Submitted:
26 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
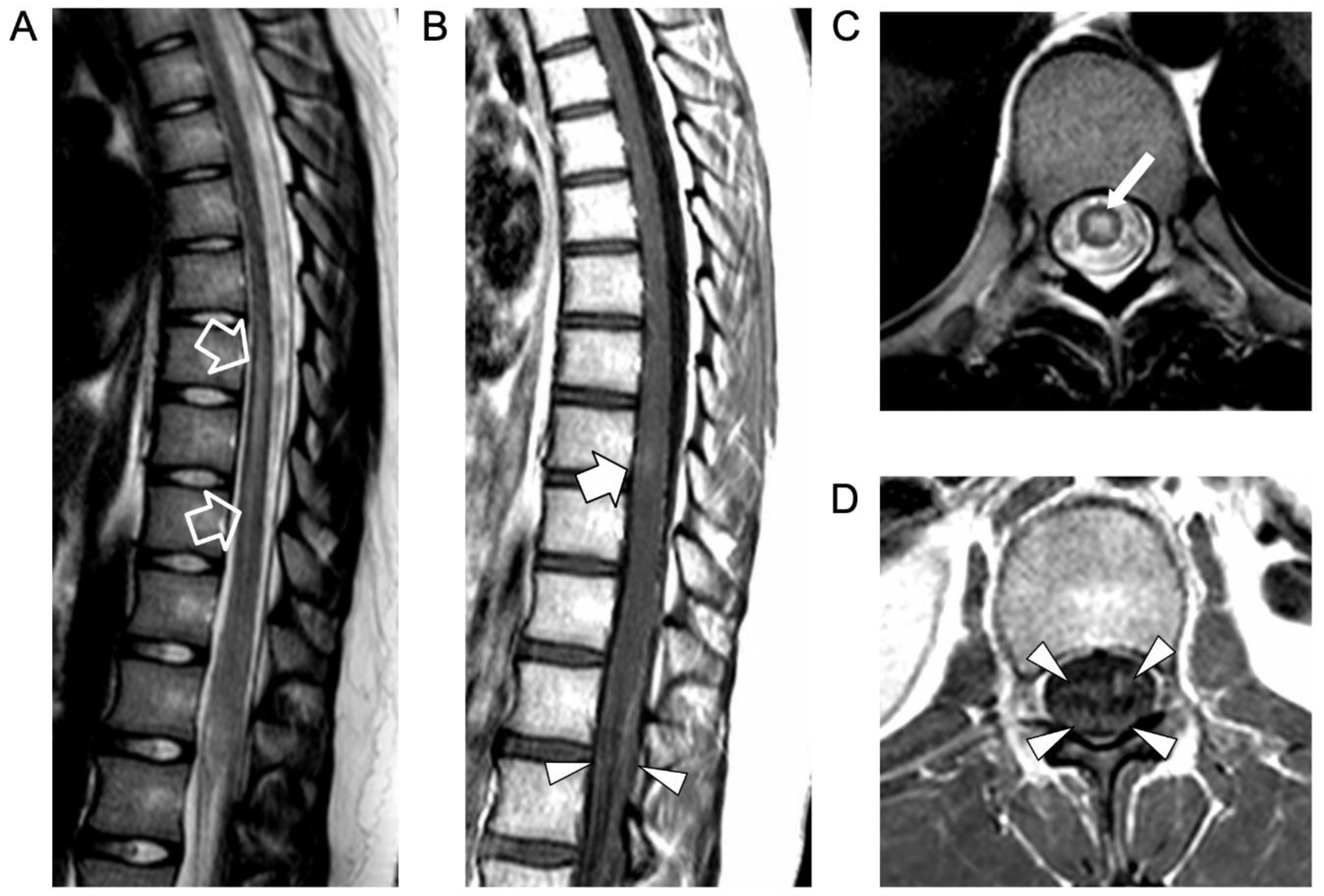
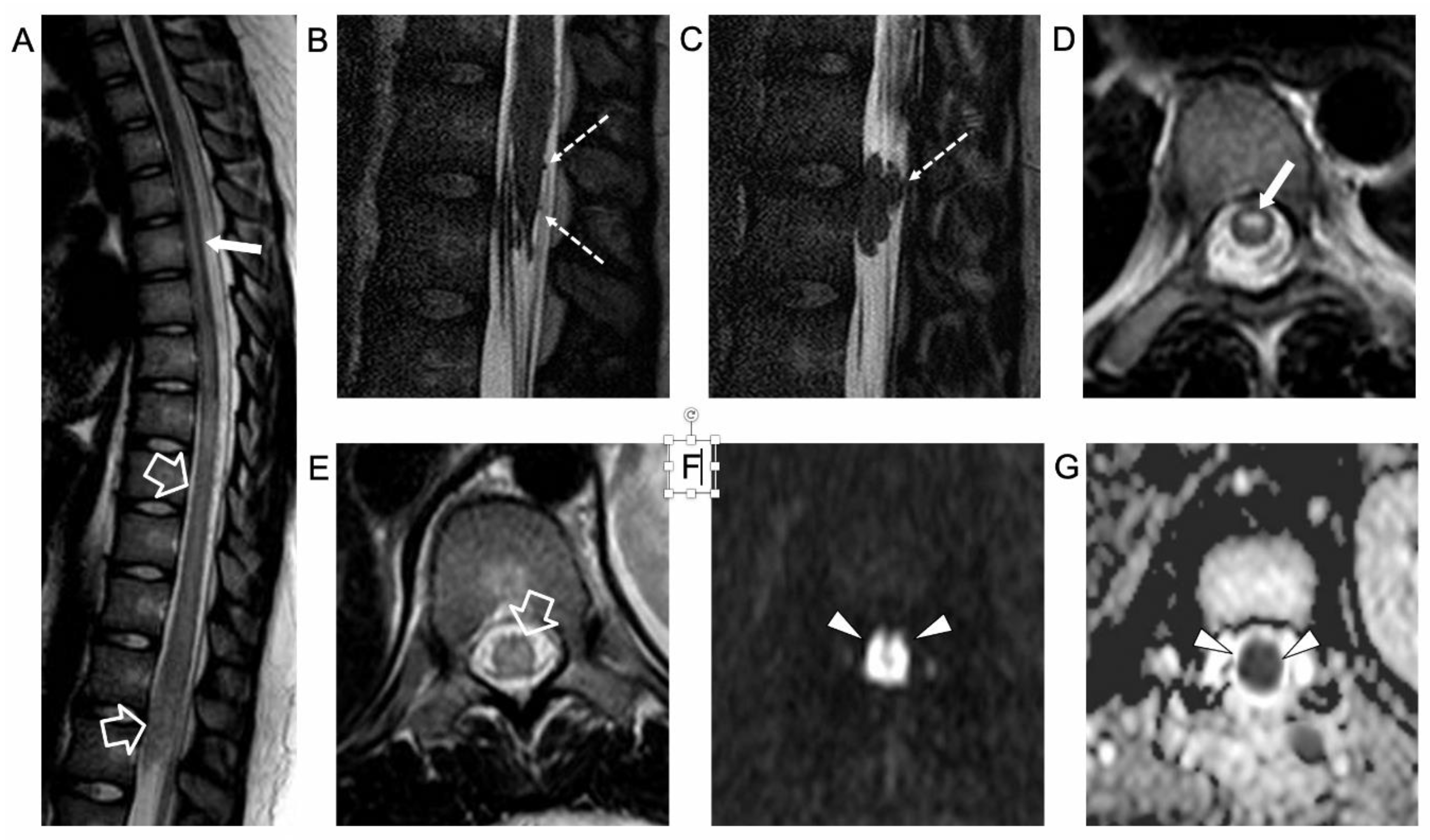
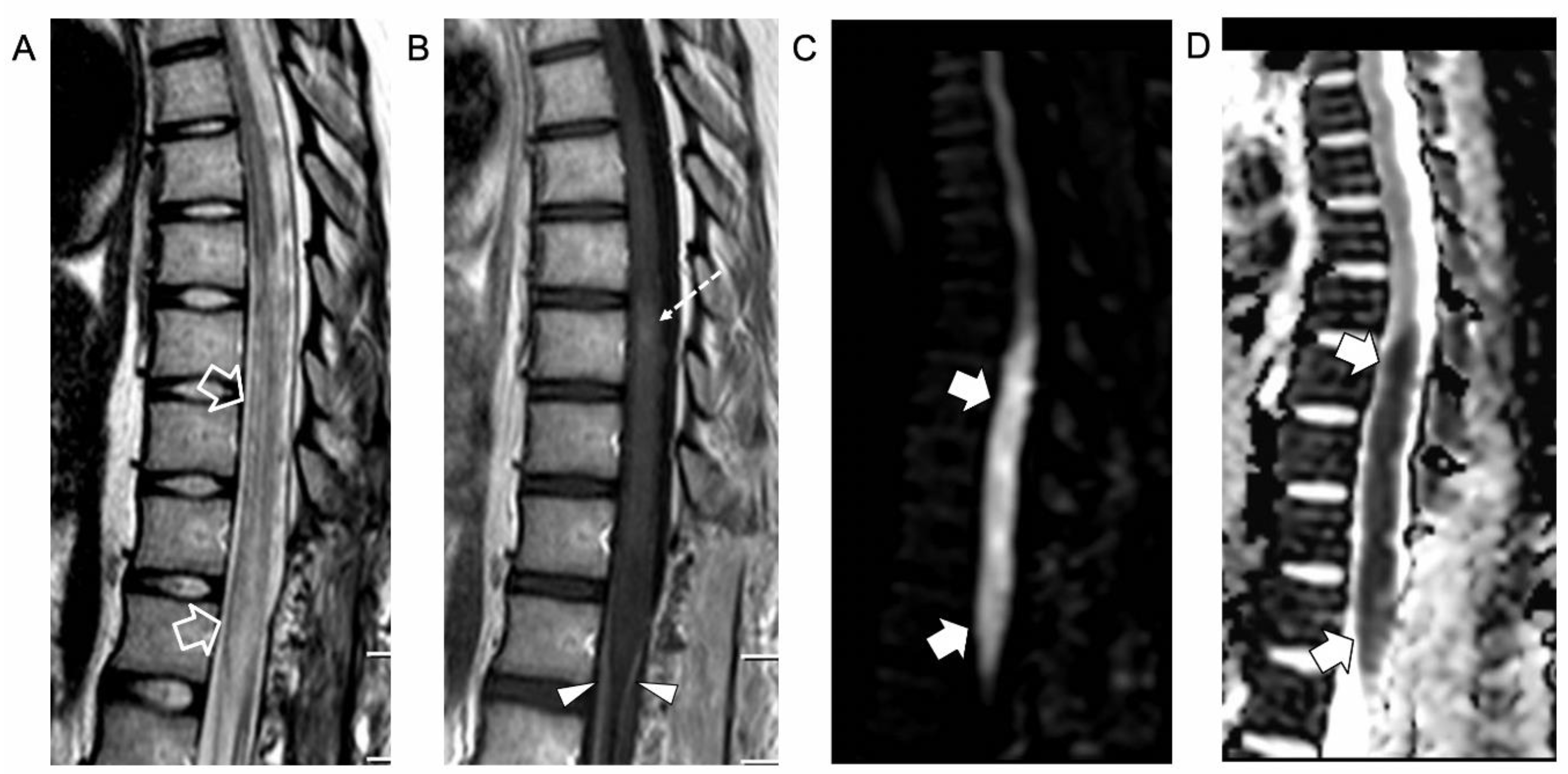
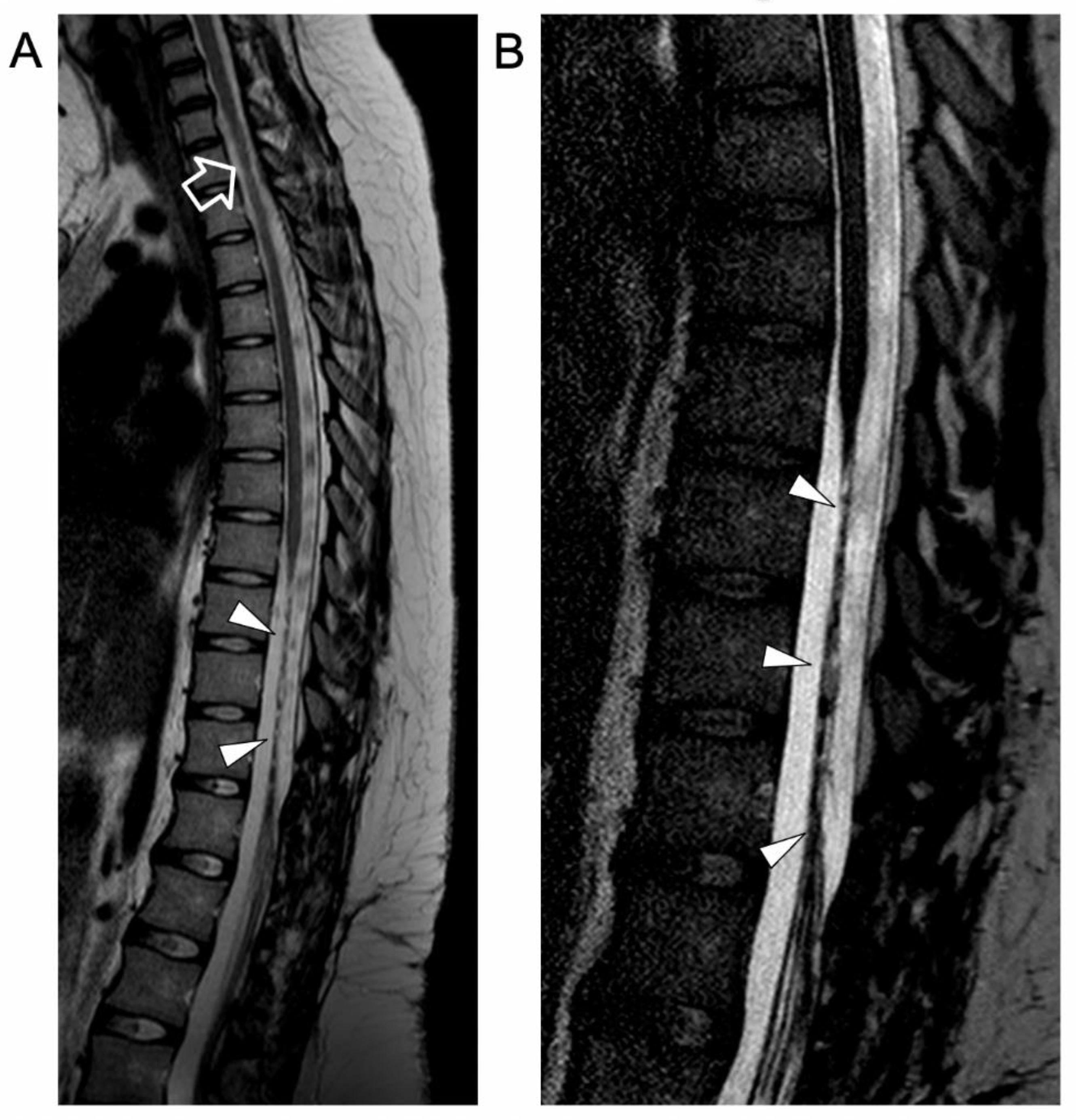
2. Case Report
3. Literature Review and Comments
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marth, T.; Moos, V.; Müller, C.; Biagi, F.; Schneider, T. Tropheryma whipplei infection and Whipple's disease. Lancet Infect. Dis. 2016, 16, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- El-Abassi, R.; Soliman, M.Y.; Williams, F.; England, J.D. Whipple’s disease. J. Neurol. Sci. 2017, 377, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Compain, C.; et al. Central nervous system involvement in whipple disease: Clinical study of 18 patients and long-term follow-up. Medicine 2013, 92, 324–330. [Google Scholar] [CrossRef]
- Jovic, N.S.; Jovic, J.Z. Neurologic disorders in Whipple’s disease. Srp. Arh. Celok. Lek. 1996, 124, 98–102. [Google Scholar] [PubMed]
- Pfeiffer, R.F. Gastroenterology and Neurology. Contin. Lifelong Learn. Neurol. 2017, 23, 744–761. [Google Scholar] [CrossRef] [PubMed]
- Messori, A.; et al. An unusual spinal presentation of Whipple disease. AJNR. Am. J. Neuroradiol. 2001, 22, 1004–1008. [Google Scholar] [PubMed]
- Helou, J.; Saliba, G.; Kolev, I.; Pierrot-Deseilligny, C. Neuro-Whipple confirmed five years after a presumptive diagnosis of a primitive CNS vasculitis. J. Neurol. 2008, 255, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.E.; Falope, Z.F.; Abdelhadi, H.A.; Franks, A.J. Cervical myelopathy caused by Whipple’s disease. Neurology 1998, 50, 1505–1506. [Google Scholar] [CrossRef] [PubMed]
- Schroter, A.; Brinkhoff, J.; Gunthner-Lengsfeld, T.; Suerbaum, S.; Reiners, K.; Messmann, H.; Naumann, M. Whipple’s disease presenting as an isolated lesion of the cervical spinal cord. Eur. J. Neurol. 2005, 12, 276–279. [Google Scholar] [CrossRef]
- Álvarez, P.; de la Tassa, G.M. Cervical myelopathy as a form of presentation of Whipple disease. Neurologia, 2019, 35, 583–585. [Google Scholar] [CrossRef]
- Kremer, S.; Besson, G.; Bonaz, B.; Pasquier, B.; Le Bas, J.F.; Grand, S. Diffuse lesions in the CNS revealed by MR imaging in a case of Whipple disease. AJNR. Am. J. Neuroradiol. 2001, 22, 493–495. [Google Scholar]
- Gerard, A.; et al. Neurologic presentation of whipple disease: Report of 12 cases and review of the literature. Medicine (Baltimore) 2002, 81, 443–457. [Google Scholar] [CrossRef]
- Balasa, M.; Gelpi, E.; Rey, M.; Vila, J.; Ramió-Torrentà, L.; Granado, A.M.Q.; Latorre, R.M.; Lepidi, H.; Raoult, D.; Saiz, A. Clinical and neuropathological variability in clinically isolated central nervous system whipple’s disease. Brain Pathol. 2013, 24, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Balducci, C.; Foresti, S.; Ciervo, A.; Mancini, F.; Nastasi, G.; Marzorati, L.; Gori, A.; Ferrarese, C.; Appollonio, I.; Peri, A.M. Primary Whipple Disease of the Central Nervous System Presenting with Rhombencephalitis. Int. J. Infect. Dis. 2019, 88, 149–151. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Fenollar, F.; Raoult, D. Acute infections caused by Tropheryma whipplei. Future Microbiol. 2017, 12, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schöniger-Hekele, M.; Petermann, D.; Weber, B.; Müller, C. Tropheryma whipplei in the environment: survey of sewage plant influxes and sewage plant workers. Appl. Environ. Microbiol. 2007, 73, 2033–2035. [Google Scholar] [CrossRef]
- Dolmans, R.A.V.; Boel, C.H.E.; Lacle, M.M.; Kusters, J.G. Clinical manifestations, treatment, and diagnosis of Tropheryma whipplei infections. Clin. Microbiol. Rev. 2017, 30, 529–555. [Google Scholar] [CrossRef]
- Raoult, D.; et al. Tropheryma whipplei in children with gastroenteritis. Emerg. Infect. Dis. 2010, 16, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Laouira, S.; Lepidi, H.; Rolain, J.; Raoult, D. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: usefulness of saliva and stool specimens for first-line screening. Clin. Infect. Dis. 2008, 47, 659–667. [Google Scholar] [CrossRef]
- Elchert, J.A.; Mansoor, E.; Abou-Saleh, M.; Cooper, G.S. Epidemiology of Whipple’s Disease in the USA Between 2012 and 2017: A Population-Based National Study. Dig. Dis. Sci. 2018, 64, 1305–1311. [Google Scholar] [CrossRef]
- Moos, V.; Schneider, T. Changing paradigms in Whipple’s disease and infection with Tropheryma whippleiEur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Keita, A.K.; Buffet, S.; Raoult, D. Intrafamilial circulation of Tropheryma whipplei, France. Emerg. Infect. Dis. 2012, 18, 949–955. [Google Scholar] [CrossRef]
- Keita, A.K.; Brouqui, P.; Badiaga, S.; Benkouiten, S.; Ratmanov, P.; Raoult, D.; Fenollar, F. Tropheryma whipplei prevalence strongly suggests human transmission in homeless shelters. Int. J. Infect. Dis. 2013, 17, e67–e68. [Google Scholar] [CrossRef] [PubMed]
- Edouard, S.; Fenollar, F.; Raoult, D. The rise of Tropheryma whipplei: a 12-year retrospective study of PCR diagnoses in our reference center. J. Clin. Microbiol. 2012, 50, 3917–3920. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Trani, M.; Davoust, B.; Salle, B.; Birg, M.; Rolain, J.; Raoult, D. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. J. Infect. Dis. 2008, 197, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Amsler, L.; Bauerfeind, P.; Nigg, C.; Maibach, R.C.; Steffen, R.; Altwegg, M. Prevalence of Tropheryma whipplei DNA in patients with various gastrointestinal diseases and in healthy controls. Infection 2003, 31, 81–85. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Pérez-Matute, P.; Blanco, J.R.; Ibarra, V.; Oteo, J.A. High prevalence of asymptomatic carriers of Tropheryma whipplei in different populations from the North of Spain. Enferm. Infecc. Microbiol. Clin. 2016, 34, 340–345. [Google Scholar] [CrossRef]
- Frickmann, H.; et al. Detection of Tropheryma whipplei in stool samples by one commercial and two in-house real-time PCR assays. Trop. Med. Int. Health 2019, 24, 101–108. [Google Scholar] [CrossRef]
- Fenollar, F.; Minodier, P.; Boutin, A.; Laporte, R.; Brémond, V.; Noël, G.; Miramont, S.; Richet, H.; Benkouiten, S.; Lagier, J.-C.; et al. Tropheryma whipplei associated with diarrhoea in young children. Clin. Microbiol. Infect. 2016, 22, 869–874. [Google Scholar] [CrossRef]
- Biagi, F.; Balduzzi, D.; Delvino, P.; Schiepatti, A.; Klersy, C.; Corazza, G.R. Prevalence of Whipple’s disease in north-western Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1347–1348. [Google Scholar] [CrossRef]
- Beltrame, A.; Ragusa, A.; Perandin, F.; Formenti, F.; Fenollar, F.; Edouard, S.; Laroche, M.; Zavarise, G.; Doro, F.; Giorli, G.; et al. Tropheryma whipplei intestinal colonization in Italian and migrant population: a retrospective observational study. Future Microbiol. 2019, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Moos, V.; Kunkel, D.; Marth, T.; Feurle, G.E.; LaScola, B.; Ignatius, R.; Zeitz, M.; Schneider, T. Reduced Peripheral and Mucosal Tropheryma whipplei -Specific Th1 Response in Patients with Whipple’s Disease. J. Immunol. 2006, 177, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Martinetti, M.; Biagi, F.; Badulli, C.; Feurle, G.E.; Müller, C.; Moos, V.; Schneider, T.; Marth, T.; Marchese, A.; Trotta, L.; et al. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple’s disease. Gastroenterology 2009, 136, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Mottola, G.; Boucherit, N.; Trouplin, V.; Barry, A.O.; Soubeyran, P.; Mege, J.-L.; Ghigo, E. Tropheryma whipplei, the agent of Whipple’s disease, affects the early to late phagosome transition and survives in a Rab5- and Rab7-positive compartment. PLOS ONE 2014, 9, e89367. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; et al. -295 T-to-C promoter region IL-16 gene polymorphism is associated with Whipple’s disease. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Fenollar, F.; Raoult, D.; Mege, J.-L. Increased Levels of Circulating IL-16 and Apoptosis Markers Are Related to the Activity of Whipple’s Disease. PLOS ONE 2007, 2, e494. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; et al. Cytokine genetic profile in Whipple’s disease. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3145–3150. [Google Scholar] [CrossRef] [PubMed]
- Kalt, A.; Schneider, T.; Ring, S.; Hoffmann, J.; Zeitz, M.; Stallmach, A.; Persing, D.H.; Marth, T. Decreased levels of interleukin-12p40 in the serum of patients with Whipple’s disease. Int. J. Color. Dis. 2005, 21, 114–120. [Google Scholar] [CrossRef]
- Ben Azzouz, E.; Boumaza, A.; Mezouar, S.; Bardou, M.; Carlini, F.; Picard, C.; Raoult, D.; Mège, J.-L.; Desnues, B. Tropheryma whipplei Increases Expression of Human Leukocyte Antigen-G on Monocytes to Reduce Tumor Necrosis Factor and Promote Bacterial Replication. Gastroenterology 2018, 155, 1553–1563. [Google Scholar] [CrossRef]
- Glaser, C.; Rieg, S.; Wiech, T.; Scholz, C.; Endres, D.; Stich, O.; Hasselblatt, P.; Geißdörfer, W.; Bogdan, C.; Serr, A.; et al. Whipple’s disease mimicking rheumatoid arthritis can cause misdiagnosis and treatment failure. Orphanet J. Rare Dis. 2017, 12, 99. [Google Scholar] [CrossRef]
- Keita, A.K.; Diatta, G.; Ratmanov, P.; Bassene, H.; Raoult, D.; Roucher, C.; Fenollar, F.; Sokhna, C.; Tall, A.; Trape, J.-F.; et al. Looking for Tropheryma whipplei source and reservoir in rural Senegal. Am. J. Trop. Med. Hyg. 2013, 88, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Trotta, L.; Corazza, G.R. Whipple’s disease. Intern. Emerg. Med. 2012, 7, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Raoult, D. Whipple’s disease and Tropheryma whipplei infections: when to suspect them and how to diagnose and treat them. Curr. Opin. Infect. Dis. 2018, 31, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Günther, U.; et al. Gastrointestinal diagnosis of classical whipple disease: Clinical, endoscopic, and histopathologic features in 191 patients. Medicine 2015, 94, e714. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D. From Whipple Disease to Tropheryma whipplei Infection. Clin. Infect. Dis. 2018, 68, 1098–1099. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.-E.; et al. Blood culture-negative endocarditis: Improving the diagnostic yield using new diagnostic tools. Medicine (Baltimore) 2017, 96, e8392. [Google Scholar] [CrossRef] [PubMed]
- Paymard, M.; Sukumaran, V.; Senanayake, S.; Watson, A.; Das, C.; Abhayaratna, W. Tropheryma Whipplei endocarditis: Case report and literature review. Hear. Views 2018, 19, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Lepidi, H.; Raoult, D.; Fenollar, F. Systemic tropheryma whipplei: Clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore) 2010, 89, 337–345. [Google Scholar] [CrossRef]
- Marth, T. Complicated Whipple’s disease and endocarditis following tumor necrosis factor inhibitors. World J. Cardiol. 2014, 6, 1278–1284. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Fenollar, F.; Lepidi, H.; Giorgi, R.; Million, M.; Raoult, D. Treatment of classic Whipple’s disease: from in vitro results to clinical outcome. J. Antimicrob. Chemother. 2013, 69, 219–227. [Google Scholar] [CrossRef]
- Chandra, S.R.; Raj, P.; Pai, A.R.; Reddy, N. A Case of Whipple’s Disease: A Very Rare Cause for Rapidly Progressive Dementia. Indian J. Psychol. Med. 2018, 40, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Panegyres, P.K.; Goh, J. Sleep disorders of Whipple’s disease of the brain. QJM 2015, 108, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.; Neil, E.; Kupsky, W.J.; Juhász, C.; Mittal, S.; Santhakumar, S. Isolated inctracranial Whipple’s disease-report of a rare case and review of the literature. J. Neurol. Sci. 2011, 308, 1–8. [Google Scholar] [CrossRef]
- Fenollar, F.; Nicoli, F.; Paquet, C.; Lepidi, H.; Cozzone, P.; Antoine, J.-C.; Pouget, J.; Raoult, D. Progressive dementia associated with ataxia or obesity in patients with Tropheryma whipplei encephalitis. BMC Infect. Dis. 2011, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Kilani, M.; Njim, L.; Nsir, A.B.; Hattab, M.N. Whipple Disease Presenting as Cystic Brain Tumor: Case Report and Review of the Literature. Turk. Neurosurg. 2018, 28, 495–499. [Google Scholar] [PubMed]
- Brönnimann, D.; Vareil, M.-O.; Sibon, I.; Lagier, J.-C.; Lepidi, H.; Puges, M.; Haneche, F.; Raoult, D.; Desclaux, A.; Neau, D.; et al. Limbic encephalitis as a relapse of Whipple’s disease with digestive involvement and spondylodiscitis. Infection 2018, 47, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Seneca, V.; Imperato, A.; Colella, G.; Cioffi, V.; Mariniello, G.; Gangemi, M. Recurrent acute obstructive hydrocephalus as clinical onset of cerebral Whipple’s disease. Clin. Neurol. Neurosurg. 2010, 112, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Tábuas-Pereira, M.; Vicente, M.; Coelho, F.; Santana, I. Prosopagnosia as the presenting symptom of whipple disease. Cogn. Behav. Neurol. 2016, 29, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Malikova, H.; Peregrin, J. Primary Whipple disease of the brain: case report with long-term clinical and MRI follow-up. Neuropsychiatr. Dis. Treat. 2015, 11, 2461–2469. [Google Scholar] [CrossRef]
- Giaccone, G.; Carella, F.; Parravicini, C.; Longhi, E.; Chiapparini, L.; Savoiardo, M.; Montano, N.; Morbin, M.; Albanese, A.; Tagliavini, F. A 52-year-old man with myoclonic jerks. Brain Pathol. 2016, 26, 291–292. [Google Scholar] [CrossRef]
- Gaddy, J.R.; Khan, Z.Z.; Chaser, B.; Scofield, R.H. Whipple’s disease diagnosis following the use of TNF-α blockade. Rheumatology 2012, 51, 946. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.A.; Moreira, R.K.; Lam-Himlin, D.; De Petris, G.; Montgomery, E. Whipple disease a century after the initial description: Increased recognition of unusual presentations, autoimmune comorbidities, and therapy effects. Am. J. Surg. Pathol. 2012, 36, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Trotta, L.; Di Stefano, M.; Balduzzi, D.; Marchese, A.; Vattiato, C.; Bianchi, P.I.; Fenollar, F.; Corazza, G.R. Previous immunosuppressive therapy is a risk factor for immune reconstitution inflammatory syndrome in Whipple’s disease. Dig. Liver Dis. 2012, 44, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G.E.; et al. The immune reconstitution inflammatory syndrome in whipple disease: a cohort study. Ann. Intern. Med. 2010, 153, 710–717. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Raoult, D. Immune reconstitution inflammatory syndrome associated with bacterial infections. Expert Opin. Drug Saf. 2014, 13, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Moos, V.; Loddenkemper, C.; Marth, T.; Fenollar, F.; Raoult, D. Whipple’s disease: new aspects of pathogenesis and treatment. Lancet Infect. Dis. 2008, 8, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G.E.; Junga, N.S.; Marth, T. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple’s disease. Gastroenterology 2010, 138, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Feurle, G.E.; et al. Intravenous ceftriaxone, followed by 12 or three months of oral treatment with trimethoprim-sulfamethoxazole in Whipple’s disease. J. Infect. 2013, 66, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Moter, A.; Janneck, M.; Wolters, M.; Iking-Konert, C.; Wiessner, A.; Loddenkemper, C.; Hartleben, B.; Lütgehetmann, M.; Schmidt, J.; Langbehn, U.; et al. Potential Role for Urine Polymerase Chain Reaction in the Diagnosis of Whipple’s Disease. Clin. Infect. Dis. 2018, 68, 1089–1097. [Google Scholar] [CrossRef]
- Tison, A.; Saraux, A. Potential Role for Urine Polymerase Chain Reaction in the Diagnosis of Whipple Disease. Clin. Infect. Dis. 2019, 69, 904–905. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Cammilleri, S.; Raoult, D. Classic Whipple’s disease diagnosed by (18)F-fluorodeoxyglucose PET. Lancet Infect. Dis. 2016, 16, 130. [Google Scholar] [CrossRef]
- Fenollar, F.; Perreal, C.; Raoult, D. Tropheryma whipplei natural resistance to trimethoprim and sulphonamides in vitro. Int. J. Antimicrob. Agents 2014, 43, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Rolain, J.-M.; Alric, L.; Papo, T.; Chauveheid, M.-P.; van de Beek, D.; Raoult, D. Resistance to trimethoprim/sulfamethoxazole and Tropheryma whipplei. Int. J. Antimicrob. Agents 2009, 34, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Lagier, J.-C.; Raoult, D. Tropheryma whipplei and Whipple’s disease. J. Infect. 2014, 69, 103–112. [Google Scholar] [CrossRef]
- Biagi, F.; Biagi, G.L.; Corazza, G.R. What is the best therapy for Whipple’s disease? Our point of view. Scand. J. Gastroenterol. 2016, 52, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Trotta, L.; Di Stefano, M.; Balduzzi, D.; Marchese, A.; Vattiato, C.; Bianchi, P.I.; Fenollar, F.; Corazza, G.R. Previous immunosuppressive therapy is a risk factor for immune reconstitution inflammatory syndrome in Whipple’s disease. Dig. Liver Dis. 2012, 44, 880–882. [Google Scholar] [CrossRef]
- Yu, C.; Gershwin, M.E.; Chang, C. Diagnostic criteria for systemic lupus erythematosus: A critical review. J. Autoimmun. 2014, 48-49, 10–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




