Submitted:
13 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Echocardiography
2.3. Statistical analysis
3. Results
3.1. Groups at baseline
3.2. Prognostic value of GLS and NT-proBNP at baseline
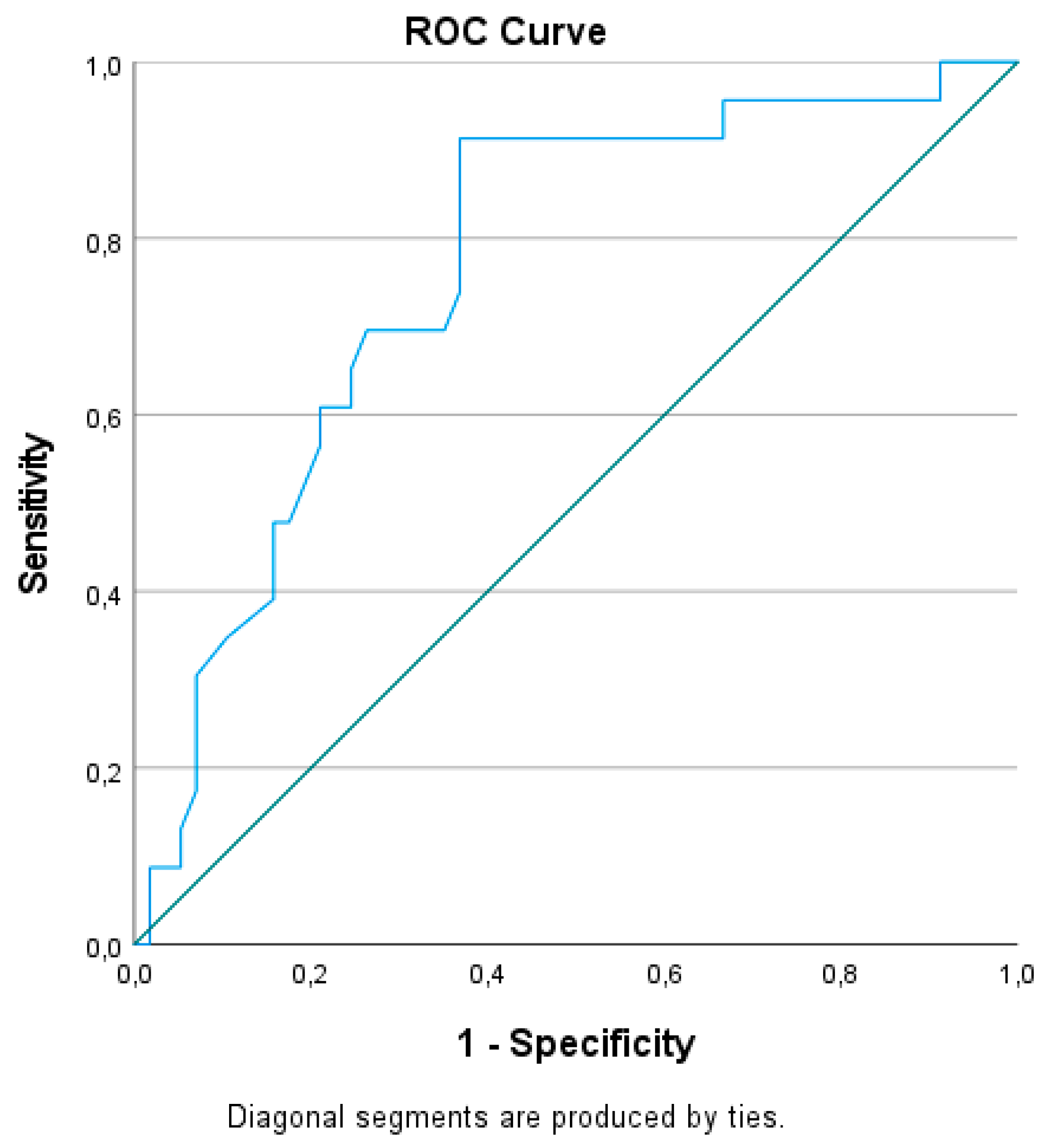
3.3. Prediction of CRT response by GLS and NT-proBNP
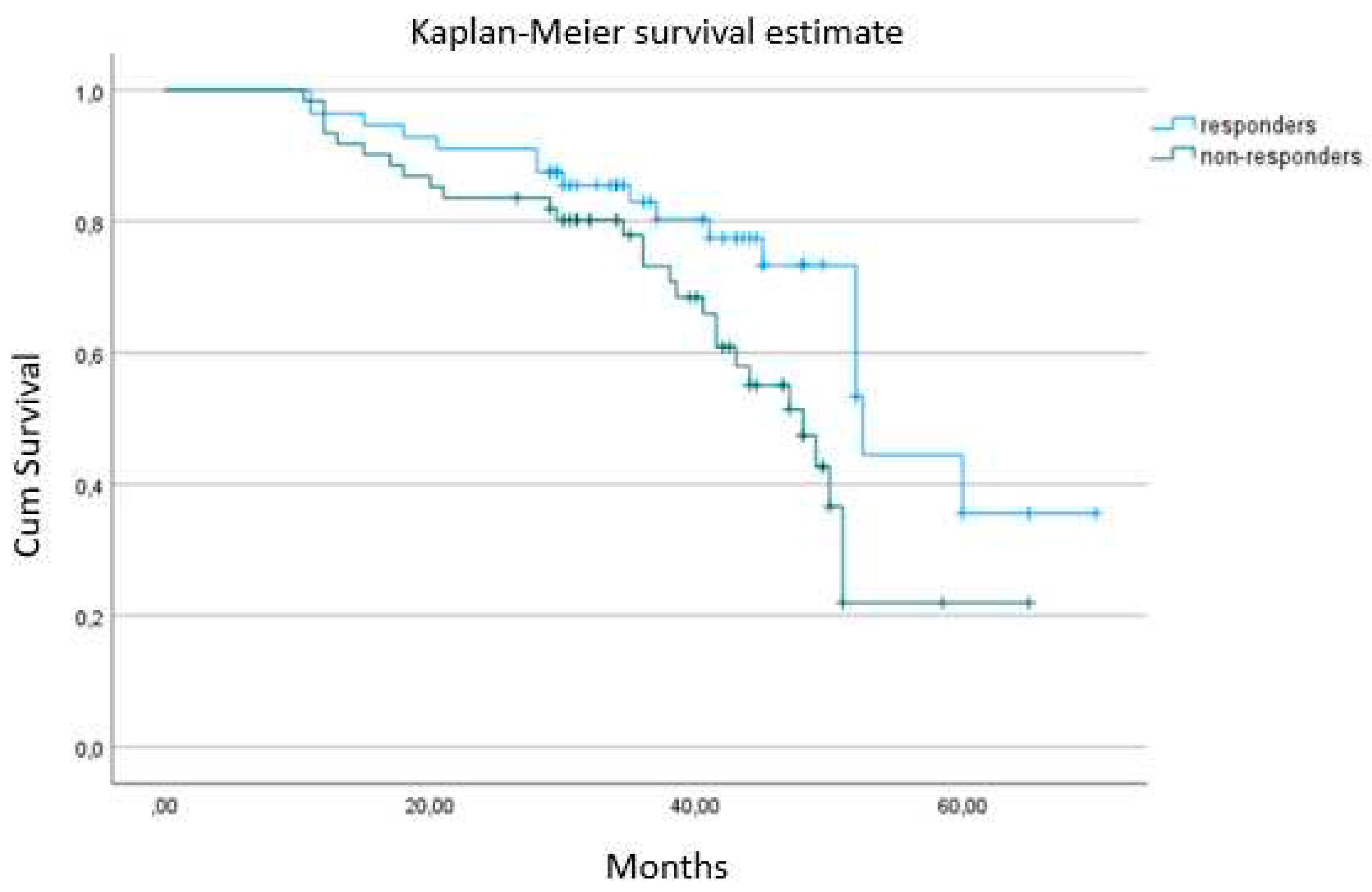
3.4. Changes of GLS and NT-proBNP with CRT and their prognostic value
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM; ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 Sep 14;42(35):3427-3520.
- Wang Z, Wu Y, Zhang J. Cardiac resynchronization therapy in heart failure patients: tough road but clear future. Heart Fail Rev. 2021 May;26(3):735-745.
- Bazoukis G, Thomopoulos C, Tse G, Tsioufis K, Nihoyannopoulos P. Global longitudinal strain predicts responders after cardiac resynchronization therapy-a systematic review and meta-analysis. Heart Fail Rev. 2021 Mar 30. [CrossRef]
- Zhu H, Zou T, Zhong Y, Yang C, Ren Y, Wang F. Prevention of non-response to cardiac resynchronization therapy: points to remember. Heart Fail Rev. 2020 Mar;25(2):269-275. [CrossRef]
- Martins R, António N, Donato H, Oliveiros B. Predictors of echocardiographic response to cardiac resynchronization therapy: A systematic review with Meta-Analysis. Int J Cardiol Heart Vasc. 2022 Feb 28;39:100979. [CrossRef]
- Mele D, Luisi GA, Malagù M, Laterza A, Ferrari R, Bertini M. Echocardiographic evaluation of cardiac dyssynchrony: Does it still matter? Echocardiography. 2018 May;35(5):707-715.
- Hu X, Xu H, Hassea SRA, Qian Z, Wang Y, Zhang X, Hou X, Zou J. Comparative efficacy of image-guided techniques in cardiac resynchronization therapy: a meta-analysis. BMC Cardiovasc Disord. 2021 May 24;21(1):255. [CrossRef]
- Bazoukis G, Naka KK, Alsheikh-Ali A, Tse G, Letsas KP, Korantzopoulos P, Liu T, Yeung C, Efremidis M, Tsioufis K, Baranchuk A, Stavrakis S. Association of QRS narrowing with response to cardiac resynchronization therapy-a systematic review and meta-analysis of observational studies. Heart Fail Rev. 2020 Sep;25(5):745-756. [CrossRef]
- Dal Ferro M, De Paris V, Collia D, Stolfo D, Caiffa T, Barbati G, Korcova R, Pinamonti B, Zovatto L, Zecchin M, Sinagra G, Pedrizzetti G. Left Ventricular Response to Cardiac Resynchronization Therapy: Insights From Hemodynamic Forces Computed by Speckle Tracking. Front Cardiovasc Med. 2019 May 14;6:59. [CrossRef]
- van der Bijl P, Kostyukevich MV, Khidir M, Ajmone Marsan N, Delgado V, Bax JJ. Left ventricular remodelling and change in left ventricular global longitudinal strain after cardiac resynchronization therapy: prognostic implications. Eur Heart J Cardiovasc Imaging. 2019 Oct 1;20(10):1112-1119. [CrossRef]
- Menet A, Guyomar Y, Ennezat PV, Graux P, Castel AL, Delelis F, Heuls S, Cuvelier E, Gevaert C, Le Goffic C, Tribouilloy C, Maréchaux S. Prognostic value of left ventricular reverse remodeling and performance improvement after cardiac resynchronization therapy: A prospective study. Int J Cardiol. 2016 Feb 1;204:6-11. [CrossRef]
- Vazquez-Montes MDLA, Debray TPA, Taylor KS, Speich B, Jones N, Collins GS, Hobbs FDRR, Magriplis E, Maruri-Aguilar H, Moons KGM, Parissis J, Perera R, Roberts N, Taylor CJ, Kadoglou NPE, Trivella M; proBHF group. UMBRELLA protocol: systematic reviews of multivariable biomarker prognostic models developed to predict clinical outcomes in patients with heart failure. Diagn Progn Res. 2020 Aug 26;4:13. [CrossRef]
- Asgardoon MH, Vasheghani-Farahani A, Sherafati A. Usefulness of Biomarkers for Predicting Response to Cardiac Resynchronization Therapy. Curr Cardiol Rev. 2020;16(2):132-140. [CrossRef]
- Debska-Kozlowska A, Ksiazczyk M, Warchol I, Lubinski A. Clinical Usefulness of N-terminal Prohormone of Brain Natriuretic Peptide and High Sensitivity Troponin T in Patients with Heart Failure Undergoing Cardiac Resynchronization Therapy. Curr Pharm Des. 2019;25(14):1671-1678. [CrossRef]
- McAloon CJ, Barwari T, Hu J, Hamborg T, Nevill A, Hyndman S, Ansell V, Musa A, Jones J, Goodby J, Banerjee P, O'Hare P, Mayr M, Randeva H, Osman F. Characterisation of circulating biomarkers before and after cardiac resynchronisation therapy and their role in predicting CRT response: the COVERT-HF study. Open Heart. 2018 Oct 18;5(2):e000899. [CrossRef]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016 Aug;18(8):891-975.
- Kato T, Harada T, Kagami K, Obokata M. The roles of global longitudinal strain imaging in contemporary clinical cardiology. J Med Ultrason (2001). 2022 Apr;49(2):175-185.
- Bakos Z, Chatterjee NC, Reitan C, Singh JP, Borgquist R. Prediction of clinical outcome in patients treated with cardiac resynchronization therapy - the role of NT-ProBNP and a combined response score. BMC Cardiovasc Disord. 2018 Apr 24;18(1):70.
- European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA), Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013 Aug;15(8):1070-118.
- Mele D, Trevisan F, Fiorencis A, Smarrazzo V, Bertini M, Ferrari R. Current Role of Echocardiography in Cardiac Resynchronization Therapy: from Cardiac Mechanics to Flow Dynamics Analysis. Curr Heart Fail Rep. 2020 Dec;17(6):384-396. [CrossRef]
- Stătescu C, Ureche C, Enachi Ș, Radu R, Sascău RA. Cardiac Resynchronization Therapy in Non-Ischemic Cardiomyopathy: Role of Multimodality Imaging. Diagnostics (Basel). 2021 Mar 30;11(4):625. [CrossRef]
- Rijks J, Ghossein MA, Wouters PC, Dural M, Maass AH, Meine M, Kloosterman M, Luermans J, Prinzen FW, Vernooy K, van Stipdonk AMW. Comparison of the relation of the ESC 2021 and ESC 2013 definitions of left bundle branch block with clinical and echocardiographic outcome in cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2023 Apr;34(4):1006-1014. [CrossRef]
- Zhu M, Chen H, Fulati Z, Liu Y, Su Y, Shu X. Left ventricular global longitudinal strain and mechanical dispersion predict response to multipoint pacing for cardiac resynchronization therapy. J Clin Ultrasound. 2019 Jul;47(6):356-365. [CrossRef]
- Khidir MJH, Abou R, Yilmaz D, Ajmone Marsan N, Delgado V, Bax JJ. Prognostic value of global longitudinal strain in heart failure patients treated with cardiac resynchronization therapy. Heart Rhythm. 2018 Oct;15(10):1533-1539. [CrossRef]
- Delgado-Montero A, Tayal B, Goda A, Ryo K, Marek JJ, Sugahara M, Qi Z, Althouse AD, Saba S, Schwartzman D, Gorcsan J 3rd. Additive Prognostic Value of Echocardiographic Global Longitudinal and Global Circumferential Strain to Electrocardiographic Criteria in Patients With Heart Failure Undergoing Cardiac Resynchronization Therapy. Circ Cardiovasc Imaging. 2016 Jun;9(6):e004241.
- Haugaa KH, Edvardsen T. Global longitudinal strain: the best biomarker for predicting prognosis in heart failure? Eur J Heart Fail. 2016 Nov;18(11):1340-1341.
- Bax JJ, Delgado V, Sogaard P, Singh JP, Abraham WT, Borer JS, Dickstein K, Gras D, Brugada J, Robertson M, Ford I, Krum H, Holzmeister J, Ruschitzka F, Gorcsan J. Prognostic implications of left ventricular global longitudinal strain in heart failure patients with narrow QRS complex treated with cardiac resynchronization therapy: a subanalysis of the randomized EchoCRT trial. Eur Heart J. 2017 Mar 7;38(10):720-726. [CrossRef]
- van der Bijl P, Kostyukevich MV, Khidir M, Ajmone Marsan N, Delgado V, Bax JJ. Left ventricular remodelling and change in left ventricular global longitudinal strain after cardiac resynchronization therapy: prognostic implications. Eur Heart J Cardiovasc Imaging. 2019 Oct 1;20(10):1112-1119. [CrossRef]
- Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L; CARE-HF Study Steering Committee and Investigators. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007 Aug;28(15):1827-34. [CrossRef]
- Cleland J, Freemantle N, Ghio S, Fruhwald F, Shankar A, Marijanowski M, Verboven Y, Tavazzi L. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol. 2008 Aug 5;52(6):438-45. [CrossRef]
- Kadoglou NPE, Parissis J, Karavidas A, Kanonidis I, Trivella M. Assessment of acute heart failure prognosis: the promising role of prognostic models and biomarkers. Heart Fail Rev. 2022 Mar;27(2):655-663. [CrossRef]
- Pitzalis MV, Iacoviello M, Di Serio F, Romito R, Guida P, De Tommasi E, Luzzi G, Anaclerio M, Varraso L, Forleo C, Pansini N. Prognostic value of brain natriuretic peptide in the management of patients receiving cardiac resynchronization therapy. Eur J Heart Fail. 2006 Aug;8(5):509-14. [CrossRef]
- Vazquez-Montes MDLA, Debray TPA, Taylor KS, Speich B, Jones N, Collins GS, Hobbs FDRR, Magriplis E, Maruri-Aguilar H, Moons KGM, Parissis J, Perera R, Roberts N, Taylor CJ, Kadoglou NPE, Trivella M; proBHF group. UMBRELLA protocol: systematic reviews of multivariable biomarker prognostic models developed to predict clinical outcomes in patients with heart failure. Diagn Progn Res. 2020 Aug 26;4:13. [CrossRef]
| No events (N=58) |
Secondary endpoint (N=84) |
p | |
|---|---|---|---|
| Age, y | 71±10 | 73±9 | 0.578 |
| Males, n | 43 (73%) | 60 (71%) | 0.789 |
| LVEF (%) | 29 ± 9 | 26 ± 10 | 0.777 |
| LVESV (ml) | 142 ± 61 | 156 ± 54 | 0.403 |
| LAVI (ml/m2) | 34 ± 8 | 38 ± 8 | 0.122 |
| GLS (%) | -8.8±2 | -6.1±2.2* | <0.001 |
| QRS duration (ms) | 157 ± 25 | 155 ± 21 | 0.061 |
| NYHA II | 18 (31%) | 21 (25%) | 0.411 |
| NYHA III | 40 (69%) | 63 (75%) | 0.173 |
| Diabetes, n | 12 (20.7%) | 23 (27.4%) | 0.125 |
| Atrial Fibrillation | 9 (15.5%) | 19 (22.6%) | 0.101 |
| CKD (stage IV-V),n | 2 (3.4%) | 4 (4.8%) | 0.535 |
| NT-proBNP (pg/ml) | 1449±288 | 2210±420 | <0.001 |
| Diuretics, n | 56 | 82 | 0923 |
| ACEIs/ARBs, n | 50 | 64 | 0.875 |
| MRA, n | 44 | 62 | 0.813 |
| B-blockers, n | 53 | 78 | 0.819 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.04 (1-1.08) | 0.891 | ||
| Male gender | 0.93 (0.77-1.13) | 0.770 | ||
| NYHA | 1.04 (0.95-1.18) | 0.441 | ||
| QRS (ms) | 1.15 (1.01-1.29) | 0.126 | ||
| Diabetes mellitus | 1.52 (1.28 – 2.05) | <0.001 | 1.27 (1.12-1.98) | 0.003 |
| Chronic kidney disease (eGFR<45mL/min/1.73 m2 ) | 1.88 (1.39-2.74) | 0.009 | 1.29 (1.10-2.12) | 0.068 |
| CRT response | 1.30 (1.03 – 1.85) | 0.033 | 1.12 (0.98 – 1.43) | 0.394 |
| GLS (absolute value %) | 0.48 (0.32-2.1) | <0.001 | 0.77 (0.51-1.91) | 0.002 |
| NT-proBNP | 1.78 (1.59-245) | <0.001 | 1.55 (1.43-2.01) | 0.002 |
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.01 (0.99-1.03) | 0.932 | ||
| Male gender | 1.05 (0.91-1.19) | 0.690 | ||
| NYHA | 1.11 (1.01-1.25) | 0.702 | ||
| QRS (ms) | 1.15 (1.01-1.29) | 0.126 | ||
| Diabetes mellitus | 1.28 (1.10 – 1.88) | 0.008 | 1.27 (1.12-1.98) | 0.087 |
| Atrial fibrillation | 1.89 (1.51 – 2.67) | <0.001 | 1.66 (1.31-2.22) | <0.001 |
| Chronic kidney disease (eGFR<45mL/min/1.73 m2 ) | 1.66 (1.33-2.55) | 0.006 | 1.41 (1.17-1.98) | <0.001 |
| CRT response | 1.91 (1.23 – 3.05) | 0.012 | 1.22 (1.15 – 2.43) | 0.132 |
| GLS (absolute value %) | 0.67 (0.56-1.05) | 0.015 | 0.96 (0.81-1.11) | 0.091 |
| NT-proBNP | 1.64 (1.23-2.22) | <0.001 | 1.23 (1.01-1.69) | <0.001 |
| CRT-responders (N=104) |
CRT non-responders (N=39) |
p | |
|---|---|---|---|
| Age, y | 70±11 | 74±7 | 0.423 |
| Males, n | 74 (71.2%) | 29 (74.4%) | 0.831 |
| LVEF (%) | 28 ± 6 | 26 ± 8 | 0.891 |
| LVESV (ml) | 148 ± 55 | 153 ± 52 | 0.790 |
| LAVI (ml/m2) | 37 ± 7 | 33 ± 5 | 0.029 |
| GLS (%) | -8.2±2.4 | -6.2±1.8* | <0.001 |
| QRS duration (ms) | 167 ± 29 | 151 ± 22 | 0.061 |
| NYHA II | 31 (30%) | 15 (38.5%) | 0.309 |
| NYHA III | 73 (70.2%) | 24 (61.5%) | 0.298 |
| Diabetes, n | 25 (24%) | 10 (25.6%) | 0.925 |
| Atrial Fibrillation | 21 (20.2%) | 7 (17.9%) | 0.881 |
| CKD (stage IV-V), n | 4 (7.7%) | 2 (5.1%) | 0.123 |
| NT-proBNP (pg/ml) | 1589±232 | 1998±308 | <0.001 |
| Diuretics, n | 101 | 37 | 0.955 |
| ACEIs/ARBs, n | 88 | 32 | 0.906 |
| MRA, n | 74 | 32 | 0.881 |
| B-blockers, n | 95 | 36 | 0.984 |
| Primary endpoint,n | 52 (50%) | 32 (82%) | <0.001 |
| Secondary endpoint, n | 35 (33.6%) | 18 (46.2%) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





