Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
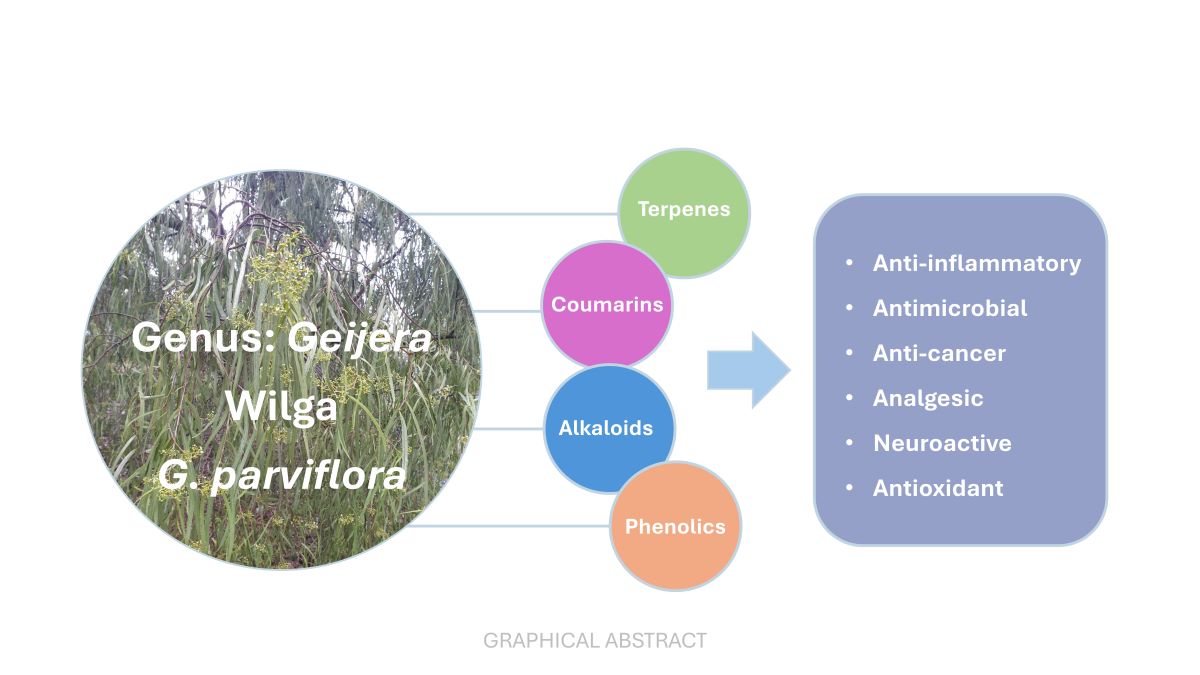
Keywords:
1. Methodology
2. Introduction
3. Chemical Constituents in Geijera Species
3.1. Coumarins
3.2. Alkaloids
3.3. Terpenes and Terpenoids
3.4. Miscellaneous Compounds Isolated
4. Pharmacological Activities of Geijera Constituents
|
|
4.1. Geijera Secondary Metabolites That Can Be Linked to Its Ethnobotanical Uses
- anti-inflammatory activity,
- analgesic/antinociceptive activity,
- antimicrobial, antifungal, and antioxidant activity, as well as
- acetylcholinesterase inhibition, monoamine oxidase inhibition, muscle relaxant activity, sedative activity, anticonvulsant activity, and psychotropic activity (from neuro- and psycho- active compounds).
4.1.1. Anti-Inflammatory, Analgesic and Antinociceptive Compounds
4.1.2. Antimicrobial, Antifungal, and Antioxidant Compounds
4.1.3. Neuroactive and Psychoactive Compounds
4.1.4. Anti-Cancer Compounds
4.1.5. Compounds That Offer Pest Resistance, Insecticidal and Semiochemical Benefits
4.2. Future Perspectives
5. Conclusion
Author Contributions
Funding
Acknowledgment of Country
Conflicts of Interest
References
- Appelhans, M.S.; Bayly, M.J.; Heslewood, M.M.; Groppo, M.; Verboom, G.A.; Forster, P.I.; Kallunki, J.A.; Duretto, M.F. A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. TAXON 2021, 70, 1035–1061. [Google Scholar] [CrossRef]
- Waterman, P.G. Alkaloids of the rutaceae: their distribution and systematic significance. Biochemical Systematics and Ecology 1975, 3, 149–180. [Google Scholar] [CrossRef]
- IPNI. International Plant Names Index. Available online: https://www.ipni.org/?f=&sort=published_desc&q=Geijera (accessed on 12 December 2023).
- POWO. Geijera Schott | Plants of the World Online | Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:35743-1 (accessed on 12 December 2023).
- ALA. Genus: Geijera. Available online: https://bie.ala.org.au/species/https://id.biodiversity.org.au/node/apni/2905474 (accessed on 12 December 2023).
- Hartley, T.G. Flora of Australia: Meliaceae, Rutaceae, Zygophyllaceae. Volume 26; ABRS/CSIRO Australia.: 2013; Volume 26.
- Bruy, D.; Lannuzel, G.; Gâteblé, G.; Munzinger, J. Three new species threatened by mining activity in New Caledonia. Phytotaxa 2023, 578, 228–240. [Google Scholar] [CrossRef]
- Kubitzki, K.; Kallunki, J.A.; Duretto, M.; Wilson, P.G. Rutaceae; Berlin, Heidelberg: Springer Berlin Heidelberg: Berlin, Heidelberg, 2011; Volume 10, pp. 276–356.
- Packer, J.; Turpin, G.; Ens, E.; Venkataya, B.; Hunter, J.; Mbabaram, C.; Yirralka, R. Building partnerships for linking biomedical science with traditional knowledge of customary medicines: a case study with two Australian Indigenous communities. Journal of Ethnobiology and Ethnomedicine 2019, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.L. The Euahlayi Tribe: A Study of Aboriginal Life in Australia; Project Gutenberg: 1905.
- Sadgrove, N.J.; Jones, G.L. Characterization and Bioactivity of Essential Oils from Geijera parviflora (Rutaceae): A Native Bush Medicine from Australia. Nat. Prod. Commun. 2013, 8, 747–751. [Google Scholar] [CrossRef]
- Banbury, L.K.; Shou, Q.; Renshaw, D.E.; Lambley, E.H.; Griesser, H.J.; Mon, H.; Wohlmuth, H. Compounds from Geijera parviflora with prostaglandin E2 inhibitory activity may explain its traditional use for pain relief. J Ethnopharmacol 2015, 163, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Cribb, A.B.; Cribb, J.W. Wild medicine in Australia; Collins: Sydney, 1981.
- Lassak, E.V.; McCarthy, T. Australian medicinal plants; New Holland: Sydney, 2001. [Google Scholar]
- Ecological Cultural Knowledge - Paakantyi (Barkindji) Knowledge shared by the Paakantyi (Barkindji) people. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0007/1419784/WLLS-Paakantyi-Booklet-PURPLE-22.pdf (accessed on 12 December 2023).
- Ecological Cultural Knowledge - Ngiyampaa Knowledge shared by the Ngiyampaa people. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0003/737625/Ngiyampaa_Booklet_WEB-updated.pdf (accessed on 12 December 2023).
- Williams, A.; Sides, T. Wiradjuri Plant Use in the Murrumbidgee Catchment. Available online: https://www.lls.nsw.gov.au/__data/assets/pdf_file/0009/1270845/Wiradjuri-Plant-Use-in-the-Murrumbidgee-Catchment.pdf (accessed on 14 December 2023).
- Brophy, J.J.; Goldsack, R.J. The Leaf Oils of Coatesia and Geijera (Rutaceae) from Australia. Journal of Essential Oil Research 2005, 17, 169–174. [Google Scholar] [CrossRef]
- Penfold, A.R. Natural Chemical Resources of Australian Plant Products. Part II. Journal of Chemical Education 1932, 9, 429–438. [Google Scholar] [CrossRef]
- Penfold, A.R. The essential oils of three species of Geijera and the occurrence of a new hydrocarbon. Part I. Journal and proceedings of the Royal Society of New South Wales 1930, v.63-64 (1929-1930).
- Penfold, A.R.; Simonsen, J.L. The essential oils of three species of Geijera and the occurrence of a new hydrocarbon, Part II. Journal and proceedings of the Royal Society of New South Wales 1932, v.65-66 (1931-1933).
- Lahey, F.N.; MacLeod, J.K. The Coumarins of Geijera Parviflora Lindl. Australian Journal of Chemistry 1967, 20, 1943–1955. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Lyddiard, D.; Jones, G.L. Bioactive volatiles from Geijera parviflora Lindl. (Rutaceae): evidence for coumarin chemotypes. 2016; pp. 145–150.
- Sadgrove, N.J.; Goncalves-Martins, M.; Jones, G.L. Chemogeography and antimicrobial activity of essential oils from Geijera parviflora and Geijera salicifolia (Rutaceae): two traditional Australian medicinal plants. Phytochemistry 2014, 104, 60–71. [Google Scholar] [CrossRef]
- Floyd, A.G. Research Note no. 30 N.S.W. Rainforest Trees Part IV Family Rutaceae. 1979, 72. [Google Scholar]
- Bodkin, F. Dharawal Pharmacopeia Collection; Western Sydney University: Penrith, N.S.W, 2021.
- Ahond, A.; Poupat, C.; Pusset, J. Geibalansine et O-acétylgeibalansine, nouveaux alcaloïdes isolés de Geijera balansae. Phytochemistry (Oxford) 1979, 18, 1415–1416. [Google Scholar] [CrossRef]
- Mitaku, S.; Skaltsounis, A.-L.; Tillequin, F.; Koch, M.; Pusset, J.; Chauviere, G. Plantes de Nouvelle-Calédonie, XCVI. Alcaloïdes de Geijera balansae. Journal of Natural Products 1985, 48, 772–777. [Google Scholar] [CrossRef]
- Jones, R.V.J.; Sutherland, M.D. Terpenoid chemistry. XV. 1,5-Dimethylcyclodeca-1,5,7-triene, the precursor of geijerene in Geijera parviflora (Lindley). Australian journal of chemistry 1968, 21, 2255. [Google Scholar] [CrossRef]
- Gray, A.I.; Waterman, P.G. Coumarins in the Rutaceae. Phytochemistry 1978, 17, 845–864. [Google Scholar] [CrossRef]
- Levin, D.A. The Chemical Defenses of Plants to Pathogens and Herbivores. Annual Review of Ecology and Systematics 1976, 7, 121–159. [Google Scholar] [CrossRef]
- Yamane, H.; Konno, K.; Sabelis, M.; Takabayashi, J.; Sassa, T.; Oikawa, H. Chemical Defence and Toxins of Plants. Elsevier: 2010; pp. 339–385.
- Kingsbury, J.M. The Evolutionary and Ecological Significance of Plant Toxins. In Handbook of natural toxins Vol. 1 Plant and Fungal Toxins, Keeler, R.F., T., T.A., Eds.; Marcel Dekker: New York, 1983.
- Shimizu, B.-I. 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Frontiers in plant science 2014, 5, 549–549. [Google Scholar] [CrossRef]
- Ritchie, E.; Taylor, W.C.; Young, L.M. Some extractives from Geijera salicifolia Schott. Australian journal of chemistry 1968, 21, 1381. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bulletin of the Faculty of Pharmacy 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. Chapter Eleven - Medicinal natural products in osteoporosis. In Annual Reports in Medicinal Chemistry, Sarker, S.D., Nahar, L., Eds.; Academic Press: 2020; Volume 55, pp. 327–372.
- Rauf, A.; Khan, R.; Khan, H.; Pervez, S.; Pirzada, A.S. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Natural Product Research 2014, 28, 1371–1374. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Current Medicinal Chemistry 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Chimichi, S.; Boccalini, M.; Salvador, A.; Dall'Acqua, F.; Basso, G.; Viola, G. Synthesis and biological evaluation of new geiparvarin derivatives. ChemMedChem 2009, 4, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Carotti, A.; Carrieri, A.; Chimichi, S.; Boccalini, M.; Cosimelli, B.; Gnerre, C.; Carotti, A.; Carrupt, P.A. Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorganic & Medicinal Chemistry Letters 2002, 12. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Lee, A. Extractives of Geijera Parviflora. Phytochemistry 1972, 11, 763–767. [Google Scholar] [CrossRef]
- Adams, D.H.; Shou, Q.; Wohlmuth, H.; Cowin, A.J. Native Australian plant extracts differentially induce Collagen I and Collagen III in vitro and could be important targets for the development of new wound healing therapies. Fitoterapia 2016, 109, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Liao, Y.-R.; Hung, H.-Y.; Chuang, C.-W.; Hwang, T.-L.; Huang, S.-C.; Shiao, Y.-J.; Kuo, D.-H.; Wu, T.-S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Padmawinata, K. Isolation and identification of cancer delaying compounds from the leaves of Geijera salicifolia. Acta Pharmaceutica (Bandung, Indonesia).
- Davis, R.A.; Vullo, D.; Maresca, A.; Supuran, C.T.; Poulsen, S.A. Natural product coumarins that inhibit human carbonic anhydrases. Bioorganic and Medicinal Chemistry 2013, 21, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Shou, Q.; Banbury, L.K.; Renshaw, D.E.; Smith, J.E.; He, X.; Dowell, A.; Griesser, H.J.; Heinrich, M.; Wohlmuth, H. Parvifloranines A and B, two 11-carbon alkaloids from Geijera parviflora. Journal of Natural Products 2013, 76, 1384–1387. [Google Scholar] [CrossRef]
- Lahey, F.N.; Wluka, D.J. Geijerin: A new coumarin from the bark of Geijera salicifolia Schott. Australian Journal of Chemistry 1955, 8, 125. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, H.P.; Kim, M.J.; Chun, W. Acetylcholinesterase inhibitors from Angelica polymorpha stem. Natural Product Sciences 2017, 23, 97–102. [Google Scholar] [CrossRef]
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the peel of citrus grown in Colombia: composition, elicitation and antifungal activity. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacological Research 2020, 160, 105170. [Google Scholar] [CrossRef] [PubMed]
- Golfakhrabadi, F.; Abdollahi, M.; Ardakani, M.R.; Saeidnia, S.; Akbarzadeh, T.; Ahmadabadi, A.N.; Ebrahimi, A.; Yousefbeyk, F.; Hassanzadeh, A.; Khanavi, M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm Biol 2014, 52, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tsai, W.J.; Wu, M.H.; Lin, L.C.; Kuo, Y.C. Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB. Br J Pharmacol 2007, 150, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Kim, C.Y.; Lee, J.K. Anti-inflammatory effects of dehydrogeijerin in LPS-stimulated murine macrophages. International Immunopharmacology 2012, 14, 734–739. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: an overview of its sources, biological activities, and modification development. Medicinal Chemistry Research 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Xu, J.; Guan, S.; Sun, D. Research of the anxiolytic effect of osthol. Chin. Med. Herald 2012, 9, 19–21. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.; Zhou, C.; Xu, D.; Qu, H.-b. Screening Antitumor Compounds Psoralen and Isopsoralen from Psoralea corylifolia L. Seeds. Evidence-based complementary and alternative medicine 2010, 2011, 363052–363052. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Lee, W.L.; Yap, W.H.; Pusparajah, P.; Low, L.E.; Tang, S.Y.; Chan, K.G.; Lee, L.H.; Goh, B.H. Angelicin—A Furocoumarin Compound With Vast Biological Potential. Frontiers in Pharmacology 2020, 11. [Google Scholar] [CrossRef]
- Wang, N.; Yan, L.U.; Lyu, Y.E.; Wei, M.I.N.; Song, P. Use of linear pyranocoumarin compound Xanthyletine as agricultural bactericide. 2017.
- Khan, A.; Kunesch, G.; Chuilon, S.; Ravisé, A. Structure and biological activity of xanthyletin : a new phytoalexin of CITRUS. Fruits 1985, 40. [Google Scholar]
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of Chemical Constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and Furochromones. International Journal of Molecular Sciences 2022, 23, 406. [Google Scholar] [CrossRef] [PubMed]
- Tuan Anh, H.L.; Kim, D.-C.; Ko, W.; Ha, T.M.; Nhiem, N.X.; Yen, P.H.; Tai, B.H.; Truong, L.H.; Long, V.N.; Gioi, T.; et al. Anti-inflammatory coumarins from Paramignya trimera. Pharmaceutical Biology 2017, 55, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Xanthoxyletin. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 14 December 2023).
- DNP. Dictionary of natural products. Available online: http://dnp.chemnetbase.com/dictionary-search.do?method=view&id=425027&si= (accessed on 15 December 23).
- Shou, Q.; Banbury, L.K.; Maccarone, A.T.; Renshaw, D.E.; Mon, H.; Griesser, S.; Griesser, H.J.; Blanksby, S.J.; Smith, J.E.; Wohlmuth, H. Antibacterial anthranilic acid derivatives from Geijera parviflora. Fitoterapia 2014, 93, 62–66. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A. Alkaloids of Geijera salicifolia Schott. (family Rutaceae): The identification of platydesmine and platydesmine acetate. Australian Journal of Chemistry 1966, 19, 1991. [Google Scholar] [CrossRef]
- Szewczyk, A.; Pęczek, F. Furoquinoline Alkaloids: Insights into Chemistry, Occurrence, and Biological Properties. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflammation research 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-d.; Zhang, D.-b.; Ren, J.; Yang, M.-j. Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor. Medicinal chemistry research 2012, 21, 722–725. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Natural product research 2019, 33, 759–762. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rojas de Arias, A.; Yaluff, G.; de Bilbao, N.V.; Nakayama, H.; Torres, S.; Schinini, A.; Guy, I.; Heinzen, H.; Fournet, A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine (Stuttgart) 2010, 17, 375–378. [Google Scholar] [CrossRef]
- De Souza, R.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.d.G.; Godoy, M.F.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A.; Pirani, J.R.J.Z.f.N.B. A new imidazole alkaloid and other constituents from Pilocarpus grandiflorus and their antifungal activity. Zeitschrift für Naturforschung B 2005, 60, 787–791. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. Journal of Ethnopharmacology 2009, 123, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Coy Barrera, C.A.; Coy Barrera, E.D.; Granados Falla, D.S.; Delgado Murcia, G.; Cuca Suarez, L.E. seco-limonoids and quinoline alkaloids from Raputia heptaphylla and their antileishmanial activity. Chem Pharm Bull (Tokyo) 2011, 59, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Guzmán, R.; Johansmann Fulks, L.C.; Radwan, M.M.; Burandt, C.L.; Ross, S.A. Chemical Constituents, Antimicrobial and Antimalarial Activities of Zanthoxylum monophyllum. Planta Med 2011, 77, 1542–1544. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. Biomed Res Int 2018, 2018, 3819714. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Natural Product Communications 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Chapter Three - Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology, Kendall, D., Alexander, S.P.H., Eds.; Academic Press: 2017; Volume 80, pp. 67–134.
- Wedman-St. Louis, B. Cannabis As Medicine; Taylor & Francis Group: Milton, 2019. [Google Scholar]
- Hazekamp, A.; Fischedick, J.T.; Díez, M.L.; Lubbe, A.; Ruhaak, R.L. 3.24 - Chemistry of Cannabis. In Comprehensive Natural Products II, Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, 2010; pp. 1033–1084. [Google Scholar]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil ofMelaleuca alternifolia. Journal of Applied Bacteriology 1995, 78, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.F.d.B.; Lopes, E.M.; de Araújo, J.M.; de Sousa, D.P.; Veras, L.M.C.; Leite, J.R.S.A.; Almeida, F.R.d.C. Involvement of Cholinergic and Opioid System in <i>γ</i>-Terpinene-Mediated Antinociception. Evidence-Based Complementary and Alternative Medicine 2015, 2015, 829414. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, T.; Pacheco de Oliveira, M.; Lima, A.; Bezerra-Santos, C.; Piuvezam, M. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta medica 2015, 81. [Google Scholar] [CrossRef]
- Menezes, I.O.; Scherf, J.R.; Martins, A.O.B.P.B.; Ramos, A.G.B.; Quintans, J.d.S.S.; Coutinho, H.D.M.; Ribeiro-Filho, J.; de Menezes, I.R.A. Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: A systematic review. Phytomedicine 2021, 93, 153768. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants – Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicology in vitro 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. Journal of the science of food and agriculture 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food and chemical toxicology 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Scientia Pharmaceutica 2022, 90, 57. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. Journal of Enzyme Inhibition and Medicinal Chemistry 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. Medicinal Plants in Australia Volume 2: Gums, Resins, Tannin and Essential Oils; Rosenberg Publishing: 2011; p. 300.
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Rios, E.; Rocha, N.; Carvalho, A.; Vasconcelos, L.; Dias, M.; Sousa, D.; Sousa, F.; Fonteles, M. TRP and ASIC channels mediate the antinociceptive effect of citronellyl acetate. Chemico-biological interactions 2013, 203. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G.J.J.B. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. 2018, 23, 346–352.
- Khayyat, S.A.; Sameeh, M.Y. Bioactive epoxides and hydroperoxides derived from naturally monoterpene geranyl acetate. Saudi Pharm J 2018, 26, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; Olivero, M.; Rouquet, V.; Moga, A.; Pagnon, A.; Cenizo, V.; Portes, P. Neryl acetate, the major component of Corsican Helichrysum italicum essential oil, mediates its biological activities on skin barrier. PLOS ONE 2023, 18, e0268384. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.S.; Denkova, Z.; Nikolova, R.; Geissler, M. Purity, Antimicrobial Activities and Olfactoric Evaluations of Geraniol/Nerol and Various of Their Derivatives. The Journal of Essential Oil Research 2007, 19, 288–291. [Google Scholar] [CrossRef]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool Odor-Induced Anxiolytic Effects in Mice. Frontiers in Behavioral Neuroscience 2018, 12, 241–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microbial Pathogenesis 2020, 141, 103980–103980. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. International Immunopharmacology 2019, 74, 105706–105706. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chemistry 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflammation Research 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 2012, 29, 415–422. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer In Vitro and In Vivo. Evidence-based complementary and alternative medicine 2011, 2012, 818261–818213. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Arancia, G.; Molinari, A.; Salvatore, G.; Mondello, F. Terpinen-4-ol, The Main Component of Melaleuca Alternifolia (Tea Tree) Oil Inhibits the In Vitro Growth of Human Melanoma Cells. Journal of Investigative Dermatology 2004, 122, 349–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Current microbiology 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respiratory Medicine 2003, 97, 250–256. [Google Scholar] [CrossRef]
- Yin, C.; Liu, B.; Wang, P.; Li, X.; Li, Y.; Zheng, X.; Tai, Y.; Wang, C.; Liu, B. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. British Journal of pharmacology 2020, 177, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon--a review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Qin, J.; Lu, W.; Wang, J. The Use of Borneol as an Enhancer for Targeting Aprotinin-Conjugated PEG-PLGA Nanoparticles to the Brain. Pharmaceutical Research 2013, 30, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Kırımer, N.; Demirci, B.; Demirci, F.; Başer, K.H.C. Composition and Antimicrobial Activity of the Essential Oils of Micromeria cristata subsp. phrygia and the Enantiomeric Distribution of Borneol. Journal of Agricultural and Food Chemistry 2001, 49, 4300–4303. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Yuzer, A.; Ince, T.; Ince, M. Anti-Cancer and Anti-Inflammatory Activities of Bromo- and Cyano-Substituted Azulene Derivatives. Inflammation 2020, 43, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.V.H.; Sutherland, M.D. Terpenoid Chemistry XV.* 1,5-Dimethylcyclodeca-1,5,7-triene the Precursor of Geijerene in Geijera Parviflora. Australian Journal of Chemistry 1968, 21, 2255–2264. [Google Scholar] [CrossRef]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Management Science 2006, 55, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.; Powell, V.; Sutherland, M.D. Constitution and biogenesis of two new sesquiterpenes. Tetrahedron Letters 1961, 2, 763–767. [Google Scholar] [CrossRef]
- Sutherland, M.D. Terpenoid chemistry. VII. The structure of geijerene. Australian journal of chemistry 1964, 17, 75–91. [Google Scholar] [CrossRef]
- Mokhtari, M.; Jackson, M.D.; Brown, A.S.; Ackerley, D.F.; Ritson, N.J.; Keyzers, R.A.; Munkacsi, A.B. Bioactivity-Guided Metabolite Profiling of Feijoa (Acca sellowiana) Cultivars Identifies 4-Cyclopentene-1,3-dione as a Potent Antifungal Inhibitor of Chitin Synthesis. Journal of Agricultural and Food Chemistry 2018, 66, 5531–5539. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chemistry 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: a rich source of germacrene D. Journal of Essential Oil Research 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Myron, K.; Clarkson, B.; Gemmill, C. New Zealand Journal of Botany Biological flora of New Zealand 16: Pittosporum kirkii Hook.f. ex Kirk, Kirk's kōhūhū, thick-leaved kohukohu. New Zealand Journal of Botany 2020, 59. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicology and Environmental Safety 2016, 133, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Park, Y.-L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.-M.; Zhao, G.-D.; Zhao, J.-J. δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. International journal of clinical and experimental pathology 2015, 8, 6046. [Google Scholar] [PubMed]
- Guo, X.; Shang, X.; Li, B.; Zhou, X.Z.; Wen, H.; Zhang, J. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, δ-cadinene against Psoroptes cuniculi. Veterinary parasitology 2017, 236, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Frontiers in Pharmacology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Weng, S.; Lv, M.; Chen, W.; Bi, Z.; Chen, H.; Luo, T.; Hu, H.; Liao, W. β-Elemene Inhibits Human Sperm Function by Affecting Sperm Vitality and Intracellular Calcium. Cellular Physiology and Biochemistry 2018, 51, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High toxicity of camphene and γ-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environmental Science and Pollution Research International 2018, 25, 10383–10391. [Google Scholar] [CrossRef]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother Res 2006, 20, 371–373. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 53 - Cannabis sativa and Hemp. In Nutraceuticals, Gupta, R.C., Ed.; Academic Press: Boston, 2016; pp. 735–754. [Google Scholar]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Medicine (Malden, MA) 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm Biol 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ishnava, K.B.; Chauhan, J.B.; Barad, M.B. Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill. Saudi J Biol Sci 2013, 20, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of Cinnamaldehyde, Ocimene, Camphene, Curcumin and Farnesene on Candida albicans</i>. Advances in Microbiology 2016, 06, 627–643. [Google Scholar] [CrossRef]
- Cugini, C.; Calfee, M.W.; Farrow, J.M.; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Molecular Microbiology 2007, 65, 896–906. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.M.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-Induced Apoptosis in Candida albicans. Antimicrobial Agents and Chemotherapy 2009, 53, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.-J.; Xie, H.-Q.; Mu, Q. Guaiol - A Naturally Occurring Insecticidal Sesquiterpene. Natural Product Communications 2013, 8, 1934578X1300801001. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Senbill, H.; Van Wyk, B.-E.; Greatrex, B.W. New Labdanes with Antimicrobial and Acaricidal Activity: Terpenes of Callitris and Widdringtonia (Cupressaceae). Antibiotics (Basel) 2020, 9, 173. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yang, Q. (-)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol Ther 2018, 19, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.F.M.; de Moura, É.P.; de Sousa, N.F.; Muratov, E.; Bezerra, A.H.R.; Scotti, M.T.; Scotti, L. Prediction of antifungal activity, cytotoxicity risks and molecular docking against Malassezia furfur of constituents of citronella essential oil (Cymbopogon winterianus). In Proceedings of the MOL2NET, International Conference Series on Multidisciplinary Sciences, UNC Chape Hill, USA; 2019. [Google Scholar]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med Vet Entomol 2005, 19, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Poblocka-Olech, L.; Zabiegala, B.; Bucinski, A.; Krauze-Baranowska, M.; Luczkiewicz, M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. Journal of Chromatography B 2018, 1086, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Natural product research 2008, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zuccolotto, T.; Bressan, J.; Lourenço, A.V.F.; Bruginski, E.; Veiga, A.; Marinho, J.V.N.; Raeski, P.A.; Heiden, G.; Salvador, M.J.; Murakami, F.S.; et al. Chemical, Antioxidant, and Antimicrobial Evaluation of Essential Oils and an Anatomical Study of the Aerial Parts fromBaccharisSpecies (Asteraceae). Chemistry & Biodiversity 2019, 16. [Google Scholar] [CrossRef]
- dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol—a Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)—Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P.; Andersson, R.; Samuelsson, G. T-cadinol: a pharmacologically active constituent of scented myrrh: introductory pharmacological characterization and high field 1H- and 13C-NMR data. Planta Med 1991, 57, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Ōmura, S.; Yamada, H. In vitro antitrypanosomal activity of plant terpenes against Trypanosoma brucei. Phytochemistry 2011, 72, 2024–2030. [Google Scholar] [CrossRef]
- Bomfim, D.S.; Ferraz, R.P.C.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Eudesmol Isomers Induce Caspase-Mediated Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells. Basic & Clinical Pharmacology & Toxicology 2013, 113, 300–306. [Google Scholar] [CrossRef]
- Asakura, K.; Kanemasa, T.; Minagawa, K.; Kagawa, K.; Yagami, T.; Nakajima, M.; Ninomiya, M. α-Eudesmol, a P/Q-type Ca2+ channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Research 2000, 873, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Misaizu, A.; Kaneko, Y.; Fukuda, T.; Miyake, M.; Miura, Y.; Okamura, H.; Yajima, J.; Tsuda, A. β-Eudesmol, an Oxygenized Sesquiterpene, Reduces the Increase in Saliva 3-Methoxy-4-Hydroxyphenylglycol After the "Trier Social Stress Test" in Healthy Humans: A Randomized, Double-Blind, Placebo-Controlled Cross-Over Study. Nutrients 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Singh, V.K.; Sharma, P.K.; Singh, V. Essential oil-based nanostructures for inflammation and rheumatoid arthritis. Journal of Drug Delivery Science and Technology 2020, 60, 101983. [Google Scholar] [CrossRef]
- Han, N.R.; Moon, P.D.; Ryu, K.J.; Jang, J.B.; Kim, H.M.; Jeong, H.J. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin Exp Pharmacol Physiol 2017, 44, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Ali, Z.; Baser, K.H.C.; Khan, I.A. Chemical Composition and Biological Activity of Four Salvia Essential Oils and Individual Compounds against Two Species of Mosquitoes. Journal of Agricultural and Food Chemistry 2015, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, L.N.F.; Nascimento, K.F.d.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.d.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. Journal of ethnopharmacology 2016, 192, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Francke, W.; Schulz, S. Pheromones. Elsevier: 1999; pp. 197–261.
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. Journal of Ethnopharmacology 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.; da, R. Guterres, Z.; Violante, I.M.P.; Lopes, T.F.S.; Garcez, W.S.; Garcez, F.R. Evaluation of mutagenic and antimicrobial properties of brown propolis essential oil from the Brazilian Cerrado biome. Toxicology Reports 2015, 2, 1482–1488. [Google Scholar] [CrossRef]
- Beattie, K. Phytochemical studies and bioactivity of Centipeda and Eremophila species. Southern Cross University, Lismore, N.S.W., 2009.
- Beattie, K.D.; Waterman, P.G.; Forster, P.I.; Thompson, D.R.; Leach, D.N. Chemical composition and cytotoxicity of oils and eremophilanes derived from various parts of Eremophila mitchellii Benth. (Myoporaceae). Phytochemistry 2011, 72, 400–408. [Google Scholar] [CrossRef]
- Ishii, T.; Shinjo, Y.; Miyagi, M.; Matsuura, H.; Abe, T.; Kikuchi, N.; Suzuki, M. Investigation of insect repellent activity of cyclocolorenone obtained from the red alga Laurencia intricata. Records of natural products 2019, 13, 81–84. [Google Scholar] [CrossRef]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer research (Chicago, Ill.) 1980, 40, 403. [Google Scholar]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytotherapy research 2002, 16, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shimura, H.; Watanabe, N.; Tamai, M.; Hanada, K.; Takahashi, A.; Tanaka, Y.; Arai, I.; Zhang, P.-L.; Rao, C.; et al. Hepatorotective Compounds from Canarium album and Euphorbia nematocypha. CHEMICAL & PHARMACEUTICAL BULLETIN 1990, 38, 2201–2203. [Google Scholar] [CrossRef]
- de Carvalho, N.C.; Neves, S.P.; Dias, R.B.; Valverde, L.d.F.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; dos Santos, E.R.; Oliveira, R.M.M.; Carlos, R.M.; et al. A novel ruthenium complex with xanthoxylin induces S-phase arrest and causes ERK1/2-mediated apoptosis in HepG2 cells through a p53-independent pathway. Cell Death & Disease 2018, 9, 79. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Yang, X.-N.; Zhu, X.; Xiao, X.-R.; Yang, X.-W.; Qin, H.-B.; Gonzalez, F.J.; Li, F. Role of Metabolic Activation in Elemicin-Induced Cellular Toxicity. Journal of Agricultural and Food Chemistry 2019, 67, 8243–8252. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T. Possible Implication of Myristicin as a Psychotropic Substance. Nature (London) 1966, 210, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Purity, Antimicrobial Activities and Olfactory Evaluations of 2-Phenylethanol and Some Derivatives. The Journal of essential oil research 2008, 20, 82–85. [Google Scholar] [CrossRef]
- Chowdary, G.V.; Ramesh, M.N.; Prapulla, S.G. Enzymic synthesis of isoamyl isovalerate using immobilized lipase from Rhizomucor miehei: a multivariate analysis. Process Biochemistry 2000, 36, 331–339. [Google Scholar] [CrossRef]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proceedings of the National Academy of Sciences 2000, 97, 9329. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J Insect Sci 2012, 12, 56–56. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y.; Kunitomi, M.; Kudo, T.; MacDonald, P.N. Endocrine Disrupting Chemicals, Phthalic Acid and Nonylphenol, Activate Pregnane X Receptor-Mediated Transcription. Molecular endocrinology (Baltimore, Md.) 2000, 14, 421–428. [Google Scholar] [CrossRef]
- Ahn, D.; Kwon, J.; Song, S.; Lee, J.; Yoon, S.; Chung, S.J. Methyl Syringate Stimulates Glucose Uptake by Inhibiting Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Life 2023, 13, 1372. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, M.J.; Park, J.-H.; Ishii, S.; Misaka, T.; Rhyu, M.-R. Methyl syringate, a low-molecular-weight phenolic ester, as an activator of the chemosensory ion channel TRPA1. Archives of Pharmacal Research 2012, 35, 2211–2218. [Google Scholar] [CrossRef]
- Phuong, N.T.; Cuong, T.T.; Quang, D.N. Anti-inflammatory activity of methyl ferulate isolated from Stemona tuberosa Lour. Asian Pac J Trop Med 2014, 7s1, S327–331. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 2012, 1822, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-x.; Wang, Y.-y.; Gao, Z.-q.; Chen, D.; Liu, G.; Wan, B.-b.; Jiang, F.-j.; Wei, M.-x.; Zuo, J.; Zhu, J.; et al. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacologica Sinica 2021, 42, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.V.M.; Coelho, A.G.; Azevedo, P.S.d.S.; da Silva, A.A.; Oliveira, F.d.A.; Nunes, L.C.C. Systematic review and technological prospection: ethyl ferulate, a phenylpropanoid with antioxidant and neuroprotective actions. Expert Opinion on Therapeutic Patents 2019, 29, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Preziuso, F.; Sharifi-Rad, M.; Marchetti, L.; Epifano, F.; Genovese, S. Auraptene and umbelliprenin: a review on their latest literature acquisitions. Phytochemistry Reviews 2020. [Google Scholar] [CrossRef]
- Guan, Z.; Hellman, J.; Schumacher, M. Contemporary views on inflammatory pain mechanisms: TRPing over innate and microglial pathways. F1000Res 2016, 5, F1000 Faculty Rev-2425. [Google Scholar] [CrossRef]
- Cho, H.-J.; Jeong, S.-G.; Park, J.-E.; Han, J.-A.; Kang, H.-R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antiviral Research 2013, 100, 75–83. [Google Scholar] [CrossRef]
- Singh, L.; Bhatti, R. Signaling Pathways Involved in the Neuroprotective Effect of Osthole: Evidence and Mechanisms. Molecular Neurobiology 2023. [Google Scholar] [CrossRef] [PubMed]
- Jerris, P.J.; Smith, A.B. Synthesis and Configurational Assignment of Geiparvarin: A Novel Antitumor Agent. Journal of Organic Chemistry 1981, 46, 577–585. [Google Scholar] [CrossRef]
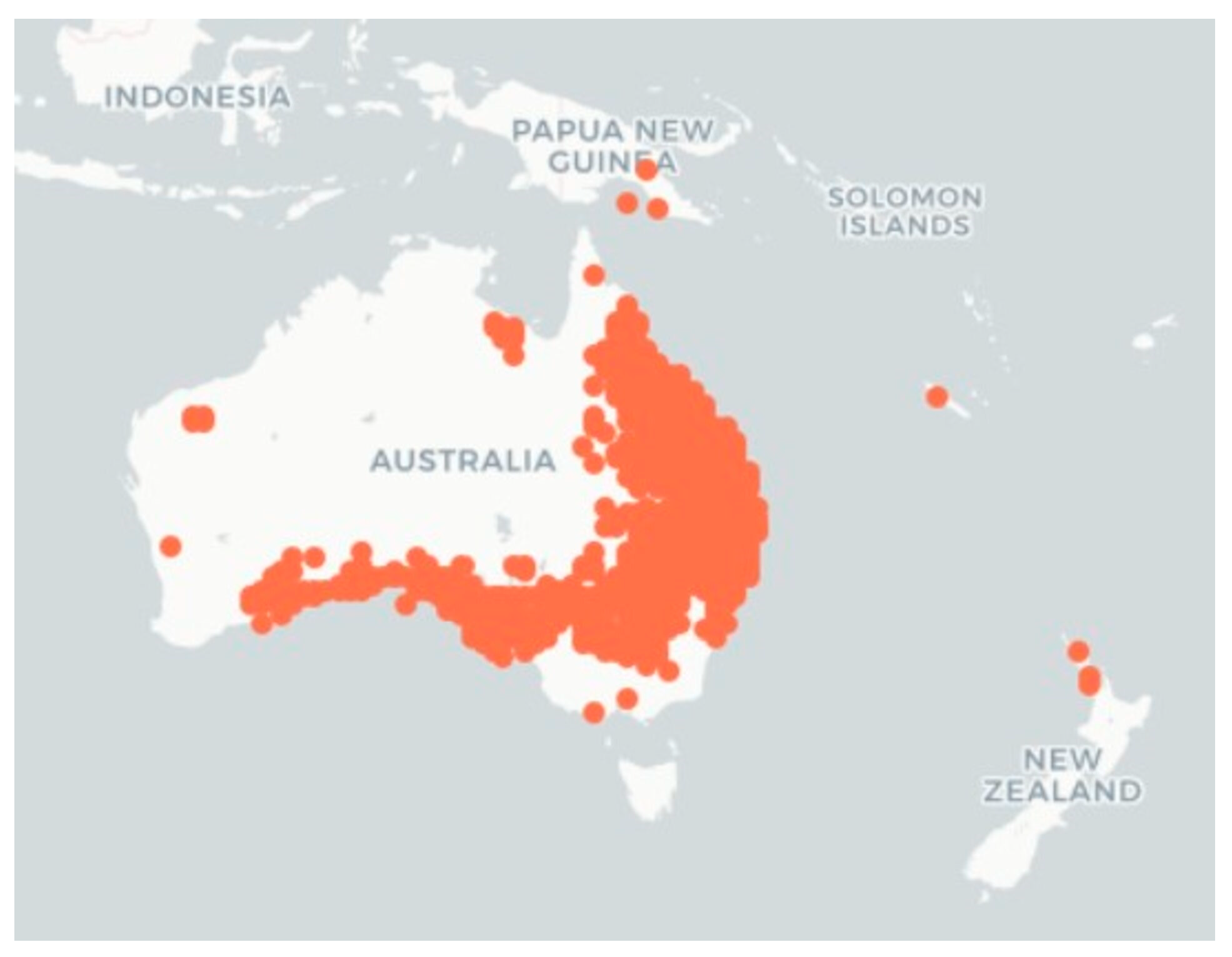
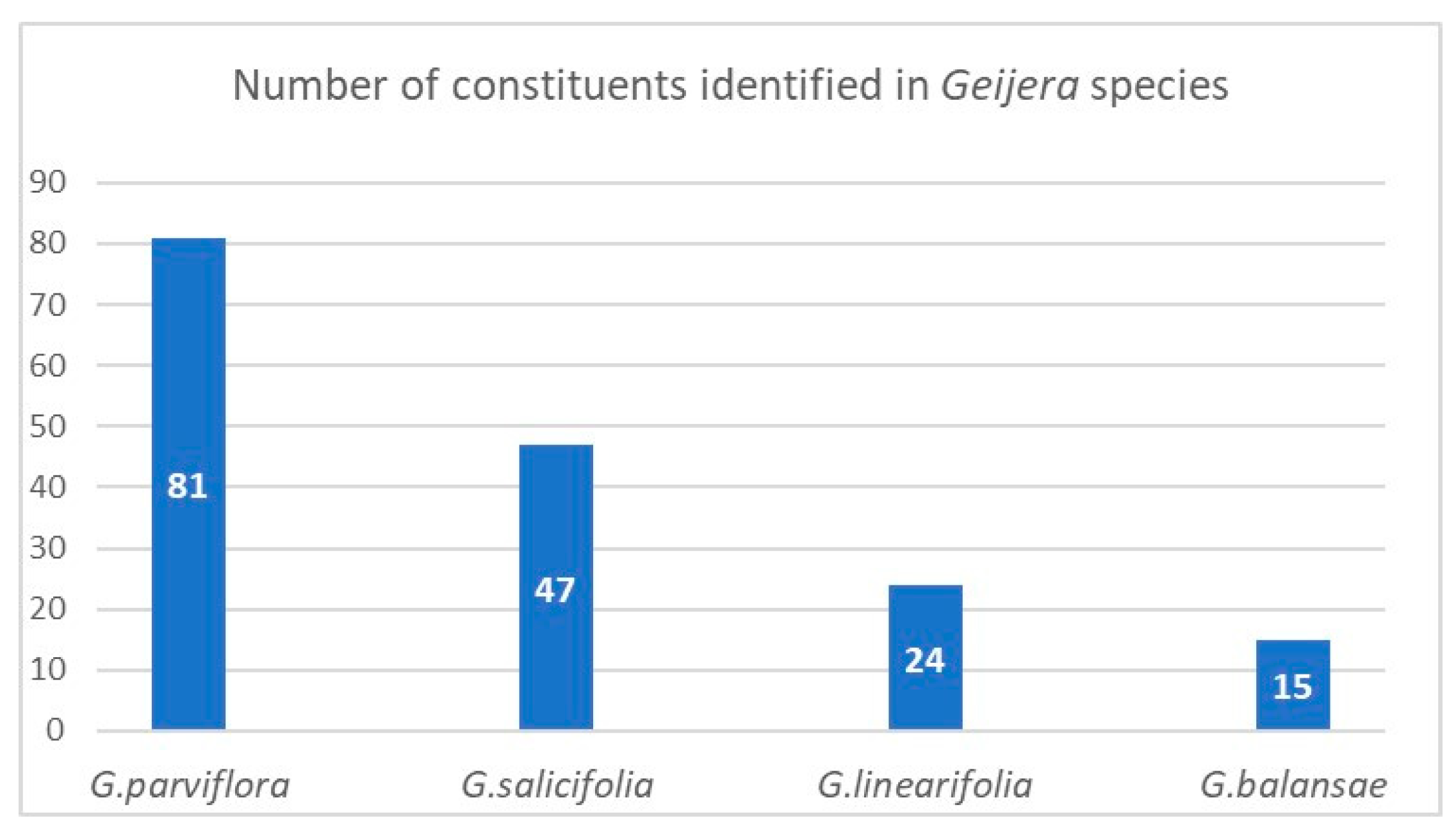
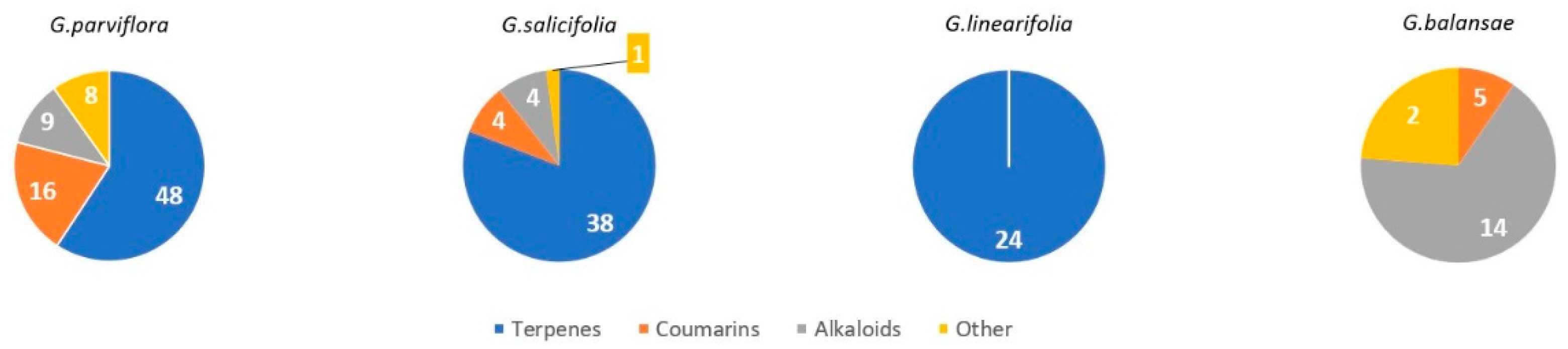
| Species (Accepted Name) | Synonyms |
|---|---|
| Geijera balansae (Baill.) Schinz & Guillaumin | Zanthoxylum balansae |
| Geijera cauliflora Baill. |
Dendrosma deplanchei Pancher & Sebert Geijera deplanchei (Pancher & Sebert) Däniker Geijera lateriflora Baill. ex Guillaumin |
| Geijera linearifolia (DC.) J.M.Black |
Geijera parviflora var. crassifolia Benth. Eriostemon linearifolius DC. Geijera linearifolia Domin |
| Geijera parviflora Lindl. |
Geijera pendula Lindl. Geijera parviflora var. parviflora Lindl. Zanthoxylum australasicum A.Juss. |
| Geijera salicifolia Schott |
Geijera salicifolia var. augustifolia Maiden Geijera salicifolia Schott var. salicifolia Geijera salicifolia var. latifolia (Lindl.) Domin Geijera salicifolia var. angustifolia Maiden & Betche Geijera latifolia Lindl. Geijera salicifolia var. typica Domin Geijera floribunda Pancher ex Guillaumin |
| Geijera tartarea T.G.Hartley ex Munzinger & Bruy | None |
| (Key: G.p - G. parviflora, G.s – G. salicifolia) | ||||
| Compound and Exact mass (Da) | Source | Method of identification | Reference | Reported pharmacological activity of compound (various sources) |
| 1 umbelliferone 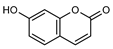 162.0316 |
G. salicifolia (leaves) | Melting point, IR and 1H NMR | Ritchie et al. 1968 [35] | Anti-inflammatory, antinociceptive, anti-hyperglycaemic, antibacterial, antifungal, inhibition of DPPH, hydroxyl, superoxide anion and ABTS radicals, molluscicide, antifeedant, anti-tumour, antimutagenic, fluorescent (sunscreen agent), bone-protective, anti-biofilm [36,37,38] |
| 2 geiparvarin 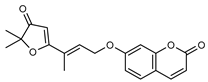 326.1154 |
G. parviflora (leaves) G. salicifolia (leaves) |
Combustion analysis, chemical derivatization, UV, IR (G.p) IR and 1H NMR (G.s) |
Lahey and MacLeod 1967; Ritchie et al. 1968 [22,35] | Anti-cancer [39,40], monoamine oxidase B inhibitor [41] |
| 3 auraptene 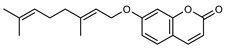 298.1568 |
G. parviflora (fruit/seeds) | IR and 1H NMR | Dreyer and Lee 1972 [42] | Increases collagen I expression [43], anti-bacterial, anti-fungal, Antileishmanial, anti-cancer and anti-oxidant [44] |
| 4 marmin 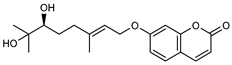 332.1623 |
G. parviflora (fruit/seeds) | IR and 1H NMR | Dreyer and Lee 1972 [42] | No significant anti-inflammatory activity reported [45] |
| 5 6’-dehydromarmin 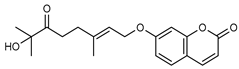 330.1467 |
G. parviflora (fruit/seeds) | IR and 1H NMR | Dreyer and Lee 1972 [42] | Anti-inflammatory, cytotoxic [12] |
| 6 2’,3’-dihydrogeiparvarin 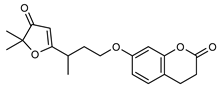 328.1310 |
G. parviflora (fruit/seeds) G. salicifolia (leaves) |
IR and 1H NMR | Dreyer and Lee 1972 [42] Padmawinata 1973 [46] |
Anti-cancer [46,47] |
| 7 G. parviflora (leaves) (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin 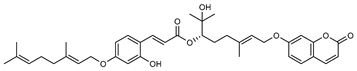 630.3193 |
2D NMR | Banbury et al. 2015 [12] | Cytotoxic, anti-inflammatory [12] | |
| 8 parvifloranine A 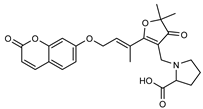 453.1787 |
G. parviflora (leaves) | 2D NMR, ECD and MS | Shou et al. 2013 [48] | Anti-inflammatory [48] |
| 9 parvifloranine B 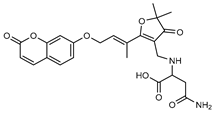 455.1692 |
G. parviflora (leaves) | 2D NMR, ECD and MS | Shou et al. 2013 [48] | No significant anti-inflammatory activity reported [48] |
| 10 geijerin 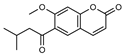 260.1048 |
G. salicifolia (bark) G. parviflora (leaves) |
Chemical derivatization, UV, and IR Melting point, IR and 1H NMR |
Lahey and Wluka 1955 [49] Ritchie et al. 1968 [35] |
Acetylcholinesterase inhibitor [50] |
| 11 scoparone 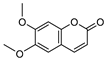 206.0579 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2016 [23] | Antifungal, anti-inflammatory, antioxidant, anti-apoptotic, anti-fibrotic and hypolipidemic [51,52] |
| 12 suberosin 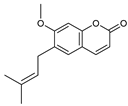 246.1256 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2016 [23] | Anti-inflammatory and anticoagulant [53,54] |
| 13 dehydrogeijerin 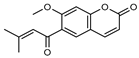 258.0892 |
G. parviflora (leaves) G. salicifolia (leaves) |
Chemical derivatization, UV, and IR (G.p) IR and 1H NMR (G.s) |
Lahey and MacLeod 1967; Ritchie et al. 1968 [22,35] | Anti-inflammatory activity, acetylcholinesterase inhibitor [50,55] |
| 14 6-(methoxyl) geiparvarin 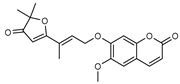 356.1260 |
G. parviflora (leaves) | 13C and 1H NMR | Banbury et al. 2015 [12] | Anti-inflammatory, cytotoxic [12] |
| 15 osthole 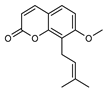 244.1099 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Antitumor, anti-inflammatory, neuroprotective, anxiolytic, osteogenic, cardiovascular protective, antimicrobial, antiparasitic [56,57] |
| 16 angelicin (isopsoralen) 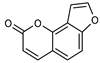 186.0317 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Anti-cancer [58], pro-osteogenic, antiviral, pro-chondrogenic, anti-inflammatory, erythroid differentiating, anti-periodontitis [59] |
| 17 xanthyletine 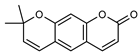 228.0786 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Antimicrobial, fungicide [60,61] |
| 18 luvangetin 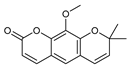 258.0892 |
G. balansae (leaves) |
UV, IR, 1H NMR, MS | Mitaku et al. 1985 [28] | Antiulcer, antifungal, anti-inflammatory, antibacterial [62,63] |
| 19 xanthoxyletin 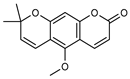 258.0892 |
G. balansae (bark) |
UV, IR, 1H NMR, MS | Mitaku et al. 1985 [28] | Anticonvulsant, anti-inflammatory, carbonic anhydrase inhibitor, anti-malaria, histone Lysine Methyltransferase G9a inhibitor [64,65] |
| (Key: G.b – G. balansae G.p - G. parviflora, G.s – G. salicifolia) | ||||
| Compound and Exact mass (Da) | Source | Method of identification |
Reference | Reported pharmacological activity of compound (various sources) |
| 20 11’-hexadecenoyl anthranilic acid 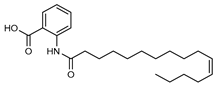 373.2617 |
G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | Shou et al. 2014 [66] |
Antibacterial vs Gram positive bacteria [66] |
| 21 9’-hexadecenoyl anthranilic acid 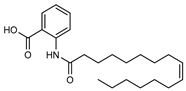 373.2617 |
G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | Shou et al. 2014 [66] |
Antibacterial vs Gram positive bacteria [66] |
| 22 7’-hexadecenoyl anthranilic acid  373.2617 |
G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | Shou et al. 2014 [66] |
Antibacterial vs Gram positive bacteria [66] |
| 23 9,12,15-octadecatrienoyl anthranilic acid 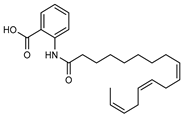 383.2460 |
G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | Shou et al. 2014 [66] |
Did not show significant antibacterial activity [66] |
| 24 hexadecanoyl anthranilic acid 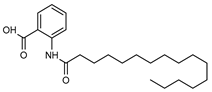 375.2773 |
G. parviflora (leaves) | HRESI-MS, IR, UV, 13C and 1H NMR | Shou et al. 2014 [66] |
Antibacterial vs Gram positive bacteria [66] |
| 25 dictamnine 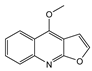 199.2090 |
G. balansae (wood/bark) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Antibacterial, antiviral, antifungal, antiprotozoal, anti-cancer, anti-inflammatory, antioxidant, cardiovascular, antiplatelet, antiosteoporosis, anti-anaphylactoid [68] |
| 26 skimmianine 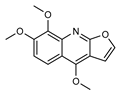 259.0845 |
G. salicifolia (leaves) G. balansae (wood/bark) |
IR, melting point (G.s) 1H NMR, IR, UV, and MS (G.b) |
Johns and Lamberton 1966; Mitaku et al. 1985 [28,67] |
Anti-inflammatory [69], acetylcholinesterase inhibitor [70], anti-cancer [71] |
| 27 γ-fagarine 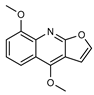 229.0739 |
G. salicifolia (leaves) G. balansae (wood/bark) |
IR , melting point (G.s) 1H NMR, IR, UV, and MS (G.b) |
Johns and Lamberton 1966; Mitaku et al. 1985 [28,67] |
Antileishmanial [72] |
| 28 platydesmine 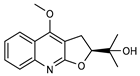 259.1208 |
G. salicifolia (leaves) G. balansae (leaves) |
Melting point, combustion analysis, chemical degradation, IR, UV and 1H NMR (G.s) 1H NMR, IR, UV, and MS (G.b) |
Johns and Lamberton 1966; Mitaku et al. 1985 [28,67] |
Antifungal [73] |
| 29 platydesmine acetate 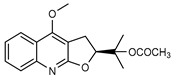 301.1314 |
G. salicifolia (leaves) | Combustion analysis, chemical degradation, IR and 1H NMR | Johns and Lamberton 1966 [67] |
No activity reported to date. |
| 30 flindersine 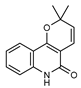 227.0946 |
G. parviflora (fruit/seeds) G. balansae (leaves) |
IR and melting point (G.p) 1H NMR, IR, UV, and MS (G.b) |
Dreyer and Lee 1972; Mitaku et al. 1985 [28,42] |
Anti-inflammatory [12], collagen III suppression [43] antibacterial, antifungal [74] |
| 31 4’-hydroxy-3’,4’-dihydroflindersine 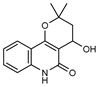 245.1052 |
G. balansae (leaves) | Chemical synthesis/derivatization, 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
No activity reported to date. |
| 32 cis- 3’, 4’- dihydroxy-3’,4’-dihydroflindersine 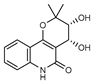 261.1001 |
G. balansae (leaves) | Chemical synthesis/ derivatization, 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
No activity reported to date. |
| 33 zanthobungeanine 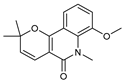 271.1208 |
G. balansae (leaves) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Leishmanicidal activity on Leishmania Viannia panamensis intracellular amastigotes (EC₅₀: 8.7 µg/ml) and promastigotes (EC₅₀: 14.3 µg/ml), respectively. [75] |
| 34 8-(methoxyl)-flindersine 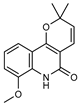 257.1052 |
G. parviflora (leaves) | UV, IR, 2D NMR and MS | Banbury et al. 2015 [12] |
No activity reported to date. |
| 35 N-(acetoxymethyl) flindersine 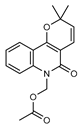 299.1158 |
G. parviflora (leaves) | UV, IR, 2D NMR and MS | Banbury et al. 2015 [12] |
Anti-inflammatory [12], collagen III suppression [43] |
| 36 haplaphine 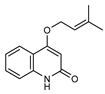 229.1103 |
G. parviflora (leaves) G. balansae (bark) |
UV, IR, 2D NMR and MS (G.p) 1H NMR, IR, UV, and MS (G.b) |
Banbury et al. 2015; Mitaku et al. 1985 [12,28] |
Anti-inflammatory, cytotoxic [12] |
| 37 4-methoxy N-methyl-2-quinolone 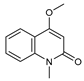 189.0790 |
G. balansae (bark) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Antimicrobial against MRSA, IC50 8.0 µM [76] |
| 38 geibalansine 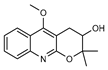 259.1208 |
G. balansae (leaves) | Chemical synthesis/ derivatization, 1H NMR, IR, UV, and MS | Ahond et al. 1979 [27] |
Antispasmodic [77] |
| 39 O-acetyl geibalansine 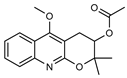 301.1314 |
G. balansae (leaves) | Chemical derivatization, 1H NMR, IR, UV, and MS | Ahond et al. 1979 [27] |
No activity reported to date. |
| 40 geijedimerine 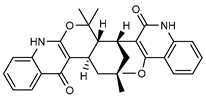 470.2206 |
G. balansae (leaves) | Chemical derivatization, 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
No activity reported to date. |
| 41 hordenine 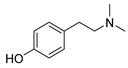 165.1154 |
G. balansae (leaves) | 1H NMR, IR, UV, and MS | Ahond et al. 1979 [27] |
Diuretic, disinfectant, antihypotensive agent. Used for treatment of dysentery. Antifeedant for grasshoppers. [65] |
| (Key: G.l – G. linearifolia G.p - G. parviflora, G.s – G. salicifolia) | ||||
| Compound and Exact mass (Da) | Source | Method of identification | Reference | Reported pharmacological activity of compound (various sources) |
| 42 (E)-β-ocimene 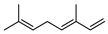 136.1252 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] |
Anticonvulsant, antifungal, antitumor, plant pest resistance and attraction of plant pollinators (semiochemical) [79] |
| 43 (Z)-β-ocimene 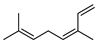 136.1252 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS |
Brophy and Goldsack 2005 [18] |
Anticonvulsant, antifungal, antitumor, plant pest resistance and attraction of plant pollinators (semiochemical) [79] |
| 44 myrcene 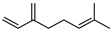 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] |
Sedative, muscle relaxant, anti-inflammatory, analgesic, anti-tumour, antioxidant, psychotropic, antibiotic, antimutagenic [80,81] |
| 45 limonene 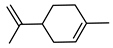 136.1252 |
G. salicifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] |
Anxiolytic, anti-carcinogenic [80] |
| 46 α-terpinene  136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] |
Antioxidant, antimicrobial, acetylcholinesterase inhibition, sedative [81,82] |
| 47 γ-terpinene 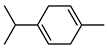 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] |
Antioxidant, antimicrobial, acetylcholinesterase inhibition, antinociceptive, anti-inflammatory [82,83,84] |
| 48 terpinolene 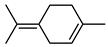 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS |
Brophy and Goldsack 2005 [18] | Antioxidant, antimicrobial, larvicide, insecticide [82,85] |
| 49 α-pinene 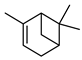 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
Chemical derivatization (G.p) GC-MS (G.s) |
Penfold 1930 [20] Brophy and Goldsack 2005 [18] |
Anti-inflammatory, anti-tumour [80] |
| 50 β-pinene 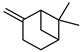 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-inflammatory, anti-tumour [80] |
| 51 camphene  136.1252 |
G. parviflora (leaves) | Chemical derivatization | Penfold 1930 [20] | Antioxidant [86] |
| 52 sabinene 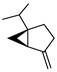 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Antioxidant, anti-inflammatory [87,88] |
| 53 α-phellandrene 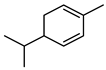 136.1252 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antinociceptive, hyperthermic, promotes immune response, anti-cancer, antimicrobial, fungicide, pesticide [89] |
| 54 β-phellandrene 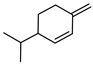 136.1252 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Acetylcholinesterase inhibitor, antifungal, expectorant [90,91] |
| 55 p-cymene 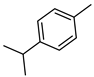 134.1096 |
G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Antioxidant, anti-inflammatory, anti-cancer, antimicrobial [92] |
| 56 citronellyl acetate 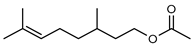 196.1619 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Pro-apoptotic activity in HepG2, fungicide, larvicide, bactericide, insect repellent/insecticide, antinociceptive [93] |
| 57 geranyl acetate 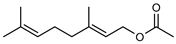 196.1463 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Anti-cancer, antifungal [94,95] |
| 58 neryl acetate 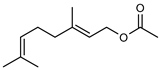 196.1463 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Fragrance and flavouring agent, strengthens skin barrier function [65,96] |
| 59 nerol 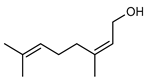 154.1357 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antimicrobial [97] |
| 60 geraniol 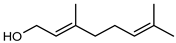 154.1357 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antimicrobial [97] |
| 61 linalool 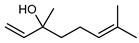 154.1357 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anxiolytic [98], antibacterial [99], anti-inflammatory [100] |
| 62 α-terpineol 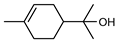 154.1357 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Antioxidant, anti-cancer, anticonvulsant, antiulcer, antihypertensive, antinociceptive, enhances skin penetration, insecticidal properties [101] |
| 63 terpinen-4-ol 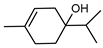 154.1357 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-inflammatory [102], antifungal [103], anti-cancer [104,105], antibacterial [106] |
| 64 1,8-cineole (eucalyptol) 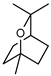 154.1357 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-inflammatory [107], antioxidant, analgesic [108], antifungal [103] |
| 65 camphor 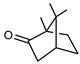 152.1201 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Sadgrove et al. 2014 [24] | Insecticidal, antimicrobial, antiviral, anticoccidial, antinociceptive, anti-cancer, antitussive, skin penetration enhancer [109] |
| 66 borneol 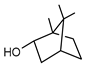 154.1357 |
G. salicifolia (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Enhances membrane permeability, antibacterial, antifungal, antispasmodic, choleretic, acesodyne, sedative [110,111] |
| 67 azulene 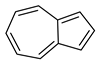 128.0626 |
G. parviflora (leaves) | Chemical derivatization | Penfold 1930 [20] | Anti-inflammatory [112] |
| 68 pregeijerene 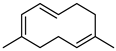 162.1408 |
G. salicifolia (leaves) G. parviflora (leaves) |
Chemical derivatization, degradative analysis, and UV | Jones and Sutherland 1968 [113] | Antifeedant, oviposition deterrence [114] |
| 69 cogeijerene 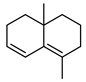 162.1408 |
G. salicifolia (leaves) G. parviflora (leaves) |
Chemical derivatization, degradative analysis, and UV (G.s) Chemical derivatization, degradative analysis, IR, and UV (G.p) |
Jones and Sutherland 1968 [113] Gough et al. 1961 [115] |
No activity reported to date. |
| 70 geijerene 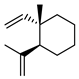 162.1408 |
G. parviflora (leaves) G. salicifolia (leaves) |
Combustion analysis, chemical derivatization, degradative analysis, IR (G.p) GC-MS (G.s) |
Penfold 1930 [20] Sutherland 1964 [116] Brophy and Goldsack 2005 [18] |
Antifeedant, oviposition deterrence [114] |
| 71 viridiflorene (ledene) 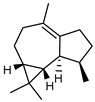 204.1878 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antifungal [117] |
| 72 α-selinene 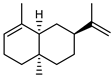 204.1878 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | No activity reported to date. |
| 73 β-selinene 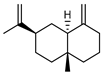 204.1878 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | No activity reported to date. |
| 74 selina-3, 7(11)-diene 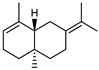 204.1878 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | No activity reported to date. |
| 75 germacrene B 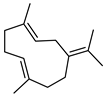 204.1878 |
G. salicifolia (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Antimicrobial activity against Gram negative bacteria [118] |
| 76 germacrene D 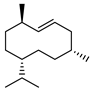 204.1878 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] Sadgrove et al. 2014 [24] |
Anti proliferative, scavenging activity towards the ABTS radical, antibacterial, antifungal, insecticidal, repels herbivores, attracts pollinators [119,120] |
| 77 bicyclogermacrene 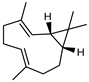 204.1878 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Larvicidal activity [121] |
| 78 α-bergamotene 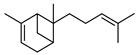 204.1878 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antifeedant [122] |
| 79 δ-cadinene 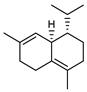 204.1878 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Acaricidal, antiproliferative and apoptotic [123,124] |
| 80 β-elemene 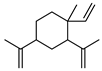 204.1878 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-cancer, antineoplastic, reproductive toxicity [125,126] |
| 81 γ-elemene 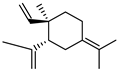 204.1878 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Larvicidal activity [127] |
| 82 α-caryophyllene (humulene) 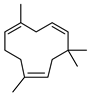 204.1878 |
G. salicifolia (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Antibacterial, anti-inflammatory, antitumor, analgesic [128,129,130] |
| 83 β-caryophyllene 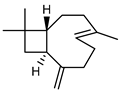 204.1878 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-inflammatory, analgesic, antimalarial, antifungal, antibacterial, anti-tumour [80,131] |
| 84 α-santalene 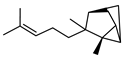 204.1878 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Insect repellent, semiochemical [122] |
| 85 aromadendrene 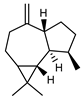 204.1878 |
G. parviflora (leaves) G. linearifolia (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] Sadgrove et al. 2014 [24] |
Antibacterial (MRSA and drug resistant pathogens) [132] |
| 86 (E,E)-α-farnesene 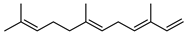 204.1878 |
G. parviflora (leaves) G. linearifolia (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Semiochemical, antibacterial, anticariogenic anti-cancer, anti-plasmodial, hepatoprotective, antioxidant, anti-inflammatory, antifungal [133,134] |
| 87 (E,E)-farnesol 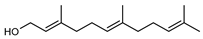 222.1983 |
G. linearifolia (leaves) | GC-MS |
Brophy and Goldsack 2005 [18] | Antibacterial [135], antifungal [136] |
| 88 guaiol 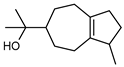 222.1983 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Sadgrove et al. 2014 [24] | Insecticide, antimicrobial, acaricidal, anti-cancer, [137,138,139] |
| 89 elemol 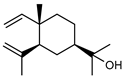 222.1983 |
G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Antifungal [140] |
| 90 palustrol 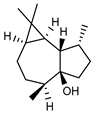 222.1983 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Semiochemical [141] |
| 91 ledol 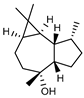 222.1983 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | Antifungal, toxic CNS effects, antitussive, expectorant [142,143] |
| 92 globulol 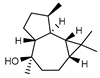 222.1983 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antimicrobial [144] |
| 93 epi-globulol 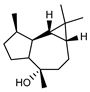 222.1983 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antimicrobial, semiochemical [145] |
| 94 τ-cadinol 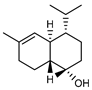 222.1983 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Antitrypanosomal, smooth muscle relaxant, inhibits effects of cholera toxins [146,147] |
| 95 α-eudesmol 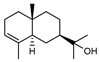 222.1983 |
G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Antitrypanosomal, anti-cancer, anti-neurogenic inflammation[148,149,150] |
| 96 β-eudesmol 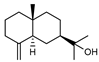 222.1983 |
G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-cancer, sedative, hepatoprotective, anti- inflammatory, diuretic, inhibits platelet aggregation, insect repellent, anti-allergy [65,149,151,152,153,154] |
| 97 γ-eudesmol 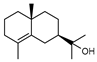 222.1983 |
G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-cancer [149] |
| 98 viridiflorol 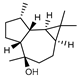 222.1983 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Anti-mycobacterial, anti-inflammatory, antioxidant [155] |
| 99 (E,E)-farnesal 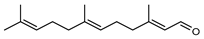 220.1827 |
G. linearifolia (leaves) | GC-MS | Brophy and Goldsack 2005 [18] | Semiochemical [156] |
| 100 caryophyllene oxide 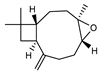 220.1827 |
G. linearifolia (leaves) G. salicifolia (leaves) G. parviflora (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] | Anti-cancer, analgesic [131] |
| 101 caryophylla-4(12), 8(13)-dien-5-ol 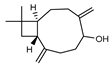 220.1827 |
G. parviflora (leaves) | GC-MS | Sadgrove et al. 2014 [24] | No activity reported to date. |
| 102 spathulenol 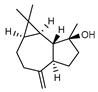 220.1827 |
G. linearifolia (leaves) G. parviflora (leaves) G. salicifolia (leaves) |
GC-MS | Brophy and Goldsack 2005 [18] Sadgrove et al. 2014 [24] |
Antioxidant, anti-inflammatory, antiproliferative, antimycobacterial, antimicrobial [157,158] |
| 103 eremophilone 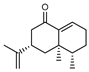 218.1670 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] Sadgrove et al. 2014 [24] |
Cytotoxic, insecticidal, insect repellent, antifeedant (against termites)[159,160] |
| 104 cyclocolorenone 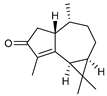 218.1670 |
G. parviflora (leaves) | GC-MS | Brophy and Goldsack 2005 [18] Sadgrove et al. 2014 [24] |
Antifeedant, antimicrobial, allelopathic, anti-inflammatory, insect repellent [161] |
| 105 β-sitosterol 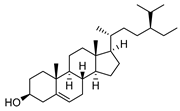 414.3861 |
G. salicifolia (leaves) | Melting point and IR | Ritchie et al. 1968 [35] |
Anti-cancer [162], anthelminthic, antimutagenic [163] |
| (Key: G.l – G. linearifolia G.p - G. parviflora, G.s – G. salicifolia) | ||||
| Compound and Exact mass (Da) | Source | Method of identification | Reference | Reported pharmacological activity of compound (various sources) |
| 106 brevifolin (xanthoxylin) 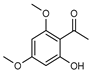 196.0735 |
G. parviflora (leaves) G. balansae (bark) G. salicifolia (leaves) |
GC-MS (G.p) 1H NMR, IR, UV, and MS (G.b) Melting point (G.s) |
Brophy and Goldsack 2005 [18] Mitaku et al. 1985 [28] Penfold 1930 [20] |
Antioxidant, hepatoprotective [164] antibacterial, antifungal, antinociceptive, antiedematogenic and antispasmodic [165] |
| 107 elemicin  208.1099 |
G. parviflora (leaves) | GC-MS | [11] | Psychotropic, antimicrobial, antioxidant, acetylcholinesterase inhibitor, antiviral [11,166,167] |
| 108 3,5,8,4’-tetrahydroxy-6,7-dimethoxyflavone 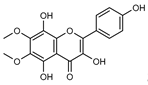 346.0689 346.0689 |
G. parviflora (leaves) | 1H and 13C NMR | [12] | No activity reported to date. |
| 109 2-phenylethyl isobutyrate 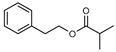 192.1150 |
G. parviflora (leaves) | 1H and 13C NMR | [12] | Odorant [168] |
| 110 isoamyl isovalerate 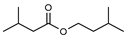 172.1463 |
G. parviflora (leaves) | 1H and 13C NMR | [12] | Flavouring/odorant [169] |
| 111 cis-jasmone 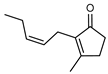 164.1201 |
G. parviflora (leaves) | GC-MS | [24] | Semiochemical [170] |
| 112 methyl eugenol 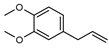 178.0993 |
G. parviflora (leaves) | GC-MS | [24] | Attracts pollinator insects (semiochemical) [171] |
| 113 phthalic acid 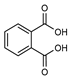 166.0266 166.0266 |
G. parviflora (leaves) | GC-MS | [24] | Endocrine disruptor [172] |
| 114 vanillin 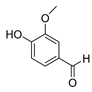 152.0473 |
G. balansae (wood) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Flavouring, pharmaceutical excipient, antioxidant, inhibits lipid peroxidation [65] |
| 115 methyl syringate 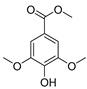 212.0685 |
G. balansae (wood) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Anti-diabetic, TRPA1 agonist [173,174] |
| 116 methyl ferulate 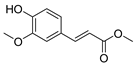 208.0736 |
G. balansae (wood) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Inhibits COX-2 expression, blocks p-p38 and p-JNK in primary bone marrow derived-macrophages [175,176] |
| 117 ethyl ferulate 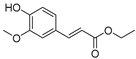 222.0892 |
G. balansae (wood) | 1H NMR, IR, UV, and MS | Mitaku et al. 1985 [28] |
Antioxidative, antiapoptotic, antirheumatic, neuroprotective and anti-inflammatory [177,178] |
| Type of activity | No. compoundso in Geijera | No. compounds in G. balansae | No. compounds in G. parviflora | No. compounds in G. salicifolia | No. compounds in G. linearifolia |
|---|---|---|---|---|---|
| Acetylcholinesterase inhibition | 7 | 1 | 6 | 6 | - |
| Anti-cancer | 41 | 4 | 32 | 26 | 13 |
| Anticonvulsant | 4 | 1 | 3 | 3 | 2 |
| Antifungal | 25 | 5 | 16 | 13 | 9 |
| Antimicrobial | 45 | 9 | 29 | 19 | 12 |
| Antioxidant | 20 | 4 | 15 | 11 | 2 |
| Increase of membrane permeability | 3 | - | 2 | 3 | - |
| Monoamine oxidase B inhibition | 1 | - | 1 | 1 | - |
| Muscle relaxant | 5 | 2 | 2 | 3 | 1 |
| Osteogenic | 3 | 1 | 2 | - | - |
| Plant pest resistance/semiochemical/ insecticide |
26 | 1 | 21 | 14 | 9 |
| Psychoactive | 3 | - | 3 | 2 | - |
| Reduction of anxiety | 7 | - | 5 | 5 | 2 |
| Reduction of inflammation |
38 | 7 | 28 | 17 | 6 |
| Reduction of pain | 12 | 1 | 8 | 10 | 3 |
| (Sources: P – G. parviflora, S – G. salicifolia, L – G. linearifolia, B – G. balansae) | |||
| umbelliferone 1 S | xanthoxyletin 19 B | sabinene 52 P,S | β-caryophyllene 83 P,S,L |
| 6’-dehydromarmin 5 P | dictamine 25 B | α-phellandrene 53 P | (E,E)-α-farnesene 86 P,S,L |
| (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin 7 P | skimmianine 26 S,B | citronellyl acetate 56 L | α-eudesmol 95 P,S,L |
| parvifloranine A 8 P | flindersine 30 P,B | linalool 61 P,S,L | β-eudesmol 96 P,S,L |
| scoparone 11 P | N-(acetoxymethyl) flindersine 35 P | α-terpineol 62 P,S | viridiflorol 98 P |
| suberosin 12 P | haplaphine 36 P,B | terpinen-4-ol 63 P,S | caryophyllene oxide 100 P,S,L |
| dehydrogeijerin 13 P,S | myrcene 44 P,S | 1,8 cineole 64 P,S | spathulenol 102 P,S,L |
| 6-(methoxyl) geiparvarin 14 P | γ-terpinene 47 P,S | camphor 65 P,S | cyclocolorenone 104 P |
| osthole 15 P | α-pinene 49 P,S | borneol 66 S | brevifolin (xanthoxylin) 106 P,S,B |
| angelicin (isopsoralen) 16 P | β-pinene 50 P,S | azulene 67 P | methyl ferulate 116 B |
| luvangetin 18 B | p-cymene 55 P,S | α-caryophyllene (humulene) 82 S | ethyl ferulate 117 B |
| (Sources: P – G. parviflora, S – G. salicifolia, L – G. linearifolia, B – G. balansae) | |||
| umbelliferone 1 S | zanthobungeanine 33 B | nerol 59 L | guaiol 88 P,S |
| auraptene 3 P | 4-methoxy N-methyl-2-quinolone 37 B | geraniol 60 L | elemol 89 P,S |
| scoparone 11 P | hordenine 41 B | linalool 61 P,S,L | ledol 91 P |
| osthole 15 P | (E)-β-ocimene 42 P,S,L | α-terpineol 62 P,S | globulol 92 P |
| angelicin (isopsoralen) 16 P | (Z)-β-ocimene 43 P,S,L | terpinen-4-ol 63 P,S | epi-globulol 93 P |
| xanthyletine 17 P | myrcene 44 P,S | 1,8 cineole 64 P,S | τ-cadinol 94 L |
| luvangetin 18 B | α-terpinene 46 P,S | camphor 65 P,S | α-eudesmol 95 P,S,L |
| xanthoxyletin 19 B | γ-terpinene 47 P,S | borneol 66 S | viridiflorol 98 P |
| 11’-hexadecanoyl anthranillic acid 20 P | terpinolene 48 P,S | viridiflorene (ledene) 71 L | spathulenol 102 P,S,L |
| 9’-hexadecenoyl anthranillic acid 21 P | camphene 51 P | germacrene B 75 S | cyclocolorenone 104 P |
| 7’-hexadecanoyl anthranillic acid 22 P | sabinene 52 P,S | germacrene D 76 P,S,L | brevifolin (xanthoxylin) 106 P,S,B |
| hexadecanoyl anthranillic acid 24 P | α-phellandrene 53 P | α-caryophyllene (humulene) 82 S | elemicin 107 P |
| dictamnine 25 B | β-phellandrene 54 P,S | β-caryophyllene 83 P,S,L | ethyl ferulate 117 B |
| γ-fagarine 27 S, B | p-cymene 53 P,S | aromadendrene 85 P,S,L | |
| platydesmine 28 S,B | citronellyl acetate 54 L | (E,E)-α-farnesene 86 P,S,L | |
| flindersine 30 P,B | geranyl acetate 57 L | (E,E)-farnesol 87 L | |
| (Sources: P – G. parviflora, S – G. salicifolia, L – G. linearifolia, B – G. balansae) | |||
|
Acetylcholinesterase inhibitors |
Anxiolytics and sedatives |
Muscle relaxants and anticonvulsants |
Psychoactive compounds |
| geijerin 10 P,S | osthole 15 P | xanthoxyletin 19 B | geiparvarin 2 P,S |
| dehydrogeijerin 13 P,S | myrcene 44 P,S | geibalansine 38 B | myrcene 44 P,S |
| skimmianine 26 S,B | limonene 45 S | (E)-β-ocimene 42 P,S,L | elemicin 107 P |
| α-terpinene 46 P,S | α-terpinene 46 P,S | (Z)-β-ocimene 43 P,S,L | |
| γ-terpinene 47 P,S | linalool 61 P,S,L | myrcene 44 P,S | |
| β-phellandrene 54 P,S | borneol 66 S | α-terpineol 62 P,S | |
| elemicin 107 P | β-eudesmol 96 P,S,L | borneol 66 S | |
| τ-cadinol 94 L | |||
| brevifolin (xanthoxylin) 106 P,S | |||
| (Sources: P – G. parviflora, S – G. salicifolia, L – G. linearifolia, B – G. balansae) | |||
| umbelliferone 1 S | dictamnine 25 B | p-cymene 55 P,S | β-caryophyllene 83 P,S,L |
| geiparvarin 2 P,S | skimmianine 26 S, B | citronellyl acetate 56 L | (E,E)-α-farnesene 86 P,S,L |
| auraptene 3 P | haplaphine 36 P,B | geranyl acetate 57 L | guaiol 88 P,S |
| 6’dehydromarmin 5 P | (E)-β-ocimene 42 P,S,L | α-terpineol 62 P,S | α-eudesmol 95 P,S,L |
| 2’,3’- dihydrogeiparvarin 6 P,S | (Z)-β-ocimene 43 P,S,L | terpinen-4-ol 63 P,S | β-eudesmol 96 P,S,L |
| (R)-6-O-(4-geranyloxy-2-hydroxy) cinnamoylmarmin 7 P | myrcene 44 P,S | camphor 65 P,S | γ-eudesmol 97 P,S,L |
| scoparone 11 P | limonene 45 S | germacrene D 76 P,S,L | caryophyllene oxide 100 P,S,L |
| 6-(methoxyl) geiparvarin 14 P | α-pinene 49 P,S | δ-cadinene 79 P | spathulenol 102 P,S,L |
| osthole 15 P | β-pinene 50 P,S | β-elemene 80 P,S,L | eremophilone 103 P |
| angelicin (isopsoralen) 16 P | α-phellandrene 53 P | α-caryophyllene (humulene) 82 S | β-sitosterol 105 S |
| xanthoxyletin 19 B | |||
| (Sources: P – G. parviflora, S – G. salicifolia, L – G. linearifolia, B – G. balansae) | |||
| Insecticides | Semiochemicals | Antifeedants | Oviposition deterrents |
| terpinolene 48 P,S | (E)-β-ocimene 42 P,S,L | umbelliferone 1 S | pregeijerene 68 S |
| α-phellandrene 53 P | (Z)-β-ocimene 43 P,S,L | hordenine 41 B | geijerene 70 S,P |
| citronellyl acetate 56 L | α-santalene 84 P | pregeijerene 68 S | |
| α-terpineol 62 P,S | (E,E)-α-farnesene 86 P,S,L | geijerene 70 S,P | |
| camphor 65 P,S | palustrol 90 L | α-bergamotene 78 P | |
| germacrene D 73 P,S,L | epi-globulol 93 P | eremophilone 103 P | |
| bicyclogermacrene 77 P,S,L | β-eudesmol 96 P,S,L | cyclocolorenone 104 P | |
| δ-cadinene 79 P | (E,E)-farnesal 99 L | ||
| γ-elemene 81 P,S | cis-jasmone 111 P | ||
| guaiol 88 P,S | methyl eugenol 112 P | ||
| β-eudesmol 96 P,S,L | |||
| eremophilone 103 P | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





