1. Introduction
After China, Italy was the first European country to be overwhelmed, at a never seen speed, by the COVID-19 pandemic, in the first year directly leading to death, subsequently to likely acceleration of neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), Creutzfeld-Jakob disease (CJD).
CJD is a fatal neurodegenerative disease caused by transmissible agents called prions which replicates in the CNS leading to characteristic neuropathological findings including spongiosis, glyosis, neuron loss and the deposition of the pathological prion protein (PrPSc). Humans transmissible spongiform encephalopathies (TSEs) include sporadic, genetic, iatrogenic and infectious forms. Surveillance of human TSEs has been inaugurated in Italy in 1993, and the sporadic Creutzfeldt–Jakob disease (sCJD) is nowadays considered the major human prion disease as well as in the Italy and worldwide. The identification and mandatory reporting of CJD in Italy has allowed to improve our understanding on the CJD pathogenesis, the variable CJD clinical phenotypes, the possible geographic clusters.
The aim of this study is to describe the rare case of the COVID-19 interplay in a genetic CJD associated with E200K mutation and to provide an ongoing insight into the disease pathogenesis of an even greater global health challenge than acute infection.
2. Materials and Methods
A 51-year-old woman with a history of arterial hypertension presented to the emergency room (ER) with rapidly progressive cognitive deterioration and functional decline, as she had become unable to maintain the upright position and to walk in few weeks. Her relatives have reported that, approximately two months before, she was diagnosed with COVID-19 through a positive reverse transcriptase polymerase chain reaction (RT-PCR) on nasopharyngeal swabs. Despite a mild course of COVID-19, in the first days after healing she developed cognitive and motor slowing, paucity of speech with paraphasic errors and hypophony, unsteady gait with ataxia, insomnia, vertical gaze palsy and blurred vision.
On ER admission, she was alerted, but quite mute, except for occasional whispered monosyllables. She was unresponsive to verbal stimulation, whereas painful stimuli evoked normal flexion of upper limbs. Bilateral direct and indirect light reflexes were normal, but she had not blink reflex in response to threat, suggesting cortical blindness. There was a right limb hyperreflexia in absence of Hoffmann and Babinski signs. In few days after the admission, she was bedridden and required enteral nutrition via percutaneous endoscopic gastrostomy (PEG) given the rapidly deterioration clinical condition.
Comprehensive blood tests, urine sample, cerebrospinal fluid (CSF) analysis and total-body computed tomography were collected to rule out differential diagnoses, including vascular, infectious, toxic-metabolic, autoimmune and systemic diseases, malignancies and paraneoplastic antibody-mediated encephalopathies. Her diagnostic assessment also included a brain magnetic resonance imaging and an electroencephalography.
3. Results
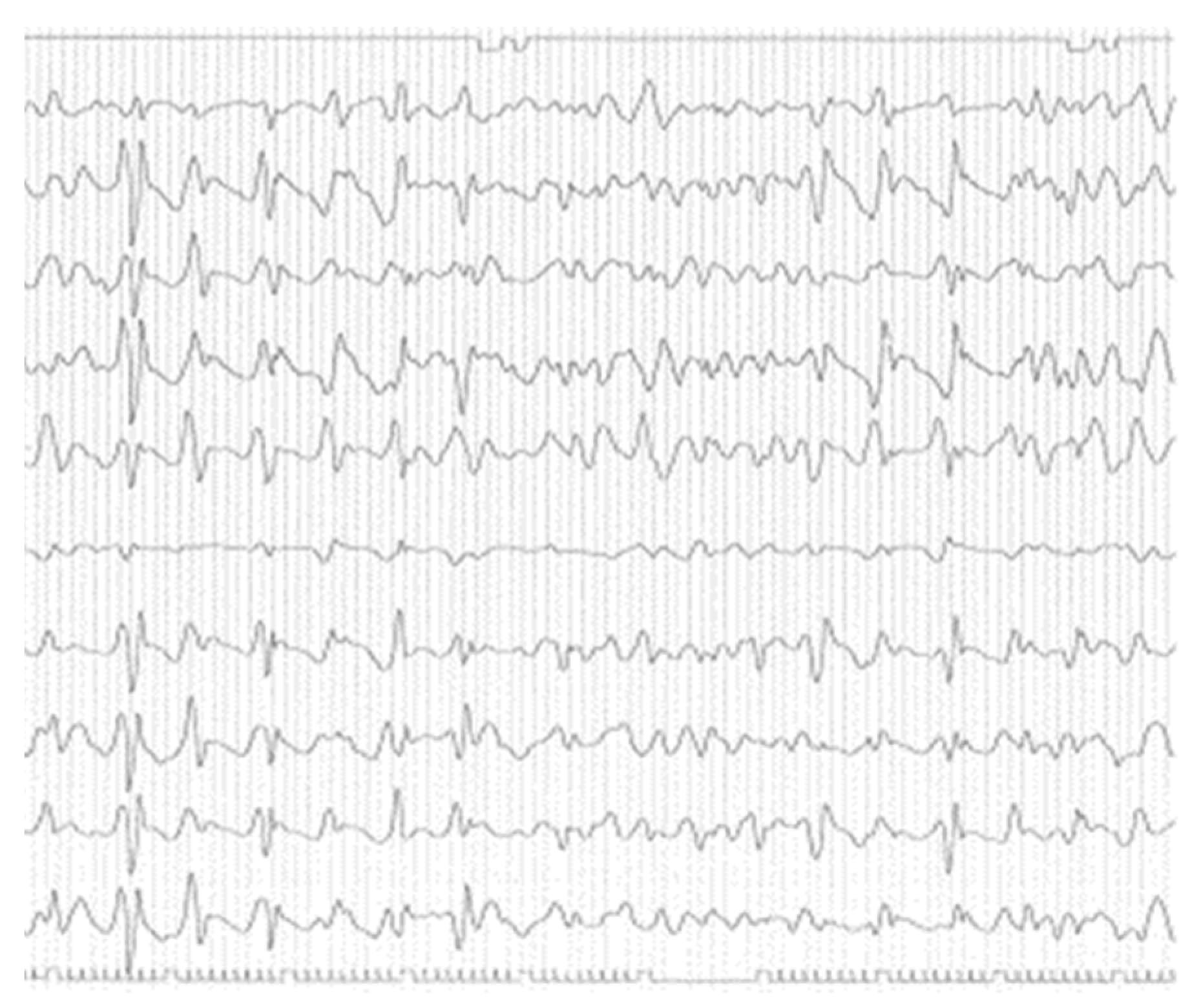
She was treated with thiamine replacement without any clinical improvement. After the electroencephalography, that have revealed diffuse pseudo-periodic sharp-waves complexes (
Figure 1), CSF real-time quaking-induced conversion (RT-QuIC) resulted positive and
PRNP sequencing on lymphocyte DNA revealed the E200K mutation with homozygosity for methionine (MET) at codon 129, thus confirming the diagnosis of Creutzfeldt-Jakob disease.
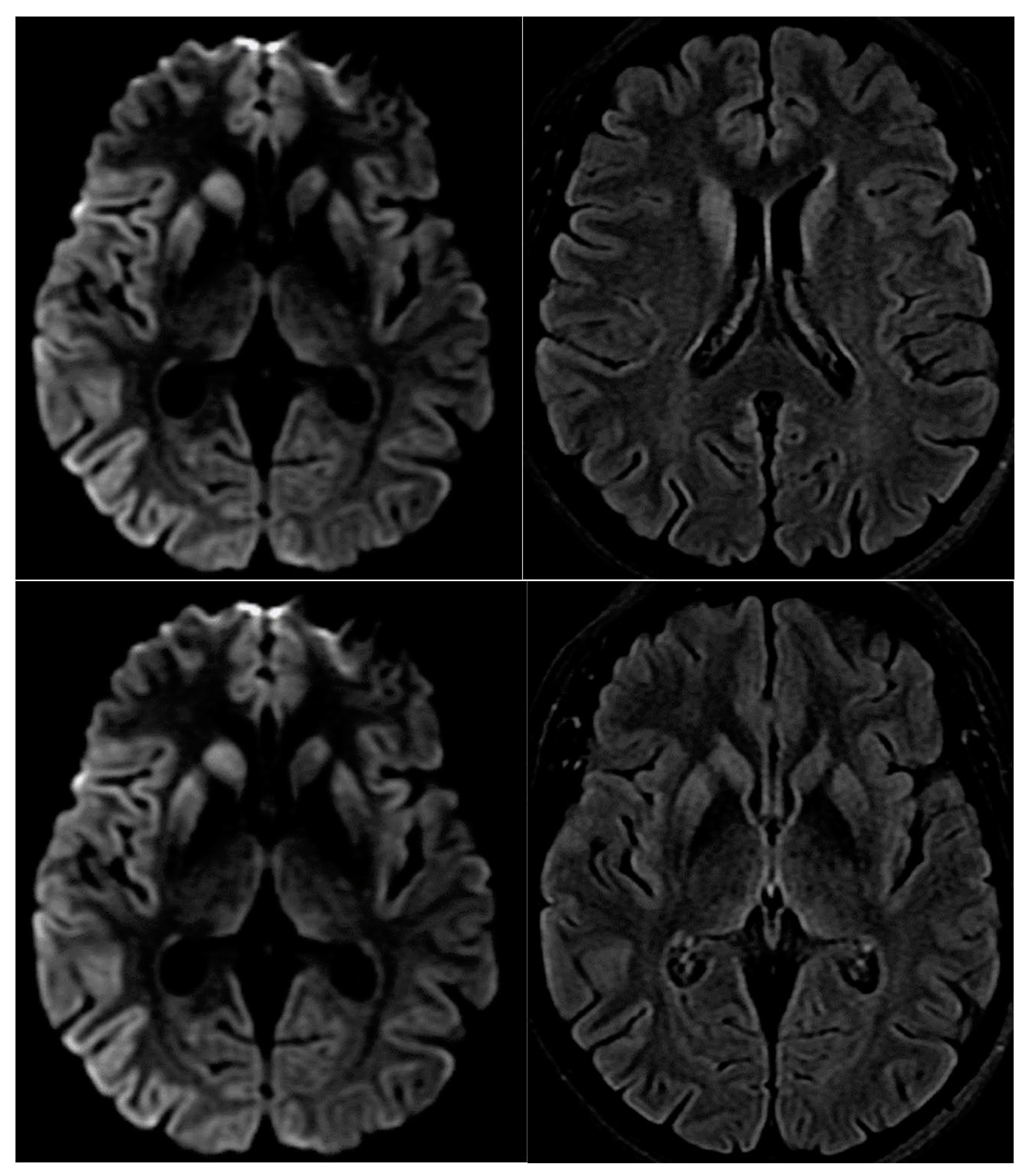
A magnetic resonance imaging (MRI) of the brain revealed restricted diffusion with corresponding FLAIR in caudate nucleus and putamen, bilaterally, and cortical ribboning in the right occipito-parietal cortex (
Figure 2).
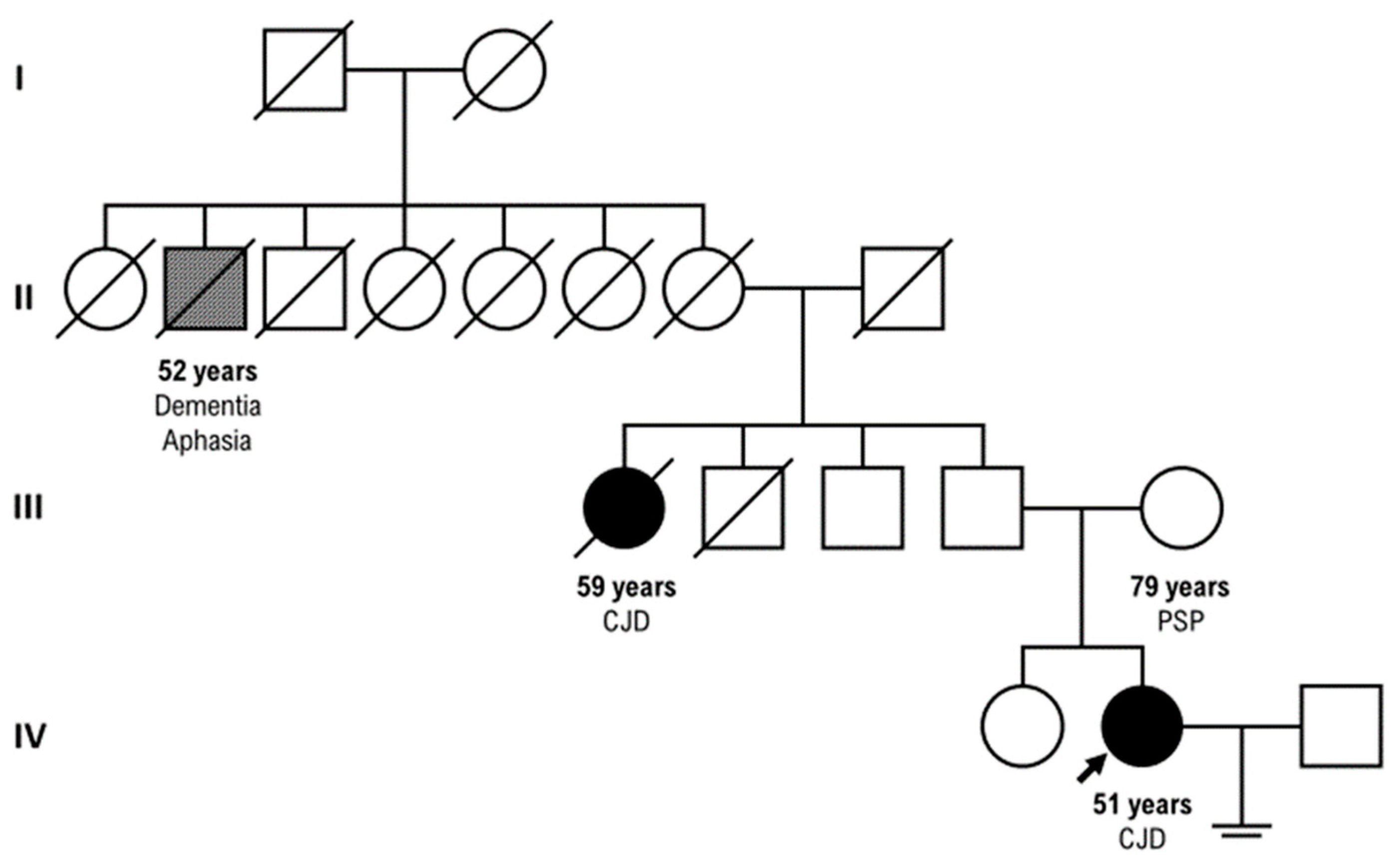
Our patient had a positive family history for CJD: her parental aunt who died at the age of 59 years with CJD (clinical course of three ys) and her paternal great-grand-uncle who died at the age of 52 years with dementia and aphasia, but
PRNP sequencing was not tested in these relatives. (
Figure 3)
4. Discussion
About 10–15% of human prion diseases or transmissible spongiform encephalopathies (TSE) are associated to
PRNP mutation, even if with variable penetrance and neuropathologic findings. [
6]
In addition, E200K is certainly described highly prevalent in European countries.[
7]
A striking example of this phenotypic variability is our family, in which we could unfortunately detect
PRNP sequencing only in our proband, who rapidly progressed and died six months after disease onset, differently from her parental aunt. Recent studies have reported that the K200 mutation of the
PRNP gene, identified especially in the frontal cortex and hippocampus of sporadic CJD patients, has manifested high penetrance, as shown in our family. [
7]
In particular, there is also evidence that the mutation at the codon 129 can be linked to different clinical phenotypes depending on whether the mutation co-segregates with methionine (FFI) or valine (genetic CJD), and mutation carriers can also develop different clinical phenotypes [
8].
It is now becoming widely accepted ([
9] that neurological manifestations, known as “neuro-COVID”, were reported to occur in 36% of COVID-19 patients and are likely favored by its neurotrophic and neuroinvasive features [
10] , inflammatory cytokine release and immunopathology [
11].
It is an intriguing question to define the interesting associations between SARS-CoV2 infection and prion neurobiology.
Human prion diseases (PrD) include an expanding spectrum of progressive, and fatal neurodegenerative disorders caused by the accumulation and aggregation of a misfolded isoform (PrPSc) from the native cellular prion protein (PrPc), associated with systemic inflammation. Previous studies described some cases developing CJD during or following SARS-CoV-2 viral invasion, supporting a significant relationship between host immune-responses to SARS-CoV-2 and an exacerbation involving a systemic inflammation, a progressive acceleration of prion-like protein spread [
12,
13].
Furthermore, the SARS-CoV-2 ‘S1′ spike proteins contain some “prion-like” regions, amyloid peptide-binding and other domains that support the formation of pathogenic plaques in the CNS [
14,
15].
Recently, after the pre-mortem diagnostic revolution represented by the disease-specific real-time quaking induced conversion assay, which detects prion seeds in cerebrospinal fluid or brain tissue, the introduction of targeted sequencing of
PRNP has allowed to diagnose the presence of protein-altering variants in
PRNP. [
16]
Our proband presented with the classical symptoms historically associated with genetic Creutzfeld-Jakob Disease: early cognitive symptoms, such as memory decline, dementia; also ataxia, myoclonus, pyramidal and extrapyramidal signs, behavioral change, psychiatric symptoms such as hallucinations, delusions, and depression. [
17]
Despite the phenotypic variability that often delays CJD diagnosis, clinicians should encourage the relatives to undertake a genetic counseling and testing, because in the era of genetically targeted therapies, it might result useful for the stratification of patients in future preventive treatment trials for prion disease, to develop novel therapeutic strategies.
5. Conclusions
Our case report confirms the importance of PRNP sequencing combined with deep phenotyping to address the genetic and preconception counseling in the CJD diseases.
Further studies will be necessary to understand how the Sars-cov2 infection can be a trigger for CJD onset, particularly for the genetic forms.
Author Contributions
E.C. designed the study. L.P and A.Pet. contributed to the acquisition of data. A.P. and L.P. drafted the manuscript. E.C. and A.P. critically revised the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The collection and scientific dissemination of data related to COVID-19 epidemics by every public health body, such as San Camillo Hospital, was authorized on February 27th, 2020, by the Italian Presidency of the Council of Ministers (Gazzetta Ufficiale Della Repubblica Italiana, Serie Generale, Parte Prima, 2020, Feb 28. Available from:
https://www.gazzettaufficiale.it/eli/gu/2020/02/28/50/sg/pdf. )
Informed Consent Statement
missing consent due to early death of the patient.
Data Availability Statement
Upon reasonable request, data presented in this study will be provided by emailing the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farheen S.; Agrawal S.; Zubair S.; Agrawal A.; Jamal F.; Altaf I.; et al.. Patho-physiology of aging and immune-senescence: possible correlates with comorbidity and mortality in middle-aged and old COVID-19 patients. Front. Aging. 2021, 2, 748591. [CrossRef]
- Fu Y. W.; Xu H. S.; Liu S. J.. COVID-19 and neurodegenerative diseases. Eur. Rev. Med. Pharmacol Sci. 2022, 26, 4535–4544. [CrossRef]
- Zhao Y; Jaber VR; Lukiw WJ. SARS-CoV-2, long COVID, prion disease and neurodegeneration. Front Neurosci. 2022;16:1002770. [CrossRef]
- McGrath A; Pai H; Clack A. Rapid progression of probable Creutzfeldt-Jakob disease with concomitant COVID-19 infection. BMJ Case Rep. 2023 1;16(11):e254402. [CrossRef]
- Ciolac D.; Racila R.; Duarte C.; Vasilieva M.; Manea D.; Gorincioi N. et al.. Clinical and radiological deterioration in a case of Creutzfeldt-Jakob Disease following SARS-CoV-2 Infection: hints to accelerated age-dependent neurodegeneration. Biomedicines. 2021, 9, 1730. 10. [CrossRef]
- Ladogana A; Gabor G; Kovacs, Chapter 13 - Genetic Creutzfeldt–Jakob disease,Editor(s): Maurizio Pocchiari, Jean Manson,Handbook of Clinical Neurology,Elsevier,Volume 153,2018,Pages 219-242,ISSN 0072-9752,ISBN 9780444639455.
- Won SY; Kim YC; Jeong BH; Elevated E200K Somatic Mutation of the Prion Protein Gene (PRNP) in the Brain Tissues of Patients with Sporadic Creutzfeldt-Jakob Disease (CJD). Int J Mol Sci. 2023, 2;24(19):14831. [CrossRef]
- Watson N; Brandel JP; Green A; Hermann P; Ladogana A;Lindsay T; Mackenzie J; Pocchiari M; Smith C; Zerr I; Pal S. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nat Rev Neurol. 2021;17(6):362-379. [CrossRef]
- Wu Z; McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 7;323(13):1239-1242. [CrossRef]
- Guedes BF. NeuroCOVID-19: a critical review. Arq Neuropsiquiatr. 2022;80(5 Suppl 1):281-289. [CrossRef]
- Frontera JA; Thorpe LE; Simon NM; de Havenon A; Yaghi S; Sabadia SB; Yang D; Lewis A; Melmed K; Balcer LJ; Wisniewski T; Galetta SL. Post-acute sequelae of COVID-19 symptom phenotypes and therapeutic strategies: A prospective, observational study. PLoS One. 2022 29;17(9):e0275274. [CrossRef]
- Pogue A. I.; Lukiw W. J.. microRNA-146a-5p, neurotropic viral infection and prion disease (PrD). Int. J. Mol. Sci. 2021, 22, 9198. [CrossRef]
- Young M. J.; O’Hare M.; Matiello M.; Schmahmann J. D.. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav. Immun. 2020, 89, 601–603. [CrossRef]
- Lukiw WJ; Jaber VR; Pogue AI; Zhao Y. SARS-CoV-2 Invasion and Pathological Links to Prion Disease. Biomolecules. 2022 7;12(9):1253. [CrossRef]
- Tetz G.; Tetz V. Prion-like domains in spike protein of SARS-CoV-2 differ across its variants and enable changes in affinity to ACE2. Microorganisms. 2022, 10, 280. [CrossRef]
- Goldman JS; Vallabh SM. Genetic counseling for prion disease: Updates and best practices, Genetics in Medicine, Volume 24, Issue 10, 2022, Pages 1993-2003, ISSN 1098-3600. [CrossRef]
- Takada L.T.; Kim M.O., Cleveland R.W. et al. Genetic prion disease: experience of a rapidly progressive dementia center in the United States and a review of the literature Am J Med Genet B Neuropsychiatr Genet, 2017, 174 (1), pp. 36-69. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).