Submitted:
23 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Experimental Mouse Models and Genotyping
Tamoxifen Administration
Muscle Injury
Ligand and Antibody Injections
µCT & HO Quantification
Cell Isolation
Flow Cytometry Analysis
Fluorescence-Activated Cell Sorting
Reciprocal Transplantation
Castration Surgery
Ovariectomy
Luciferase Proliferation Assay
Statistics
Results
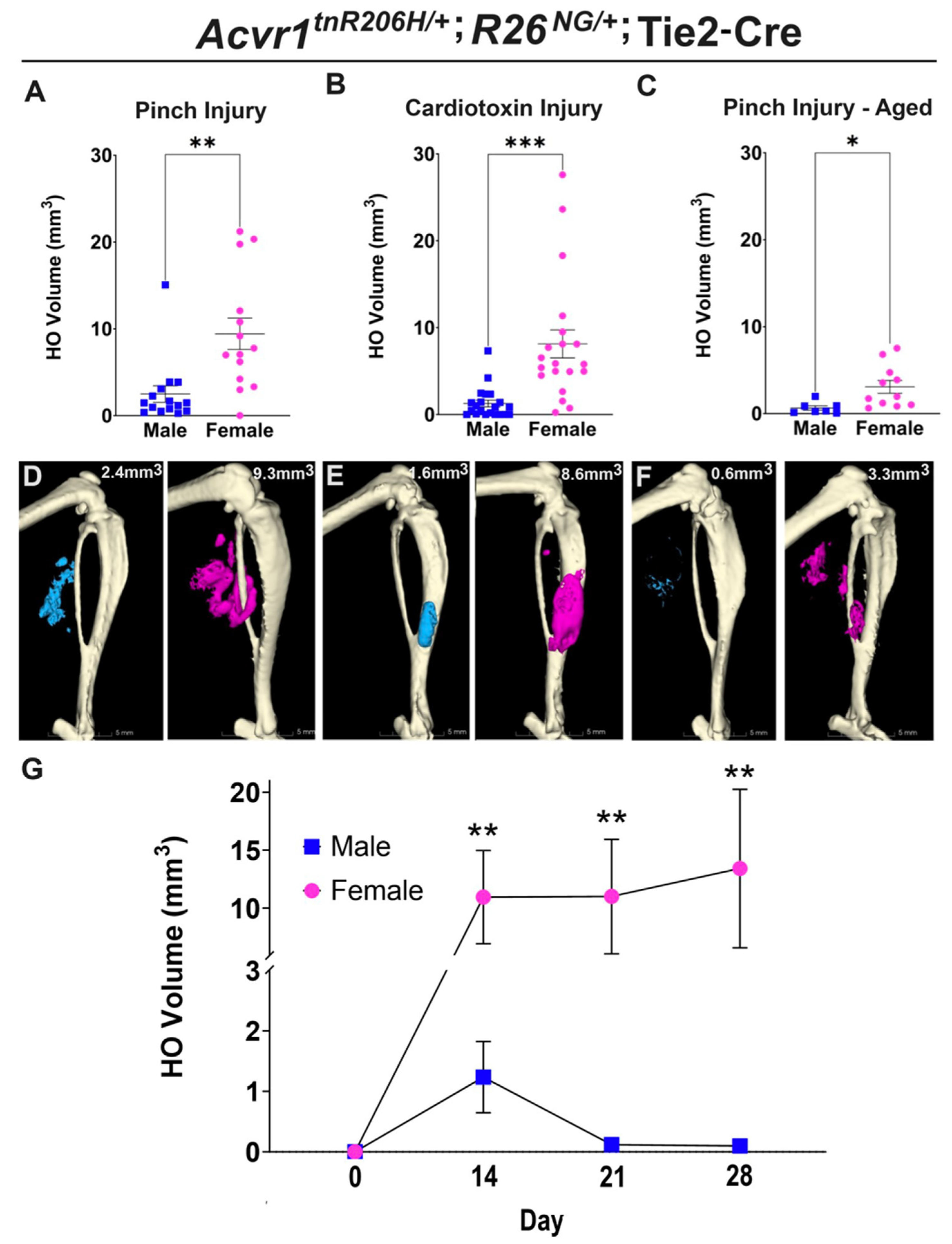
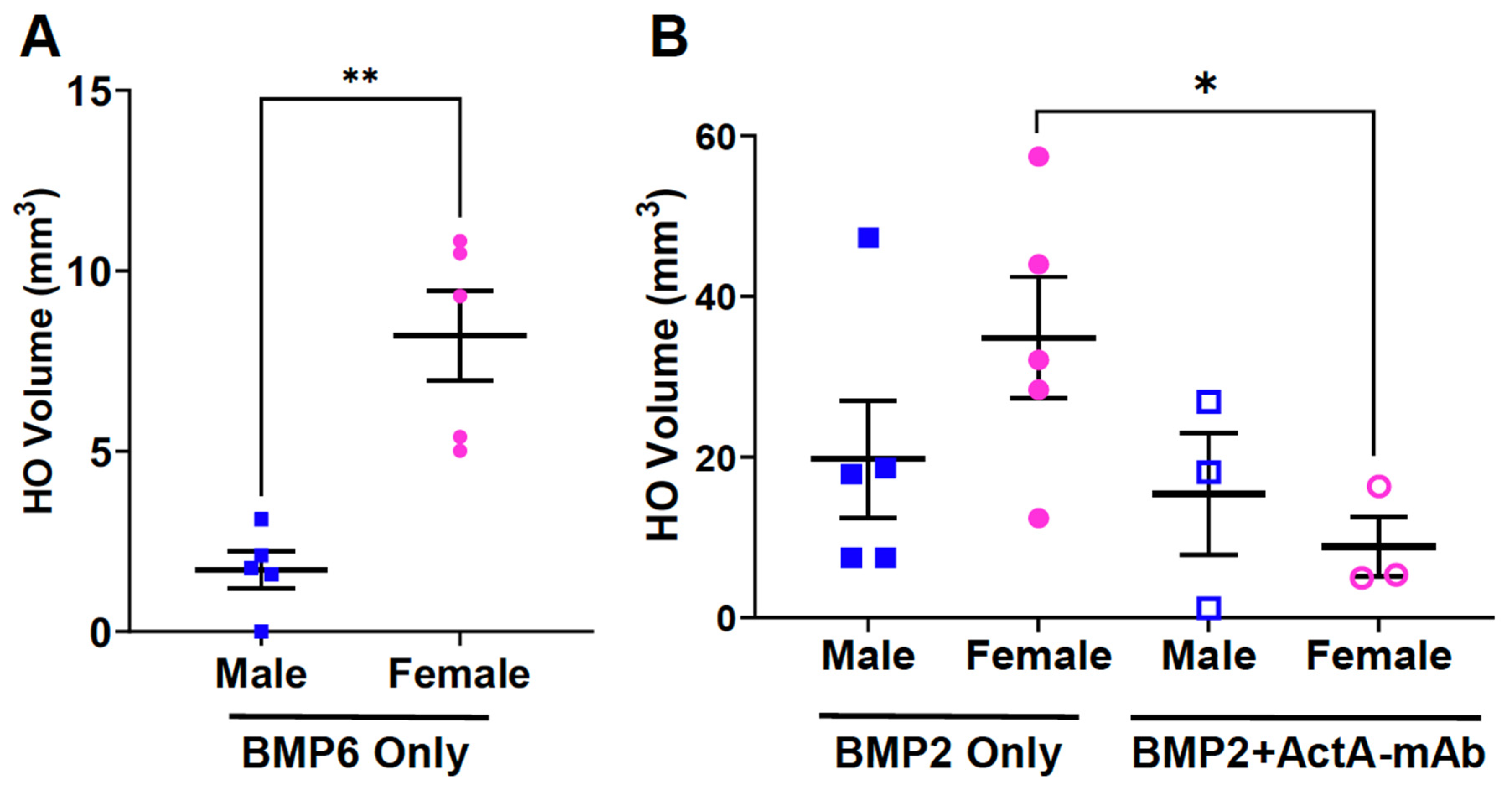
Female FOP mice develop more heterotopic bone and exhibit a more variable response to muscle injury than males
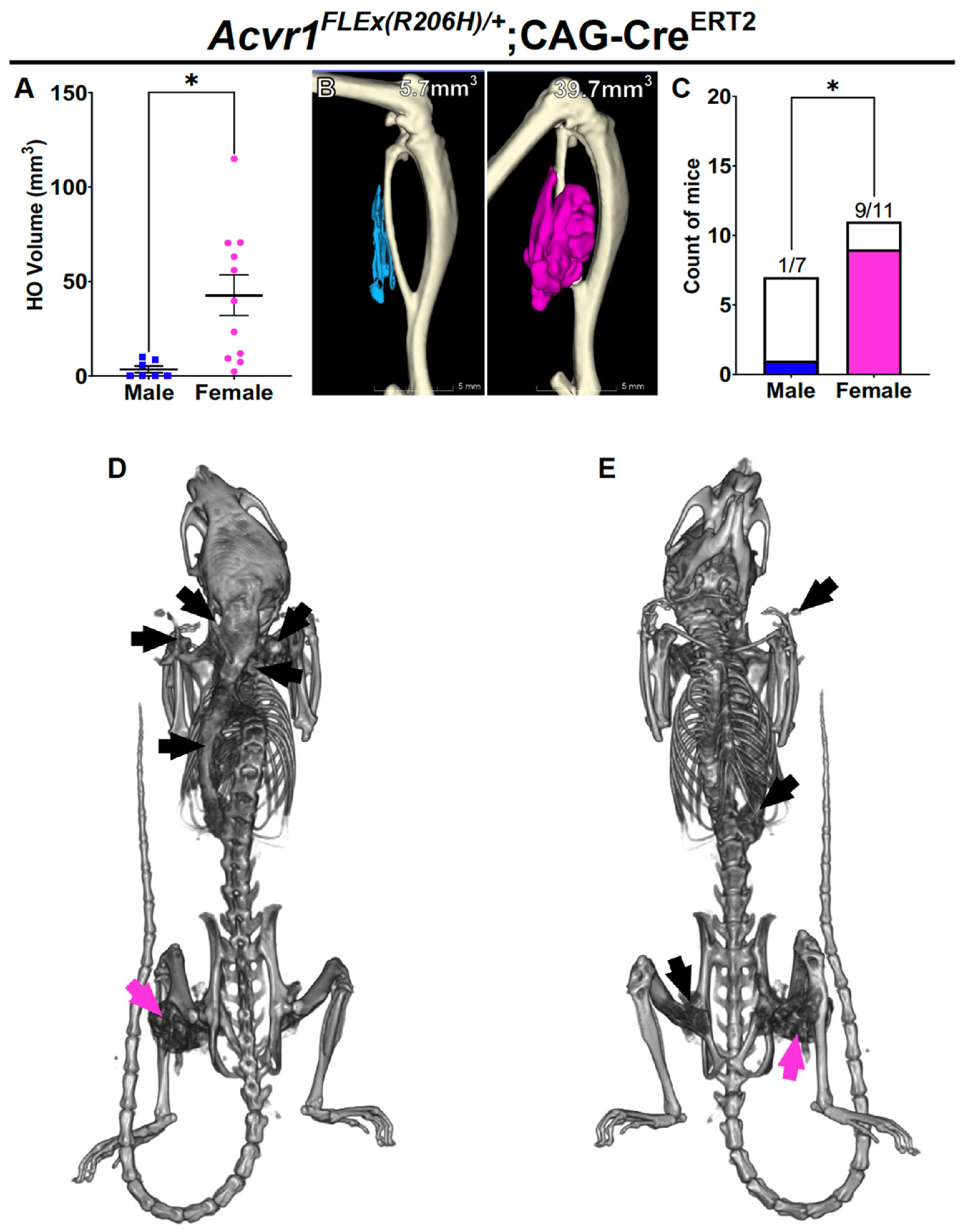
Female bias in both injury-induced and spontaneous HO in an independently derived FOP mouse model
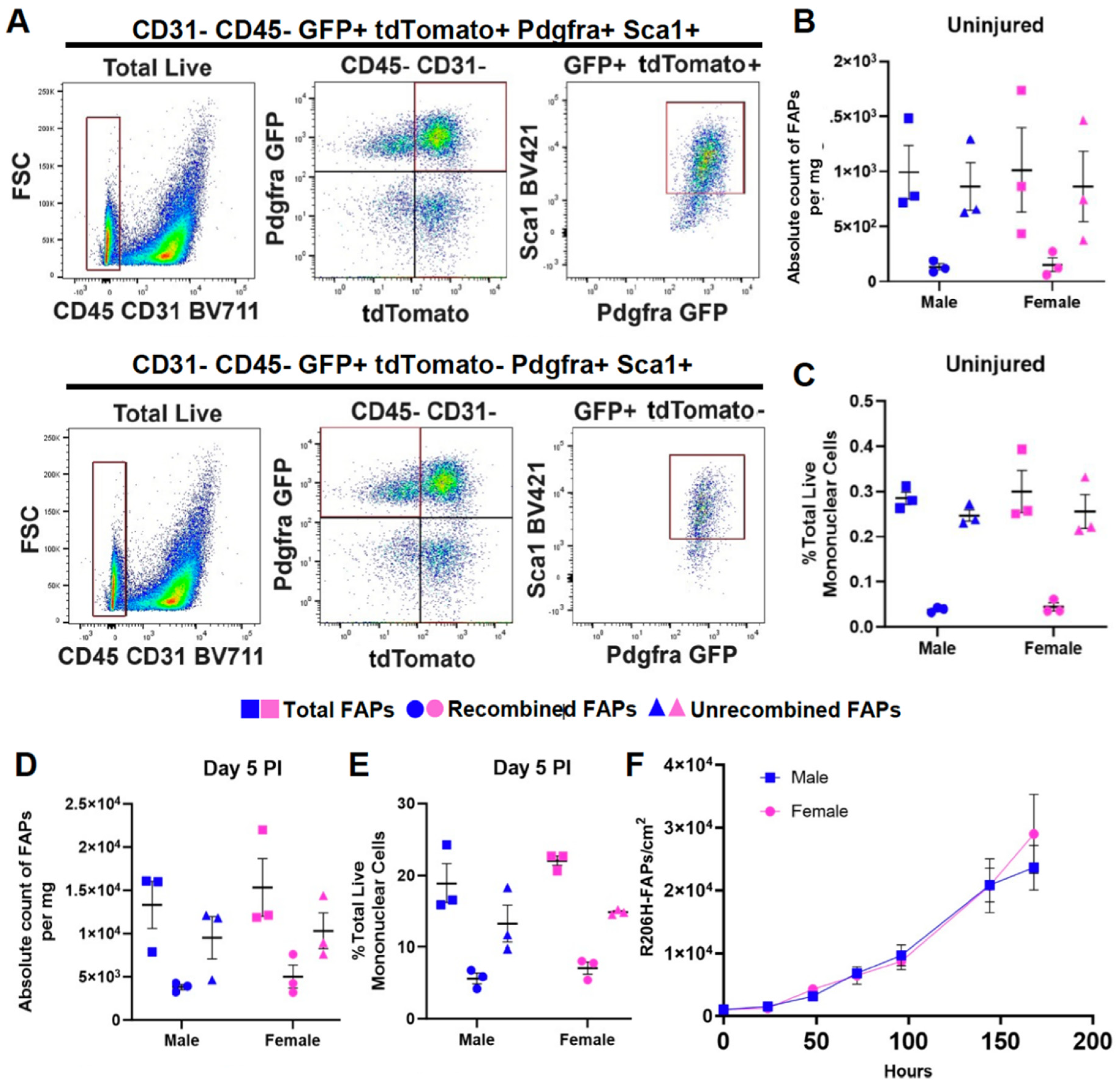
The female sex bias in HO is not due to a difference in the abundance of FAPs or to a greater efficiency of Cre-dependent recombination of the Acvr1tnR206H allele
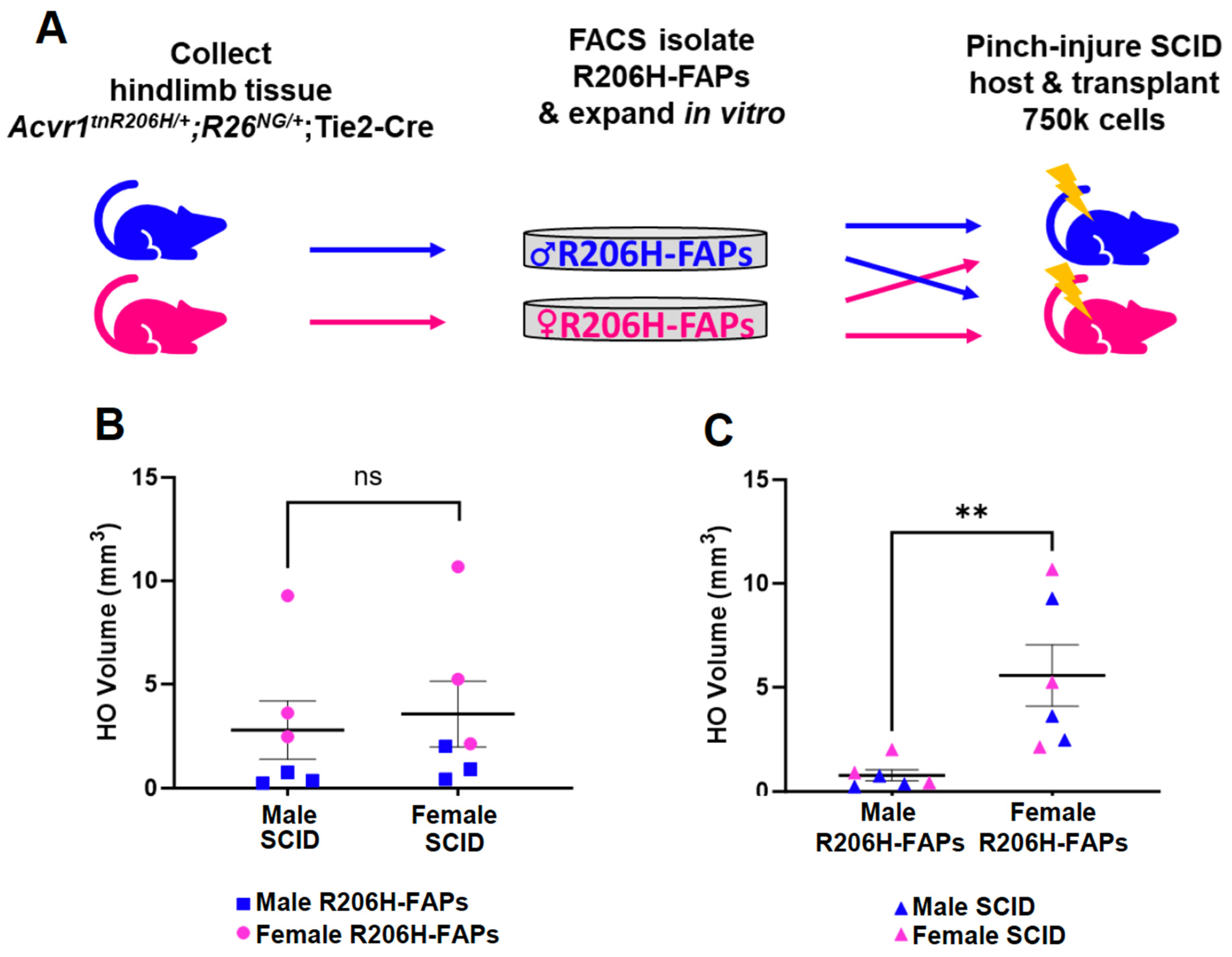
The female bias in FAP-directed HO is driven by cell-autonomous factors
Activin A inhibition attenuates HO formation and reduces the stability of nascent HO in female FOP mice
Biological sex impacts BMP-induced HO and the effect of activin A inhibition
Discussion
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Acknowledgements
Conflict of Interest
References
- Lee, S.K. Sex as an Important Biological Variable in Biomedical Research. BMB Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Morris, M.E.; Lee, H.-J.; Predko, L.M. Gender Differences in the Membrane Transport of Endogenous and Exogenous Compounds. Pharmacol. Rev. 2003, 55, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- Health, N.I. of NOT-OD-15-102: Consideration of Sex as a Biological Variable in NIH-Funded Research. Available online: https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (accessed on 18 September 2023).
- Bongetta, D.; Bua, M.; Bruno, R.; Colombo, E.V.; Laurentis, C. de; Versace, A.; Locatelli, M.; Assietti, R. Is Gender a Factor Affecting Long-Term Heterotopic Ossification Incidence After Single-Level Cervical Disc Arthroplasty? World Neurosurg. 2022, 165, 6–12. [Google Scholar] [CrossRef]
- Leung, C.; Casey, A.Th.; Goffin, J.; Kehr, P.; Liebig, K.; Lind, B.; Logroscino, C.; Pointillart, V. Clinical Significance of Heterotopic Ossification in Cervical Disc Replacement: A Prospective Multicenter Clinical Trial. Neurosurgery 2005, 57, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Shin, D.A.; Kim, K.N.; Choi, G.; Shin, H.C.; Kim, K.S.; Yoon, D.H. The Predisposing Factors for the Heterotopic Ossification after Cervical Artificial Disc Replacement. Spine J. 2013, 13, 1048–1054. [Google Scholar] [CrossRef]
- Kjægaard-Andersen, P.; Steinke, M.S.; Hougaard, K.; Søjbjerg, J.O.; Jensen, J. Heterotopic Bone Formation Following Hip Arthroplasty: A Retrospective Study of 65 Bilateral Cases. Acta Orthop. Scand. 2009, 62, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, F.; Chen, W.; Zhang, Q.; Liu, S.; Zhang, Y. Incidence and Risk Factors for Heterotopic Ossification after Total Hip Arthroplasty: A Meta-Analysis. Arch. Orthop. Trauma Surg. 2015, 135, 1307–1314. [Google Scholar] [CrossRef]
- Steinberg; Charles Heterotopic Ossification after Femoral Intramedullary Rodding. Journal of Orthopaedic Trauma 1993, 7, 536–542. [CrossRef]
- Bargellesi, S.; Cavasin, L.; Scarponi, F.; Tanti, A.D.; Bonaiuti, D.; Bartolo, M.; Boldrini, P.; Estraneo, A.; * H.O.C.S.S. group (HOCSS). Occurrence and Predictive Factors of Heterotopic Ossification in Severe Acquired Brain Injured Patients during Rehabilitation Stay: Cross-Sectional Survey. Clin. Rehabilitation 2017, 32, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Sun, Z.; Li, F.; Jiang, C.; Yan, W.; Sun, Y. Burn-Induced Heterotopic Ossification from Incidence to Therapy: Key Signaling Pathways Underlying Ectopic Bone Formation. Cell. Mol. Biol. Lett. 2021, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Papanagiotou, M.; Dailiana, Z.H.; Karachalios, T.; Varitimidis, S.; Hantes, M.; Dimakopoulos, G.; Vlychou, M.; Malizos, K.N. Heterotopic Ossification after the Use of Recombinant Human Bone Morphogenetic Protein-7. World J. Orthop. 2017, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Salemi, P.; Olson, J.M.S.; Dickson, L.E.; Germain-Lee, E.L. Ossifications in Albright Hereditary Osteodystrophy: Role of Genotype, Inheritance, Sex, Age, Hormonal Status, and BMI. J. Clin. Endocrinol. Metab. 2017, 103, 158–168. [Google Scholar] [CrossRef]
- Huso, D.L.; Edie, S.; Levine, M.A.; Schwindinger, W.; Wang, Y.; Jüppner, H.; Germain-Lee, E.L. Heterotopic Ossifications in a Mouse Model of Albright Hereditary Osteodystrophy. PLoS ONE 2011, 6, e21755. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Peterson, J.; Agarwal, S.; Oluwatobi, E.; Loder, S.; Forsberg, J.A.; Davis, T.A.; Buchman, S.R.; Wang, S.C.; Levi, B. Role of Gender in Burn-Induced Heterotopic Ossification and Mesenchymal Cell Osteogenic Differentiation. Plast. Reconstr. Surg. 2015, 135, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Corsi, K.A.; Pollett, J.B.; Phillippi, J.A.; Usas, A.; Li, G.; Huard, J. Osteogenic Potential of Postnatal Skeletal Muscle–Derived Stem Cells Is Influenced by Donor Sex. J. Bone Miner. Res. 2007, 22, 1592–1602. [Google Scholar] [CrossRef]
- Wang, L.; Carroll, D.O.; Liu, X.; Roth, T.; Kim, H.; Halloran, B.; Nissenson, R.A. Effects of Blockade of Endogenous Gi Signaling in Tie2-expressing Cells on Bone Formation in a Mouse Model of Heterotopic Ossification. J. Orthop. Res. 2015, 33, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.-J.; Choi, I.H.; Connor, J.M.; Delai, P.; Glaser, D.L.; LeMerrer, M.; et al. A Recurrent Mutation in the BMP Type I Receptor ACVR1 Causes Inherited and Sporadic Fibrodysplasia Ossificans Progressiva. Nature Genetics 2006, 38, 525–527. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Xu, M.; Seemann, P.; Connor, J.M.; Glaser, D.L.; Carroll, L.; Delai, P.; Fastnacht-Urban, E.; Forman, S.J.; Gillessen-Kaesbach, G.; et al. Classic and Atypical Fibrodysplasia Ossificans Progressiva (FOP) Phenotypes Are Caused by Mutations in the Bone Morphogenetic Protein (BMP) Type I Receptor ACVR1. Human Mutation 2009, 30, 379–390. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H Receptor Mutation Causes Fibrodysplasia Ossificans Progressiva by Imparting Responsiveness to Activin A. Sci. Transl. Med. 2015, 7, 303ra137–303ra137. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in Fibrodysplasia Ossificans Progressiva. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 15438–15443. [Google Scholar] [CrossRef] [PubMed]
- Mantick, N.; Bachman, E.; Baujat, G.; Brown, M.; Collins, O.; Cunto, C.D.; Delai, P.; Eekhoff, M.; Felde, R. zum; Grogan, D.R.; et al. The FOP Connection Registry: Design of an International Patient-Sponsored Registry for Fibrodysplasia Ossificans Progressiva. Bone 2018, 109, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Cheung, K.; Lee, A.; Sieberg, C.; Borsook, D.; Upadhyay, J. Longitudinal Evaluation of Pain, Flare-Up, and Emotional Health in Fibrodysplasia Ossificans Progressiva: Analyses of the International FOP Registry. JBMR Plus 2019, 3, e10181. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Hsiao, E.C.; Baujat, G.; Lapidus, D.; Sherman, A.; Kaplan, F.S. Prevalence of Fibrodysplasia Ossificans Progressiva (FOP) in the United States: Estimate from Three Treatment Centers and a Patient Organization. Orphanet J Rare Dis 2021, 16, 350. [Google Scholar] [CrossRef]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Grand, F.L.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors That Facilitate Myogenesis. Nat Cell Biol 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal Progenitors Distinct from Satellite Cells Contribute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat Cell Biol 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Eisner, C.; Cummings, M.; Johnston, G.; Tung, L.W.; Groppa, E.; Chang, C.; Rossi, F.M. Murine Tissue-Resident PDGFRα+ Fibro-Adipogenic Progenitors Spontaneously Acquire Osteogenic Phenotype in an Altered Inflammatory Environment. J Bone Miner Res 2020. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.-A.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J.; Cummins, S.M.; Legendre, N.P.; et al. Activin-Dependent Signaling in Fibro/Adipogenic Progenitors Causes Fibrodysplasia Ossificans Progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Nicholas, S.-A.E.; Stoessel, S.J.; Devarakonda, P.M.; Schneider, M.J.; Yamamoto, M.; Goldhamer, D.J. Palovarotene Reduces Heterotopic Ossification in Juvenile FOP Mice but Exhibits Pronounced Skeletal Toxicity. eLife 2018, 7, 305. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Biswas, A.A.; Cogswell, C.A.; Goldhamer, D.J. Multipotent Progenitors Resident in the Skeletal Muscle Interstitium Exhibit Robust BMP-Dependent Osteogenic Activity and Mediate Heterotopic Ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef]
- Yamamoto, M.; Stoessel, S.J.; Yamamoto, S.; Goldhamer, D.J. Overexpression of Wild-Type ACVR1 in Fibrodysplasia Ossificans Progressiva Mice Rescues Perinatal Lethality and Inhibits Heterotopic Ossification. J Bone Miner Res 2022, 37, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis in Vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.G.; Klinghoffer, R.A.; Corrin, P.D.; Soriano, P. Evolutionary Divergence of Platelet-Derived Growth Factor Alpha Receptor Signaling Mechanisms. Mol. Cell. Biol. 2003, 23, 4013–4025. [Google Scholar] [CrossRef]
- Yamamoto, M.; Shook, N.A.; Kanisicak, O.; Yamamoto, S.; Wosczyna, M.N.; Camp, J.R.; Goldhamer, D.J. A Multifunctional Reporter Mouse Line for Cre- and FLP-Dependent Lineage Analysis. genesis 2009, 47, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Kim, W.Y.; Kung, A.L.; Horner, J.W.; DePinho, R.A.; Kaelin, W.G. Mouse Reporter Strain for Noninvasive Bioluminescent Imaging of Cells That Have Undergone Cre-Mediated Recombination. Mol. Imaging 2003, 2, 15353500200303154. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; McMahon, A.P. Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Lees-Shepard, J.B.; Stoessel, S.J.; Chandler, J.T.; Bouchard, K.; Bento, P.; Apuzzo, L.N.; Devarakonda, P.M.; Hunter, J.W.; Goldhamer, D.J. An Anti-ACVR1 Antibody Exacerbates Heterotopic Ossification by Fibro-Adipogenic Progenitors in Fibrodysplasia Ossificans Progressiva Mice. J Clin Invest 2022, 132, e153795. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.A.; Goldhamer, D.J. FACS Fractionation and Differentiation of Skeletal-Muscle Resident Multipotent Tie2+ Progenitors. Methods Mol. Biol. 2016, 1460, 255–267. [Google Scholar] [CrossRef]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Castration. Cold Spring Harb. Protoc. 2006, 2006, pdb–prot4387. [Google Scholar] [CrossRef]
- Souza, V.R.; Mendes, E.; Casaro, M.; Antiorio, A.T.F.B.; Oliveira, F.A.; Ferreira, C.M. Pre-Clinical Models, Techniques and Protocols. Methods Mol. Biol. 2018, 1916, 303–309. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; Maidment, A.D.A.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of Progenitor Cells That Contribute to Heterotopic Skeletogenesis. The Journal of bone and joint surgery. American volume 2009, 91, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Bagarova, J.; Hatsell, S.J.; Armstrong, K.A.; Huang, L.; Ermann, J.; Vonner, A.J.; Shen, Y.; Mohedas, A.H.; Lee, A.; et al. Two Tissue-Resident Progenitor Lineages Drive Distinct Phenotypes of Heterotopic Ossification. Science translational medicine 2016, 8, 366ra163–366ra163. [Google Scholar] [CrossRef]
- Rowe, R.W.; Goldspink, G. Muscle Fibre Growth in Five Different Muscles in Both Sexes of Mice. J. Anat. 1969, 104, 519–530. [Google Scholar] [PubMed]
- Griffin, G.E.; Goldspink, G. The Increase in Skeletal Muscle Mass in Male and Female Mice. Anat. Rec. 1973, 177, 465–469. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthröm, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2016, 31, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Xie, L.; Huang, L.; Das, N.; Stewart, R.C.; Lyon, M.C.; Palmer, K.; Rajamani, S.; Graul, C.; Lobo, M.; et al. The Expansion of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva Is Activin A-Dependent. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2017, 38, 525. [Google Scholar] [CrossRef]
- Aykul, S.; Huang, L.; Wang, L.; Das, N.M.; Reisman, S.; Ray, Y.; Zhang, Q.; Rothman, N.; Nannuru, K.C.; Kamat, V.; et al. Anti-ACVR1 Antibodies Exacerbate Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva (FOP) by Activating FOP-Mutant ACVR1. J Clin Investigation 2022, 132, e153792. [Google Scholar] [CrossRef] [PubMed]
- Mundy, C.; Yao, L.; Sinha, S.; Chung, J.; Rux, D.; Catheline, S.E.; Koyama, E.; Qin, L.; Pacifici, M. Activin A Promotes the Development of Acquired Heterotopic Ossification and Is an Effective Target for Disease Attenuation in Mice. Sci Signal 2021, 14, eabd0536. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Shaughnessy, K.A.; Saunders, C.; Shore, E.M.; Koyama, E.; Pacifici, M. Palovarotene Action Against Heterotopic Ossification Includes a Reduction of Local Participating Activin A-Expressing Cell Populations. JBMR Plus 2023. [Google Scholar] [CrossRef]
- Lin, S.; Svoboda, K.K.H.; Feng, J.Q.; Jiang, X. The Biological Function of Type I Receptors of Bone Morphogenetic Protein in Bone. Bone Res. 2016, 4, 16005. [Google Scholar] [CrossRef]
- Nickel, J.; Mueller, T.D. Specification of BMP Signaling. Cells 2019, 8, 1579. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Kitterman, J.A.; Strober, J.B.; Kan, L.; Rocke, D.M.; Cali, A.; Peeper, J.; Snow, J.; Delai, P.L.R.; Morhart, R.; Pignolo, R.J.; et al. Neurological Symptoms in Individuals with Fibrodysplasia Ossificans Progressiva. J. Neurol. 2012, 259, 2636–2643. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Cheung, K.; Kile, S.; Fitzpatrick, M.A.; Cunto, C.D.; Mukaddam, M.A.; Hsiao, E.C.; Baujat, G.; Delai, P.; Eekhoff, E.M.W.; et al. Self-Reported Baseline Phenotypes from the International Fibrodysplasia Ossificans Progressiva (FOP) Association Global Registry. Bone 2020, 134, 115274. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Baujat, G.; Brown, M.A.; Cunto, C.D.; DiRocco, M.; Hsiao, E.C.; Keen, R.; Mukaddam, M.A.; Sang, K.-H.L.Q.; Wilson, A.; et al. Natural History of Fibrodysplasia Ossificans Progressiva: Cross-Sectional Analysis of Annotated Baseline Phenotypes. Orphanet J. Rare Dis. 2019, 14, 98. [Google Scholar] [CrossRef]
- Chakkalakal, S.A.; Uchibe, K.; Convente, M.R.; Zhang, D.; Economides, A.N.; Kaplan, F.S.; Pacifici, M.; Iwamoto, M.; Shore, E.M. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1(R206H) Fibrodysplasia Ossificans Progressiva (FOP) Mutation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2016, n/a-n/a. [CrossRef]
- Goldhamer, D.J.; Lees-Shepard, J.B. Response to Comment on “Palovarotene Reduces Heterotopic Ossification in Juvenile FOP Mice but Exhibits Pronounced Skeletal Toxicity”. eLife 2019, 8, e43928. [Google Scholar] [CrossRef]
- Katagiri, T.; Tsukamoto, S.; Kuratani, M.; Tsuji, S.; Nakamura, K.; Ohte, S.; Kawaguchi, Y.; Takaishi, K. A Blocking Monoclonal Antibody Reveals Dimerization of Intracellular Domains of ALK2 Associated with Genetic Disorders. Nat Commun 2023, 14, 2960. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Lin, C.; Ma, H.; Xie, J.; Kaplan, F.S.; Gao, G.; Shim, J.-H. AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva. Biomolecules 2023, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Kim, J.-M.; Xie, J.; Chaugule, S.; Lin, C.; Ma, H.; Hsiao, E.; Hong, J.; Chun, H.; Shore, E.M.; et al. Suppression of Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva Using AAV Gene Delivery. Nat Commun 2022, 13, 6175. [Google Scholar] [CrossRef] [PubMed]
- Malecova, B.; Gatto, S.; Etxaniz, U.; Passafaro, M.; Cortez, A.; Nicoletti, C.; Giordani, L.; Torcinaro, A.; Bardi, M.D.; Bicciato, S.; et al. Dynamics of Cellular States of Fibro-Adipogenic Progenitors during Myogenesis and Muscular Dystrophy. Nat. Commun. 2018, 9, 3670. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Goldhamer, D.J. Stem Cells and Heterotopic Ossification: Lessons from Animal Models. Bone 2018, 109, 178–186. [Google Scholar] [CrossRef]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a Novel Population of Muscle Stem Cells in Mice. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef]
- Stanley, A.; Tichy, E.D.; Kocan, J.; Roberts, D.W.; Shore, E.M.; Mourkioti, F. Dynamics of Skeletal Muscle-Resident Stem Cells during Myogenesis in Fibrodysplasia Ossificans Progressiva. Npj Regen Medicine 2022, 7, 5. [Google Scholar] [CrossRef]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.-K.; Lee, S.T.; Fiore, D.; Zhang, R.-H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib Reduces Muscle Fibrosis in Chronic Muscle Injury by Promoting TNF-Mediated Apoptosis of Fibro/Adipogenic Progenitors. Nat Med 2015, 21, 786–794. [Google Scholar] [CrossRef]
- Compston, J.E. Sex Steroids and Bone. Physiol. Rev. 2001, 81, 419–447. [Google Scholar] [CrossRef]
- Deasy, B.M.; Lu, A.; Tebbets, J.C.; Feduska, J.M.; Schugar, R.C.; Pollett, J.B.; Sun, B.; Urish, K.L.; Gharaibeh, B.M.; Cao, B.; et al. A Role for Cell Sex in Stem Cell-Mediated Skeletal Muscle Regeneration: Female Cells Have Higher Muscle Regeneration Efficiency. J Cell Biol 2007, 177, 73–86. [Google Scholar] [CrossRef]
- Kaipia, A.; Toppari, J.; Huhtaniemi, I.; Paranko, J. Sex Difference in the Action of Activin-A on Cell Proliferation of Differentiating Rat Gonad. Endocrinology 1994, 134, 2165–2170. [Google Scholar] [CrossRef]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex Specificity of Pancreatic Cancer Cachexia Phenotypes, Mechanisms, and Treatment in Mice and Humans: Role of Activin. J. Cachexia, Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef]
- Aykul, S.; Corpina, R.A.; Goebel, E.J.; Cunanan, C.J.; Dimitriou, A.; Kim, H.; Zhang, Q.; Rafique, A.; Leidich, R.; Wang, X.; et al. Activin A Forms a Non-Signaling Complex with ACVR1 and Type II Activin/BMP Receptors via Its Finger 2 Tip Loop. Elife 2020, 9, e54582. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Xie, L.; Crane, J.; Zhen, G.; Mishina, Y.; Deng, R.; Gao, B.; Chen, H.; Liu, S.; et al. Inhibition of Overactive TGF-β Attenuates Progression of Heterotopic Ossification in Mice. Nat Commun 2018, 9, 551. [Google Scholar] [CrossRef]
- Bloise, E.; Ciarmela, P.; Cruz, C.D.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




