Submitted:
22 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Lentiviral Transduction of Ca Ski Cells and Isolation of Clones Expressing RT_A and GFP Variants
2.2. Determination of the Number of Copies of the Provirus in the Genome of the Transduced Lines
2.3. Confirmation of RT Production by Western Blotting
2.4. Isolation of Nucleic Acids, Reverse Transcription, and Semiquantitative PCR
2.5. Cell Culture and Microscopic Quantitation of Proliferation
2.6. Measurement of Glycolysis and Mitochondrial Respiration
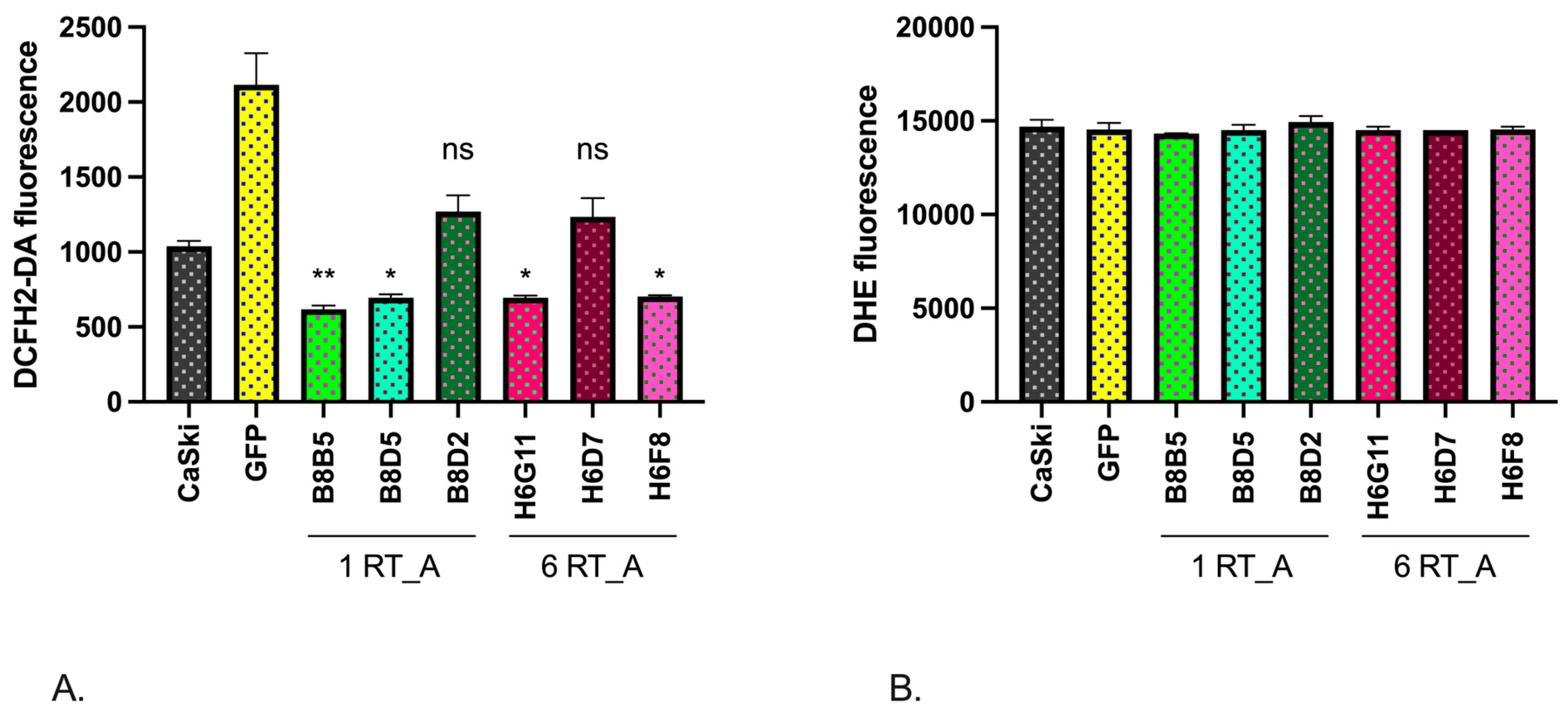
2.7. Assessment of the Production of Reactive Oxygen Species (ROS)
2.8. Wound Healing Assay
2.9. Clonogenic assay
2.10. Cell Cycle Analysis
2.11. Assessment of Tumorigenicity of Ca Ski Derivative Clones
2.12. Statistical Analysis
3. Results
3.1. Lentivirally Transduced Ca Ski Cells Express HIV-1 Reverse Transcriptase
3.2. RT Increases the Expression of HPV16 E6*I Isoform
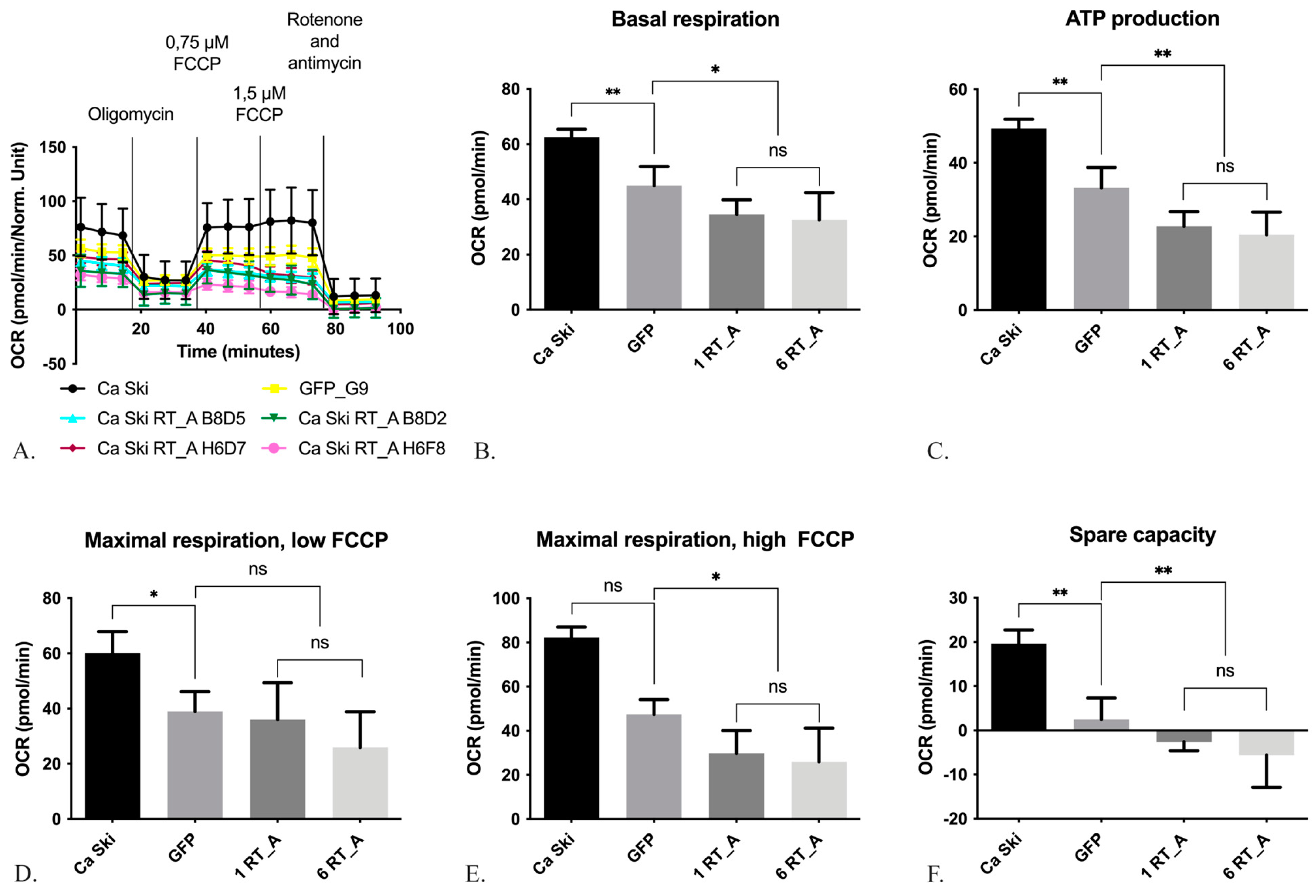
3.3. HIV-1 RT Expression Increases the Extracellular Acidification Rate and Decreases the Oxygen Consumption rate of Ca SKI CELLS
3.3.1. HIV-1 RT Expression in Ca Ski Cells Increases the Glycolysis
3.3.2. HIV-1 RT Expression in Ca Ski Cells Suppresses Mitochondrial Respiration
3.4. Expression of HIV-1 RT Did Not Lead to the Changes in the Cytoskeleton
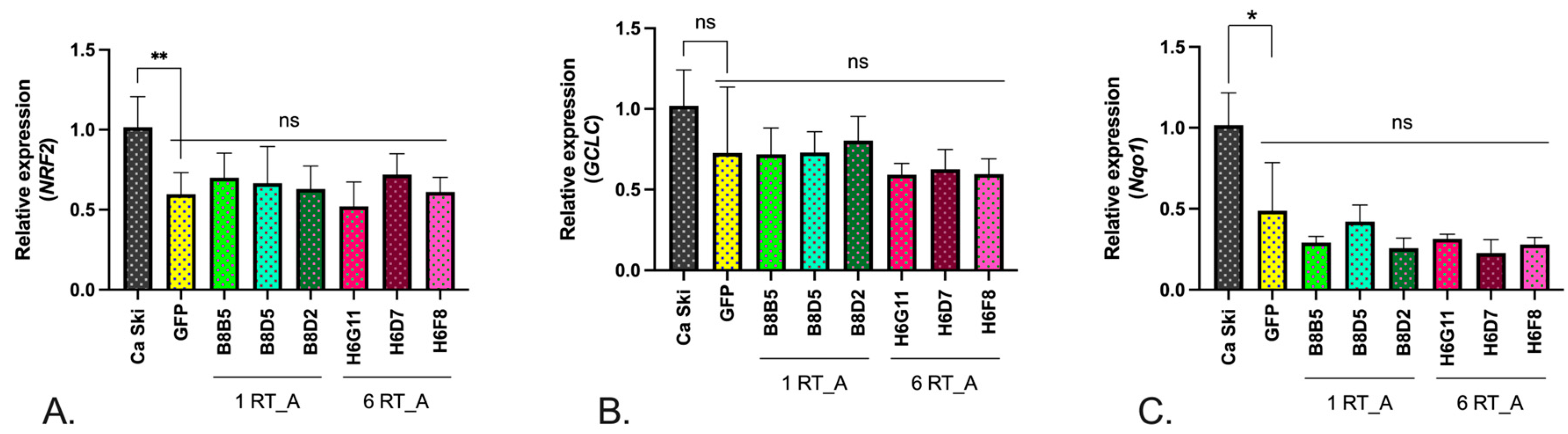
3.5. Expression of HIV-1 RT in Epithelial Ca Ski Cells Does Not Induce Oxidative Stress
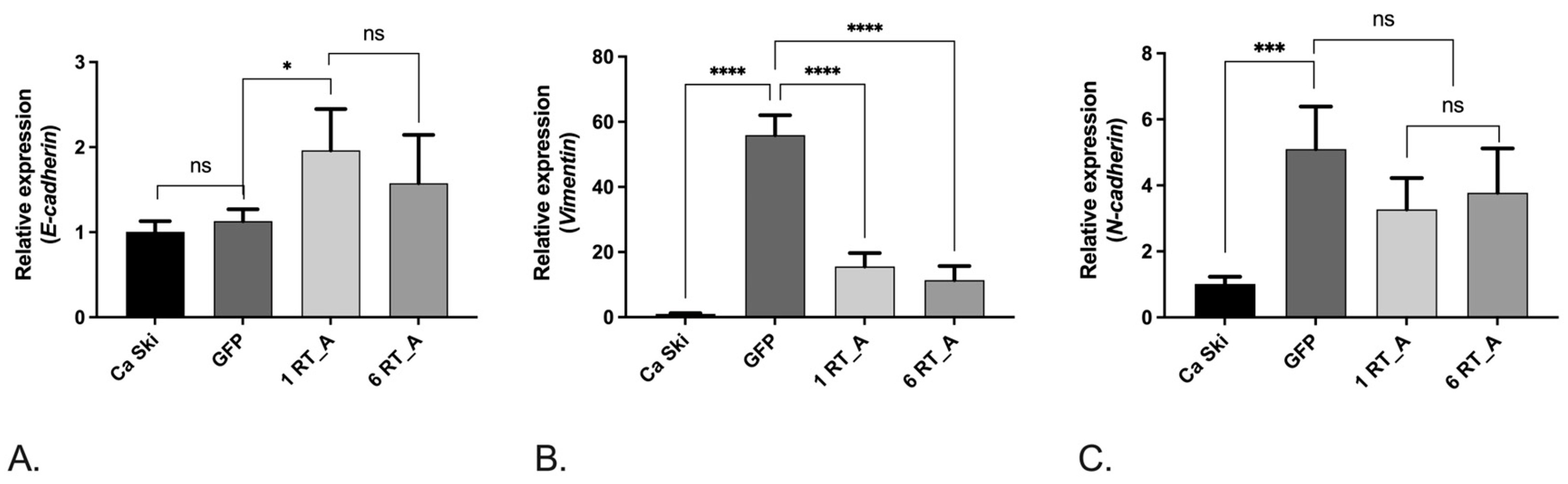
3.6. Lentivirus-Transduced Ca Ski Express Reduced Levels of Factors Associated with Epithelial-Mesenchymal Transition
3.7. Effects of HIV-1 RT Expression on Phenotypical Properties of Ca Ski Cells—Motility and Clonogenic Activity
3.7.1. Expression of RT_A Causes an Increase in Cell Doubling Time, but Does Not Affect Cell Cycle Progression
3.7.2. Overexpression of HIV-1 RT Reverses Inhibitory Effect of Lentiviral Transduction on Cell Mobility
| Cells | Doubling time, h (n=12) * | % of cells in cell cycle phase (n=3) ** | Migration rate in WHA, μm/h (n=6) *** | ||
| G1/G0, % | S, % | G2/M, % | |||
| Ca Ski | 25,90±3,26 | 50±3,90 | 24,43±4,90 | 18,47±1,25 | 23,84±1,56 |
| GFP | 35,95±7,78 | 38,57±2,63 | 27,67±3,62 | 22,3±3,60 | 11,61±2,06 |
| B8B5 | 32,59±6,05 | 45,57±1,01 | 28,17±5,92 | 16,67±4,88 | 13,57±1,42 |
| B8D5 | 31,05±7,80 | 50,23±0,94 | 24,1±2,68 | 18,17±1,97 | 15,87±0,91 |
| B8D2 | 31,04±7,57 | 47,8±4,07 | 23,87±6,53 | 19,53±4,96 | 31,12±3,35 |
| H6G11 | 35,04±7,83 | 41,03±3,27 | 27,53±2,87 | 23,03±1,79 | 20,71±2,61 |
| H6D7 | 30,47±6,17 | 43,77±0,25 | 23,77±1,15 | 24,27±1,50 | 27,55±1,04 |
| H6F8 | 38,60±9,45 | 44,37±3,73 | 26,33±1,59 | 21,93±1,80 | 15,84±1,72 |
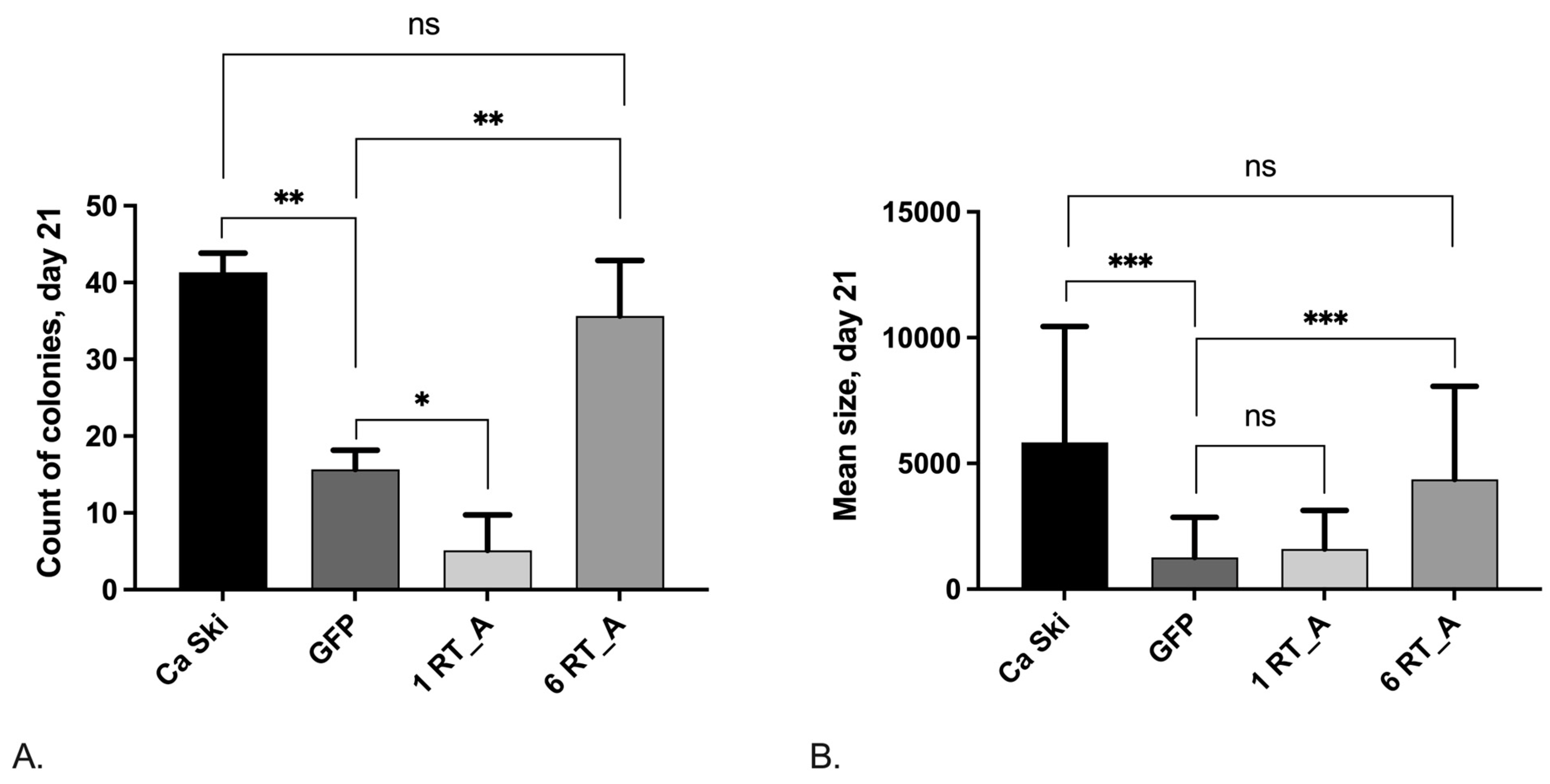
3.7.3. Loss of Clonogenic Activity after Retroviral Transduction Is Compensated in Ca Ski Cells Expressing High Levels of HIV-1 RT
3.8. The Relationship between the Levels of mRNA/Protein Production RT_A and E6*I and Various Specific Cell Characteristics
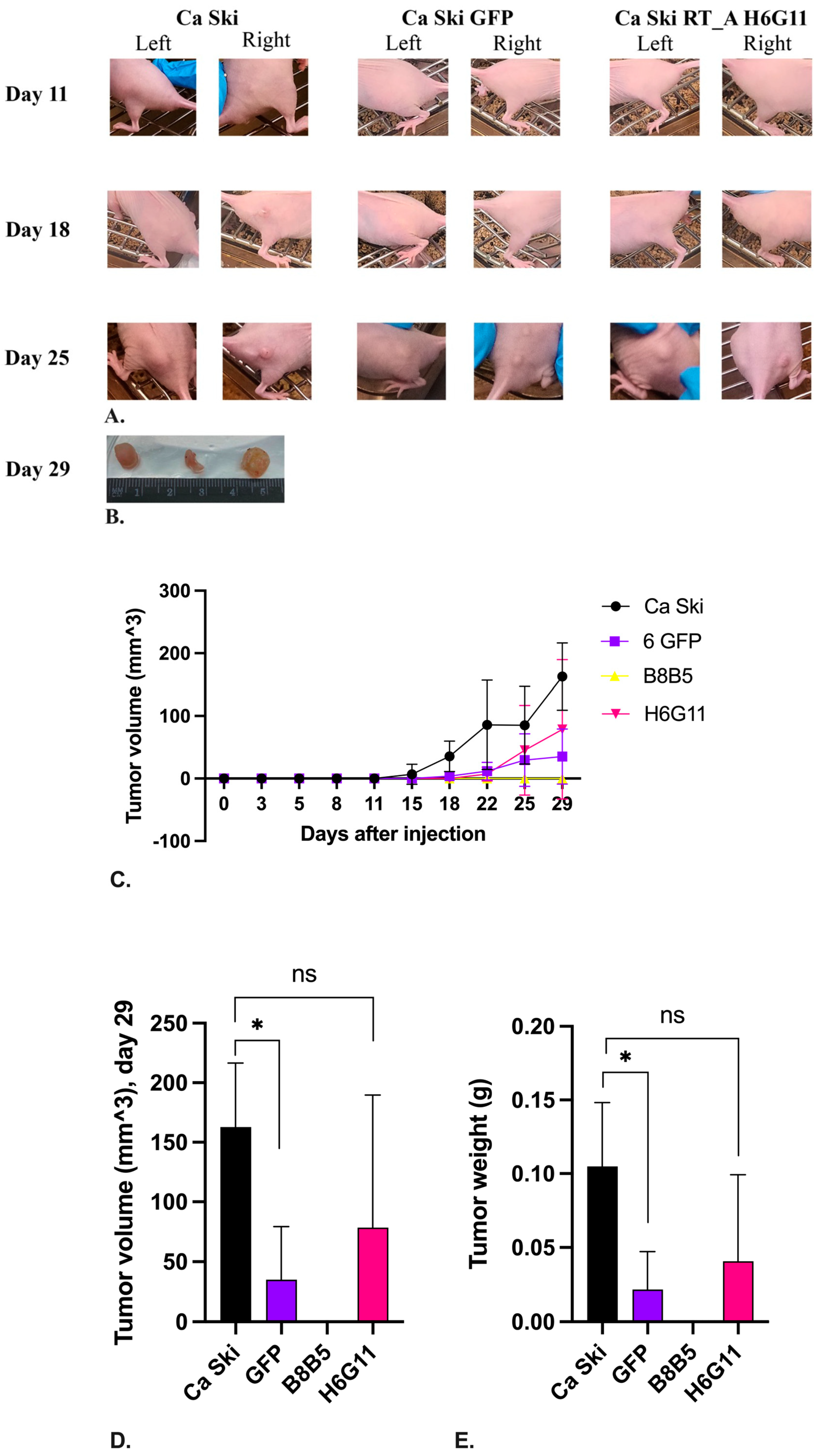
3.9. Overexpression of RT Rescues Tumorigenic Activity of Ca SKI SUBCLONES AFFECTED by the Retroviral Transduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers 2021, 13, 305. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxidative Medicine and Cellular Longevity 2016, 2016, e8910396. [Google Scholar] [CrossRef]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat Protein Induces DNA Damage in Human Peripheral Blood B-Lymphocytes via Mitochondrial ROS Production. Redox Biol 2017, 15, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Bayurova, E.; Jansons, J.; Skrastina, D.; Smirnova, O.; Mezale, D.; Kostyusheva, A.; Kostyushev, D.; Petkov, S.; Podschwadt, P.; Valuev-Elliston, V.; et al. HIV-1 Reverse Transcriptase Promotes Tumor Growth and Metastasis Formation via ROS-Dependent Upregulation of Twist. Oxidative Medicine and Cellular Longevity 2019, 2019, 1–28. [Google Scholar] [CrossRef]
- Riedel, D.J.; Tang, L.S.; Rositch, A.F. The Role of Viral Co-Infection in HIV-Associated Non-AIDS Related Cancers. Curr HIV/AIDS Rep 2015, 12, 362–372. [Google Scholar] [CrossRef]
- Frisch, M. Association of Cancer With AIDS-Related Immunosuppression in Adults. JAMA 2001, 285, 1736. [Google Scholar] [CrossRef]
- Clifford, G.M.; Polesel, J.; Rickenbach, M.; on behalf of the Swiss HIV Cohort Study; Dal Maso, L.; Keiser, O.; Kofler, A.; Rapiti, E.; Levi, F.; Jundt, G.; et al. Cancer Risk in the Swiss HIV Cohort Study: Associations With Immunodeficiency, Smoking, and Highly Active Antiretroviral Therapy. JNCI Journal of the National Cancer Institute 2005, 97, 425–432. [CrossRef]
- Palefsky, J.M.; Holly, E.A. Chapter 6: Immunosuppression and Co-Infection with HIV. JNCI Monographs 2003, 2003, 41–46. [Google Scholar] [CrossRef] [PubMed]
- International Collaboration on HIV and Cancer Highly Active Antiretroviral Therapy and Incidence of Cancer in Human Immunodeficiency Virus-Infected Adults. Journal of the National Cancer Institute 2000, 92, 1823–1830. [CrossRef] [PubMed]
- Frisch, M. Human Papillomavirus-Associated Cancers in Patients With Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Syndrome. Journal of the National Cancer Institute 2000, 92, 1500–1510. [Google Scholar] [CrossRef]
- Palefsky, J.M. Human Papillomavirus Infection and Anogenital Neoplasia in Human Immunodeficiency Virus-Positive Men and Women. JNCI Monographs 1998, 1998, 15–20. [Google Scholar] [CrossRef]
- Gillison, M.L.; Shah, K.V. Chapter 9: Role of Mucosal Human Papillomavirus in Nongenital Cancers. J Natl Cancer Inst Monogr 2003, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F. Chapter 2: The Burden of HPV-Related Cancers. Vaccine 2006, 24, S11–S25. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int J Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Ibrahim Khalil, A.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the Global Burden of Cervical Cancer Associated with HIV. Lancet Glob Health 2021, 9, e161–e169. [Google Scholar] [CrossRef]
- Dryden-Peterson, S.; Bvochora-Nsingo, M.; Suneja, G.; Efstathiou, J.A.; Grover, S.; Chiyapo, S.; Ramogola-Masire, D.; Kebabonye-Pusoentsi, M.; Clayman, R.; Mapes, A.C.; et al. HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol 2016, 34, 3749–3757. [Google Scholar] [CrossRef]
- Shiels, M.S.; Cole, S.R.; Kirk, G.D.; Poole, C. A Meta-Analysis of the Incidence of Non-AIDS Cancers in HIV-Infected Individuals. J Acquir Immune Defic Syndr 2009, 52, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, P.; Singhal, S.; Desai, D.; Parsi, M.; Potdar, R. HIV-Associated Anal Cancer. Cureus 13, e14834. [CrossRef]
- Piketty, C.; Selinger-Leneman, H.; Grabar, S.; Duvivier, C.; Bonmarchand, M.; Abramowitz, L.; Costagliola, D.; Mary-Krause, M.; Co 4, on behalf of the F.-A. Marked Increase in the Incidence of Invasive Anal Cancer among HIV-Infected Patients despite Treatment with Combination Antiretroviral Therapy. AIDS 2008, 22, 1203. [CrossRef]
- Pereira, A.C.C.; Lacerda, H.R. de; Barros, R.C. do R. Diagnostic Methods for Prevention of Anal Cancer and Characteristics of Anal Lesions Caused by HPV in Men with HIV/AIDS. Braz J Infect Dis 2008, 12, 293–299. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; Ceccarelli, M.; Rullo, E.V.; Facciolà, A.; D’Aleo, F.; Cacopardo, B.; Iacobello, C.; Costa, A.; Altavilla, G.; Pellicanò, G.F.; et al. Cancer Screening in HIV-Infected Patients- Early Diagnosis in a High-Risk Population.
- Ceccarelli, M.; Condorelli, F.; Rullo, E.V.; Pellicanò, G.F. Editorial – Improving Access and Adherence to Screening Tests for Cancers.
- Tanaka, L.F.; Latorre, M. do R.D.O.; Gutierrez, E.B.; Curado, M.P.; Dal Maso, L.; Herbinger, K.-H.; Froeschl, G.; Heumann, C. Cancer Survival in People with AIDS: A Population-Based Study from São Paulo, Brazil. Int J Cancer 2018, 142, 524–533. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human Papillomavirus Molecular Biology and Disease Association. Reviews in Medical Virology 2015, 25, 2–23. [Google Scholar] [CrossRef]
- Schiller, J.T.; Day, P.M.; Kines, R.C. Current Understanding of the Mechanism of HPV Infection. Gynecologic Oncology 2010, 118, S12–S17. [Google Scholar] [CrossRef]
- Carias, A.M.; McCoombe, S.; McRaven, M.; Anderson, M.; Galloway, N.; Vandergrift, N.; Fought, A.J.; Lurain, J.; Duplantis, M.; Veazey, R.S.; et al. Defining the Interaction of HIV-1 with the Mucosal Barriers of the Female Reproductive Tract. J Virol 2013, 87, 11388–11400. [Google Scholar] [CrossRef]
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission | PLOS Pathogens. [CrossRef]
- Joag, S.V.; Adany, I.; Li, Z.; Foresman, L.; Pinson, D.M.; Wang, C.; Stephens, E.B.; Raghavan, R.; Narayan, O. Animal Model of Mucosally Transmitted Human Immunodeficiency Virus Type 1 Disease: Intravaginal and Oral Deposition of Simian/Human Immunodeficiency Virus in Macaques Results in Systemic Infection, Elimination of CD4+ T Cells, and AIDS. J Virol 1997, 71, 4016–4023. [Google Scholar] [CrossRef]
- Girard, M.; Mahoney, J.; Wei, Q.; van der Ryst, E.; Muchmore, E.; Barré-Sinoussi, F.; Fultz, P.N. Genital Infection of Female Chimpanzees with Human Immunodeficiency Virus Type 1. AIDS Res Hum Retroviruses 1998, 14, 1357–1367. [Google Scholar] [CrossRef]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV Internalization into Oral and Genital Epithelial Cells by Endocytosis and Macropinocytosis Leads to Viral Sequestration in the Vesicles. Virology 2018, 515, 92–107. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef Is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4+ T Cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Nobile, C.; Rudnicka, D.; Hasan, M.; Aulner, N.; Porrot, F.; Machu, C.; Renaud, O.; Prévost, M.-C.; Hivroz, C.; Schwartz, O.; et al. HIV-1 Nef Inhibits Ruffles, Induces Filopodia, and Modulates Migration of Infected Lymphocytes. J Virol 2010, 84, 2282–2293. [Google Scholar] [CrossRef]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 Evades Virus-Specific IgG2 and IgA Responses by Targeting Systemic and Intestinal B Cells via Long-Range Intercellular Conduits. Nat Immunol 2009, 10, 1008–1017. [Google Scholar] [CrossRef]
- Pyeon, D.; Rojas, V.K.; Price, L.; Kim, S.; Singh, M.; Park, I.-W. HIV-1 Impairment via UBE3A and HIV-1 Nef Interactions Utilizing the Ubiquitin Proteasome System. Viruses 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.L.; Keiger, K.E.; Lee, C.J.; Huibregtse, J.M. The Global Transcriptional Effects of the Human Papillomavirus E6 Protein in Cervical Carcinoma Cell Lines Are Mediated by the E6AP Ubiquitin Ligase. J Virol 2005, 79, 3737–3747. [Google Scholar] [CrossRef]
- Talis, A.L.; Huibregtse, J.M.; Howley, P.M. The Role of E6AP in the Regulation of P53 Protein Levels in Human Papillomavirus (HPV)-Positive and HPV-Negative Cells*. Journal of Biological Chemistry 1998, 273, 6439–6445. [Google Scholar] [CrossRef]
- Massimi, P.; Shai, A.; Lambert, P.; Banks, L. HPV E6 Degradation of P53 and PDZ Containing Substrates in an E6AP Null Background. Oncogene 2008, 27, 1800–1804. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Buonaguro, F.M.; Beth-Giraldo, E.; Giraldo, G. Human Immunodeficiency Virus Type 1 Tat Gene Enhances Human Papillomavirus Early Gene Expression. Intervirology 2008, 36, 57–64. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L.; Del Gaudio, E.; Beth-Giraldo, E.; Giraldo, G. Role of HIV as Cofactor in HPV Oncogenesis: In Vitro Evidences of Virus Interactions. Antibiot Chemother (1971) 1994, 46, 102–109. [Google Scholar] [CrossRef]
- Kim, R.H.; Yochim, J.M.; Kang, M.K.; Shin, K.-H.; Christensen, R.; Park, N.-H. HIV-1 Tat Enhances Replicative Potential of Human Oral Keratinocytes Harboring HPV-16 Genome. Int J Oncol 2008, 33, 777–782. [Google Scholar]
- Barillari, G.; Palladino, C.; Bacigalupo, I.; Leone, P.; Falchi, M.; Ensoli, B. Entrance of the Tat Protein of HIV-1 into Human Uterine Cervical Carcinoma Cells Causes Upregulation of HPV-E6 Expression and a Decrease in P53 Protein Levels. Oncol Lett 2016, 12, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Hart, C.E.; Reeves, W.C.; Icenogle, J.P. The HIV-1 Tat Protein Enhances E2-Dependent Human Papillomavirus 16 Transcription. Virus Research 1993, 27, 133–145. [Google Scholar] [CrossRef]
- Tan, W.; Felber, B.K.; Zolotukhin, A.S.; Pavlakis, G.N.; Schwartz, S. Efficient Expression of the Human Papillomavirus Type 16 L1 Protein in Epithelial Cells by Using Rev and the Rev-Responsive Element of Human Immunodeficiency Virus or the Cis-Acting Transactivation Element of Simian Retrovirus Type 1. J Virol 1995, 69, 5607–5620. [Google Scholar] [CrossRef]
- Nuclear Import and Cell Cycle Arrest Functions of the HIV-1 Vpr Protein Are Encoded by Two Separate Genes in HIV-2/SIV(SM). | The EMBO Journal. Available online: https://www.embopress.org/doi/abs/10.1002/j.1460-2075.1996.tb01003.x (accessed on 7 June 2023).
- Toy, E.P.; Rodríguez-Rodríguez, L.; McCance, D.; Ludlow, J.; Planelles, V. Induction of Cell-Cycle Arrest in Cervical Cancer Cells by the Human Immunodeficiency Virus Type 1 Viral Protein R11Named a Searle-Donald F. Richardson Prize Paper after Presentation at the ACOG District II Junior Fellows Annual Meeting, New York, October 1998, and Annual Clinical Meeting of ACOG, Philadelphia, May 1999. Obstetrics & Gynecology 2000, 95, 141–146. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Palefsky, J.M.; Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Palefsky, J.M. 46. HIV-Induced Epithelial–Mesenchymal Transition in Mucosal Epithelium Facilitates HPV Paracellular Penetration. Sex. Health 2013, 10, 592–592. [Google Scholar] [CrossRef]
- Lien, K.; Mayer, W.; Herrera, R.; Padilla, N.T.; Cai, X.; Lin, V.; Pholcharoenchit, R.; Palefsky, J.; Tugizov, S.M. HIV-1 Proteins Gp120 and Tat Promote Epithelial-Mesenchymal Transition and Invasiveness of HPV-Positive and HPV-Negative Neoplastic Genital and Oral Epithelial Cells. Microbiol Spectr 2022, 10, e0362222. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Garson, K.; Li, L.; Vanderhyden, B.C. Optimization of Lentiviral Vector Production Using Polyethylenimine-Mediated Transfection. Oncol Lett 2015, 9, 55–62. [Google Scholar] [CrossRef]
- Giry-Laterrière, M.; Verhoeyen, E.; Salmon, P. Lentiviral Vectors. Methods Mol Biol 2011, 737, 183–209. [Google Scholar] [CrossRef]
- Isaguliants, M.G.; Belikov, S.V.; Starodubova, E.S.; Gizatullin, R.Z.; Rollman, E.; Zuber, B.; Zuber, A.K.; Grishchenko, O.I.A.; Rytting, A.-S.; Källander, C.F.R.; et al. Mutations Conferring Drug Resistance Affect Eukaryotic Expression of HIV Type 1 Reverse Transcriptase. AIDS Res Hum Retroviruses 2004, 20, 191–201. [Google Scholar] [CrossRef]
- Zhang, J.; Ruschhaupt, M.; Biczok, R. ddCt Method for qRT–PCR Data Analysis.; 2010.
- Golikov, M.V.; Karpenko, I.L.; Lipatova, A.V.; Ivanova, O.N.; Fedyakina, I.T.; Larichev, V.F.; Zakirova, N.F.; Leonova, O.G.; Popenko, V.I.; Bartosch, B.; et al. Cultivation of Cells in a Physiological Plasmax Medium Increases Mitochondrial Respiratory Capacity and Reduces Replication Levels of RNA Viruses. Antioxidants 2022, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Smirnova, O.A.; Ivanova, O.N.; Masalova, O.V.; Kochetkov, S.N.; Isaguliants, M.G. Hepatitis C Virus Proteins Activate NRF2/ARE Pathway by Distinct ROS-Dependent and Independent Mechanisms in HUH7 Cells. PLOS ONE 2011, 6, e24957. [Google Scholar] [CrossRef]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An Introduction to the Wound Healing Assay Using Live-Cell Microscopy. Cell Adh Migr 2014, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F. Jab1 Inhibition by Methanolic Extract of Moringa Oleifera Leaves in Cervical Cancer Cells: A Potent Targeted Therapeutic Approach. Nutr Cancer 2021, 73, 2411–2419. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of Subcutaneous Tumor Size in Athymic (Nude) Mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, R.A.; Hussa, R.O.; Story, M.T.; Ruckert, A.C.F.; Shalaby, M.R.; Mattingly, R.F. Tumor Antigen and Human Chorionic Gonadotropin in CaSki Cells: A New Epidermoid Cervical Cancer Cell Line. Science 1977, 196, 1456–1458. [Google Scholar] [CrossRef]
- Isaguliants, M.G.; Gudima, S.O.; Ivanova, O.V.; Levi, M.; Hinkula, J.; Garaev, M.M.; Kochetkov, S.N.; Wahren, B. Immunogenic Properties of Reverse Transcriptase of HIV Type 1 Assessed by DNA and Protein Immunization of Rabbits. AIDS Res Hum Retroviruses 2000, 16, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Shearer, R.F.; Saunders, D.N. Experimental Design for Stable Genetic Manipulation in Mammalian Cell Lines: Lentivirus and Alternatives. Genes Cells 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.C.D.; Summers, D.K. The Quiescent-Cell Expression System for Protein Synthesis in Escherichia Coli. Appl Environ Microbiol 1999, 65, 2710–2715. [Google Scholar] [CrossRef]
- Filippova, M.; Johnson, M.M.; Bautista, M.; Filippov, V.; Fodor, N.; Tungteakkhun, S.S.; Williams, K.; Duerksen-Hughes, P.J. The Large and Small Isoforms of Human Papillomavirus Type 16 E6 Bind to and Differentially Affect Procaspase 8 Stability and Activity. Journal of Virology 2007, 81, 4116–4129. [Google Scholar] [CrossRef] [PubMed]
- Pim, D.; Tomaić, V.; Banks, L. The Human Papillomavirus (HPV) E6* Proteins from High-Risk, Mucosal HPVs Can Direct Degradation of Cellular Proteins in the Absence of Full-Length E6 Protein. Journal of Virology 2009, 83, 9863–9874. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Contreras-Paredes, A.; Lizano, M. The Role of E6 Spliced Isoforms (E6*) in Human Papillomavirus-Induced Carcinogenesis. Viruses 2018, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, T.; Han, C.; Xuan, Y.; Jiang, D.; Sun, Y.; Zhang, X.; Zhang, W.; Xu, Y.; Liu, Y.; et al. HPV E6/E7 Promotes Aerobic Glycolysis in Cervical Cancer by Regulating IGF2BP2 to Stabilize m6A-MYC Expression. Int J Biol Sci 2022, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying Intracellular Rates of Glycolytic and Oxidative ATP Production and Consumption Using Extracellular Flux Measurements. Journal of Biological Chemistry 2017, 292, 7189–7207. [Google Scholar] [CrossRef]
- Petrovskaya, A.V.; Tverskoi, A.M.; Barykin, E.P.; Varshavskaya, K.B.; Dalina, A.A.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Distinct Effects of Beta-Amyloid, Its Isomerized and Phosphorylated Forms on the Redox Status and Mitochondrial Functioning of the Blood–Brain Barrier Endothelium. International Journal of Molecular Sciences 2023, 24, 183. [Google Scholar] [CrossRef]
- Rodic, S.; Vincent, M.D. Reactive Oxygen Species (ROS) Are a Key Determinant of Cancer’s Metabolic Phenotype. International Journal of Cancer 2018, 142, 440–448. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem Res Toxicol 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Robinson, J.P.; Carter, W.O.; Narayanan, P.K. Chapter 28 Oxidative Product Formation Analysis by Flow Cytometry. In Methods in Cell Biology; Darzynkiewicz, Z., Paul Robinson, J., Crissman, H.A., Eds.; Flow Cytometry Second Edition, Part A; Academic Press, 1994; Vol. 41, pp. 437–447.
- Zakirova, N.F.; Kondrashova, A.S.; Golikov, M.V.; Ivanova, O.N.; Ivanov, A.V.; Isaguliants, M.G.; Bayurova, E.O. [Expression of HIV-1 Reverse Transcriptase in Murine Cancer Cells Increases Mitochondrial Respiration]. Mol Biol (Mosk) 2022, 56, 795–807. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Developmental Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Mishra, A.K.; Campanale, J.P.; Mondo, J.A.; Montell, D.J. Cell Interactions in Collective Cell Migration. Development 2019, 146, dev172056. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, P.; Changtam, C.; Patumraj, S. Effects of Tetrahydrocurcumin on Tumor Growth and Cellular Signaling in Cervical Cancer Xenografts in Nude Mice. BioMed Research International 2016, 2016, e1781208. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Chen, R.; Chen, J.; Qi, Q.; Pan, Y.; Du, L.; Xiao, G.; Jiang, S. Combining Metformin and Nelfinavir Exhibits Synergistic Effects against the Growth of Human Cervical Cancer Cells and Xenograft in Nude Mice. Sci Rep 2017, 7, 43373. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res Treat 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Purohit, V.; Balakrishnan, M.; Kim, B.; Bambara, R.A. Evidence That HIV-1 Reverse Transcriptase Employs the DNA 3′ End-Directed Primary/Secondary RNase H Cleavage Mechanism during Synthesis and Strand Transfer*. Journal of Biological Chemistry 2005, 280, 40534–40543. [Google Scholar] [CrossRef]
- Anyanwu, S.I.; Doherty, A.; Powell, M.D.; Obialo, C.; Huang, M.B.; Quarshie, A.; Mitchell, C.; Bashir, K.; Newman, G.W. Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV+ Patients. Adv Virol 2018, 2018, 7863412. [Google Scholar] [CrossRef]
- Latanova, A.; Petkov, S.; Kuzmenko, Y.; Kilpeläinen, A.; Ivanov, A.; Smirnova, O.; Krotova, O.; Korolev, S.; Hinkula, J.; Karpov, V.; et al. Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice. J Immunol Res 2017, 2017, 7407136. [Google Scholar] [CrossRef]
- Isaguliants, M.; Smirnova, O.; Ivanov, A.V.; Kilpelainen, A.; Kuzmenko, Y.; Petkov, S.; Latanova, A.; Krotova, O.; Engström, G.; Karpov, V.; et al. Oxidative Stress Induced by HIV-1 Reverse Transcriptase Modulates the Enzyme’s Performance in Gene Immunization. Hum Vaccin Immunother 2013, 9, 2111–2119. [Google Scholar] [CrossRef]
- Hu, W.-S.; Hughes, S.H. HIV-1 Reverse Transcription. Cold Spring Harb Perspect Med 2012, 2, a006882. [Google Scholar] [CrossRef]
- Ferris, A.L.; Hizi, A.; Showalter, S.D.; Pichuantes, S.; Babe, L.; Craik, C.S.; Hughes, S.H. Immunologic and Proteolytic Analysis of HIV-1 Reverse Transcriptase Structure. Virology 1990, 175, 456–464. [Google Scholar] [CrossRef]
- Boer, R.J.D.; Ribeiro, R.M.; Perelson, A.S. Current Estimates for HIV-1 Production Imply Rapid Viral Clearance in Lymphoid Tissues. PLOS Computational Biology 2010, 6, e1000906. [Google Scholar] [CrossRef] [PubMed]
- Hockett, R.D.; Michael Kilby, J.; Derdeyn, C.A.; Saag, M.S.; Sillers, M.; Squires, K.; Chiz, S.; Nowak, M.A.; Shaw, G.M.; Bucy, R.P. Constant Mean Viral Copy Number per Infected Cell in Tissues Regardless of High, Low, or Undetectable Plasma HIV RNA. J Exp Med 1999, 189, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, C.; Wang, B.; Wang, F.; Pei, B.; Cheng, C.; Yang, W.; Zhao, Z. Transduction with Lentiviral Vectors Altered the Expression Profile of Host MicroRNAs. Journal of Virology 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bhuniya, A.; Pattarayan, D.; Yang, D. Lentiviral Vector Transduction Provides Nonspecific Immunogenicity for Syngeneic Tumor Models. Molecular Carcinogenesis 2022, 61, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Filippova, M.; Evans, W.; Aragon, R.; Filippov, V.; Williams, V.M.; Hong, L.; Reeves, M.E.; Duerksen-Hughes, P. The Small Splice Variant of HPV16 E6, E6⁎, Reduces Tumor Formation in Cervical Carcinoma Xenografts. Virology 2014, 450–451, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, I.; Carrillo-García, A.; Contreras-Paredes, A.; Ortiz-Sánchez, E.; Cruz-Gregorio, A.; Lizano, M. Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses. International Journal of Molecular Sciences 2018, 19, 1839. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Huang, Y.; Song, S. Inhibiting the HPV16 Oncogene-Mediated Glycolysis Sensitizes Human Cervical Carcinoma Cells to 5-Fluorouracil. Onco Targets Ther 2019, 12, 6711–6720. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem J 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Arjona, S.P.; Allen, C.N.S.; Santerre, M.; Gross, S.; Soboloff, J.; Booze, R.; Sawaya, B.E. Disruption of Mitochondrial-Associated ER Membranes by HIV-1 Tat Protein Contributes to Premature Brain Aging. CNS Neuroscience & Therapeutics 2023, 29, 365–377. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Coronado-Martínez, I.; Pedraza-Chaverri, J.; Lizano, M. E6 Oncoproteins from High-Risk Human Papillomavirus Induce Mitochondrial Metabolism in a Head and Neck Squamous Cell Carcinoma Model. Biomolecules 2019, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.H.; Grefte, S. Interactions between Mitochondrial Reactive Oxygen Species and Cellular Glucose Metabolism. Arch Toxicol 2015, 89, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress. In Innovative Medicine: Basic Research and Development; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, 2015 ISBN 978-4-431-55650-3.
- Wu, W.; Papagiannakopoulos, T. The Pleiotropic Role of the KEAP1/NRF2 Pathway in Cancer. Annual Review of Cancer Biology 2020, 4, 413–435. [Google Scholar] [CrossRef]
- Liu, A.; Lv, Z.; Yan, Z.; Wu, X.; Yan, L.; Sun, L.; Yuan, Y.; Xu, Q. Association of Mitochondrial Homeostasis and Dynamic Balance with Malignant Biological Behaviors of Gastrointestinal Cancer. Journal of Translational Medicine 2023, 21, 27. [Google Scholar] [CrossRef]
- ABEL, E.D. MITOCHONDRIAL DYNAMICS AND METABOLIC REGULATION IN CARDIAC AND SKELETAL MUSCLE. Trans Am Clin Climatol Assoc 2018, 129, 266–278.
- Binarová, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.; Warrilow, D.; Meredith, L.; Harrich, D. Reverse Transcriptase and Cellular Factors: Regulators of HIV-1 Reverse Transcription. Viruses 2009, 1, 873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Merrill, R.A.; Strack, S. A-Kinase Anchoring Protein 1: Emerging Roles in Regulating Mitochondrial Form and Function in Health and Disease. Cells 2020, 9, 298. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gabrovsek, L.; Langeberg, L.K.; Golkowski, M.; Ong, S.-E.; Smith, F.D.; Scott, J.D. Depletion of dAKAP1–Protein Kinase A Signaling Islands from the Outer Mitochondrial Membrane Alters Breast Cancer Cell Metabolism and Motility. J Biol Chem 2019, 294, 3152–3168. [Google Scholar] [CrossRef]
- Schwager, S.C.; Mosier, J.A.; Padmanabhan, R.S.; White, A.; Xing, Q.; Hapach, L.A.; Taufalele, P.V.; Ortiz, I.; Reinhart-King, C.A. Link between Glucose Metabolism and Epithelial-to-Mesenchymal Transition Drives Triple-Negative Breast Cancer Migratory Heterogeneity. iScience 2022, 25, 105190. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers (Basel) 2021, 13, 4985. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Aboukameel, A.; Kong, D.; Wang, Z.; Sethi, S.; Chen, W.; Sarkar, F.H.; Raz, A. Phosphoglucose Isomerase/Autocrine Motility Factor Mediates Epithelial-Mesenchymal Transition Regulated by miR-200 in Breast Cancer Cells. Cancer Res 2011, 71, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.; Liang, J.; Li, W.; Zhu, Y.; Zhang, M.; Wang, C.; Hou, J. Deregulation of Hexokinase II Is Associated with Glycolysis, Autophagy, and the Epithelial-Mesenchymal Transition in Tongue Squamous Cell Carcinoma under Hypoxia. BioMed Research International 2018, 2018, e8480762. [Google Scholar] [CrossRef]
- Funasaka, T.; Hogan, V.; Raz, A. Phosphoglucose Isomerase/Autocrine Motility Factor Mediates Epithelial and Mesenchymal Phenotype Conversions in Breast Cancer. Cancer Res 2009, 69, 5349–5356. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Lee, S.; Youn, H.; Youn, B. Role of Metabolic Reprogramming in Epithelial−Mesenchymal Transition (EMT). Int J Mol Sci 2019, 20, 2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tang, Z.; Huang, A.; Chen, P.; Liu, P.; Yang, J.; Lu, W.; Liao, J.; Sun, Y.; Wen, S.; et al. Glyceraldehyde-3-Phosphate Dehydrogenase Promotes Cancer Growth and Metastasis through Upregulation of SNAIL Expression. International Journal of Oncology 2017, 50, 252–262. [Google Scholar] [CrossRef]
- Bhattacharya, S. Metabolic Reprogramming and Cancer: 2022. Qeios 2022. [Google Scholar] [CrossRef]
- Song, G.-L.; Jin, C.-C.; Zhao, W.; Tang, Y.; Wang, Y.-L.; Li, M.; Xiao, M.; Li, X.; Li, Q.-S.; Lin, X.; et al. Regulation of the RhoA/ROCK/AKT/β-Catenin Pathway by Arginine-Specific ADP-Ribosytransferases 1 Promotes Migration and Epithelial-Mesenchymal Transition in Colon Carcinoma. International Journal of Oncology 2016, 49, 646–656. [Google Scholar] [CrossRef]
- Nieto, M. Nieto MA.. The Snail Superfamily of Zinc-Finger Transcription Factors. Nat Rev Mol Cell Biol 3: 155-166. Nature reviews. Molecular cell biology 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Elloul, S.; Bukholt Elstrand, M.; Nesland, J.M.; Tropé, C.G.; Kvalheim, G.; Goldberg, I.; Reich, R.; Davidson, B. Snail, Slug, and Smad-Interacting Protein 1 as Novel Parameters of Disease Aggressiveness in Metastatic Ovarian and Breast Carcinoma. Cancer 2005, 103, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Storci, G.; Sansone, P.; Trere, D.; Tavolari, S.; Taffurelli, M.; Ceccarelli, C.; Guarnieri, T.; Paterini, P.; Pariali, M.; Montanaro, L.; et al. The Basal-like Breast Carcinoma Phenotype Is Regulated by SLUG Gene Expression. J Pathol 2008, 214, 25–37. [Google Scholar] [CrossRef]
- Canciello, A.; Cerveró-Varona, A.; Peserico, A.; Mauro, A.; Russo, V.; Morrione, A.; Giordano, A.; Barboni, B. “In Medio Stat Virtus”: Insights into Hybrid E/M Phenotype Attitudes. Frontiers in Cell and Developmental Biology 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Sample, R.A.; Nogueira, M.F.; Mitra, R.D.; Puram, S.V. Epigenetic Regulation of Hybrid Epithelial-Mesenchymal Cell States in Cancer. Oncogene 2023, 42, 2237–2248. [Google Scholar] [CrossRef]
- Shah, S.; Philipp, L.-M.; Giaimo, S.; Sebens, S.; Traulsen, A.; Raatz, M. Understanding and Leveraging Phenotypic Plasticity during Metastasis Formation. NPJ Syst Biol Appl 2023, 9, 48. [Google Scholar] [CrossRef]
- Cai, D.; Chen, S.-C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J.K.; Danuser, G.; Montell, D.J. Mechanical Feedback through E-Cadherin Promotes Direction Sensing during Collective Cell Migration. Cell 2014, 157, 1146–1159. [Google Scholar] [CrossRef]
- Nieman, M.T.; Prudoff, R.S.; Johnson, K.R.; Wheelock, M.J. N-Cadherin Promotes Motility in Human Breast Cancer Cells Regardless of Their E-Cadherin Expression. J Cell Biol 1999, 147, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.; Yamada, S. N-Cadherin as a Key Regulator of Collective Cell Migration in a 3D Environment. Cell Adh Migr 2012, 6, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Na, T.-Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The Functional Activity of E-Cadherin Controls Tumor Cell Metastasis at Multiple Steps. Proceedings of the National Academy of Sciences 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Park, S.-Y.; Jang, H.; Kim, S.-Y.; Kim, D.; Park, Y.; Kee, S.-H. Expression of E-Cadherin in Epithelial Cancer Cells Increases Cell Motility and Directionality through the Localization of ZO-1 during Collective Cell Migration. Bioengineering 2021, 8, 65. [Google Scholar] [CrossRef]
- Derycke, L.D.M.; Bracke, M.E. N-Cadherin in the Spotlight of Cell-Cell Adhesion, Differentiation, Embryogenesis, Invasion and Signalling. Int J Dev Biol 2004, 48, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-Cadherin in Cancer Metastasis, Its Emerging Role in Haematological Malignancies and Potential as a Therapeutic Target in Cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Radice, G.L. N-Cadherin-Mediated Adhesion and Signaling from Development to Disease: Lessons from Mice. Prog Mol Biol Transl Sci 2013, 116, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Duchesne, L.; Sankara Narayana, G.H.N.; Boggetto, N.; Fernig, D.D.; Uttamrao Murade, C.; Ladoux, B.; Mège, R.-M. Enhanced Cell-Cell Contact Stability and Decreased N-Cadherin-Mediated Migration upon Fibroblast Growth Factor Receptor-N-Cadherin Cross Talk. Oncogene 2019, 38, 6283–6300. [Google Scholar] [CrossRef]
- Epshtein, A.; Jackman, A.; Gonen, P.; Sherman, L. HPV16 E6 Oncoprotein Increases Cell Adhesion in Human Keratinocytes. Arch Virol 2009, 154, 55–63. [Google Scholar] [CrossRef]
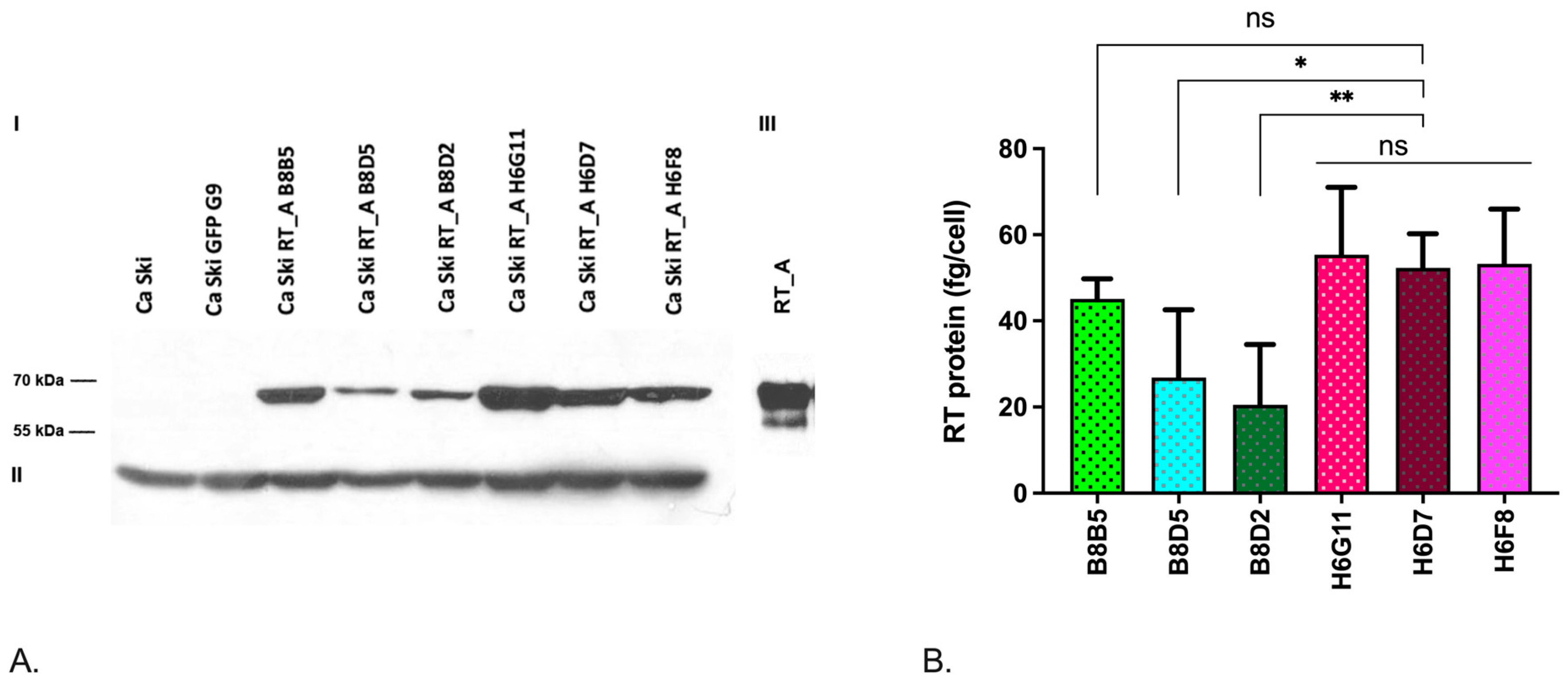
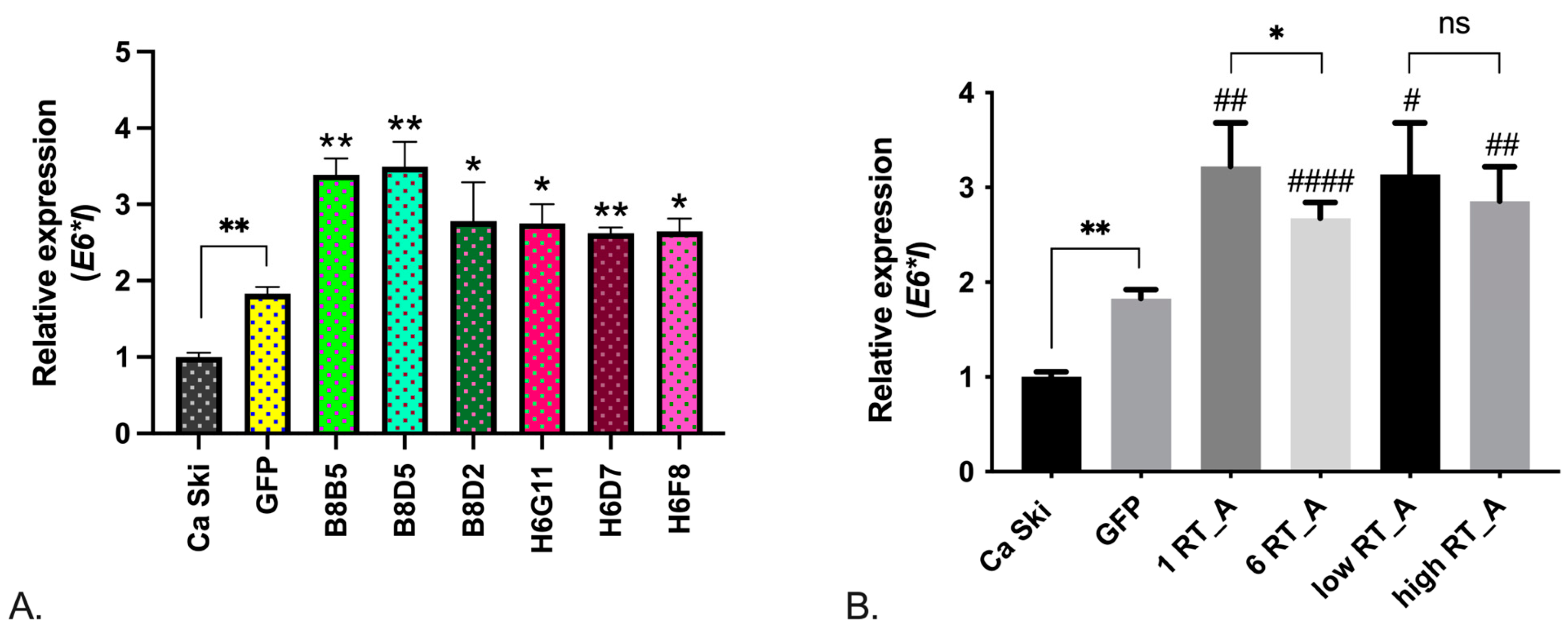
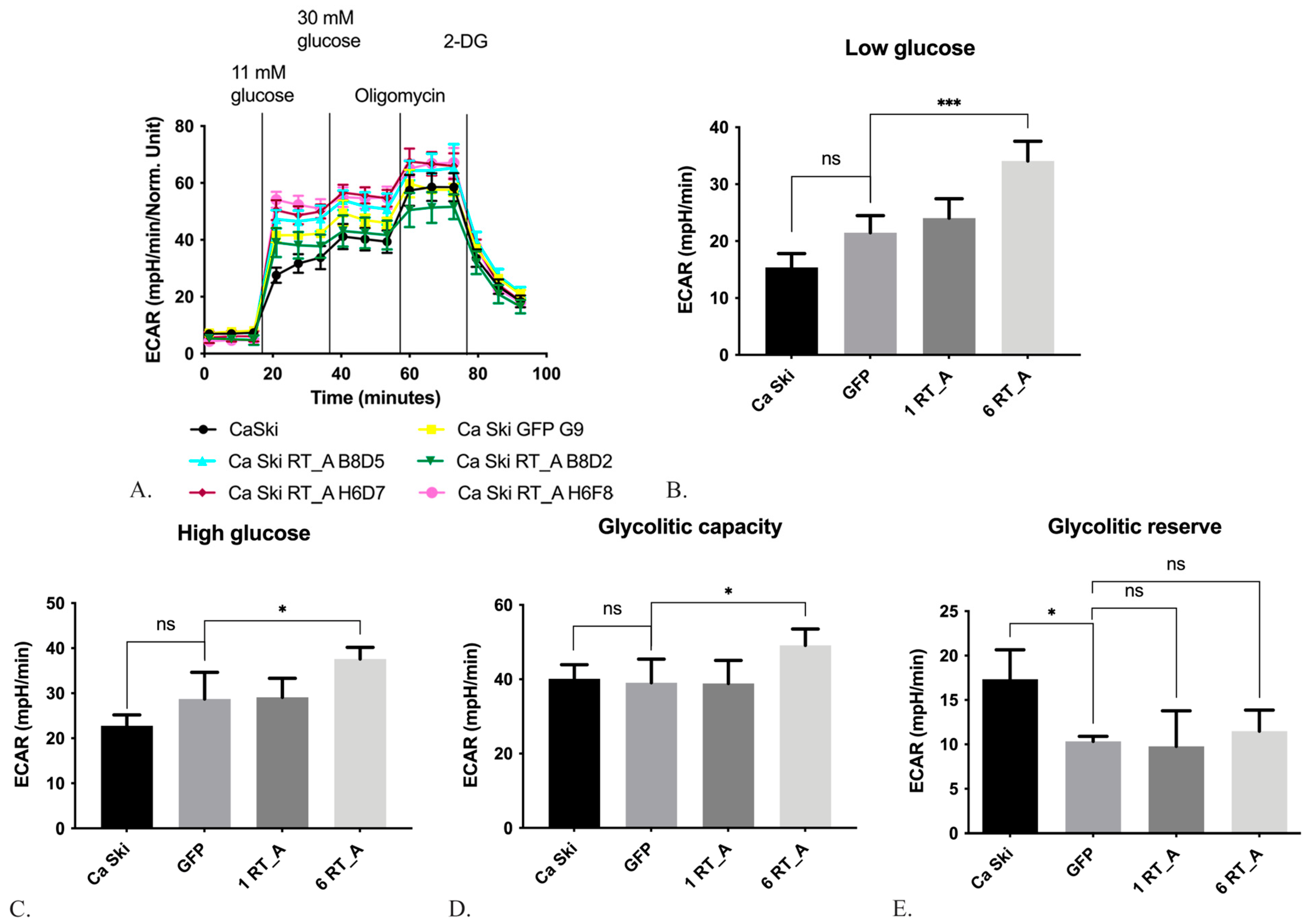
| Transgene | Monoclonal cell line | MOI | The number of copies per genome | Series | Relative expression of mRNA (RT), 2^-ddCt |
Quantification of RT_A protein expression (fg/cell) |
|---|---|---|---|---|---|---|
| B8B5 | 5 | 1 | 2,40±0,19 | 45,13±4,0 | ||
| B8D5 | 5 | 1 | 1 RT_A | 1±0,53 | 26,82±13,66 | |
| RT_A | B8D2 | 5 | 1 | 1,99±0,19 | 20,50±12,13 | |
| H6G11 | 10 | 6 | 12,61±0,09 | 55,35±12,82 | ||
| H6D7 | 10 | 6 | 6 RT_A | 12,64±0,13 | 52,30±6,87 | |
| H6F8 | 10 | 6 | 14,25±0,32 | 53,26±10,99 |
| B* | Standard Error of b* |
b | Standard Error of b |
T(8) | p-Value | |
|---|---|---|---|---|---|---|
| Intercept | 26,5005 | 9,009944 | 2,94125 | 0,018674 | ||
| RT, fg/cell | 0,50138 | 0,181762 | 9,2949 | 3,369607 | 2,75845 | 0,024734 |
| mRNA E-cadherin, 2DDCt | 2,09612 | 0,277794 | 29,0282 | 3,847040 | 7,54560 | 0,000066 |
| mRNA Twist1, 2DCt | 1,02590 | 0,217805 | 7,0439 | 1,495474 | 4,71017 | 0,001521 |
| mRNA N-cadherin, 2DCt | -1,67859 | 0,273251 | -10,5204 | 1,712571 | -6,14302 | 0,000276 |
| mRNA E6*I, 2DCt | -2,12317 | 0,323863 | -25,7346 | 3,925501 | -6,55575 | 0,000177 |
| ECAR at high glucose, mpH/min | 0,71069 | 0,190328 | 0,9383 | 0,251297 | 3,73403 | 0,005754 |
| ECAR, low glucose, mpH/min | ECAR, high glucose, mpH/min | Basal respiration, pmol/min | ATP production, pmol/min | Maximal respiration, low FCCP, pmol/min | Maximal respiration, high FCCP, pmol/min | Relative intensity of DCFH2-DA | mRNA E-cadherin, 2DCt | Colony nn, day 21 | Colony area, day 21 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| RT_A mRNA, 2DCt | 0,802027 | 0,696912 | -0,677992 | -0,798874 | -0,651713 | -0,778902 | -0,385992 | 0,408336 | 0,218884 | 0,216436 |
| RT_A, fg/cell | 0,804130 | 0,762084 | -0,604411 | -0,737907 | -0,561314 | -0,673787 | -0,447330 | 0,263754 | 0,163998 | 0,166489 |
| mRNA E6*I, 2DCt | 0,467492 | 0,399381 | -0,585139 | -0,603715 | -0,498452 | -0,669763 | -0,674783 | 0,757391 | -0,655339 | -0,413913 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





