetative state in the body, which manifests after several years [
8]. Viral entry into neurons is facilitated by CD46 and protein F, once inside the neuron, the virus evades the host immune response and continues viral replication.
The clinical presentation of SSPE varies from person to person, thus it is difficult to diagnose. Visual symptoms usually precede the disease onset by 2 years. Ocular features range from retinal haemorrhage to complete visual loss. The most characteristic ophthalmological lesion is necrotizing retinitis. SSPE has four stages with the following clinical features [
9]:
Stage 1- personality/behavioural changes like irritability, dementia, speech regression, etc.
Stage 2- progressive decline in motor function i.e., myoclonus, dyskinesia, dystonia, ataxia, jerking movements, muscle spasms, etc.
Stage 3- jerking movements are replaced with twisting movements and rigidity.
Stage 4- complete neuronal loss resulting in autonomic failure and the person may end in a vegetative state.
There is a specific diagnostic criteria used to diagnose SSPE [
10]:
| MAJOR |
- 1.
Elevated CSF measles antibody titers |
- 2.
-
Typical or atypical clinical history:
- •
Typical: acute progressive; subacute progressive; chronic progressive; chronic relapsing-remitting - •
Atypical: seizures, prolonged stage 1, unusual age.
|
| MINOR |
- 1.
Typical EEG |
- 2.
Increased CSF fluid IgG |
- 3.
Brain biopsy |
- 4.
Molecular diagnostic test to identify measles virus mutated genome |
| Requirement: 2 major plus 1 minor criterion |
The cerebrospinal fluid analysis shows pleocytosis with increased immunoglobulins and normal glucose and protein levels. CSF ELISA shows the presence of the measles virus. The typical EEG shows the periodic high amplitude of delta wave complexes [
11]. The imaging findings play a supportive role, but they are not used as an investigation of choice because MRI findings may be normal in the early stages. The characteristic MRI finding is the presence of high signal within white matter especially in the parietal and temporal lobes and bilateral asymmetric subcortical lesions. With further disease progression, there is more extensive white matter, corpus callosum, and basal ganglia involvement [
12]. The magnetic resonance spectroscopy shows decreased N-acetyl-aspartate and increased choline in the early stages, this is suggestive of demyelination [
13]. The ratio alters in later stages indicating reduced brain volumes. The brain biopsy shows the presence of inclusion bodies called Dawson body, thus the name of the disease, and neurofibrillary tangles are found in some cases [
14].
There is no cure for SSPE, only symptomatic treatment is given which reduces disease progression and prolongs survival. The following drugs are given [
5,
15]:
Inosine pranobex (aka Isoprinosine)- an oral antiviral used to halt viral replication.
Interferon alpha- an immunomodulator that is given intrathecally.
Ribavirin- provides mild benefits.
Ketogenic diet- neuroprotective, given in refractory cases, has been shown to reduce myoclonus symptoms.
Conclusion
Dawson disease, aka Subacute Sclerosing Panencephalitis (SSPE), is a rare, neurological disorder occurring as a late complication of the measles infection. Due to varied clinical presentations and a smaller number of cases, its diagnosis is often missed. The cerebrospinal fluid analysis and electroencephalography findings aid in disease diagnosis. Due to the lack of a definitive treatment, a complete cure is not possible. The prognosis of SSPE is poor with a 90% risk of death. Only symptomatic treatment is given. Therefore, to reduce the incidence of this condition, effective strategies for immunisation should be implemented. The parents should be educated about the importance of immunisation and the severity of this life-threatening complication.
Funding
None of the authors are financially interested in any of the products, devices or drugs mentioned in this manuscript.
Institutional Review Board Statement
Being a case report study, there were no ethical issues and the IRB was notified about the topic and the case. Still, no formal permission was required as this was a record-based case report. Permission from the patient for the article has been acquired and ensured that their information or identity is not disclosed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papetti L, Amodeo ME, Sabatini L, Baggieri M, Capuano A, Graziola F, et al. Subacute Sclerosing Panencephalitis in Children: The Archetype of Non-Vaccination. Viruses [Internet]. 2022 Apr 1 [cited 2023 Dec 25];14(4). Available from: /pmc/articles/PMC9029616/. [CrossRef]
- Serap B, Neslihan Ö, Duygu Güner Ö, Gülen Gül M, Faruk İ, M Özlem H. Atypic subacute sclerosing panensefalitis in a six-year-old male. Journal of Advanced Pediatrics and Child Health. 2022 Nov 23;5(1):039–41. Avaiable from: https://www.pediatricshealthjournal.com/articles/japch-aid1051.php. [CrossRef]
- Simkhada N, Adhikari P, Pathak BD, Dhakal B, Mahat K. A Rare Case of Subacute Sclerosing Panencephalitis Presenting As Generalized Seizure. Cureus [Internet]. 2021 May 18 [cited 2023 Dec 25];13(5). Available from: /pmc/articles/PMC8212923/. [CrossRef]
- Hayman DTS. Measles vaccination in an increasingly immunized and developed world. Hum Vaccin Immunother [Internet]. 2019 Jan 2 [cited 2023 Dec 25];15(1):28. Available from: /pmc/articles/PMC6363159/. [CrossRef]
- Samia P, Oyieke K, Tunje D, Udwadia-Hegde A, Feemster K, Oncel I, et al. Options in the Treatment of Subacute Sclerosing Panencephalitis: Implications for Low Resource Areas. Curr Treat Options Neurol [Internet]. 2022 Mar 1 [cited 2023 Dec 25];24(3):99. Available from: /pmc/articles/PMC8933242/. [CrossRef]
- Lizarraga KJ, Gutierrez J, Singer C. Subacute Sclerosing Panencephalitis. The Curated Reference Collection in Neuroscience and Biobehavioral Psychology [Internet]. 2023 May 19 [cited 2023 Dec 25];187–9. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560673/.
- Bankamp B, Takeda M, Zhang Y, Xu W, Rota PA. Genetic Characterization of Measles Vaccine Strains. J Infect Dis [Internet]. 2011 Jul 1 [cited 2023 Dec 25];204(suppl_1):S533–48. Available from: https://dx.doi.org/10.1093/infdis/jir097. [CrossRef]
- Griffin DE. Measles virus persistence and its consequences. Curr Opin Virol [Internet]. 2020 Apr 1 [cited 2023 Dec 25];41:46. Available from: /pmc/articles/PMC7492426/. [CrossRef]
- Jafri SK, Kumar R, Ibrahim S. Subacute sclerosing panencephalitis – current perspectives. Pediatric Health Med Ther [Internet]. 2018 Jun [cited 2023 Dec 25];9:67. Available from: /pmc/articles/PMC6027681/. [CrossRef]
- Jagtap SA, Nair MD, Kambale HJ. Subacute sclerosing panencephalitis: A clinical appraisal. Ann Indian Acad Neurol [Internet]. 2013 Oct [cited 2023 Dec 25];16(4):631. Available from: /pmc/articles/PMC3841616/. [CrossRef]
- Ali S, Kumar H, Ullah S, Haq MAU, Gul NG, Kumar J. Electroencephalography Patterns of Subacute Sclerosing Panencephalitis. Cureus [Internet]. 2021 Jun 18 [cited 2023 Dec 25];13(6). Available from: /pmc/articles/PMC8286205/. [CrossRef]
- Knipe H, Gaillard F. Subacute sclerosing panencephalitis. Radiopaedia.org [Internet]. 2009 Nov 3 [cited 2023 Dec 25]; Available from: http://radiopaedia.org/articles/7471.
- Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol [Internet]. 2010 Oct 1 [cited 2023 Dec 25];52(10):901–7. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1469-8749.2010.03717.x. [CrossRef]
- Jafri SK, Kumar R, Ibrahim S. Subacute sclerosing panencephalitis – current perspectives. Pediatric Health Med Ther [Internet]. 2018 Jun [cited 2023 Dec 25];9:67. Available from: /pmc/articles/PMC6027681/. [CrossRef]
- Gascon GG, Anlar B, Güven A, Haspolat S, Köse G, Yalaz K, et al. Randomized Treatment Study of Inosiplex Versus Combined Inosiplex and Intraventricular Interferon-α in Subacute Sclerosing Panencephalitis (SSPE): International Multicenter Study. http://dx.doi.org/101177/088307380301801201 [Internet]. 2003 Dec 1 [cited 2023 Dec 25];18(12):819–27. Available from: https://journals.sagepub.com/doi/abs/10.1177/088307380301801201. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).