Introduction:
Megalencephalic Leukoencephalopathy with Subcortical Cysts also known as Van der Knaap disease, is a rare autosomal recessive disorder [
1] characterized by neurological deterioration, macrocephaly, and subcortical cyst formation within the brain. It was first described in 1995 by Van der Knaap [
2] and has involved many clinicians and researchers in this neurological disorder due to its clinical features and radiological features. Van Der Knaap disease is an autosomal recessive disorder involving a mutation in the MLC1 gene which is located on 22q13 chromosomes [
3].
The main clinical features of this disease include neurological deterioration, macrocephaly, and the formation of subcortical cysts within the brain that can be detected by MRI scans [
4]. This disease mostly appears in childhood in the form of developmental delay where symptoms appear as motor and cognitive functions begin to decline [
5]. Initial mental and motor development is normal in most individuals. Cognitive deterioration occurs later in the course of the disease and is usually mild in severity. Disease severity varies, with some individuals being able to walk independently for only a few years from disease onset to other individuals continuing to independently walk in the later part of life
The presented case here of a 14-year-old female shows the clinical scenario with challenges associated with the diagnosis and management of this rare neurological condition.
Case Presentation:
A 14 yr. female born of a consensus marriage with no previous comorbidity with normal early developmental milestones and poor scholastic performance since the age of 5 years presented with complaints of Weakness of all four limbs since the age of 5 years of age. The patient was asymptomatic before 5 years of age then her mother noticed that she had difficulty walking in a form that she used to walk on the lateral border of her foot. She had difficulty in wearing chappals and complained of slippage of chappals with her awareness since the age of 5 years.
Over time, the patient faced challenges in performing activities such as squatting, climbing stairs where she required 1 person's support for the same, and fine motor tasks involving both hands in the form of not buttoning and unbuttoning.
There are no complaints of Tingling, numbness, burning sensation, or pain. The patient’s clinical scenario is not associated with headache, diplopia, vomiting, fever, dripping, or dragging of the foot. There is no difficulty in swallowing, chewing, or facial asymmetry.
There is no History of any seizure or sleep alteration, loss of consciousness, or any involuntary movements. Negative History of Alteration in smell, vision, eye movement, ptosis or any sensory abnormality on the face, any difficulty in hearing, tinnitus, vertigo, or diuresis, Regurgitation of food or fluid & and no nasal twang of voice.
Physical Examination
Physical examination showed a temperature of 98.6 F, pulse rate of 86/min, blood pressure of 110/82, SpO2 97% on room air, and RBS of 106 mg/dl. Respiratory examination showed a respiratory rate of 18/min and bilateral equal air entry was present. S1S2 were present on cardiovascular examination. The patient was oriented and responding to verbal commands. On fundal examination, there is Bilateral optic disk pallor present.
On Neurological examination, the patient's general condition was good. She was conscious and well oriented to time place and person. Examination revealed mild spasticity, muscle weakness, areflexia, ill-sustained clonus in both lower limbs, and sensory deficits. Her pupils were Bilaterally equal in size and diameter and Reactive to Light with no ptosis. The hand grip, lumbrical, and interossei muscles were weak in both hands with hypothenar atrophy (left > right). There was also deformity in both feet in the form of Pes Cavus with Left > Right.
History of past illness
The patient had no previous medical history.
Investigations:
Laboratory examinations
Blood examination revealed a hemoglobin level of 110 g/L (normal range > 120 g/L) with a normal leukocyte count and platelet count. The serum C-reactive protein level and the prothrombin and partial thromboplastin times, the blood biochemistry, and urine analyses were normal. The electrocardiogram result was also normal.
Blood Investigation
| CBC |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| HEMOGLOBIN |
11 |
mg/dl |
12.3–17 mg/dL |
| WBC |
7000 |
cells/μL |
4,500 to 11,000 cells/μL |
| PLATELET |
2.05 |
lakh/μL |
1.5-4.5 cells/μL |
| LFT |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| SGPT |
11 |
units/liter |
7-56 units/liter |
| RFT |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| CREATININE |
0.49 |
mg/dl |
0.74 to 1.35 mg/dL |
| SODIUM |
140 |
mmol/L |
135-145 mmol/L |
| POTASSIUM |
4.40 |
mmol/L |
3.5-5 mmol/L |
Radiological Examination:
A chest X-ray and USG abdomen were done but it turned out to be normal. Hence MRI Brain Contrast was done for further evaluation of the patient's condition.
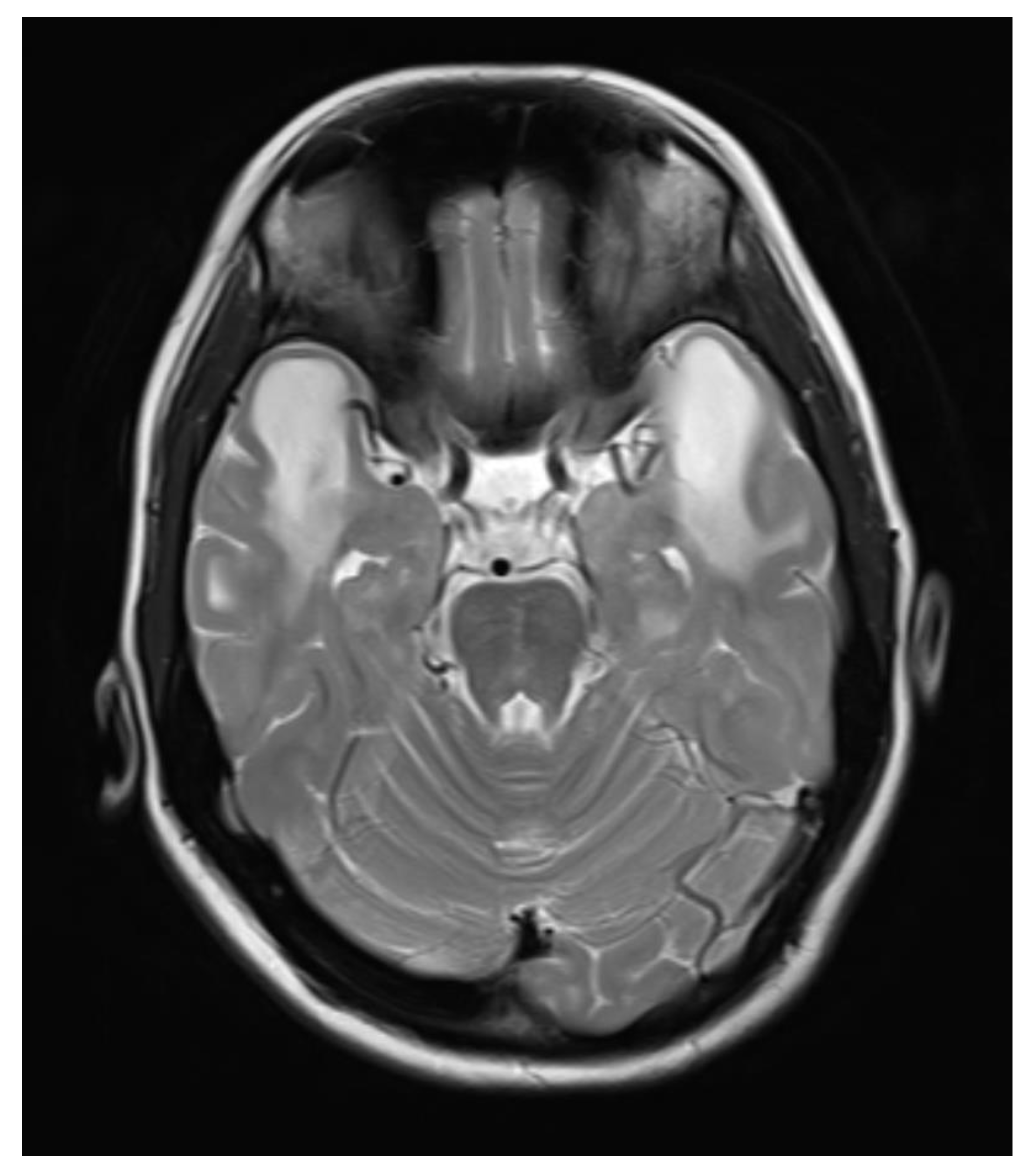
A contrast-enhanced MRI of the brain was performed, bilateral symmetrical diffuse abnormal T2WI/FLAIR hyperintensity involving the subcortical and periventricular deep white matter of bilateral cerebral hemispheres and corpus callosum with areas of diffuse restriction in bilateral centrum semi-ovale restriction, subcortical cysts, and cerebral atrophy were seen with possibility of Megalencephalic leukoencephalopathy with subcortical cysts also known as Van der knap disease. Post-contrast imaging showed normal enhancement, ruling out vascular abnormalities or abnormal enhancement patterns.
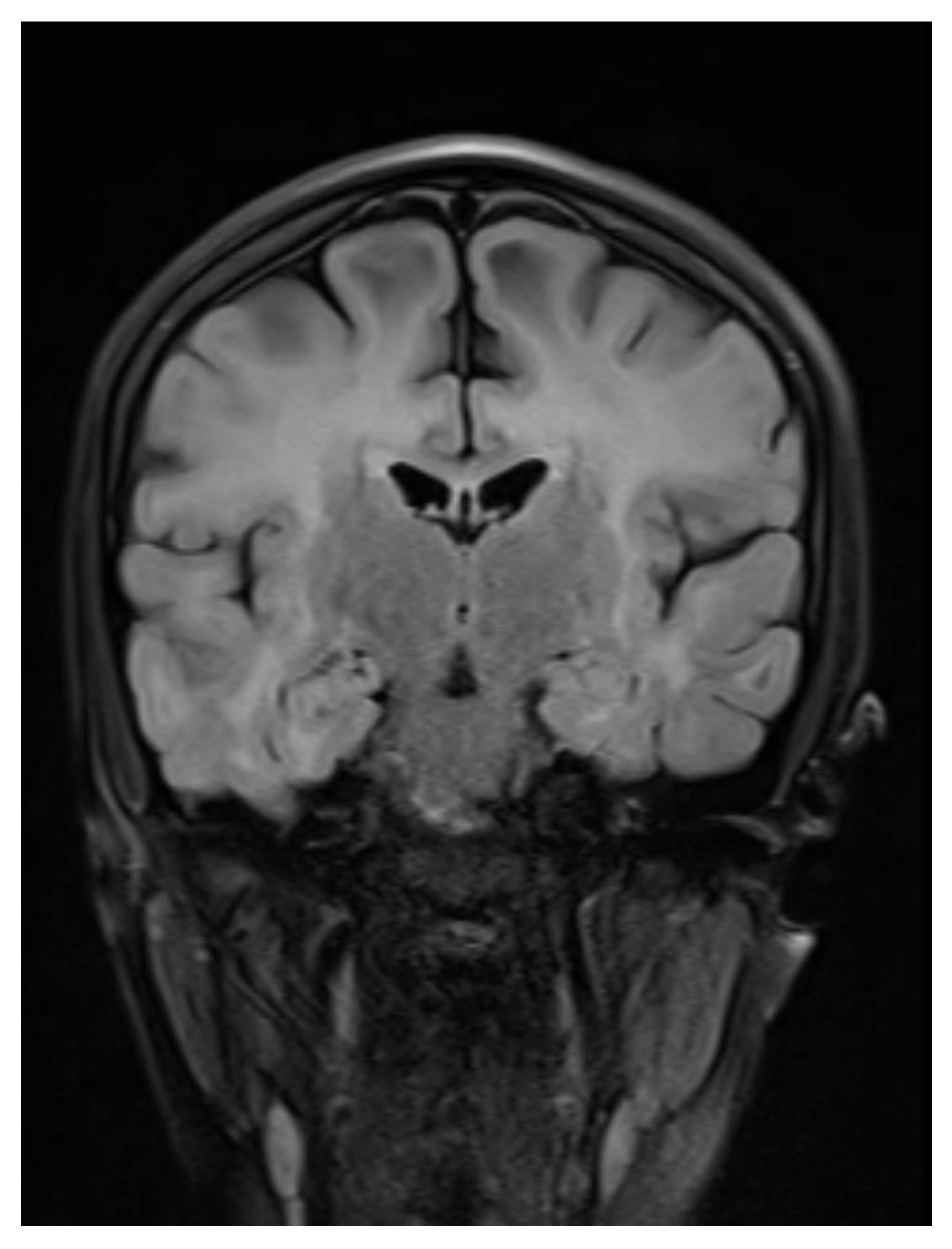
Figure 1.
Coronal Flair image showing Bilateral symmetric white matter hyperintensity.
Figure 1.
Coronal Flair image showing Bilateral symmetric white matter hyperintensity.
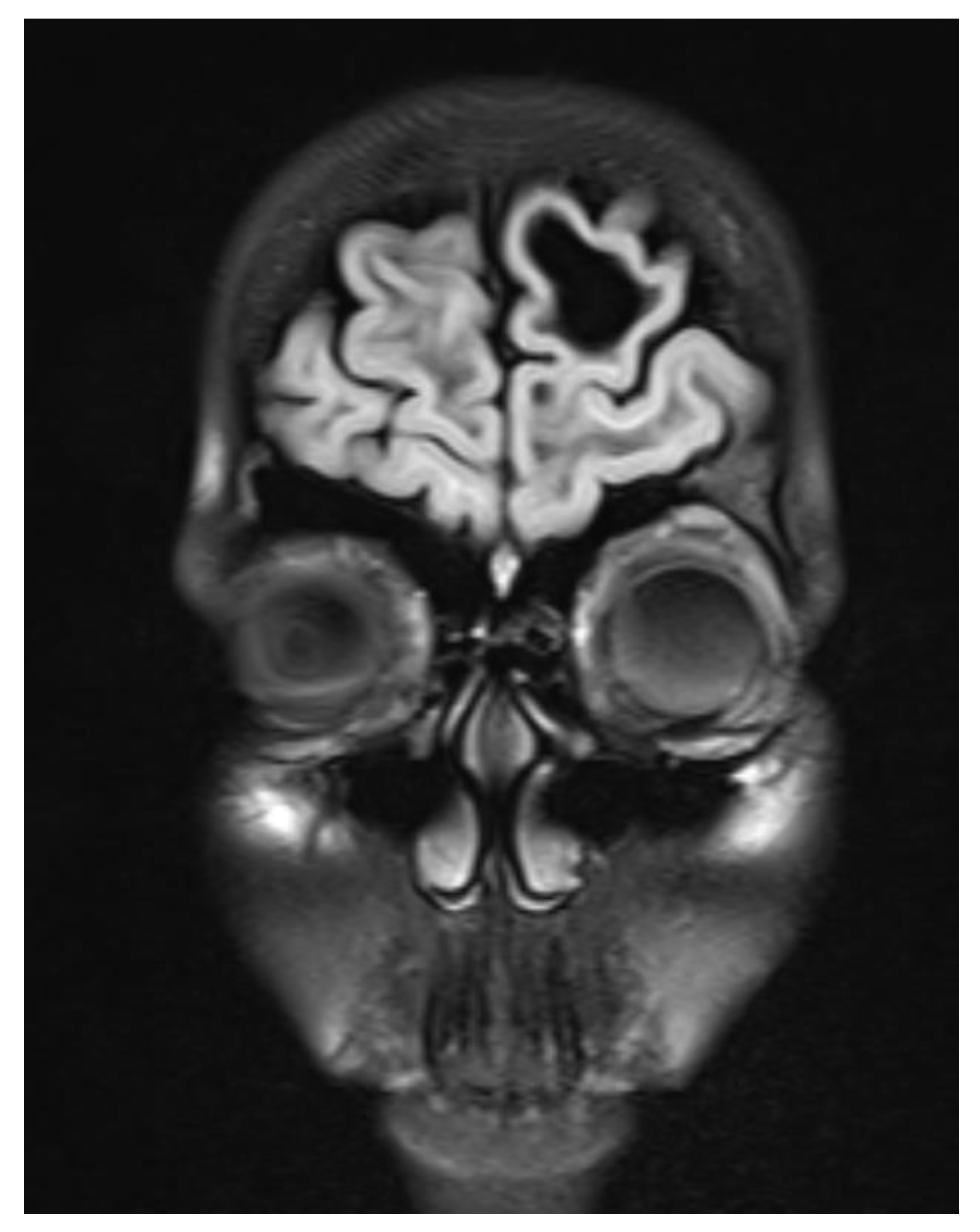
Figure 2.
Subcortical cyst formation in the left frontal lobe.
Figure 2.
Subcortical cyst formation in the left frontal lobe.
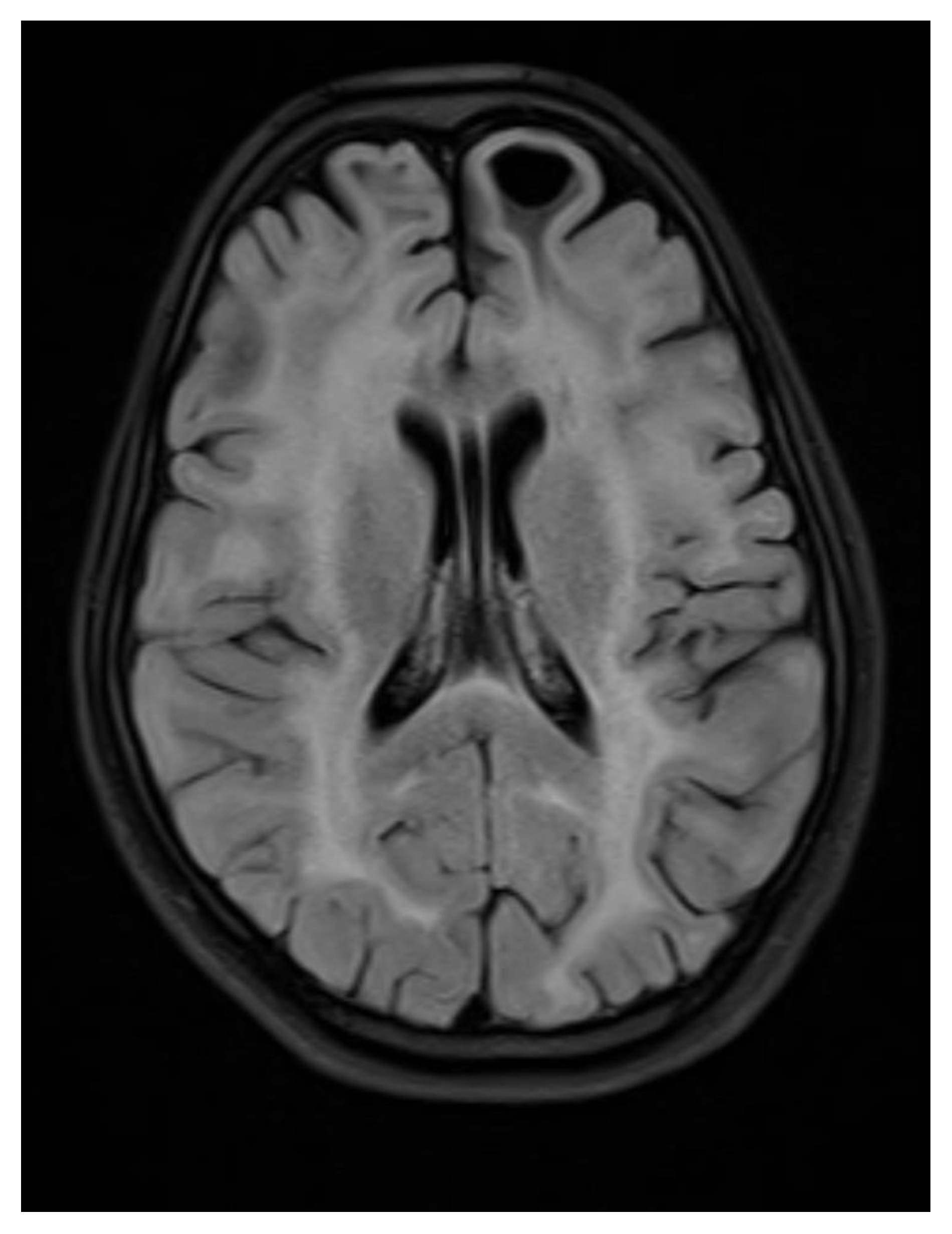
Figure 3.
Axial flair images showing Bilateral symmetrical white matter hyperintensity with subcortical cyst formation in the left frontal lobe.
Figure 3.
Axial flair images showing Bilateral symmetrical white matter hyperintensity with subcortical cyst formation in the left frontal lobe.
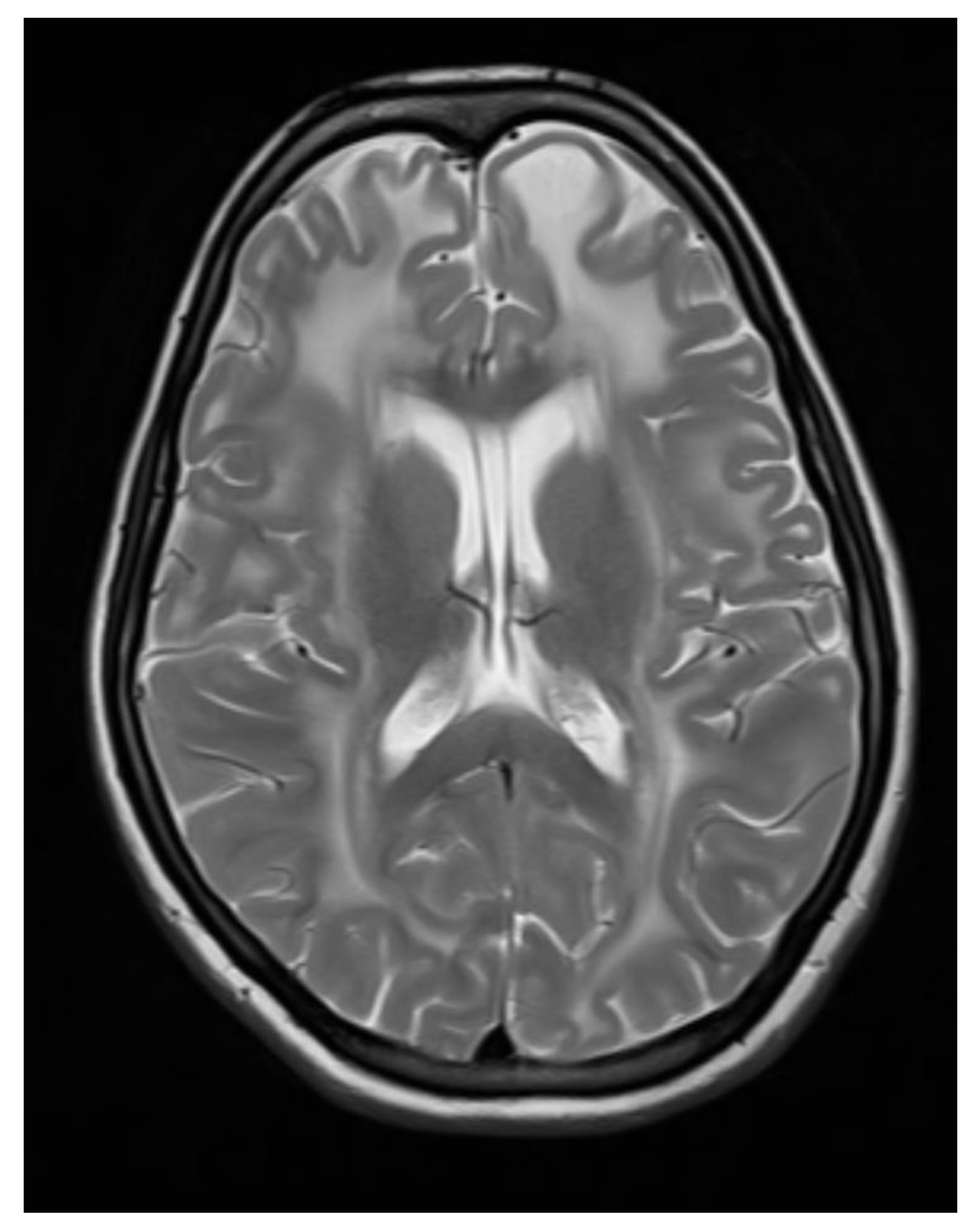
Figure 4.
Axial T2 WI images showing Bilateral symmetrical white matter hyperintensity with subcortical cyst formation in the left frontal lobe.
Figure 4.
Axial T2 WI images showing Bilateral symmetrical white matter hyperintensity with subcortical cyst formation in the left frontal lobe.
Figure 5.
Axial T2WI images showing Subcortical and periventricular white matter hyperintensity with subcortical cyst formation in the bilateral anterior temporal lobe.
Figure 5.
Axial T2WI images showing Subcortical and periventricular white matter hyperintensity with subcortical cyst formation in the bilateral anterior temporal lobe.
Diagnosis:
The clinical and radiological findings were consistent with megalencephalic leukoencephalopathy with subcortical cysts, also known as Van der Knaap disease.
Discussion:
Van Deer Knap disease, Megalencephalic Leukoencephalopathy with subcortical cysts (MLC), is a rare autosomal recessive neurological disorder. This disorder was first described in the year 1995 showing abnormal enlargement of the brain i.e. megalocephaly which is a hallmark feature of MLC, primarily affecting white matter of the brain [
6] which can be identified through neuroimaging techniques such as magnetic resonance imaging (MRI). There is progressive neurological deterioration along with fluid field cysts in subcortical regions which is accompanied by enlarged brain size [
7]. The combination of megalocephaly and subcortical cysts distinguishes MLC from other leukodystrophies. MLC occurs due to a mutation in genes that is inherited from both parents to show clinical symptoms. Common genes include MLC1 and HEPACAM genes, which are important in the development and maintenance of myelin sheath around the nerve fibers which are needed for efficient nerve transmission [
8].
The pathological mechanism of MLC is yet to be fully understood, but defective myelin sheath formation and maintenance is the main culprit of MLC and is the central aspect of the disease. Myelin sheath is important in the insulation of nerve fibers and its defective nature results in impaired signal transmission between different neurons [
9]. Along with this accumulation of fluid-filled cysts in the subcortical region further damage the white matter and exacerbates neurological symptoms in patients.
Clinically, MLC includes a group of neurological symptoms seen in early childhood, including motor dysfunction, walking difficulties, speech abnormalities, and cognitive decline [
10]. Affected individuals also reported seizures as a common manifestation of this disease. As there is disease progression individuals show a decline in cognitive function which leads to severe intellectual disability.
The diagnosis of MLC ( a.k.a Van Deer Knap) disease is based on clinical and radiological imaging features. Radiological imaging plays a crucial role in diagnosing Van der Knaap's disease [
11]. MRI (Magnetic Resonance Imaging) is the best imaging method which is used to detect abnormality in this disease. The characteristic findings on MRI include hypomyelination particularly in the cerebral white matter, along with atrophy of the basal ganglia and cerebellum and signal abnormalities in the affected regions.
Currently, there is no cure for Van Deer Knaap's Disease but supportive management strategies can help to alleviate symptoms and improve patients' condition. Supportive management includes speech therapy, and physical and occupational therapy can help to improve motor function and improve the life quality of individuals [
12]. Patients who show seizures can be treated with antiepileptic medication to manage the seizures. Medications may be prescribed to manage spasticity and improve quality of life.
Conclusion:
This case shows the importance of the clinical and radiological features of Van der Knaap's disease, a rare leukodystrophy affecting the white matter of the brain with macrocephaly and subcortical cysts which are fluid-filled, allowing for early diagnosis and appropriate management. Clinically this disease involves motor deterioration, cognitive decline, and spasticity. As there is no cure for this condition, early diagnosis and supportive treatment are needed. Genetic counseling is important to prevent these diseases at best. Additional research and studies are needed to have different therapeutic interventions for this rare leukodystrophy.
Patient Consent:
The patient in the study was provided with detailed information about the purpose, procedures, risks, benefits, and confidentiality measures associated with the research. The patient was given ample time to review the informed consent, ask questions, and make an informed decision regarding their participation. The patient obtained Written informed consent before their involvement in the study.
Ethical Statement
Being a case report study, there were no ethical issues and the IRB was notified about the topic and the case. Still, no formal permission was required as this was a record-based case report. Permission from the patient for the article has been acquired and ensured that their information or identity is not disclosed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bokhari, M.R.; Inayat, F.; Sardar, J.; A Bokhari, S.R. Van der Knaap Disease. J. Coll. Physicians Surg. Pak. 2018, 28, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Joshi, B.; Ganguly, G. Van der Knaap disease: A rare disease with atypical features. BMJ Case Rep. 2015, 2015, bcr2015209831. [Google Scholar] [CrossRef] [PubMed]
- Khalaf-Nazzal, R.; Dweikat, I.; Maree, M.; Alawneh, M.; Barahmeh, M.; Doulani, R.T.; Qrareya, M.; Qadi, M.; Dudin, A. Prevalent MLC1 mutation causing autosomal recessive megalencephalic leukoencephalopathy in consanguineous Palestinian families. Brain Dev. 2022, 44, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.K.; Singh, B.B. Megalencephalic leukoencephalopathy with subcortical cysts in all three siblings of a non-Aggarwal Indian family. Ann. Indian Acad. Neurol. 2012, 15, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Batla, A.; Pandey, S.; Nehru, R. Megalencephalic leukoencephalopathy with subcortical cysts: A report of four cases. J. Pediatr. Neurosci. 2011, 6, 74–77. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, M.S.; Bugiani, M. Leukodystrophies: A proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 2017, 134, 351–382. [Google Scholar] [CrossRef] [PubMed]
- Cogollos, V.B.; García, M.M.; Pérez-Gramunt, M.A.; Sanchís, E.M.; Mombiela, R.M.; Martínez, J.C.M.; Vila, M.T.; Castellano, F.M. Leucoencefalopatía megalencefálica con quistes: Importancia de la descripción clínica en la era genética. 2020, 71, 373–376. Rev. Neurol. 2020, 71, 373–376. [Google Scholar] [CrossRef]
- Arnedo, T.; López-Hernández, T.; Jeworutzki, E.; Capdevila-Nortes, X.; Sirisi, S.; Pusch, M.; Estévez, R. Functional Analyses of Mutations inHEPACAMCausing Megalencephalic Leukoencephalopathy. Hum. Mutat. 2014, 35, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Myelin damage and repair in pathologic CNS: Challenges and prospects. Front. Mol. Neurosci. 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qu, H.; Yao, Q.; Cai, X.; He, T.; Zhang, X. Case report: Analysis of a gene variant and prenatal diagnosis in a family with megalencephalic leukoencephalopathy with subcortical cysts. Front. Neurol. 2023, 14, 1253398. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, M.R.; Inayat, F.; Sardar, J.; A Bokhari, S.R. Van der Knaap Disease. J. Coll. Physicians Surg. Pak. 2018, 28, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Perry, A.; Bilney, B.; Curran, A.; Dodd, K.; Wittwer, J.E.; Dalton, G.W. Outcomes of Physical Therapy, Speech Pathology, and Occupational Therapy for People with Motor Neuron Disease: A Systematic Review. Neurorehabilit. Neural Repair 2006, 20, 424–434. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).