Submitted:
25 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
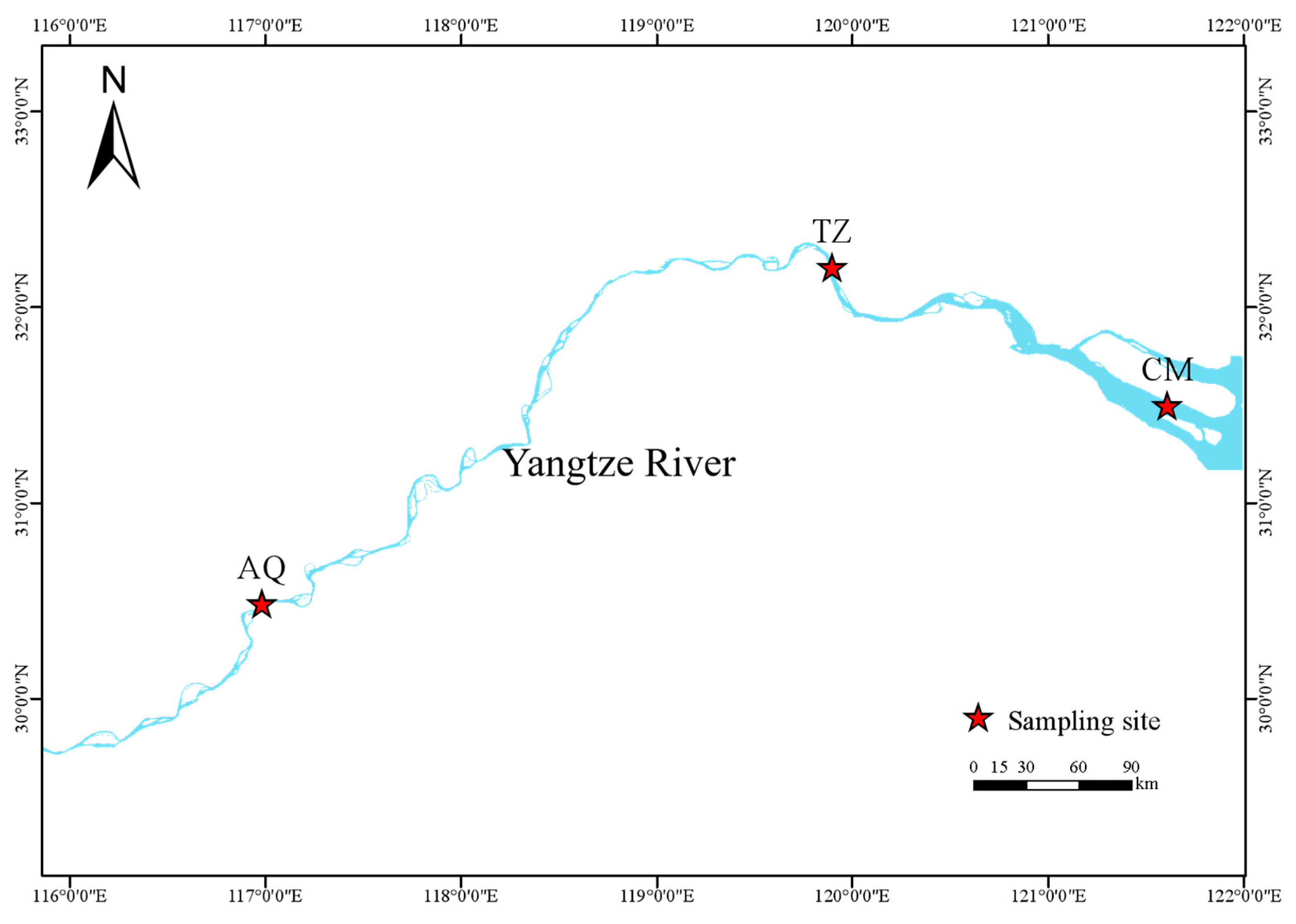
2.1. Survey Sample Areas and Methods
2.2. Sample Collection and Processing
2.3. Data processing and analysis
3. Results
3.1. Biological Characteristics of C. nasus
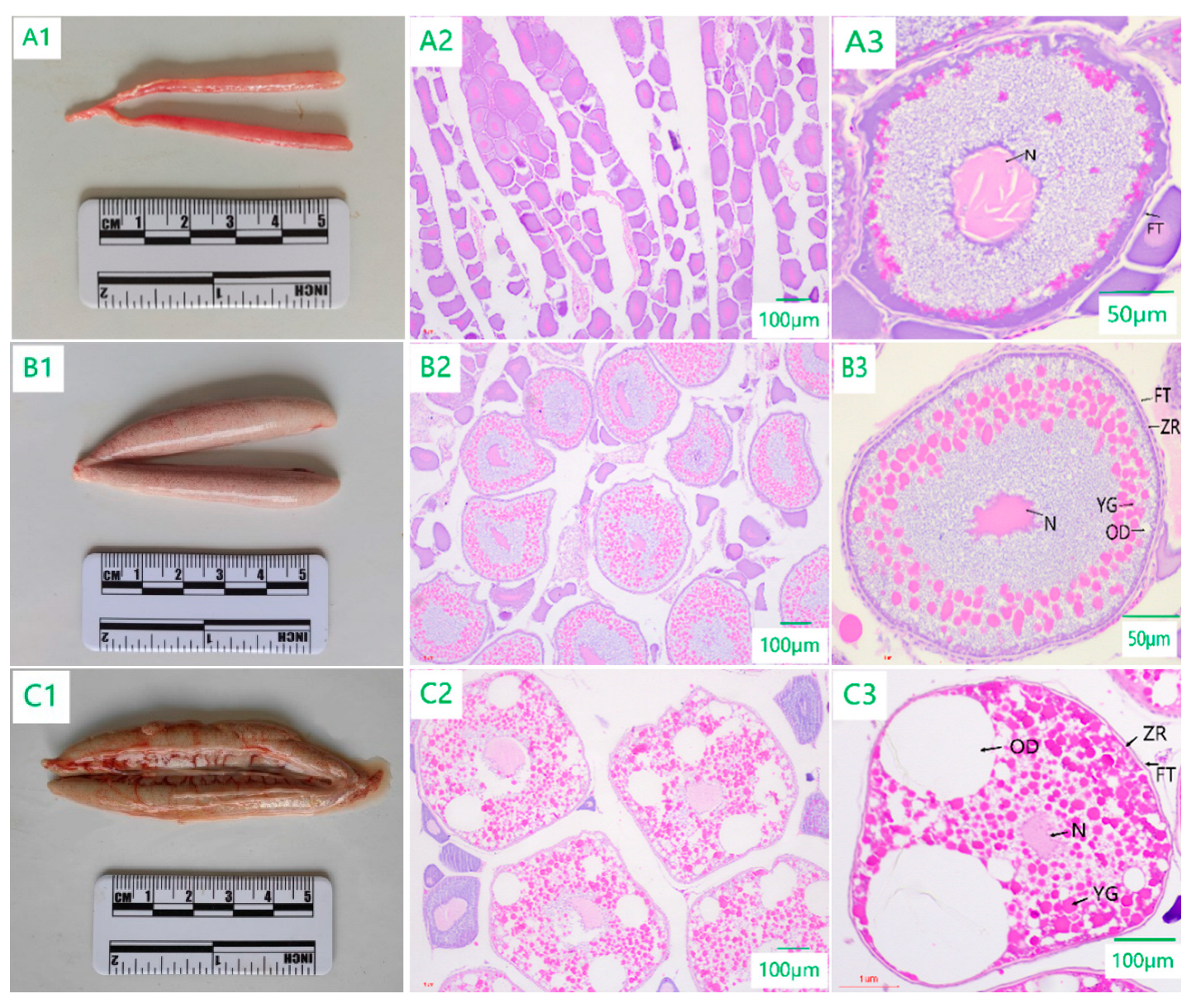
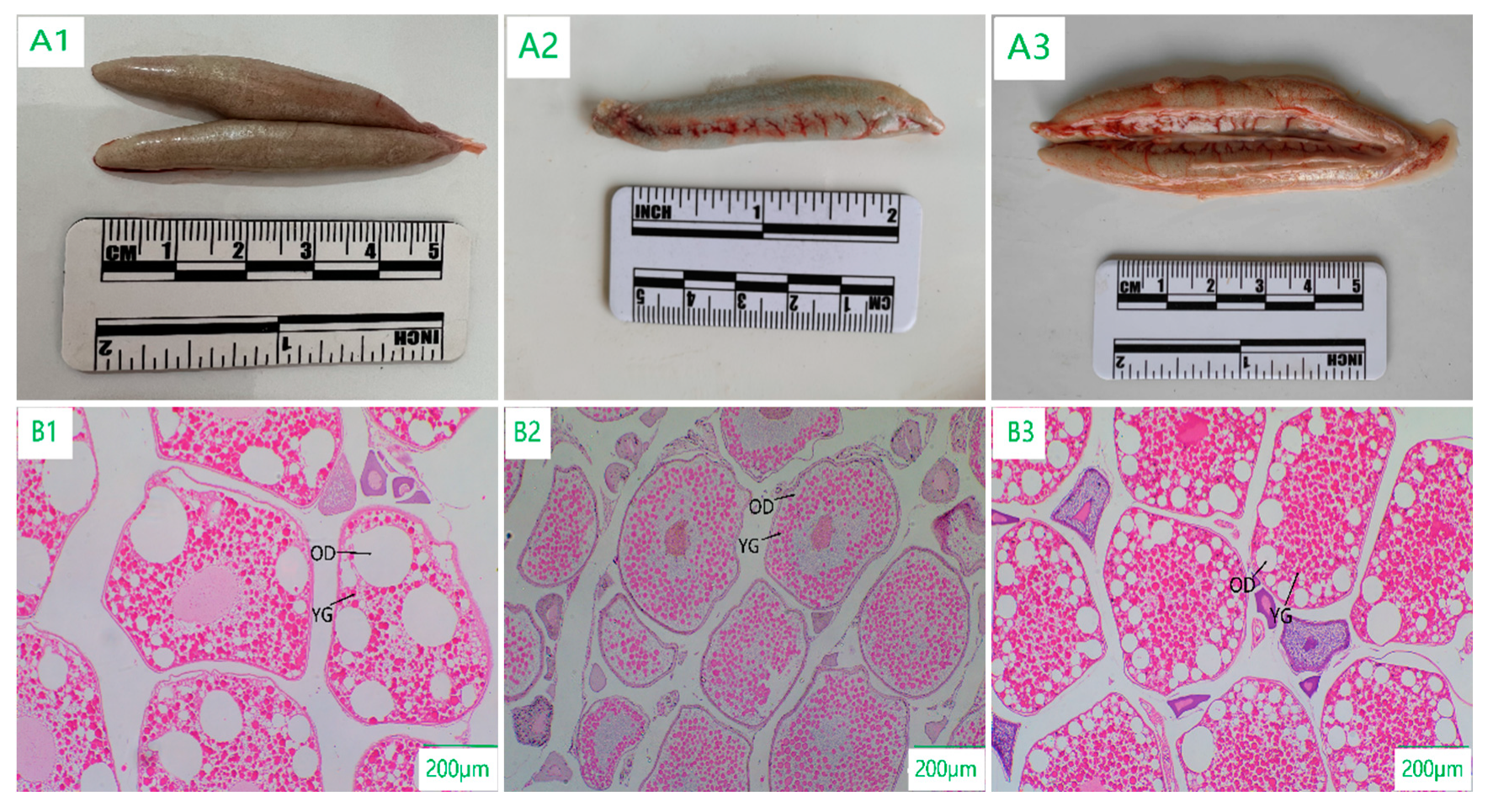
3.2. Morphologic and Histologic Sections of the Ovary
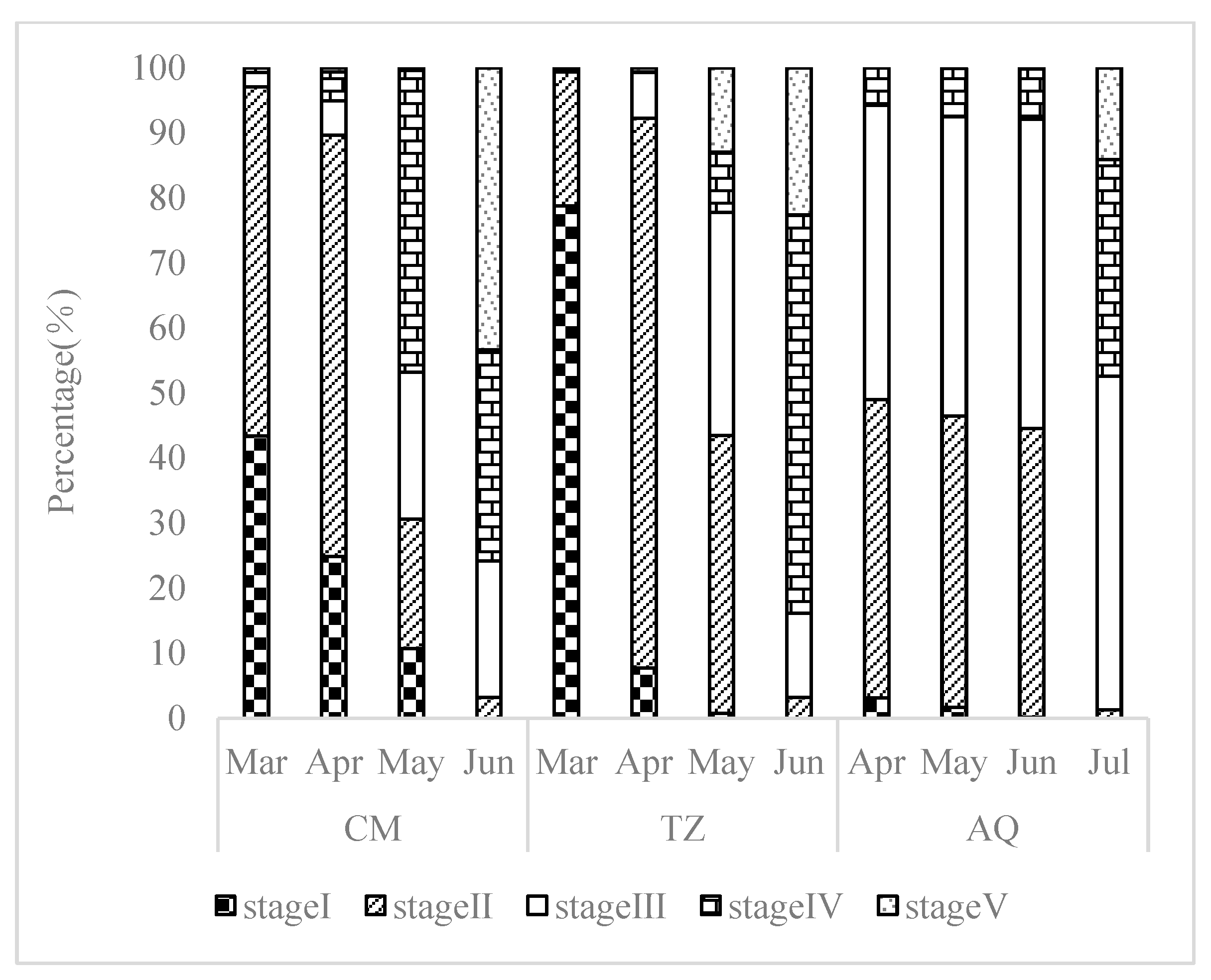
3.2.1. Developmental Stage Characteristics of Ovarian Tissue Sections
3.2.2. Spatial Characteristics of Ovarian Tissue Sections
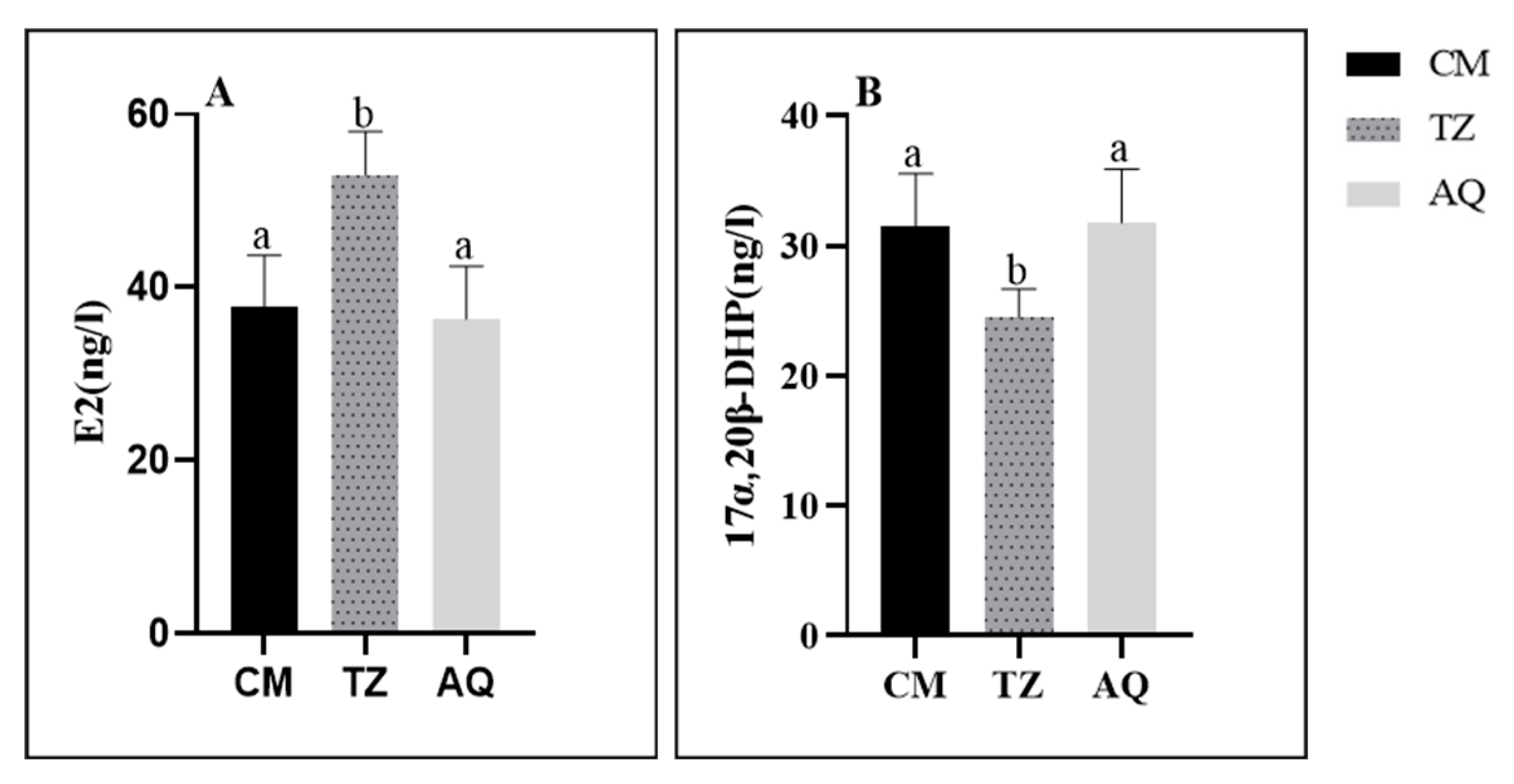
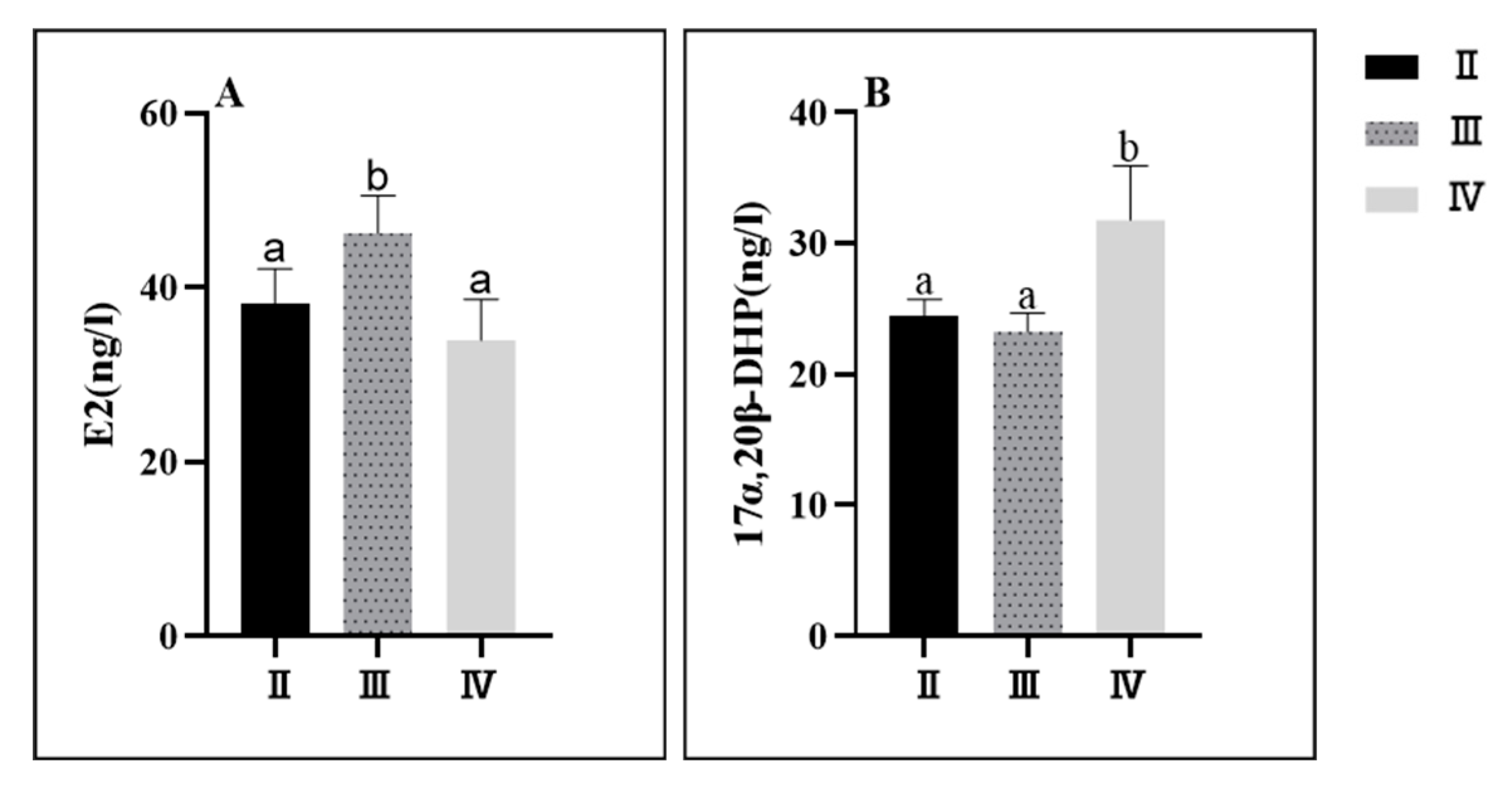
3.3. Serum Neutral Steroid Hormone Levels
3.3.1. Characterization of the Developmental Phase of Sex Steroid Hormones
3.3.2. Spatial Characterization of Sex Steroid Hormones
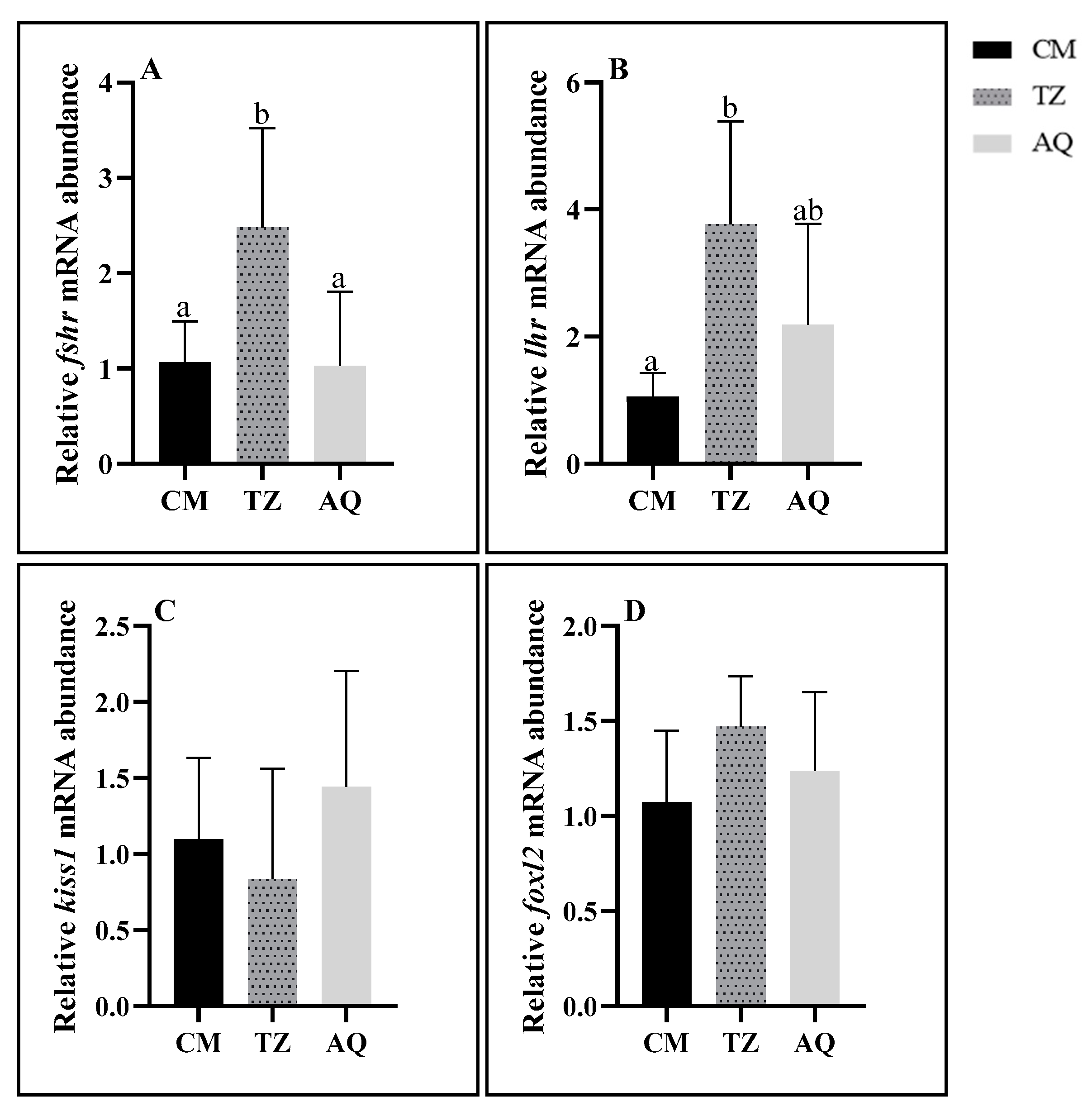
3.4. Expression Patterns of Ovarian Development-Related Genes
3.4.1. Characterization of Developmental Stages of Gene Expression
3.4.2. Spatial Characterization of Gene Expression
4. Discussion
4.1. Characterization of Ovarian Development in C. nasus
4.2. Characterization of Changes in Serum Neutral Steroid Hormones
4.3. Characterization of Gene Expression Related to Ovarian Development
Author Contributions
Funding
Institutional Review Board Statement
Conflict of Interest
References
- Sang, H.; Lam, H.; Hy, L.; Ky, P.; Minh, T. Changes in plasma and ovarian steroid hormone level in wild female blue tang fish Paracanthurus hepatus during a reproductive cycle. Animals 2019, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Tenugu, S.; Pranoty, A9.; Mamta, S.; Senthilkumaran, B. Development and organisation of gonadal steroidogenesis in bony fishes-a review. Aquaculture and Fisheries 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Moreira, R.; Honji, R.; Melo, R.; Moraes, N.; Amaral, J.; Carvalho, A.; Hilsdorf, A. The involvement of gonadotropins and gonadal steroids in the ovulatory dysfunction of the potamodromous Salminus hilarii (Teleostei: Characidae) in captivity. Fish physiology and biochemistry 2015, 41, 1435–1447. [Google Scholar] [CrossRef]
- Tokumoto T; Tokumoto M; Horiguchi R; Ishikawa K; Nagahama Y. Diethylstilbestrol induces fish oocyte maturation. Proceedings of the National Academy of Sciences 2004, 101, 3686–3690. [Google Scholar] [CrossRef]
- Brzuska, E.; Socha, M.; Chyb, J.; Sokołowska-Mikołajczyk, M.; Inglot, M. The Effect of [(D-Ala6, Pro9NEt) mGnRH-α+ Metoclopramide] (Ovopel) on Propagation Effectiveness of Two Breeding Lines of Common Carp (Cyprinus carpio L.) and on Luteinizing Hormone and 17α, 20β-Dihydroxyprogesterone Levels in Females during Ovulation Induction. Animals 2023, 13, 14–28. [Google Scholar]
- Luo, M. Effects of heat stress on the function and related genes expression in mice ovarian granulosa cells. Nanjing Agricultural University, 2016.
- Menon, K.; Munshi, U.; Clouser, C.; Nair, A. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biology of reproduction 2004, 70, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Y.; Rao, J.; Liu, Z. The expression of three gonadotropin subunits in response to 17α-ethynylestadiol in male pelteobagrus fulvidraco. Acta Hydrobiologica Sinica 2015, 39, 1117–1125. [Google Scholar]
- Nie, Z.; Zhao, N.; Zhao, H.; Fu, Z.; Ma, Z.; Wei, J. Cloning, Expression Analysis and SNP Screening of the kiss1 Gene in Male Schizothorax biddulphi. Genes 2023, 14, 862. [Google Scholar] [CrossRef] [PubMed]
- Kuohung, W.; Kaiser, U. GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Reviews in Endocrine and Metabolic Disorders 2006, 7, 257–263. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, M.; Jiang, D.; Zhang, J.; Hou, X.; Zhu, C. Induction of Premature Reversal in Plectropomus leopardus by Letrozole. Journal of Zhanjiang Ocean University 2023, 43, 19–25. [Google Scholar]
- Fan, Z.; Zou, Y.; Liang, D.; Tan, X.; Jiao, S.; Wu, Z.; Li, J.; Zhang, P.; You, F. Roles of forkhead box protein L2 (foxl2) during gonad differentiation and maintenance in a fish, the olive flounder (Paralichthys olivaceus). Reproduction, Fertility and Development 2019, 31, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, Z.; Hu, Y.; Huang, C.; Tian, S.; Wan, R.; Pauly, D. Assessment of Coilia mystus and C. nasus in the Yangtze River Estuary, China, using a length-based approach. Fishes 2022, 7, 95. [Google Scholar] [CrossRef]
- Liu, K.; Duan, J.; Xu, D.; Zhang, M.; Fang, D.; Shi, W. Present situation of Coilia nasus population features and yield in Yangtze River estuary waters in fishing season. Journal of Ecology 2012, 31, 3138–3143. [Google Scholar]
- Yuan, C. Spawning migration of Coilia nasus. Bulletin of Biology 1987, 12, 1–3. [Google Scholar]
- He, W.; Li, J.; Jiang, Z. Cytological observations on the gonad of Coilia ectenes in the Yangtze River. Journal of Shanghai Fisheries University 2006, 15, 292–295. [Google Scholar]
- He, W.; Li, J. Histological studies of gonadal development of gonad of Coilia ectenes in the Changjiang River. Journal of fisheries of China 2006, 6, 773–777. [Google Scholar]
- Xu, G.; Wan, J.; Gu, R.; Zhang, C.; Xu, P. Morphological and histological studies on ovary development of Coilia nasus under artificial farming conditions. Journal of Fishery Sciences of China 2011, 18, 537–546. [Google Scholar] [CrossRef]
- Yin, D.; Lin, D.; Ying, C.; Ma, F.; Yang, Y.; Wang, Y.; Tan, J.; Liu, K. Metabolic mechanisms of Coilia nasus in the natural food intake state during migration. Genomics 2020, 112, 3294–3305. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, G.; Xu, P. Whole-genome resequencing of three Coilia nasus population reveals genetic variations in genes related to immune, vision, migration, and osmoregulation. BMC genomics 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Liu, K.; Yin, D.; Shu, Y.; Dai, P.; Yang, Y.; Wu, H. Transcriptome and metabolome analyses of Coilia nasus in response to Anisakidae parasite infection. Fish & shellfish immunology 2019, 87, 235–242. [Google Scholar]
- Li, L.; Tang, W.; Zhang, Y. Changes of fatty acid content and its components in different tissues during spawning migration processes of female Coilia nasus in the lower reaches of the Yangtze River. Journal of Fisheries of China 2019, 43, 790–800. [Google Scholar]
- Guo, W. Fatty acid composition and energy utilization of Coilia nasus during spawning migration in Yangtze River. Shanghai Ocean University, 2021.
- Ma, F.; Guo, W.; Ying, C.; Yang, Y.; Xu, P.; Liu, K.; Yin, G. Changes of fatty acid components and content of Coilia nasus in the Yangtze River during spawning migration. Acta Hydrobiologica Sinica 2023, 47, 156–167. [Google Scholar]
- Wang, M.; Xu, P.; Zhu, Z. Regulation of signal transduction in Coilia nasus during migration. Genomics 2020, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X. Study on the development and annual change in the ovary of Pelteobagrus fulvidraco. Jour Nat Scie Hunan Norm Uni 2003, 26, 73–78. [Google Scholar]
- Ma, F.; Yang, Y.; Jiang, M.; Yin, D.; Liu, K. Digestive enzyme activity of the Japanese grenadier anchovy Coilia nasus during spawning migration: influence of the migration distance and the water temperature. Journal of fish biology 2019, 95, 1311–1319. [Google Scholar] [CrossRef]
- Xue, X.; Peng, Y.; Fang, D.; Xu, D.; Wang, X.; Ren, K. Preliminary study of Coilia nasus spawning grouds at Sutong section in the lower reaches of the Yangtze River. Journal of Fisheries of China 2022, 46, 1377–1388. [Google Scholar]
- Dai, P.; Yan, Y.; Zhu, X.; Tian, J.; Ma, F.; Liu, K. Status of Coilia nasus resources in the National Aquatic Germplasm Resources Conservation Area in the Anqing Section of the Yangtze River. Journal of Fishery Sciences of China 2020, 27, 1267–1276. [Google Scholar]
- He, W.; Li, J. Study on the gonadal development pattern of the Coilia nasus in the Yangtze River. China Fisheries 2006, 05, 70–72. [Google Scholar]
- Qu, H.; Liu, Y.; Yang, Y.; Li, S.; Hu, M.; Guan, M.; Lu, X.; Wen, Z.; Guo, W. Histological change in annual development of ovary in gudgeon Rhinogobio ventralis. Fishery Sciences 2015, 34, 32–37. [Google Scholar]
- Zhu, L.; Sun, M.; Zhang, D.; Yuan, J.; Xu, S. Histological study on the ovary development of Girella leonine. Journal of Applied Oceanography 2018, 37, 255–262. [Google Scholar]
- Liu, M.; Xiong, B.; Lv, G. Histological observation of ovary development in Xenocypris microlepis. Fisheries Science 2010, 29, 125–130. [Google Scholar]
- Ma, F.; Yang, Y.; Fang, D.; Ying, C.; Xu, P.; Liu, K.; Yin, G. Characteristics of Coilia nasus resources after fishingban in the Yangtze River. Acta Hydrobiologica Sinica 2022, 46, 1580–1590. [Google Scholar]
- Gu, H.; Feng, Y.; Yang, Z.; Wang, J. Histological observation of gonadal development of white bream Parabramis pekinensis. Journal of Fisheries of China 2022, 35, 44–50. [Google Scholar]
- Chen, L.; Qin, G.; Wang, X.; Liu, Y.; Xiao, W.; Lin, Q. Distinct structures of gonads and germ cell development of lined seahorse, Hippocampus erectus. Journal of tropical oceanography 2020, 39, 93–102. [Google Scholar]
- Zhao, H. Study on long-term sperm storage and sperm energy metabolism of Sebastes schlegelii. University of Chinese Acedemy of Science, 2020.
- Li, Y. Study on the function and regulatory mechanism of transcription factor Sox3 in oogenesis of Nile tilapia(Oreochromis niloticus). Southwest University, 2021.
- Qiu, Y.; Ding, X.; Li, Z.; Duan, P.; Wang, X.; Li, L.; Wang, L.; Liu, Y.; Ma, W.; Pang, Z.; Li, S.; Tian, Y. Comparative analysis of ovarian development and sex steroid hormone levels in hybrid Jinhu grouper (Epinephelus fuscoguttatus ♀ ×Epinephelus tukula ♂) and Epinephelus fuscoguttatus. Journal of Fishery Sciences of China 2023, 30, 457–467. [Google Scholar]
- Zhao, H.; Zhao, N.; Li, L.; Qiang, Z.; Nie, Z.; Wei, J. Gonadal development and changes inconcentration of serum sex hormones in Schizothorax irregularis. Fisheries Science 2023, 42, 233–240. [Google Scholar]
- Liu, C. Effects of photoperiod on fecundity, plasma hormone content and gene expression related to ovarian development in zebrafish. Shanghai Ocean University, 2022.
- Tokumoto, T.; Yamaguchi, T.; Ii, S.; Tokumoto, M. In vivo induction of oocyte maturation and ovulation in zebrafish. PloS one 2011, 6, e25206. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tang, Y.; Zhang, J.; Soyano, K.; Li, B. Annual ovarian development and changes in the concentration of serum sex hormones in blacktip grouper Epinephelus fasciatus. Journal of Dalian Ocean University 2020, 35, 657–662. [Google Scholar]
- Li, P.; Wang, J.; Lu, W.; Tang, F.; Liu, W. Reproduction related endocrine hormone level and gonadal decelopment of Oncorhynchus ketain pre-growth period. Guizhou Agricultural Sciences 2020, 48, 54–59. [Google Scholar]
- Dai, P.; Ma, F.; Tian, J.; Wang, Y.; Yang, Y.; Liu, K. Community structure and infection characteristics of nematodes in the Coilia nasus Anqing section of the Yangtze River. Acta Hydrobiologica Sinica 2023, 47, 917–923. [Google Scholar]
- Hu, Y.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Otolith microchemical “fingerprints” of Coilia nasus from the Taizhou section of Changjiang River in Jiangsu Province. Chinese Journal of Ecology 2023, 1–10. [Google Scholar]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, EL.; Gómez, A.; Lareyre, J. Perspectives on fish gonadotropins and their receptors. General and comparative endocrinology 2010, 165, 412–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Effects of gonadotropin and receptor on oocytes of Micropterus salmoides. Henan Normal University, 2022.
- Sambroni, E.; Gac, L.; Breton, B.; Lareyre, J. Functional specificity of the rainbow trout (Oncorhynchus mykiss) gonadotropin receptors as assayed in a mammalian cell line. Journal of Endocrinology 2007, 195, 213–228. [Google Scholar] [CrossRef] [PubMed]
- She, D.; Chen, D.; Wang, Y.; Zhou, Y.; Liu, Q.; Li, J. Molecular gene cloning and expression of gonadotropin receptors from Ctenopharyngodon idella. Life Science Research 2015, 19, 39–43. [Google Scholar]
- Lee, E.; Chakravarthi, V.; Wolfe, M.; Rumi, M. ERβ regulation of gonadotropin responses during folliculogenesis. International journal of molecular sciences 2021, 22, 10348. [Google Scholar] [CrossRef]
- Miao, X. Estradiol-induced sexual reversal and its effects on gonadal differentiation and development in mandarin fish (Siniperca chuatsi). Southwest University, 2023.
- Wang, S.; Huang, J; Liang, L; Su, B; Zhang, Y.; Liew, HJ.; Sun, B.; Zhang, L.; Chang, Y. Distinctive metabolite profiles in migrating Amur ide (Leuciscus waleckii) reveal changes in osmotic pressure, gonadal development, and energy allocation strategies. Frontiers in Environmental Science 2022, 10, 1–15. [Google Scholar]

| Sampling area | Sampling point | Sampling period | Net type | Net secification |
|---|---|---|---|---|
| CM | 31° 29′ 52″ N, 121° 36′ 36″ E | March- June | Throwing gill net | Length 150 m, height 12 m, mesh size 4 cm |
| TZ | 32° 12′ 14″ N, 119° 53′ 40″ E | March- June | Gill net | Length 180 m, height 4 m, mesh size 4 cm |
| AQ | 30° 29′ 11″ N, 116° 59′ 39″ E | April- July | Gill net | Length 180 m, height 4 m, mesh size 4 cm |
| Gene | Primers sequence(5’-3’) | Amplicon size(bp) |
|---|---|---|
| Coilia β-actin-F | GAGCCTCCGATCCAGACAGAG | |
| Coilia β-actin-R | CATGAAGTGTGATGTCGACATCC | |
| fshr-F | GATGCCAACCTCACATACCC | 120 |
| fshr-R | GAAGACGGGCTCCTCCAG | |
| lhr-F | ACCTCAGCAGCCTTCCCA | 113 |
| lhr-R | ATTACCGATGACAGCAGACCC | |
| kissr1-F | CTTGTTGGGCTTCTGGGTAA | 228 |
| kissr1-R | TGTCCTGTGGCGGAGTGA | |
| foxl2-F | CCGGCATGGTGAACTCTTAC | 119 |
| foxl2-R | GTTAGCGGAGGGGACAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




