Submitted:
21 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Analytical methods for the analysis of organic UVFs
2.1. Benzophenones
2.1.1. Environmental matrices
2.1.2. Biota marine matrices
2.2. Dibenzoyl methane derivatives
2.2.1. Environmental and biota marine matrices
2.3. Benzotriazoles
2.3.1. Environmental matrices
2.3.2. Biota marine matrices
2.4. Cinnamates
2.4.1. Environmental and biota marine matrices
2.5. Salicylates
2.5.1. Environmental and biota marine matrices
2.6. Crylenes
2.6.1. Environmental and biota marine matrices
2.7. p-Aminobenzoic acid (PABA) derivatives
2.7.1. Environmental and biota marine matrices
2.8. Camphor derivatives
2.8.1. Environmental and biota marine matrices
2.9. Triazine derivatives
2.9.1. Environmental and biota marine matrices
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022. [CrossRef] [PubMed]
- Chen, L.L.; Wang, S.Q. Nanotechnology in Photoprotection. In Nanoscience in Dermatology; 2016 ISBN 9780128029459.
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.; Klotz, K.; Meyer, S.; Uter, W.; Hof, K.; Greiner, A.; Göen, T.; Drexler, H. Systemic Availability of Lipophilic Organic UV Filters through Dermal Sunscreen Exposure. Environ. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in Analytical Methods and Occurrence of Organic UV-Filters in the Environment - A Review. Sci. Total Environ. 2015. [CrossRef] [PubMed]
- Wang, J.; Xiao, X.; Chen, T.; Liu, T.; Tao, H.; He, J. High-Performance Liquid Chromatography - Ultraviolet Method for the Determination of Total Specific Migration of Nine Ultraviolet Absorbers in Food Simulants Based on 1,1,3,3-Tetramethylguanidine and Organic Phase Anion Exchange Solid Phase Extraction To. J. Chromatogr. A 2016. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tian, M.; Feng, W.; He, H.; Wang, Y.; Yang, L. Sensitive Detection of Benzophenone-Type Ultraviolet Filters in Plastic Food Packaging Materials by Sheathless Capillary Electrophoresis–Electrospray Ionization–Tandem Mass Spectrometry. J. Chromatogr. A 2019. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, L.H.; Wang, F.; Gao, C.J.; Chen, D.; Guo, Y. Several Environmental Endocrine Disruptors in Beverages from South China: Occurrence and Human Exposure. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, Y.; Zhang, Y.; Feng, X.; Zhang, F. Synthesis of a Magnetic Covalent Organic Framework for Extraction and Separation of Ultraviolet Filters in Beverage Samples. Food Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV Filter Concentrations in Marine Mussels from French Coastal Regions. Sci. Total Environ. 2012. [Google Scholar] [CrossRef]
- Giraldo, A.; Montes, R.; Rodil, R.; Quintana, J.B.; Vidal-Liñán, L.; Beiras, R. Ecotoxicological Evaluation of the UV Filters Ethylhexyl Dimethyl P-Aminobenzoic Acid and Octocrylene Using Marine Organisms Isochrysis Galbana, Mytilus Galloprovincialis and Paracentrotus Lividus. Arch. Environ. Contam. Toxicol. 2017. [Google Scholar] [CrossRef]
- Caloni, S.; Durazzano, T.; Franci, G.; Marsili, L. Sunscreens’ Uv Filters Risk for Coastal Marine Environment Biodiversity: A Review. Diversity 2021, 13, 374. [Google Scholar] [CrossRef]
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and Toxicological Effects of Uv-Filters on Marine Species. In Handbook of Environmental Chemistry; 2020.
- Cunha, S.C.; Trabalón, L.; Jacobs, S.; Castro, M.; Fernandez-Tejedor, M.; Granby, K.; Verbeke, W.; Kwadijk, C.; Ferrari, F.; Robbens, J.; et al. UV-Filters and Musk Fragrances in Seafood Commercialized in Europe Union: Occurrence, Risk and Exposure Assessment. Environ. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV Filters Bioaccumulation in Fish from Iberian River Basins. Sci. Total Environ. 2015. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Zafra-Gómez, A.; Hidalgo, F.; Ibáñez-Yuste, A.J.; Alonso, E.; Vilchez, J.L. Multi-Residue Analysis of 36 Priority and Emerging Pollutants in Marine Echinoderms (Holothuria Tubulosa) and Marine Sediments by Solid-Liquid Extraction Followed by Dispersive Solid Phase Extraction and Liquid Chromatography–Tandem Mass Spectrometry Anal. Talanta 2017. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Petersen-Thiery, M. Sustainable Sunscreens: A Challenge between Performance, Animal Testing Ban, and Human and Environmental Safety. In Handbook of Environmental Chemistry; 2020.
- Valle-Sistac, J.; Molins-Delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Silvia Díaz-Cruz, M. Determination of Parabens and Benzophenone-Type UV Filters in Human Placenta: First Description of the Existence of Benzyl Paraben and Benzophenone-4. Environ. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, A.; Egeberg, D.L.; Almstrup, K.; Petersen, J.H.; Dissing, S.; Skakkebæk, N.E. EDC IMPACT: Chemical UV Filters Can Affect Human Sperm Function in a Progesterone-like Manner. Endocr. Connect. 2018. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; Barceló, D. Chemical Analysis and Ecotoxicological Effects of Organic UV-Absorbing Compounds in Aquatic Ecosystems. TrAC - Trends Anal. Chem. 2009. [CrossRef]
- Lukić, J.; Đurkić, T.; Onjia, A. Dispersive Liquid–Liquid Microextraction and Monte Carlo Simulation of Margin of Safety for Octocrylene, EHMC, 2ES, and Homosalate in Sunscreens. Biomed. Chromatogr. 2023. [Google Scholar] [CrossRef] [PubMed]
- The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 11th Revision, 30-31 March 2021, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021. [CrossRef]
- Raslan, R.; Hassim, M.H.; Chemmangattuvalappil, N.G.; Ng, D.K.S.; Ten, J.Y. Safety and Health Risk Assessment Methodology of Dermal and Inhalation Exposure to Formulated Products Ingredients. Regul. Toxicol. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Chisvert, A.; Benedé, J.L.; Salvador, A. Current Trends on the Determination of Organic UV Filters in Environmental Water Samples Based on Microextraction Techniques – A Review. Anal. Chim. Acta 2018. [CrossRef]
- Adoamnei, E.; Mendiola, J.; Moñino-García, M.; Vela-Soria, F.; Iribarne-Durán, L.M.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary Concentrations of Benzophenone-Type Ultra Violet Light Filters and Reproductive Parameters in Young Men. Int. J. Hyg. Environ. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Narloch, I.; Wejnerowska, G. An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples. Molecules 2021. [CrossRef] [PubMed]
- European Commission Regulation (EC) No 1223/2009. Off. J. Eur. Union 2009.
- Health Products and Food Branch- Primary Sunscreen Monograph. Heal. Canada 2022.
- US Food and Drug Administration Sunscreen Drug Products for Over-The-Counter Human Use; Proposal to Amend and Lift Stay on Monograph. U.S. Dep. Heal. Hum. Serv. 2019.
- China Food and Drug Administration Safety and Technical Standards for Cosmetics 2022; 2022.
- Ministry of Health, L. and W. Standards for Cosmetics; Ministry of Health and Welfare Notification No. 331/2000. 2001.
- European Commission Regulation (EC) No 1176/2022. Off. J. Eur. Union 2022.
- Fivenson, D.; Sabzevari, N.; Qiblawi, S.; Blitz, J.; Norton, B.B.; Norton, S.A. Sunscreens: UV Filters to Protect Us: Part 2-Increasing Awareness of UV Filters and Their Potential Toxicities to Us and Our Environment. Int. J. Women’s Dermatology 2021. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J. V.; Taylor, R.; Kitzen, J.M. Sunscreen Bans: Coral Reefs and Skin Cancer. J. Clin. Pharm. Ther. 2019. [CrossRef] [PubMed]
- IARC IARC Handbooks of Cancer Prevention Volume 5 Sunscreens. IARC Press 2001.
- Krause, M.; Frederiksen, H.; Sundberg, K.; Jørgensen, F.S.; Jensen, L.N.; Nørgaard, P.; Jørgensen, C.; Ertberg, P.; Juul, A.; Drzewiecki, K.T.; et al. Presence of Benzophenones Commonly Used as UV Filters and Absorbers in Paired Maternal and Fetal Samples. Environ. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Klotz, K.; Hof, K.; Hiller, J.; Göen, T.; Drexler, H. Quantification of Prominent Organic UV Filters and Their Metabolites in Human Urine and Plasma Samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of Organic UV Filters: A Review of Environmental and Human Health Concern Studies. Sci. Total Environ. 2021. [CrossRef] [PubMed]
- Lorigo, M.; Mariana, M.; Cairrao, E. Photoprotection of Ultraviolet-B Filters: Updated Review of Endocrine Disrupting Properties. Steroids 2018. [CrossRef] [PubMed]
- Ekstein, S.F.; Hylwa, S. Sunscreens: A Review of UV Filters and Their Allergic Potential. Dermatitis 2023. [CrossRef] [PubMed]
- Vela-Soria, F.; Rodríguez, I.; Ballesteros, O.; Zafra-Gómez, A.; Ballesteros, L.; Cela, R.; Navalón, A. Simplified Matrix Solid Phase Dispersion Procedure for the Determination of Parabens and Benzophenone-Ultraviolet Filters in Human Placental Tissue Samples. J. Chromatogr. A 2014. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kojima, H.; Takeuchi, S.; Uramaru, N.; Sanoh, S.; Sugihara, K.; Kitamura, S.; Ohta, S. Metabolism of UV-Filter Benzophenone-3 by Rat and Human Liver Microsomes and Its Effect on Endocrine-Disrupting Activity. Toxicol. Appl. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; An, Y.; Wang, D.; Zhao, J.; Zhan, M.; Xu, W.; Lu, L.; Gao, Y. Concentrations of Bisphenols, Benzophenone-Type Ultraviolet Filters, Triclosan, and Triclocarban in the Paired Urine and Blood Samples from Young Adults: Partitioning between Urine and Blood. Chemosphere 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kannan, K. Characteristic Profiles of Benzonphenone-3 and Its Derivatives in Urine of Children and Adults from the United States and China. Environ. Sci. Technol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; Molins-Delgado, D.; Serra-Roig, M.P.; Kalogianni, E.; Skoulikidis, N.T.; Barceló, D. Personal Care Products Reconnaissance in EVROTAS River (Greece): Water-Sediment Partition and Bioaccumulation in Fish. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- EC Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Parliam. 2000. [CrossRef]
- Gago-Ferrero, P.; Mastroianni, N.; Díaz-Cruz, M.S.; Barceló, D. Fully Automated Determination of Nine Ultraviolet Filters and Transformation Products in Natural Waters and Wastewaters by On-Line Solid Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2013. [Google Scholar] [CrossRef]
- Força-Lima, M.; Pacheco, W.F.; Cassella, R.J. Evaluation of a Semi-Permeable Membrane Device (SPMD) for Passive Sampling of Solar Filters from Swimming Pool Waters and Determination by HPLC-DAD. J. Chromatogr. A 2019. [Google Scholar] [CrossRef]
- Horricks, R.A.; Tabin, S.K.; Edwards, J.J.; Lumsden, J.S.; Marancik, D.P. Organic Ultraviolet Filters in Nearshore Waters and in the Invasive Lionfish (Pterois Volitans) in Grenada, West Indies. PLoS One 2019. [Google Scholar] [CrossRef]
- Kharbouche, L.; Gil García, M.D.; Lozano, A.; Hamaizi, H.; Martínez Galera, M. Determination of Personal Care Products in Water Using UHPLC–MS after Solid Phase Extraction with Mesoporous Silica-Based MCM-41 Functionalized with Cyanopropyl Groups. J. Sep. Sci. 2020. [Google Scholar] [CrossRef]
- Mallappa Gopal, C.; Bhat, K.; Praveenkumarreddy, Y.; Shailesh; Kumar, V.; Basu, H.; Joshua, D.I.; Singhal, R.K.; Balakrishna, K. Evaluation of Selected Pharmaceuticals and Personal Care Products in Water Matrix Using Ion Trap Mass Spectrometry: A Simple Weighted Calibration Curve Approach. J. Pharm. Biomed. Anal. 2020. [CrossRef]
- Feitosa-Felizzola, J.; Temime, B.; Chiron, S. Evaluating On-Line Solid-Phase Extraction Coupled to Liquid Chromatography-Ion Trap Mass Spectrometry for Reliable Quantification and Confirmation of Several Classes of Antibiotics in Urban Wastewaters. J. Chromatogr. A 2007. [Google Scholar] [CrossRef]
- Batt, A.L.; Aga, D.S. Simultaneous Analysis of Multiple Classes of Antibiotics by Ion Trap LC/MS/MS for Assessing Surface Water and Groundwater Contamination. Anal. Chem. 2005. [Google Scholar] [CrossRef]
- Harron, D.W.G. ICH Harmonised Tripartite Guideline; Validation of Analytical Procedures; 2013.
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and Environmental Hazard of Organic UV Filters in Seawater and Wastewater from Gran Canaria Island (Canary Islands, Spain). Environ. Pollut. 2022. [Google Scholar] [CrossRef]
- Fisch, K.; Zhang, R.; Zhou, M.; Schulz-Bull, D.E.; Waniek, J.J. PPCPs - A Human and Veterinary Fingerprint in the Pearl River Delta and Northern South China Sea. Emerg. Contam. 2021. [Google Scholar] [CrossRef]
- Fenni, F.; Sunyer-Caldú, A.; Ben Mansour, H.; Diaz-Cruz, M.S. Contaminants of Emerging Concern in Marine Areas: First Evidence of UV Filters and Paraben Preservatives in Seawater and Sediment on the Eastern Coast of Tunisia. Environ. Pollut. 2022. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Fast Pressurized Liquid Extraction with In-Cell Purification and Analysis by Liquid Chromatography Tandem Mass Spectrometry for the Determination of UV Filters and Their Degradation Products in Sediments. Anal. Bioanal. Chem. 2011. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; López-Manía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Multiclass Determination of Sunscreen Chemicals in Water Samples by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2008. [Google Scholar] [CrossRef]
- Mokh, S.; Nassar, R.; Berry, A.; Khatib, M. El; Doumiati, S.; Taha, M.; Ezzeddine, R.; Al Iskandarani, M. Chromatographic Methods for the Determination of a Broad Spectrum of UV Filters in Swimming Pool Water. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Farenhorst, A. Quantitation of Canadian Organic Ultraviolet Filters Using Polarity Switching and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2023. [Google Scholar] [CrossRef] [PubMed]
- Votani, A.; Chisvert, A.; Giokas, D.L. On-Line Extraction Coupled to Liquid Chromatographic Analysis of Hydrophobic Organic Compounds from Complex Solid Samples—Application to the Analysis of UV Filters in Soils and Sediments. J. Chromatogr. A 2020. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.G.; Leme, G.M.; Cavalheiro, A.J.; Funari, C.S. Online Extraction Coupled to Liquid Chromatography Analysis (OLE-LC): Eliminating Traditional Sample Preparation Steps in the Investigation of Solid Complex Matrices. Anal. Chem. 2016. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Pino, Á.S. Del; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Assessment of Anthropogenic Pollution by UV Filters Using Macrophytes as Bioindicators. Sci. Total Environ. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Rodríguez, A.; Rodrigo Sanz, M.; Betancort Rodríguez, J.R. Occurrence of Eight UV Filters in Beaches of Gran Canaria (Canary Islands). An Approach to Environmental Risk Assessment. Chemosphere 2015. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.T.; Bavcon Kralj, M.; Polyakova, O. V.; Detenchuk, E.A.; Pokryshkin, S.A.; Trebše, P. Identification of Avobenzone By-Products Formed by Various Disinfectants in Different Types of Swimming Pool Waters. Environ. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Seyer, A.; Mlynek, F.; Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Investigations on the Uptake and Transformation of Sunscreen Ingredients in Duckweed (Lemna Gibba) and Cyperus Alternifolius Using High-Performance Liquid Chromatography Drift-Tube Ion-Mobility Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2020. [Google Scholar] [CrossRef]
- Parks, N. UV-Stabilizing Chemicals Contaminating Japan’s Marine Environment. Environ. Sci. Technol. 2009. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Du, T.; Kou, H.; Du, X.; Lu, X. Determination of Six Benzotriazole Ultraviolet Filters in Water and Cosmetic Samples by Graphene Sponge-Based Solid-Phase Extraction Followed by High-Performance Liquid Chromatography. Anal. Bioanal. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Di, S.; Su, X.; Wang, J.; Ning, T.; Yang, H.; Zhu, S. Preparation of Beta-Cyclodextrin Based Nanocomposite for Magnetic Solid-Phase Extraction of Organic Ultraviolet Filters. J. Chromatogr. A 2022. [Google Scholar] [CrossRef]
- Mulliken, J.S.; Russak, J.E.; Rigel, D.S. The Effect of Sunscreen on Melanoma Risk. Dermatol. Clin. 2012. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, K.; Yamamoto, H.; Kamata, R.; Shiraishi, F.; Koda, T.; Morita, M. Estrogenic Activity of 37 Components of Commercial Sunscreen Lotions Evaluated by in Vitro Assays. Toxicol. Vitr. 2005. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Fent, K. Multiple Hormonal Activities of UV Filters and Comparison of in Vivo and in Vitro Estrogenic Activity of Ethyl-4-Aminobenzoate in Fish. Aquat. Toxicol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; He, Y.; Liang, B.; Li, J.; Luo, D.; Chen, H.; Liu, L.Y.; Guo, Y.; Zeng, L. Identification of Triazine UV Filters as an Emerging Class of Abundant, Ubiquitous Pollutants in Indoor Dust and Air from South China: Call for More Concerns on Their Occurrence and Human Exposure. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Mánez, M.; Andreu, A.; Hiraldo, F.; Eljarrat, E.; Barceló, D.; Díaz-Cruz, M.S. A Potential New Threat to Wild Life: Presence of UV Filters in Bird Eggs from a Preserved Area. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Moualek, F.; Babin, M.; Parent, G.J.; Ponton, D.E.; Senay, C.; Amyot, M.; Robert, D.; Lu, Z. Organic UV Absorbents in the Deepwater Redfish (Sebastes Mentella) from the St. Lawrence Estuary and Gulf: Distribution and Human Health Risk Assessment. Sci. Total Environ. 2024, 906. [Google Scholar] [CrossRef]
- Núñez, O.; Gallart-Ayala, H.; Martins, C.P.B.; Lucci, P. New Trends in Fast Liquid Chromatography for Food and Environmental Analysis. J. Chromatogr. A 2012. [Google Scholar] [CrossRef]
| Classes | Chemical name | INCI name | Code | Chemical structure |
|---|---|---|---|---|
| BENZOPHENONES | Benzophenone | Benzophenone | BP | 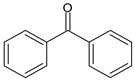 |
| 2,4-Dihydroxybenzophenone | Benzophenone-1 | BP-1 | 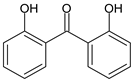 |
|
| 2,2',4,4'-Tetrahydroxy benzophenone |
Benzophenone-2 | BP-2 | 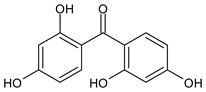 |
|
| 2-Hydroxy-4-methoxy benzophenone (Oxybenzone) |
Benzophenone-3 | BP-3 | 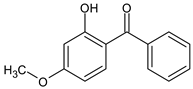 |
|
| 2-Hydroxy-4-methoxy benzophenone-5-sulfonic acid (Sulisobenzone) |
Benzophenone-4 | BP-4 | 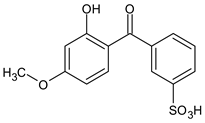 |
|
| 2,2'-Dihydroxy-4-methoxybenzophenone | Benzophenone-8 | BP-8 | 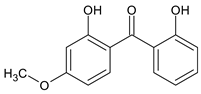 |
|
| 2,2'-Dihydroxy-4,4'-dimethoxybenzophenone-5,5'-disulfonic acid disodium salt | Benzophenone-9 | BP-9 |  |
|
| 4-Hydroxybenzophenone | 4-HBP | 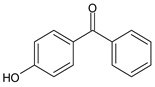 |
||
| 4,4′-Dihydroxybenzophenone | 4,4′-DHBP | 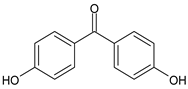 |
||
| DIBENZOYL METHANE DERIVATIVES |
4-tert-Butyl-4'-methoxy- dibenzoylmethane |
Avobenzone | AVO | 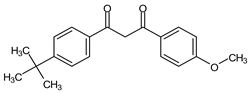 |
| BENZOTRIAZOLES | 1H--Benzotriazole | Benzotriazole | 1HBT | 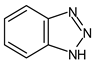 |
| 5-Methyl-1H-benzotriazole | MeBT | 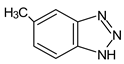 |
||
| 5,6-Dimethyl-1H-benzotriazole | DMeBT | 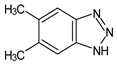 |
||
| 2-(5-tert-Butyl-2-hydroxyphenyl) benzotriazole | TBHPBT | 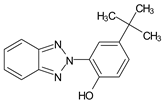 |
||
| 2-(2'-Hydroxy-5'-methylphenyl) benzotriazole | Drometizole | UV-P | 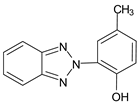 |
|
| 2-(benzotriazol-2-yl)-4-methyl-6-[2-methyl-3-[methyl-bis(trimethylsilyloxy)silyl] propyl] phenol |
Drometizole trisiloxane | DTS | 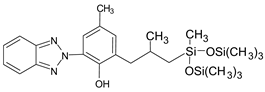 |
|
| 2-[2-Hydroxy-3,5-bis (1,1-dimethylbenzyl) phenyl] benzotriazole |
UV-234 | 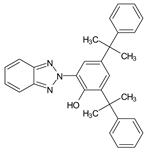 |
||
| 2-(2′-Hydroxy-3′-tert-butyl- 5′-methylphenyl)- 5-chlorobenzotriazole |
Bumetrizole | UV-326 | 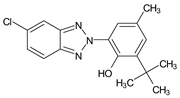 |
|
| 2-(2′-Hydroxy- 3′,5′-di-tert-butylphenyl)- 5-chlorobenzotriazole |
UV-327 | 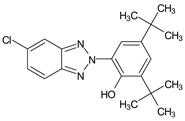 |
||
| 2-(2′-Hydroxy-3′,5′-di-tert-amylphenyl) benzotriazole | UV-328 | 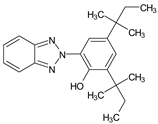 |
||
| 2-(2′-Hydroxy-5′- (1,1,3,3-tetramethylbutyl) phenyl) benzotriazole |
Octrizole | UV-329 | 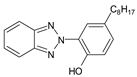 |
|
| Methylene bis- Benzotriazolyl tetramethyl butylphenol |
Bisoctrizole | UV-360 | 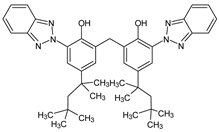 |
|
| CYNNAMATES | 2-Ethylhexyl 4-methoxy cinnamate (Octyl methoxy cinnamate) |
Octinoxate | EMC | 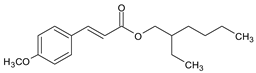 |
| Isoamyl 4-methoxycinnamate | Amiloxate | IMC | 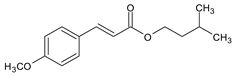 |
|
| SALYCILATES | 2-Ethylhexyl salicylate | Octisalate | EHS | 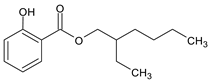 |
| 3,3,5-Trimethylciclohexyl salicylate Homomethyl salicylate |
Homosalate | HMS | 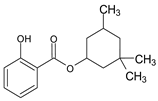 |
|
| CRYLENES | 2-Ethylhexyl 2-cyano-3,3- diphenyl acrylate |
Octocrilene | OCR | 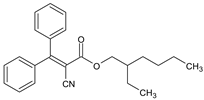 |
| PABA DERIVATES | Ethyl-4-(dimethyl-amino) benzoate |
EtPABA |  |
|
| 2-Ethylhexyl 4-(dimethyl-amino) benzoate (Padimate-O) |
ODPABA | 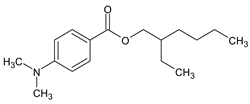 |
||
| CHAMPHOR DERIVATIVES | 4-Methylbenzylidene camphor | 4-MBC | 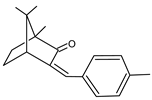 |
|
| TRIAZINE DERIVATIVES | Bis-ethylhexyloxyphenol methoxyphenyl triazine (Bemotrizinol) |
BEMT | 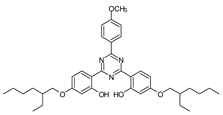 |
|
| Diethylhexyl butamido triazone (Iscotrizinol) |
BDT | 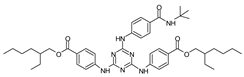 |
||
| Ethylhexyl triazone | EHT | 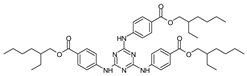 |
| Class | UVF | Detection | Matrices | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|
| BENZOPHENONES | BP | HPLC-UV | pool water | 0.2 µg/L | 0.7 µg/L | [48] |
| BP-1 | LC-MS/MS | river water, sediment, pool water river water river water seawater, sediment fish |
/ / 0.9 µg/L 0.01-1.7 ng/L 0.3-10 ng/L / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.05 µg/L 3.0 µg/L 0.02- 2.4 ng/L 1.0-33.3 ng/L 0.33-5.91 ng/g dw |
[45] [50] [51] [56] [57] [45] |
|
| BP-2 | LC-MS/MS | river water, sediment river water seawater, sediment fish |
/ 0.01-1.7 ng/L 0.3-10 ng/L / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.02- 2.4 ng/L 1.0-33.3 ng/L 0.33-5.91 ng/g dw |
[45] [56] [57] [45] |
|
| BP-3 | HPLC-UV LC-MS/MS |
pool water soil, sediment river water, sediment seawater seawater, wastewater river water seawater, sediment pool water fish seawater, lionfish seaweeds and seagrass |
0.2 µg/L / / / 11.9- 29.1 ng/L 0.01-1.7 ng/L 0.3-10 ng/L 0.31 µg/L / / 22.81 ng/L |
0.7 µg/L / 0.2-6 ng/L, 0.02-0.2 ng/g dw / 39.8-97.1 ng/L 0.02- 2.4 ng/L 1.0-33.3 ng/L 1.0 µg/L 0.33-5.91 ng/g dw / 76.05 ng/L |
[48] [62] [45] [49] [55] [56] [57] [61] [45] [49] [64] |
|
| BP-4 | LC-MS/MS | river water seawater, sediment pool water pool water |
0.01-1.7 ng/L 0.3-10 ng/L 12.5 ng/L 1.7 ng/L |
0.02- 2.4 ng/L 1.0-33.3 ng/L / 5.8 ng/L |
[56] [57] [60] [61] |
|
| BP-8 | LC-MS/MS | river water, sediment seawater, sediment pool water fish |
/ 0.3-10 ng/L 0.15 µg/L / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 1.0-33.3 ng/L 0.49 µg/L 0.33-5.91 ng/g dw |
[45] [57] [61] [45] |
|
| 4-HBP | LC-MS/MS | river water, sediment pool water seawater, sediment fish |
/ / 0.3-10 ng/L / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.01 µg/L 1.0-33.3 ng/L 0.33-5.91 ng/g dw |
[45] [50] [57] [45] |
|
| 4,4′-DHBP | LC-MS/MS |
river water, sediment pool water river water seawater, sediment fish |
/ / 0.01-1.7 ng/L 0.3-10 ng/L / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.01 µg/L 0.02- 2.4 ng/L 1.0-33.3 ng/L 0.33-5.91 ng/g dw |
[45] [50] [56] [57] [45] |
|
| DIBENZOYL METHANE DERIVATIVES |
AVO | LC-MS/MS LC-QTOF-MS |
seawater seaweeds and seagrass seawater, sediment pool water pool water marine plants |
10.8 ng/L 40.43 ng/L 0.93 ng/L, 0.51 ng/g dw 2.5 ng/L 1.1 ng/L / |
35.9 ng/L 134.77 ng/L 3.09 ng/L, 1.69 ng/g dw / 3.6 ng/L / |
[55] [64] [57] [60] [61] [67] |
| BENZOTRIAZOLES | 1HBT | LC-MS/MS | river water, sediment fish |
/ / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw |
[45] [45] |
| MeBT | LC-MS/MS | river water, sediment fish seawater, sediment |
/ / 0.73 ng/L |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 2.42 ng/L |
[45] [45] [57] |
|
| DMeBT | LC-MS/MS | river water, sediment fish |
/ / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw |
[45] [45] |
|
| TBHPBT | LC-MS/MS | river water, sediment fish |
/ / |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw |
[45] [45] |
|
| UV-P | HPLC-UV LC-MS/MS |
water river water, sediment fish seawater, sediment river water |
0.02-0.08 μg/L / / 1.03 ng/L 0.12-1.4 µg/L |
0.07-0.26 μg/L 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 3.42 ng/L 0.46–4.6 µg/L |
[69] [45] [45] [57] [70] |
|
| DTS | LC-MS/MS | seawater seaweeds and seagrass |
12.1 ng/L 6.84 ng/L |
40.4 ng/L 22.79 ng/L |
[55] [64] |
|
| UV-234 | HPLC-UV | water river water |
0.02-0.08 μg/L 0.12-1.4 µg/L |
0.07-0.26 μg/L 0.46–4.6 µg/L |
[69] [70] |
|
| UV-326 | HPLC-UV LC-MS/MS |
water river water, sediment fish |
0.02-0.08 μg/L / / |
0.07-0.26 μg/L 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw |
[69] [45] [45] |
|
| UV-327 | HPLC-UV LC-MS/MS |
water river water, sediment fish river water |
0.02-0.08 μg/L / / 0.12-1.4 µg/L |
0.07-0.26 μg/L 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 0.46–4.6 µg/L |
[69] [45] [45] [70] |
|
| UV-328 | HPLC-UV LC-MS/MS |
water river water, sediment fish river water |
0.02-0.08 μg/L / / 0.12-1.4 µg/L |
0.07-0.26 μg/L 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 0.46–4.6 µg/L |
[69] [45] [45] [70] |
|
| UV-329 | HPLC-UV LC-MS/MS |
water river water, sediment fish river water |
0.02-0.08 μg/L / / 0.12-1.4 µg/L |
0.07-0.26 μg/L 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 0.46–4.6 µg/L |
[69] [45] [45] [70] |
|
| UV-360 | LC-MS/MS | seawater seaweeds and seagrass |
36.4 ng/L 140.34 ng/L |
121.3 ng/L 467.81 ng/L |
[55] [64] |
|
| CYNNAMATES | EMC | HPLC-UV LC-MS/MS |
pool water soil, sediment river water, sediment fish seawater, lionfish river water seawater, sediments pool water |
13 µg/L / / / / 16.1 ng/L 1.71 ng/L, 1.43 ng/g dw 1.0 ng/L |
44 µg/L / 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw / 48.8 ng/L 5.69 ng/L, 4.78 ng/g dw 3.4 ng/L |
[48] [62] [45] [45] [49] [56] [57] [61] |
| IMC | HPLC-UV | soil, sediment seawater seaweeds and seagrass |
/ 13.7 ng/L 16.30 ng/L |
/ 45.6 ng/L 54.35 ng/L |
[62] [55] [64] |
|
| SALYCILATES | EHS | HPLC-UV LC-MS/MS LC-QTOF-MS |
pool water sediment river water pool water marine plants |
0.4 µg/L / 0.80 µg/L 0.33 ng/L / |
1.4 µg/L / 2.6 µg/L 1.1 ng/L / |
[48] [62] [70] [61] [67] |
| HMS | HPLC-UV LC-MS/MS |
pool water sediment seawater seaweeds and seagrass pool water |
0.9 µg/L / 11.3 ng/L 11.4 ng/L 0.74 ng/L |
3.2 µg/L / 37.7 ng/L 38.0 ng/L 2.5 ng/L |
[48] [62] [55] [64] [61] |
|
| CRYLENES | OCR | HPLC-UV LC-MS/MS LC-QTOF-MS |
sediment river water, sediment fish seawater seaweeds and seagrass river water pool water marine plants |
/ / / 12.7 ng/L 20.8 ng/L 1.3 ng/L 2.0 ng/L / |
/ 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 42.4 ng/L 69.2 ng/L 3.9 ng/L 6.7 ng/L / |
[62] [45] [45] [55] [64] [56] [61] [67] |
| PABA DERIVATES | EtPABA | LC-MS/MS | river water, sediment fish seawater, sediments |
/ / 1.71 ng/L, 1.43 ng/g dw |
0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw 5.69 ng/L, 4.78 ng/g dw |
[45] [45] [57] |
| ODPABA | HPLC-UV LC-MS/MS |
soil, sediment river water river water, sediment fish |
1.3 µg/L / / / |
4.0 µg/L / 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw |
[62] [70] [45] [45] |
|
| CHAMPHOR DERIVATIVES |
4-MBC | HPLC-UV LC-MS/MS |
pool water sediment river water, sediment fish seawater, lionfish seawater seaweeds and seagrass river water seawater, sediments |
0.4 µg/L / / / / 12.9 ng/L 51.41 ng/L 1.0 ng/L 0.40 ng/L, 1.19 ng/g dw |
1.4 µg/L / 0.2-6 ng/L, 0.02-0.2 ng/g dw 0.33-5.91 ng/g dw / 43.1 ng/L 171.35 ng/L 1.6 ng/L 1.32 ng/L, 3.97 ng/g dw |
[48] [62] [45] [45] [49] [55] [64] [56] [57] |
| TRIAZINES | BEMT | LC-MS/MS | pool water | 50 ng/L | / | [60] |
| BDT | LC-MS/MS | pool water | 50 ng/L | / | [60] | |
| EHT | LC-MS/MS | pool water | 50 ng/L | / | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





