Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Sample collection and processing
| Species | Number of sample | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| P. pawak | 417 | 226 | 579 | 284 |
| P. anea | 317 | 241 | 788 | 388 |
2.2. Stomach contents analysis
2.3. Carbon and nitrogen stable isotope analysis
2.4. Spatial niche
2.5. Data processing
3. Results
3.1. Feeding habit
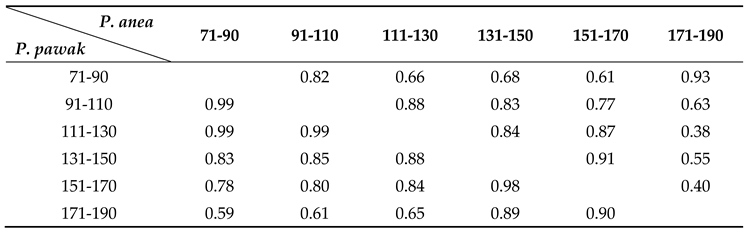
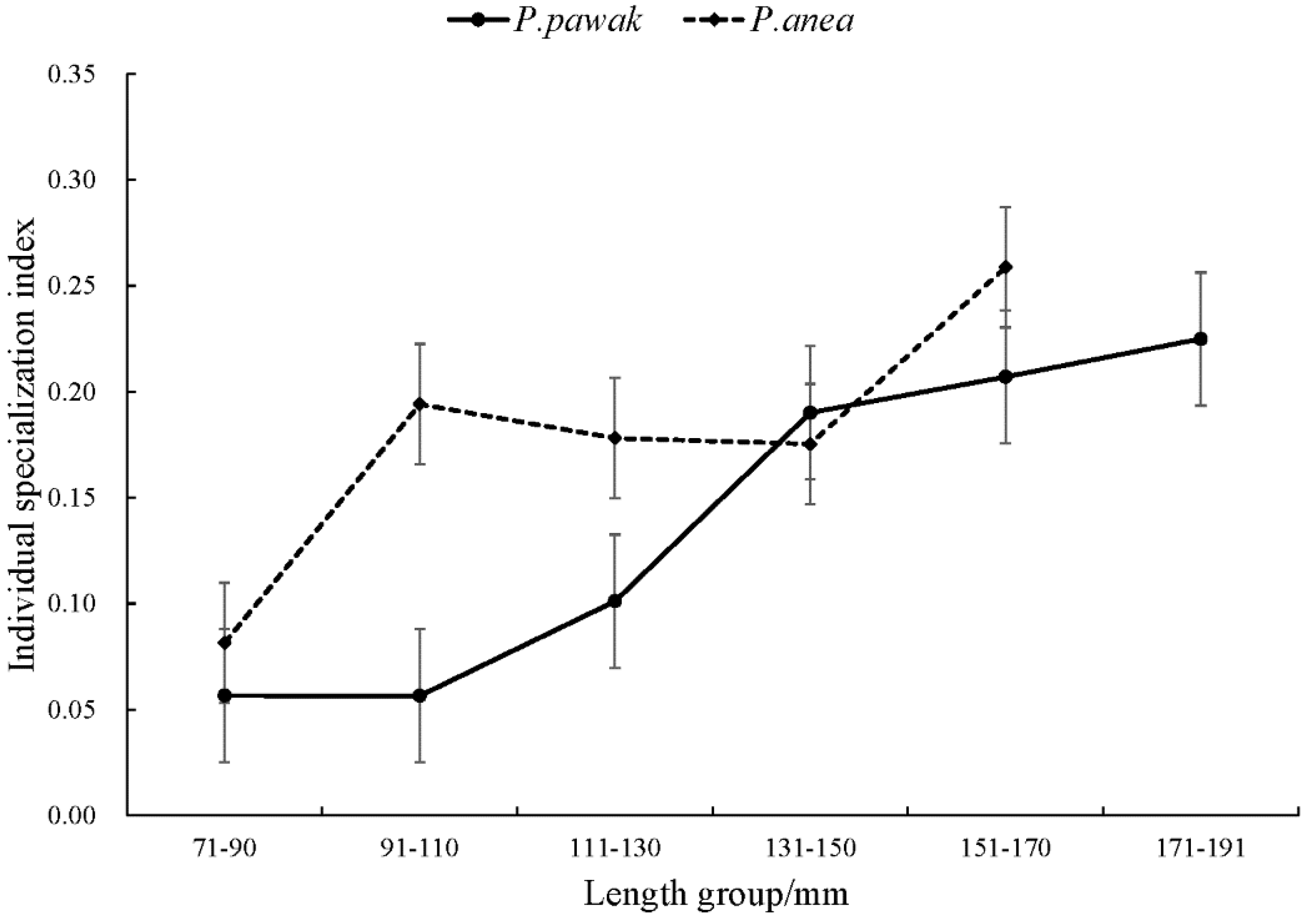
3.1.1. Differences in feeding habits within species
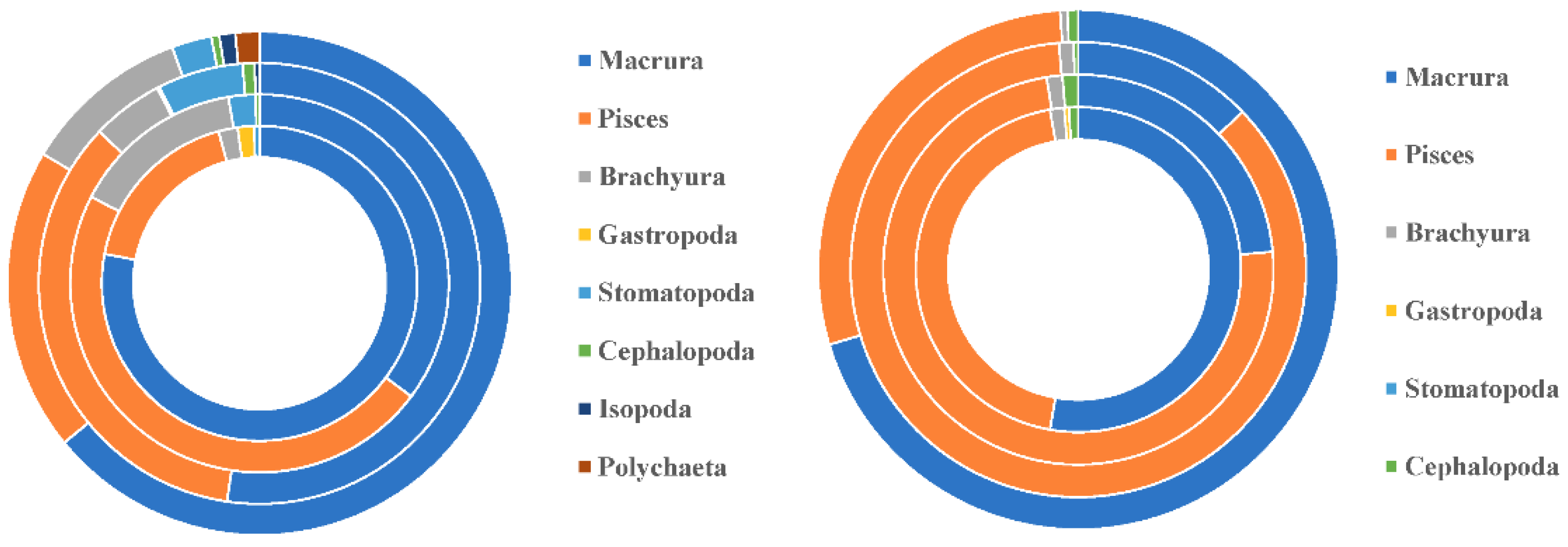
3.1.2. Differences in feeding habits between species
| Prey | P. pawak | P. anea | ||||||
|---|---|---|---|---|---|---|---|---|
| Science name | W% | N% | F% | IRI% | W% | N% | F% | IRI% |
| Pisces | ||||||||
| Leiognathidae | 0.46 | 0.22 | 0.56 | 0.02 | 0.42 | 0.92 | 3.86 | 0.03 |
| Trichiuridae | 0.87 | 0.88 | 2.22 | 0.18 | 0.47 | 0.23 | 0.97 | + |
| Thrissa dussumieri | 2.90 | 0.44 | 1.11 | 0.17 | 3.02 | 0.69 | 2.90 | 0.07 |
| Champsodon atridorsalis | 0.35 | 0.66 | 1.67 | 0.08 | 0.10 | 0.12 | 0.48 | + |
| Photopectoralis bindus | 1.98 | 2.84 | 7.22 | 1.60 | 1.09 | 0.69 | 2.90 | 0.03 |
| Stolephorus heterolobas | 0.66 | 0.44 | 1.11 | 0.06 | 0.23 | 0.12 | 0.48 | + |
| Secutor ruconius | 0.23 | 0.22 | 0.56 | 0.01 | 0.83 | 0.35 | 1.45 | 0.01 |
| Bregmaceros rarisquamosus | 1.54 | 6.35 | 16.11 | 5.82 | 8.07 | 23.44 | 98.07 | 19.14 |
| Apogonidae | 4.52 | 6.13 | 15.56 | 7.60 | 0.21 | 0.12 | 0.48 | + |
| Bregmaceros | 3.25 | 7.88 | 20.00 | 10.20 | 9.07 | 28.52 | 119.32 | 27.79 |
| Gobiidae | 2.44 | 3.50 | 8.89 | 2.42 | 0.05 | 0.12 | 0.48 | + |
| Atherinidae | 0.33 | 0.22 | 0.56 | 0.01 | 1.55 | 0.46 | 1.93 | 0.02 |
| Bregmaceros nectabanus | 3.08 | 3.94 | 10.00 | 3.22 | 6.20 | 7.51 | 31.40 | 2.67 |
| Stolephorus indicus | 0.23 | 0.22 | 0.56 | 0.01 | 1.26 | 0.35 | 1.45 | 0.01 |
| Stolephorus | 2.75 | 2.41 | 6.11 | 1.45 | 47.16 | 26.10 | 109.18 | 49.55 |
| Champsodon | 0.63 | 0.88 | 2.22 | 0.15 | - | - | - | - |
| Jaydia striata | 0.11 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Trypauchen vagina | 0.94 | 0.66 | 1.67 | 0.12 | - | - | - | - |
| Thryssa | 0.21 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Bregmaceros macclellandii | 0.59 | 0.22 | 0.56 | 0.02 | - | - | - | - |
| Diaphus knappi | 0.11 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Parachaeturichthys polynema | 2.56 | 1.53 | 3.89 | 0.73 | - | - | - | - |
| Carangidae | 0.54 | 0.22 | 0.56 | 0.02 | - | - | - | - |
| Leiognathus berbis | 0.67 | 0.22 | 0.56 | 0.02 | - | - | - | - |
| Sirembo imberbis | 0.22 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Callionymus octostigmatus | 1.15 | 0.22 | 0.56 | 0.03 | - | - | - | - |
| Sirembo | 0.04 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Hypoatherina valenciennei | - | - | - | - | 0.64 | 0.12 | 0.48 | + |
| Leiognathus nuchalis | - | - | - | - | 0.08 | 0.12 | 0.48 | + |
| Stolephorus commersonnii | - | - | - | - | 4.59 | 1.62 | 6.76 | 0.26 |
| Stolephorus zollingeri | - | - | - | - | 0.60 | 0.23 | 0.97 | + |
| Sardinella | - | - | - | - | 6.18 | 0.92 | 3.86 | 0.17 |
| Stolephorus chinensis | - | - | - | - | 0.56 | 0.35 | 1.45 | 0.01 |
| Macrura | ||||||||
| Alpheus | 5.16 | 16.19 | 41.11 | 40.26 | 1.05 | 1.50 | 6.28 | 0.10 |
| Metapenaeopsis palmensis | 0.85 | 1.09 | 2.78 | 0.25 | 0.48 | 0.23 | 0.97 | + |
| Alpheus bisincisus | 3.57 | 5.47 | 13.89 | 5.76 | 0.70 | 0.35 | 1.45 | 0.01 |
| Metapenaeopsis barbata | 9.47 | 7.00 | 17.78 | 13.43 | 0.52 | 0.12 | 0.48 | - |
| Solenocera crassicornis | 2.55 | 1.75 | 4.44 | 0.88 | 0.46 | 0.12 | 0.48 | - |
| Metapenaeopsis | 0.70 | 1.31 | 3.33 | 0.31 | - | - | - | - |
| Alpheus brevicristatus | 0.84 | 1.31 | 3.33 | 0.33 | - | - | - | - |
| Penaeidae | 1.59 | 1.31 | 3.33 | 0.44 | - | - | - | - |
| Parapenaeopsis | 0.10 | 0.44 | 1.11 | 0.03 | - | - | - | - |
| Parapenaeopsis | 0.51 | 0.22 | 0.56 | 0.02 | - | - | - | - |
| Metapenaeopsis acclivis | 0.56 | 0.22 | 0.56 | 0.02 | - | - | - | - |
| Parapenaeus sextuberculatus | 0.43 | 0.44 | 1.11 | 0.04 | - | - | - | - |
| Parapenaeus | 0.17 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Alpheus japonicus | 0.22 | 0.44 | 1.11 | 0.03 | - | - | - | - |
| Alpheus distinguendus | 1.21 | 1.75 | 4.44 | 0.60 | - | - | - | - |
| Metapenaeus | 0.06 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Trachypenaeus curvirostris | 0.26 | 0.44 | 1.11 | 0.04 | - | - | - | - |
| Palaemonidae | 0.03 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Miyadiella podophthalmus | 0.02 | 0.44 | 1.11 | 0.02 | - | - | - | - |
| Parapenaeus longipes | 0.68 | 0.88 | 2.22 | 0.16 | - | - | - | |
| Parapenaeopsis cornuta | - | - | - | - | 0.54 | 0.46 | 1.93 | 0.01 |
| Trachypenaeus pescadoreensis | - | - | - | - | 0.52 | 0.12 | 0.48 | + |
| Parapenaeopsis incisa | - | - | - | - | 0.21 | 0.23 | 0.97 | + |
| Brachyura | ||||||||
| Portunus | 0.18 | 1.31 | 3.33 | 0.23 | 0.03 | 0.12 | 0.48 | - |
| Eucrate alcocki | 0.06 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Typhlocarcinops canaliculata | 0.05 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Charybdis hellerii | 0.21 | 0.44 | 1.11 | 0.03 | - | - | - | - |
| Charybdis variegata brevispinosa | 0.55 | 0.66 | 1.67 | 0.09 | - | - | - | - |
| Lissocarcinus laevis | 0.03 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Portunus hastatoides | 0.58 | 2.19 | 5.56 | 0.70 | - | - | - | - |
| Jonas | 0.04 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Eucrate solaris | 0.12 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Charybdis | 0.25 | 0.88 | 2.22 | 0.11 | - | - | - | - |
| Petrolisthes | 0.05 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Portunus argentatus | 1.43 | 2.41 | 6.11 | 1.07 | - | - | - | - |
| Charybdis truncata | 0.07 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Carcinoplax purpurea | 0.10 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Charybdis vadorum | 0.06 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Charybdis variegata | 0.10 | 0.44 | 1.11 | 0.03 | - | - | - | - |
| Typhlocarcinus villosusStimpson | - | - | - | - | 0.03 | 0.12 | 0.48 | - |
| Stomatopoda | ||||||||
| Gryllotalpidae | 0.16 | 0.22 | 0.56 | 0.01 | 0.09 | 0.12 | 0.48 | + |
| Oratosquillina interrupta | 0.48 | 0.44 | 1.11 | 0.05 | - | - | - | - |
| Oratosquilla oratoria | 0.35 | 0.44 | 1.11 | 0.04 | - | - | - | - |
| Anchisquilla fasciata | 0.16 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Oratosquillina | 0.28 | 0.44 | 1.11 | 0.04 | - | - | - | - |
| kempina stridulans | 0.77 | 0.66 | 1.67 | 0.11 | - | - | - | - |
| Squillidae | - | - | - | - | 0.23 | 0.92 | 3.86 | 0.03 |
| Cephalopoda | ||||||||
| Cephalopoda | 0.17 | 1.53 | 3.89 | 0.30 | 2.00 | 0.69 | 2.90 | 0.05 |
| Isopoda | ||||||||
| isopoda | 0.15 | 0.88 | 2.22 | 0.10 | 0.04 | 0.23 | 0.97 | + |
| Gastropoda | ||||||||
| Turritella terebra | 0.32 | 1.53 | 3.89 | 0.33 | - | - | - | - |
| Polychaeta | ||||||||
| Nereis | 0.14 | 0.22 | 0.56 | 0.01 | - | - | - | - |
| Sipunculs nudus | 0.23 | 0.44 | 1.11 | 0.03 | - | - | - | - |
3.2. Isotopic characteristics and trophic levels
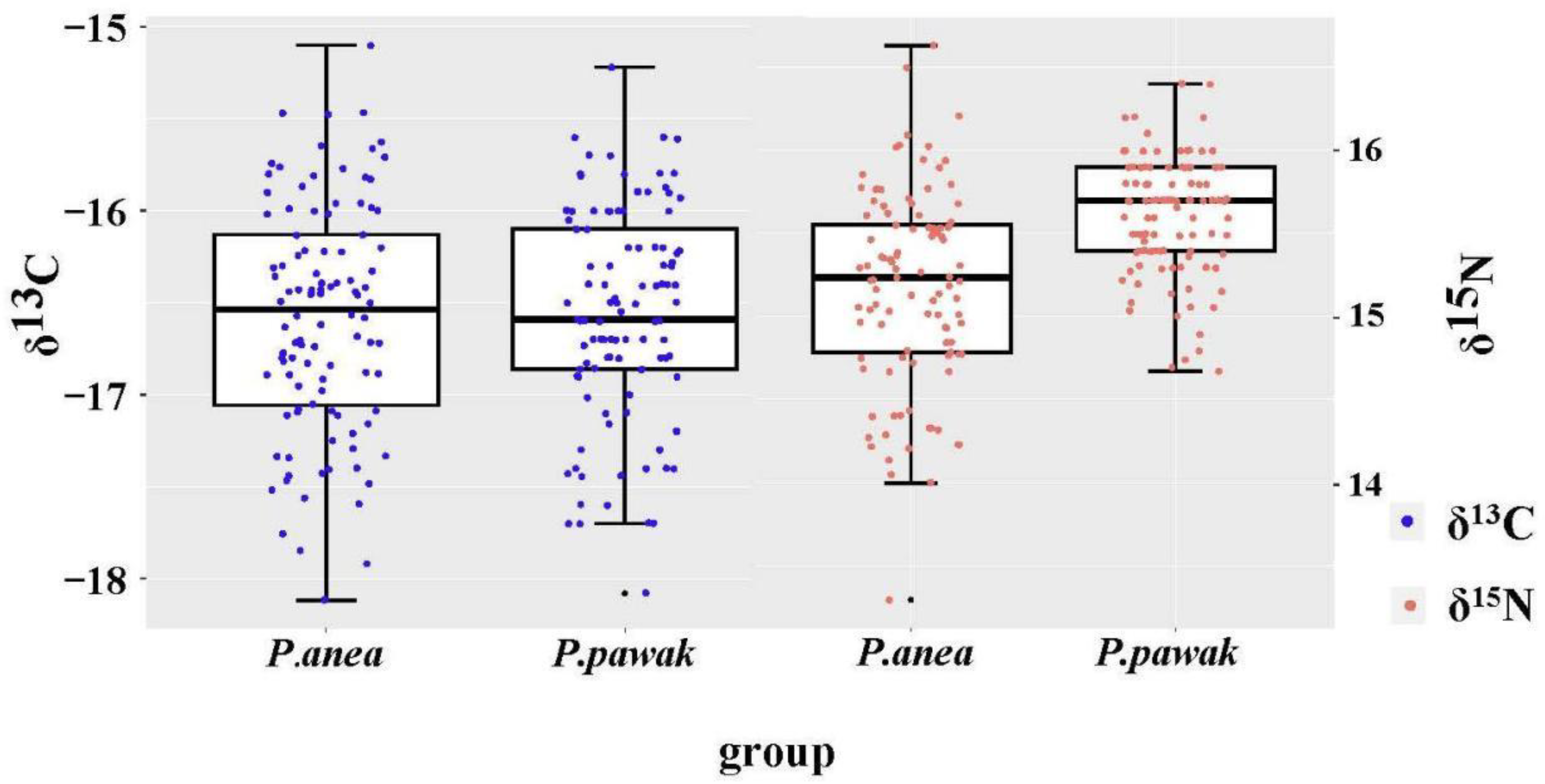
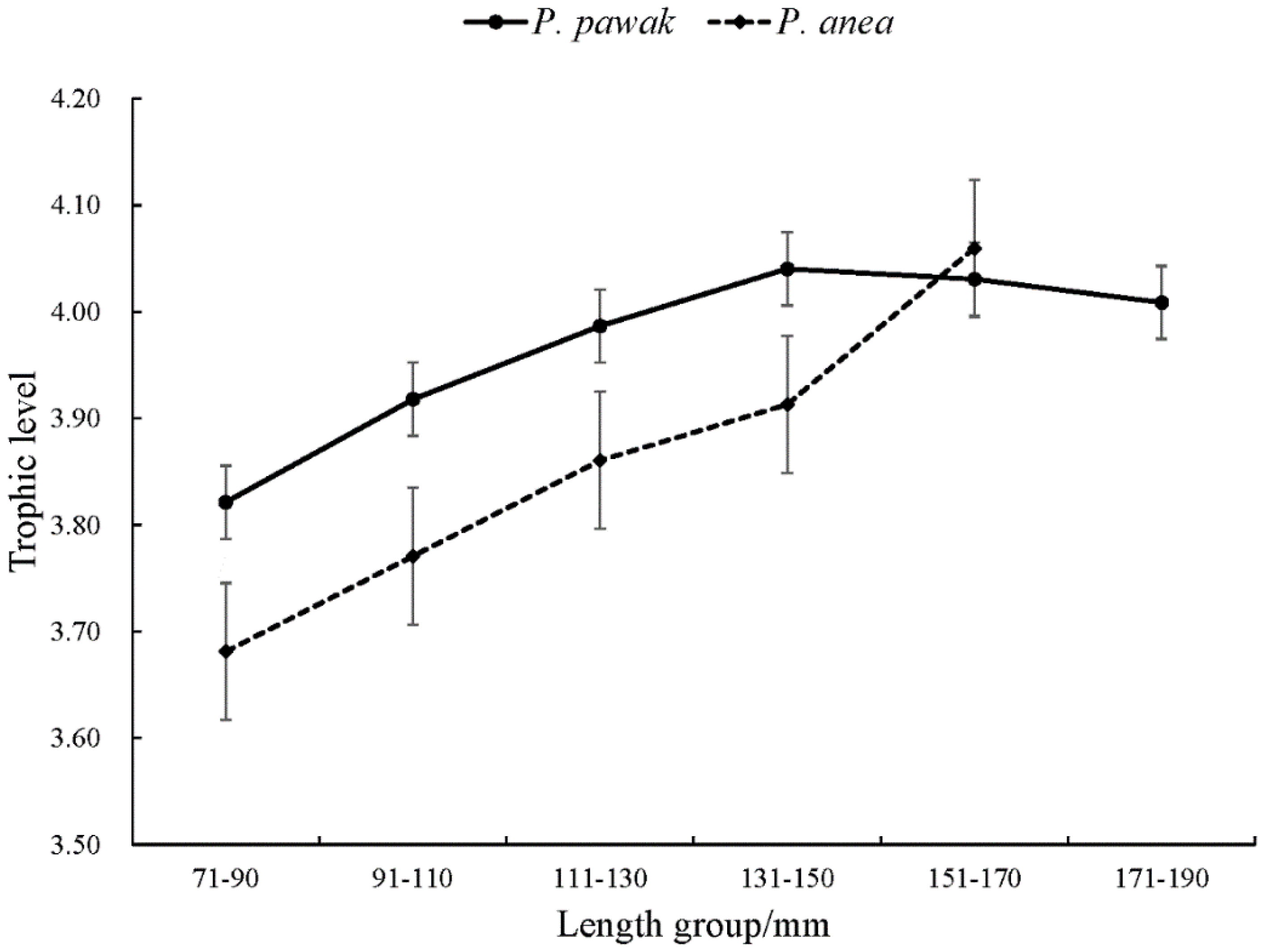
3.3. Trophic niche

| Species | CD | CR | NR | MNND | SDNND | SEAc | TA | Overlap index |
|---|---|---|---|---|---|---|---|---|
| P. pawak | 0.59 | 2.86 | 1.72 | 0.09 | 0.09 | 0.65 | 3.70 | 0.29 |
| P. anea | 0.76 | 3.02 | 3.32 | 0.13 | 0.15 | 1.16 | 6.09 |
3.4. Spatial niche
| Total | Before SCS | After SCS | ||
|---|---|---|---|---|
| Spatial niche width | P. pawak | 1.19 | 0.10 | 1.22 |
| P. anea | 0.58 | 0.85 | 0.50 | |
| Overlap index | 0.20 | 0.16 | 0.13 |
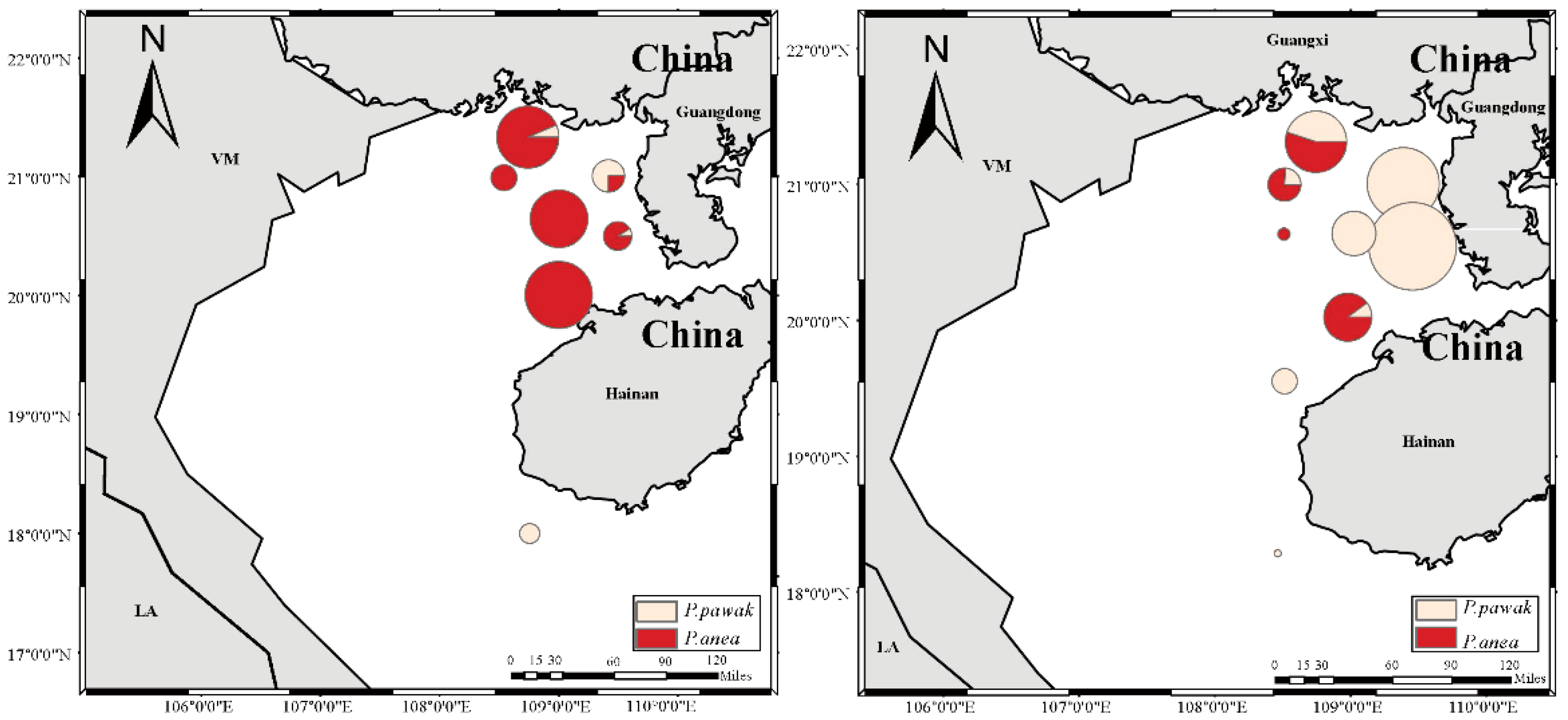
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J. Advanced aquatic biology; Science Press: Beijing, China, 1999.
- Brönmark, C., Hansson, L. The biology of lakes and ponds; Oxford University Press: New York, USA, 2005.
- Tang, Q. Strategies of research on marine food web and trophodynamics between high trophic levels. Progress in Fishery Sciences 1999, 2: 1-6.
- Schoener, T. Resource partitioning in ecological communities: research on how similar species divide resources helpsreveal the natural regulation of species diversity. Science 1974, 185: 27-39.
- Wang, J., Su, Y., Liu, J., et al. The feeding habits of five sciaenids fishes in Louyuan Bay. Journal of Jimei University (Natural Science) 1994, 2: 34-39.
- Li, Z., Dai, Q., Zuo, T., et al. Studies on food competition between Pseudosciaena polyactis and Johnius belengerii from changjian estuary and adjacent southern yellow sea in autumn. Journal of Hydroecology 2009, 30: 67-72.
- Matley, J., Heupel, M., Fisk, A., et al. Measuring niche overlap between co-occurring plectropomus spp. Using acoustic telemetry and stable isotopes. Mar Freshwater Res 2017, 68: 1468. [CrossRef]
- Wang, Q., Liu, M., Zhu, F., et al. Comparative study of three species of schizothoracine on feeding and digestive organs in Upper Nujiang River. Chinese Journal of Zoology 2019, 54: 207-221.
- Zhang, Y., Xu, B., Zhang, C., et al. Spatial heterogeneity in the feeding habits and feeding ground distribution of johnius belangerii in Haizhou Bay during spring. Journal of Fishery Sciences of China 2020, 27: 315-326.
- Olesen, J. M., Bascompte, J., Dupont, Y. L., et al. The modularity of pollination networks. P Natl Acad Sci USA 2007, 104: 19891-19896. [CrossRef]
- Raul, C. P., MaRcio, S. A., Souza, F. L., et al. Competition and resource breadth shape niche variation and overlap in multiple trophic dimensions. Proc Biol Sci 2019, 1902: 20190369.
- Li, Y., Wang, H., Chen, X., et al. Trophic niches and interspecific relationships between closely related ommastrephidae species, dosidicus gigas and sthenoteuthis oualaniensis. Acta Ecologica Sinica 2020, 40: 5418-5423.
- Xiao, Y., Jiang, R., Yin, R., et al. Trophic niche and interspecific relationship of five eels in the waters of the zhoushan islands. Journal of Fisheries of China 2023, 47: 65-74.
- Yang, Z., Chen, X., Zhao, N., et al. The effect of different habitat types and ontogenetic stages on the diet shift of a critically endangered fish species, coreius guichenoti (sauvage and dabry de thiersant, 1874). Int J Environ Res Public Health 2018, 15: 2204. [CrossRef]
- Hyslop, E. J. Stomach contents analysis-a review of methods and their application. J Fish Biol 1980, 17: 411-429. [CrossRef]
- Xue, Y., Jin, X. Review of the study on feeding habits of fishes and food webs. Progress in Fishery Sciences 2003, 2: 76-87.
- Dixon, H., Dempson, J., Sheehan, T., et al. Assessing the diet of North American Atlantic salmon (Salmo salar l.) off the West Greenland coast using gut content and stable isotope analyses. Fisheries Oceanography 2017, 26: 555-568. [CrossRef]
- Wang, X., Qiu, Y., Du, F., et al. Dynamics of demersal fish species diversity and biomass of dominant species in autumn in the Beibu Gulf, northwestern South Shina Sea. Acta Ecologica Sinica 2012, 32: 333-342.
- Yi, X., Qiu, K., Zhou, X., et al. Analysis of fishery biology of pennahia pawak in Beibu Gulf. Journal of Shanghai Ocean University 2021, 30: 515-524.
- Wang, X., Du, F., Qiu, Y. Length-weight relationships of important commercial fishes in Northern South China Sea. Journal of Applied Oceanography 2006, 2: 262-266.
- Wagiyo, K., Tirtadanu, Chodriyah, U. Biology characteristic, abundance index and fishing aspect of donkey croaker (pennahia anea bloch,1793) in the Tangerang Waters. E3S Web of Conferences 2020, 4: 1011.
- Tuuli, C., de Mitcheson, Y., Liu, M. Reproductive biology of the greyfin croaker pennahia anea in the northern South China Sea. Ichthyol Res 2011, 58: 302-309. [CrossRef]
- He, X., Tao, Y., Hou, G., et al. Population structure and spatio-temporal distribution of pennahia pawak in the Beibu Gulf, South China Sea. Journal of Guangdong Ocean University 2015, 35: 35-42.
- Yan, Y., Hou, G., Lu, H., et al. Age and growth of pawak croaker Pennahia pawak in Beibu Gulf. Journal of Fishery Sciences of China 2011, 18: 145-155. [CrossRef]
- Yi, X. Feeding ecology of pawak croaker (pennahia pawak) in the Beibu Gulf. Master Thesis, Guangdong Ocean University, Zhanjiang, China, 2021.
- Third Institute of Oceanography State Oceanic Administration. Specifications for oceanographic survey—part 6: marine biological survey; State General Administration of the People"s Republic of China for Quality Supervision and Inspection and Quarantine; Standardization Administration of the People'sRepublic of China: Beijing, China, 2007.
- Figueiredo, M., Morato, T., Barreiros, J. P., et al. Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the azores. Fish Res 2005, 75: 107-119. [CrossRef]
- Dou, S. Fish-stomach content analysis: methods and application. Marine Science Bulletin 1992, 2: 28-31.
- Pianka, E. The structure of lizard communities. Annu Rev Ecol Evol S 1973, 4: 53-74. [CrossRef]
- Shannon, C. The mathematical theory of communication. 1963. Md Comput 1997, 14: 306-317.
- Roughgarden, J. Evolution of niche width. The American Naturalist 1972, 106: 683-718.
- Bolnick, D. I., Yang, L. H., Fordyce, J. A., et al. Measuring individual-level resource specialization. Ecology 2002, 83: 2936-2941.
- McKinney, C., McCrea, J., Epstein, S., et al. Improvements in mass spectrometers for the measurement of small differences in isotope abundance ratios. Rev Sci Instrum 1950, 21: 724-730. [CrossRef]
- Post, D. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology (Durham), 2002, 83: 703-718.
- He, X., Zhu, D., Zhao, C., et al. Feeding habit of asian moon scallop (amusium pleuronectes) and as an isotopic baseline indicator in the Beibu Gulf, South China Sea. J Shellfish Res 2019, 38: 245-252. [CrossRef]
- Jackson, A., Inger, R., Parnell, A., et al. Comparing isotopic niche widths among and within communities: siber - stable isotope bayesian ellipses in R. J Anim Ecol 2011, 80: 595-602. [CrossRef]
- Schoener, T. W. Sizes of feeding territories among birds. Ecology 1968, 49: 123-141. [CrossRef]
- Zhan, B. Fishery resources assessment; China Agriculture Press: Beijing, China, 1995, pp 26-27.
- Krebs, C. Ecological methodology; Harper Collins Publishers: New York, USA, 1989.
- Layman, C., Post, D. Can stable isotope ratios provide for community-wide measures of trophic structure? Reply. Ecology 2008, 89: 2358-2359. [CrossRef]
- Ward, A., Webster, M., Hart, P. Intraspecific food competition in fishes. Fish Fish, 2006,7(4):231-261. [CrossRef]
- Deng, J., Jiang, W., Yang, J., et al. Species interaction and food web of major predatory species in the Bohai Sea. Journal of Fishery Sciences of China 1997, 4: 2-8.
- Tang, Q., Fan, Y., Lin, H. Initial inquiring into the developmental strategy of chinese ocean ecosystem dynamics research. Advances in Earth Science 1996, 2: 160-168.
- Svanbäck, R., Bolnick, D. I. Intraspecific competition affects the strength of individual specialization: an optimal diet theory method. Evol Ecol Res 2005, 7: 993-1012.
- Xia, Y., Li, Y., Zhu, S., et al. Individual dietary specialization reduces intraspecific competition, rather than feeding activity, in black amur bream (megalobrama terminalis). Sci Rep 2020, 10: 17961. [CrossRef]
- Zhang, B., Yuan, W., Dai, F. Study on feeding ecology of fish community in Laoshan Bay during summer using stable carbon and nitrogen isotopes. Journal of Fisheries of China 2016, 40: 585-594.
- Ji, W., Chen, X., Jiang, Y., et al. Stable isotope analysis of some representative nektonic organisms in the central and northern part of East China Sea. Marine Fisheries 2011, 33: 241-250.
- Gao, Y., Sui, H., Ren, X., et al. Feeding habits of saurida elongata in Haizhou Bay, Shandong, China, based on stomach contents and stable isotope. Chinese Journal of Applied Ecology 2020, 31: 4277-4283.
- Wang, F., Ju, R., Li, Z., et al. Niche concept and its application in insect ecology. Chinese Journal of Ecology 2006, 10: 1280-1284.
- Yang, X., Ma, J. A review on some terms related to niche and their measurements. Chinese Journal of Ecology 1992, 2: 46-51.
- Hurlbert, S. H. The measurement of niche overlap and some relatives. Ecology 1978, 1: 66-77. [CrossRef]
- Abrams, P. Some comments on measuring niche overlap. Ecology 1980, 61: 44-49. [CrossRef]
- Yu, Z., Jin, X., Li, X. Analysis of ecological niche for major fish species in the central and southern Yellow Sea. Progress in Fishery Sciences 2010, 31: 1-8.
- Han, D., Xue, Y., Ji, Y., et al. Trophic and spatial niche of five gobiid fishes in Jiaozhou Bay. Journal of Fishery Sciences of China 2013, 20: 148-156. [CrossRef]
- Cachera, M., Ernande, B., Villanueva, M. C., et al. Individual diet variation in a marine fish assemblage: optimal foraging theory, niche variation hypothesis and functional identity. J Sea Res 2017, 120: 60-71. [CrossRef]
- Svanback, R., Bolnick, D. I. Intraspecific competition drives increased resource use diversity within a natural population. Proc Biol Sci 2007, 274: 839-844. [CrossRef]
- Ross, S. Resource partitioning in fish assemblages: a review of field studies. Copeia 1986, 2: 352-388. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





