Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction:
Insights of how striatal DA signaling affects locomotor function have reached a plateau
Dissecting the impact of the 5 components of DA neurotransmission on locomotor function
Approaches and outcomes needed to discern role of striatal and nigral DA signaling
Autonomy of DA biosynthesis in SN and impact on motor function in aging and PD
Autonomy of post-synaptic DA signaling in SN and impact on motor function
Upstream regulators of DA signaling: the role of GDNF signaling in SN
Conclusions:
Future Directions:
References
- Glowinski, J.; Axelrod, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain, I.V. Effects of drugs on the disposition and metabolism of H3-norepinephrine and H3-dopamine. J. Pharmaco.l Exp. Ther. 1966, 153, 30–41. [Google Scholar]
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat brain, I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 1966, 13, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J. Noradrenaline: Fate and Control of its biosynthesis. Science 1971, 173, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J. 1973. Dopaminergic terminals in the rat cortex. Science 182: 499-501. 4- Coyle JT, Axelrod J. 1971. Development of the uptake and storage of L [3H] norepinephrine in the rat brain. J Neurchem 18: 2061-2075 .
- Carlsson, A.; Dahlstroem, A.; Fuxe, K.; Lindqvist, M. HISTOCHEMICAL AND BIOCHEMICAL DETECTION OF MONOAMINE RELEASE FROM BRAIN NEURONS. Life Sci. 1965, 4, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Anden, N.E.; Carlsson, A.; Dahlstroem, A.; Fuxe, K.; Hillarp, N.A.; Larsson, K. DEMONSTRATION AND MAPPING OUT OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS. Life Sci. 1964, 3, 523–530. [Google Scholar]
- Rech, R.H.; Borys, H.K.; Moore, K.E. Alterations in behavior and brain catecholamine levels in rats treated with alpha-methyltyrosine. J. Pharmacol. Exp. Ther. 1966, 153, 412–419, ISSN 0022-3565. [Google Scholar] [PubMed]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Trans. 2019, 126, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kumer, S.C.; Vrana, K.E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef]
- Reed, X.; Bandrés-Ciga, S.; Blauwendraat, C.; Cookson, M.R. The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol. Dis. 2019, 124, 230–239. [Google Scholar] [CrossRef]
- Nishioka, K.; Imai, Y.; Yoshino, H.; Li, Y.; Funayama, M.; Hattori, N. Clinical Manifestations and Molecular Backgrounds of Parkinson's Disease Regarding Genes Identified From Familial and Population Studies. Front. Neurol. 2022, 13, 764917. [Google Scholar] [CrossRef] [PubMed]
- Chotibut, T.; Davis, R.W.; Arnold, J.C.; Frenchek, Z.; Gurwara, S.; Bondada, V.; Geddes, J.W.; Salvatore, M.F. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson's model. Mol. Neurobiol. 2014, 49, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Pickel, V.M.; Beckley, S.C.; Joh, T.H.; Reis, D.J. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981, 225, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Chotibut, T.; Apple, D.M.; Jefferis, R.; Salvatore, M.F. Dopamine transporter loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine uptake. PLoS ONE 2012, 7, e52322. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Xu, M.; Bohlen, J.K.; Meshul, C.K. Differential ultrastructural alterations in the Vglut2 glutamatergic input to the substantia nigra pars compacta/pars reticulata following nigrostriatal dopamine loss in a progressive mouse model of Parkinson’s disease. Eur. J. Neurosci. 2020, 53, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzato, E.; Antonini, A.; Bisiachhi, P.; Weis, L.; Biundo, R. Asymmetric Dopamine Transporter Loss Affects Cognitive and Motor Progression in Parkinson's Disease. Mov. Disord. 2021, 36, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, L.C.; Dore, V.; Villemagne, V.L.; Xu, S.; Finkelstein, D.; Barnham, K.J.; Rowe, C. Utilizing 18F-AV-133 VMAT2 PET Imaging to Monitor Progressive Nigrostriatal Degeneration in Parkinson Disease. Neurology 2023. [CrossRef]
- Salvatore, MF. ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J. Neurochem. 2014, 129, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Dovero, S.; Prunier, C.; Ravenscroft, P.; Chalon, S.; Guilloteau, D.; Crossman, A.R.; Bioulac, B.; Brotchie, J.M.; Gross, C.E. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned Macaque model of Parkinson's disease. J. Neurosci. 2001, 21, 6853–6861. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A Role for alpha -Synuclein in the Regulation of Dopamine Biosynthesis. J. Neurosci. 2002, 22, 3090–3099, ISSN 0270-6474. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, E.A.; Han, Y.; Shifflet, M.K.; Navarrete, W.; McManus, R.; Parry, C.; Barahona, A.; Nejtek, V.A.; Manfredsson, F.P.; Kordower, J.H.; Richardson, J.R.; Salvatore, M.F. Nigral-specific increase in ser31 phosphorylation compensates for tyrosine hydroxylase protein and nigrostriatal neuron loss: Implications for delaying parkinsonian signs. Exp. Neurol. 2023, 368, 114509. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Salvatore, M.F.; Maiolo, S.A.; Bobrovskaya, L. Tyrosine hydroxylase as a sentinel for central and peripheral tissue responses in Parkinson's progression: Evidence from clinical studies and neurotoxin models. Prog. Neurobiol. 2018, 165–167, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, J.; Double, K.I.; Murphy, K.E.; Bobrovskaya, L.; Reyes, L.; Dunkely, P.R.; Halliday, G.M.; Dickson, P.W. Expression of tyrosine hydroxylase isoforms and phosphorylation at serine 40 in the human nigrostriatal system in Parkinson's disease. Neurobiol. Dis. 2019, 130, 104524. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Mori, K.; Kaneko, Y.S.; Hayashi, N.; Nagatsu, T.; Ota, A. Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Biochem. Biophys. Res. Commun. 2011, 407, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Kolacheva, A.; Alekperova, L.; Pavlova, E.; Bannikova, A.; Ugrumov, M.V. Changes in tyrosine hydroxylase activity and dopamine synthesis in the nigrostriatal system of mice in an acute model of Parkinson’s disease as a manifestation of neurodegeneration and neuroplasticity. Brain Sci. 2022, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W.; Haycock, D.A. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals: Multiple-site phosphorylation in vivo and in synaptosomes. J. Biol. Chem. 1991, 266, 5650–5657, ISSN 0021-9258. [Google Scholar] [CrossRef]
- Morgenroth, V.H.; Hegstrand, L.R.; Roth, R.H.; Greengard, P. Evidence for involvement of protein kinase in the activation by adenosine 3':5'-monophosphate of brain tyrosine 3-monooxygenase. J. Biol. Chem. 1975, 250, 1946–1948, ISSN 0021-9258. [Google Scholar] [CrossRef]
- Willard, A.M.; Islett, B.R.; Whalen, T.C.; Mastro, K.J.; Ki, C.S.; Mao, X.; Gittis, A.H. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive depletion in mice. eLife 2019, 8, e42746. [Google Scholar] [CrossRef]
- Kliem, M.A.; Maidment, N.T.; Axkerson, L.C.; Chen, S.; Smith, Y.; Wichmann, T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J. Neurophysiol. 2007, 98, 489–1500. [Google Scholar] [CrossRef]
- Dagra, A.; Miller, D.R.; Lin, M.; Gopinath, A.; Shaerzadeh, F.; Harris, S.; Sorrentino, Z.A.; Stoier, J.F.; Velasco, S.; Azar, J.; Alonge, A.R.; Lebowitz, J.J.; Ulm, B.; Bu, M.; Hansen, C.A.; Urs, N.; Giasson, B.I.; Khoshbouei, H. α-Synuclein-induced dysregulation of neuronal activity contributes to murine dopamine neuron vulnerability. npj Parkinsons Dis. 2021, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Matschke, L.A.; Komadowski, M.A.; Stohr, A.; Lee, B.; Henrick, M.T.; Griesbach, M.; Rinne, S.; Geibl, F.F.; Chiu, W.H.; Koprich, J.B.; Brotchie, J.M.; Kiper, A.K.; Dolga, A.M.; Oertel, W.H.; Decher, N. Enhanced firing of locus coeruleus neurons and SK channel dysfunction are conserved in distinct models of prodromal Parkinson's disease. Sci. Rep. 2022, 12, 3180. [Google Scholar] [CrossRef] [PubMed]
- Ellens, D.J.; Leventhal, D.K. Electrophysiology of Basal Ganglia and Cortex in Models of Parkinson Disease. J. Parkinson’s Dis. 2013, 3, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Mckie, P.M.; Shaerzadeh, F.; Gamble-George, J.; Miller, D.R.; Martyniuk, C.J.; Khoshbouei, H. In Parkinson's patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition. Acta Neuropathologica Commun. 2021, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Matuskey, D.; Tinaz, S.; Wilcox, K.C.; Naganawa, M.; Toyonaga, T.; Dias, M.; Henry, S.; Pittman, B.; Ropchan, J.; Nabulsi, N.; Suridjan, I.; Comley, R.A.; Huang, Y.; Finnema, S.J.; Carson, R.E. Synaptic Changes in Parkinson Disease Assessed with in vivo Imaging. Ann. Neurol. 2020, 87, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.; Kivinen, K.; Gardberg, M.; Joutsa, J.; Noponen, T.; Kaasinen, V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 2017, 88, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Creed, R.B.; Menallel, L.; Casey, B.; Dave, K.D.; Janssens, H.B.; Veinbergs, I.; van der Hart, M.; Rassoulpour, A.; Goldberg, M.S. Basal and Evoked Neurotransmitter Levels in Parkin, DJ-1, PINK1 and LRRK2 Knockout Rat Striatum. Neuroscience 2019, 409, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chotibut, T.; Fields, V.; Salvatore, M.F. Norepinephrine transporter inhibition with desipramine exacerbates L-DOPA-induced dyskinesia: role for synaptic dopamine regulation in denervated nigrostriatal terminals. Mol. Pharmacol. 2014, 86, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Sarre, S.; Vandeneede, D.; Ebinger, G.; Michotte, Y. Biotransformation of L-DOPA to dopamine in the substantia nigra of freely moving rats: effect of dopamine receptor agonists and antagonists. J. Neurochem. 1990, 70, 1730–1739. [Google Scholar] [CrossRef]
- Perez, X.A.; Parameswaran, N.; Huang, L.Z.; O’Leary, K.T.; Wuik, M. Pre-synaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J. Neurochem. 2008, 105, 1861–1872. [Google Scholar] [CrossRef]
- Mela, F.; Marti, M.; Bido, S.; Cenci, M.A.; Morari, M. In Vivo evidence for a differential contribution of striatal and nigral D1 and D2 receptors to l-DOPA induced dyskinesia and the accompanying surge of nigral amino acid levels. Neurobiol. Dis. 2012, 45, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kliem, M.A.; Pare, J.F.; Khan, Z.U.; Wichmann, T.; Smith, Y. Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur. J. Neurosci. 2010, 31, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Mailman, R.B.; Yang, Y.; Huang, X. D.1.; not, D.2.; dopamine receptor activation dramatically improves MPTP-induced parkinsonism unresponsive to levodopa. Eur. J. Pharmacol. 2021, 892, 173760. [Google Scholar] [CrossRef]
- Trevitt, J.T.; Carlson, B.B.; Nowend, K.; Salamone, J.D. Substantia nigra pars reticulate is a highly potent site of action for the behavioral effects of the D1 antagonist SCH23390 in rat. Psychopharmacol. 2001, 156, 32–41. [Google Scholar]
- Tang, P.; Knight, W.C.; Li, H.; Guo, Y.; Perlmutter, J.S.; Benzinger, T.L.S.; Morris, J.C.; Xu, J. Dopamine D1 + D3 receptor density may correlate with parkinson disease clinical features. Ann. Clin. Transl. Neurol. 2021, 8, 224–237. [Google Scholar]
- Roedter, A.; Winkler, C.; Samil, M.; Walter, G.; Brandis, A.; Nikkhah., *!!! REPLACE !!!*. Comparison of unilateral and bilateral intrastriatal 6-hydroxydopamine-induced axon terminal lesions: Evidence for interhemispheric functional coupling of the two nigrostriatal pathways. J. Comp. Neurol. 2001, 432, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Radnikow, G. Misgeld U Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata, J. Neurosci. 1998, 18, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Dorval, A.D.; Grill, W.M. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 2014, 111, 1949–1959. [Google Scholar] [CrossRef]
- DeLong, M.R.; Wichmann, T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol. 2015, 72, 1354–1360. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit mechanisms of Parkinson’s disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Di Filippo, M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 2014, 17, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Foffani, G.; Dehay, B.; Bezard, E.; Obeso, J.A. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat. Rev. Neurosci. 2022, 23, 115–128. [Google Scholar] [CrossRef]
- Zaman, V.; Boger, H.A.; Granholm, A.C.; Rohrer, B.; Moore, A.; Buhusi, M.; Gerhardt, G.A.; Hoffer, B.J.; Middaugh, L.D. The nigrostriatal dopamine system of aging GFRalpha-1 heterozygous mice: neurochemistry morphology behaviorEur, J. Neurosci. 2008, 28, 1557–1568. [Google Scholar]
- Pruett, B.S.; Salvatore, M.F. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol. Neurobiol. 2013, 47, 988–999. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O'Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Grondin, R.; Cass, W.A.; Zhang, Z.; Stanford, J.A.; Gash, D.M.; Gerhardt, G.A. Glial Cell Line -Derived Neurotrophic Factor Increases Stimulus-Evoked Dopamine Release and Motor Speed in Aged Rhesus Monkeys. J. Neurosci. 2003, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; Hoffer, B.J. Gerhardt GA Functional recovery in parkinsonian monkeys treated with, G. D.N.F. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Cass, W.A.; Huettl, P.; Brock, S.; Zhang, Z.; Gash, D.M. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999, 817, 163–171. [Google Scholar] [CrossRef]
- Hoffer, B.J.; Hoffman, A.F.; Bowenkamp, K.E.; Huettl, P.; Hudson, J.; Martin, D.; Lin, L.F.; Gerhardt, G.A. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994, 182, 107–111. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; Brodsky, M.A.; Burchiel, K.; Kelly, P.; Dalvi, A.; Scott, B.; Stacy, M.; Turner, D.; Wooten, G.F.; Elias, W.J.; Laws, E.R.; Dhawan, V.; Stoessl, A.J.; Matcham, J.; Coffey, R.J.; Traub, M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson's disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Salvatore, M.F.; Zhang, J.L.; Large, D.M.; Wilson, P.E.; Gash, C.R.; Thomas, T.C.; Haycock, J.W.; Bing, G.; Stanford, J.A.; Gash, D.M.; Gerhardt, G.A. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in rat striatum and substantia nigra. J. Neurochem. 2004, 90, 245–254. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Gerhardt, G.A.; Dayton, R.D.; Klein, R.L.; Stanford, J.A. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp. Neurol. 2009, 219, 197–207. [Google Scholar] [CrossRef]
- Kasanga, E.A.; Han, Y.; Navarrete, W.; McManus, R.; Shifflet, M.K.; Parry, C.; Barahona, A.; Manfredsson, F.P.; Nejtek, V.A.; Richardson, J.R.; Salvatore, M.F. Differential expression of RET and GDNF family receptor, GFR-α1, between striatum and substantia nigra following nigrostriatal lesion: A case for diminished GDNF-signaling. Exp. Neurol. 2023, 366, 114435. [Google Scholar] [CrossRef]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson's disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef]
- Kordower, J.H.; Goetz, C.G.; Chu, Y.; Halliday, G.M.; Nicholson, D.A.; Musial, T.F.; Marmion, D.J.; Stoessl, A.J.; Freeman, T.B.; Olanow, C.W. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann. Neurol. 2017, 81, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Shima, A.; Kambe, D.; Nishida, A.; et al. Motor progression and nigrostriatal neurodegeneration in Parkinson’s disease. Ann. Neurol. 2022, 92, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.K.; Tian, L.; Flores, H.; Su, Y.; Tabbal, S.D.; Loftin, S.K.; Moerlin, S.M.; Perlmutter, J.S. Validation of nigrostriatal positron emission tomography measures: critical limits. Ann. Neurol. 2013, 73, 390–396. [Google Scholar] [CrossRef]
- Perlmuttter, J.S.; Norris, S.A. Neuroimaging biomarkers for Parkinson’s disease: fact and fantasy. Ann. Neurol. 2014, 76, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Schröter, N.; Rijntjes, M.; Urbach, H.; Weiller, C.; Treppner, M.; Kellner, E.; Jost, W.H.; Sajonz, B.E.A.; Reisert, M.; Hosp, J.A.; Rau, A. Disentangling nigral and putaminal contribution to motor impairment and levodopa response in Parkinson’s disease. npj Parkinson’s Dis. 2022, 8, 132. [Google Scholar] [CrossRef]
- Pérez-Taboada, I.; Alberquilla, S.; Martin, E.D.; Anand, R.; Vietti-Michelina, S.; Tebeka, N.N.; Cantley, J.; Cragg, S.J.; Moratalla, R.; Vallejo, M. Diabetes Causes Dysfunctional Dopamine Neurotransmission Favoring Nigrostriatal Degeneration in Mice. Mov. Disord. 2020, 35, 1636–1648. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.D.; De Silva, S.; et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol. Dis. 2014, 70, 190–203. [Google Scholar] [CrossRef]
- Blesa, J.; Pifl, C.; Sanchez-Gonzalez, M.A.; Juri, C.; Garcia-Cabezas, M.A.; Adanez, R.; Iglesias, E.; et al. The nigrostriatal system in the presymptomatic and symptomatic stages in the MPTP monkey model: A PET, histological, and biochemical study. Neurobiol. Dis. 2012, 48, 79–91. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Walsh, J.P.; Akopian, G.; Hogg, E.; Abernathy, A.; Arevalo, P.; Turnquist, P.; Vuckovic, M.; Fisher, B.E.; Togasaki, D.M.; Jakowec, M.W. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007, 27, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, S.J.; Gross, N.B.; Fricks, A.N.; Casiano, B.D.; Nguyen, T.B.; Marshall, J.F. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience 2007, 144, 1141–1151. [Google Scholar] [CrossRef]
- Churchill, M.J.; Pflibsen, L.; Sconce, M.D.; Moore, C.; Kim, K.; Meshul, C.K. Exercise in an animal model of Parkinson’s disease: Motor recovery but not restoration of the nigrostriatal pathway, Neuroscience. 2017, 359, 224–247.
- Robertson, G.S.; Robertson, H.A. Evidence that L-DOPA-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 1999, 9, 3326–3331. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.S.; Robertson, H.A. Evidence that the substantia nigra is a site of action for, L.-D.O.P.A. Neurosci. Lett. 1988, 89, 204–208. [Google Scholar] [CrossRef]
- Jackson, E.A.; Kelly, P.H. Role of nigral dopamine in amphetamine-induced locomotor activity. Brain Res. 1983, 278, 366–369. [Google Scholar] [CrossRef]
- Bradbury, A.J.; Costall, B.; Kelly, M.E.; Naylor, R.J.; Smith, J.A. Biochemical correlates of motor changes caused by the manipulation of dopamine function in the substantia nigra of the mouse. Neuropharmacology 1985, 24, 1155–1161. [Google Scholar] [CrossRef]
- Jackson, E.A.; Kelly, P.H. Effects of intranigral injections of dopamine agonists and antagonists, glycine, muscimol and N- methyl-D,L-aspartate on locomotor activity. Brain Res. Bull. 1984, 13, 309–317. [Google Scholar] [CrossRef]
- Ahlenius, S.; Anden, N.E.; Engel, J. Restoration of locomotor activity in mice by low L-DOPA doses after suppression by alpha-methyltyrosine but not by reserpine. Brain Res. 1973, 62, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Jenner, P.; Marsden, C.D. The relative importance of dopamine and noradrenaline receptor stimulation for the restoration of motor activity in reserpine or alpha-methyl-p-tyrosine pre-treated mice. Pharmacol. Biochem. Behav. 1976, 4, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Pruett, B.S. Dichotomy of Tyrosine Hydroxylase and Dopamine Regulation between Somatodendritic and Terminal Field Areas of Nigrostriatal and Mesoaccumbens Pathways. PLoS ONE 2012, 7, e29867. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Mura, A.; Hengerer, B.; Feldon, J.; Ferger, B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav. Brain Res. 2005, 154, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.A.; Marsh, S.T.; Hutchings, J.E.; Castañeda, E. Amphetamine-evoked rotation requires newly synthesized dopamine at 14 days but not 1 day after intranigral 6-OHDA and is consistently dissociated from sensorimotor behavior. Behav. Brain Res. 2009, 200, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ankenman, R.; Salvatore, M.F. Low dose alpha-methyl-para-tyrosine (AMPT) in the treatment of dystonia and dyskinesia. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, O.J.N.; de Koning, M.B.; Boot, E.; Booij, J.; van Amelsvoort, T.A. Challenge and Therapeutic Studies Using Alpha-Methyl-para-Tyrosine (AMPT) in Neuropsychiatric Disorders: A Review. Central Nervous System agents in Medicinal Chemistry. 2008, 8, 249–256. [Google Scholar] [CrossRef]
- Rubinstein, M.; Gershanik, O.; Stefano, F.J. Different roles of D-1 and D-2 dopamine receptors involved in locomotor activity of supersensitive mice. Eur. J. Pharmacol. 1988, 148, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Meurer, Y.S.R.; Bioni, V.S.; Cunha, D.M.G.; Goncalves, N.; Lopes-Silva, L.B.; Becegato, M.; Soares, M.B.L.; Marinho, G.F.; Santos, J.R.; Silva, R.H. Female Rats Are Resistant to Cognitive, Motor and Dopaminergic Deficits in the Reserpine-Induced Progressive Model of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 757714. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- May, R.H.; Voegele, G.E. Parkinsonian reactions following chlorpromazine and reserpine; similar reactions in the same patients. AMA Arch. Neurol. Psychiatry 1956, 75, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, M.J.; Chan, J.; Liu, Y.; Edwards, R.H.; Pickel, V.M. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J. Neurosci. 1996, 16, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.M.; Salvatore, M.F.; Pruett, B.S.; Guerin, G.F.; Goeders, N.E. Biphasic dopamine regulation in mesoaccumbens pathway in response to non-contingent binge and escalating methamphetamine regimens in the Wistar rat. Psychopharmacology 2011, 215, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Nissbrandt, H.; Sundström, E.; Jonsson, G.; Hjorth, S.; Carolsson, A. Synthesis and Release of Dopamine in Rat Brain: Comparison Between Substantia Nigra Pars Compacta, Pars Reticulata, and Striatum. J. Neurochem. 1989, 52, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, M.J.; Abercrombie, E.D. Biochemistry of Somatodendritic Dopamine Release in Substantia Nigra: An In Vivo Comparison with Striatal Dopamine Release. J. Neurochem. 1995, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Westerink, B.H.C. Characterization and Pharmacological Responsiveness of Dopamine Release Recorded by Microdialysis in the Substantia Nigra of Conscious Rats. J Neurochem. 1991, 57, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.G.; Forbes, B.; Cheung, P.Y.; Martini, A.; Burrell, M.H.; Freestone, P.S.; Lipski, J. Action potential and calcium dependence of tonic somatodendritic dopamine release in the Substantia Nigra pars compacta. J. Neurochem. 2018, 148, 462–479. [Google Scholar] [CrossRef]
- Cragg, S.J.; Rice, M.E. Dancing past the DAT at a DA synapse. Trends Neurosci. 2004, 27, 270–277. [Google Scholar] [CrossRef]
- Kaasinen, V.; Vahlberg, T.; Stoessl, J.A.; Strafella, A.P.; Antonini, A. Dopamine receptors in Parkinson’s disease: A meta-analysis of imaging studies. Mov. Disord. 2021, 36, 1781–1791. [Google Scholar] [CrossRef]
- Biswas, B.; Carlsson, A. Potentiation by Neuroleptic Agents of the Inhibitory Action of Intraperitoneally Administered GABA on the Locomotor Activity of Mice. Pharmacol. Biochem. Behav. 1978, 6, 651–654. [Google Scholar] [CrossRef]
- Hillegaart, V.; Ahlenius, S. Effects of raclopride on exploratory locomotor activity, treadmill locomotion, conditioned avoidance behaviour and catalepsy in rats: behavioural profile comparisons between raclopride, haloperidol and preclamol. Pharmacol. Toxicol. 1987, 60, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Löschmann, P.A.; Smith, L.A.; Lange, K.W.; Jaehnig, P.; Jenner, P.; Marsden, C.D. Motor activity following the administration of selective D-1 and D-2 dopaminergic drugs to normal common marmosets. Psychopharmacology 1991, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ericson, H.; Radesäter, A.C.; Servin, E.; Magnusson, O.; Mohringe, B. Effects of intermittent and continuous subchronic administration of raclopride on motor activity, dopamine turnover and receptor occupancy in the rat. Pharmacol. Toxicol. 1996, 79, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.C.; Beninger, R.J. The D1 dopamine receptor antagonist, SCH 23390 reduces locomotor activity and rearing in rat. Pharmacol Biochem Behav 1985, 22, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.W.; Caramona, G.N. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol. Biochem. Behav. 2002, 72, 857–863. [Google Scholar] [CrossRef]
- Svensson, K.A.; Heinz, B.A.; Schaus, J.M.; Beck, J.P.; Hao, J.; Krushinski, J.H.; et al. An Allosteric Potentiator of the Dopamine D1 Receptor Increases Locomotor Activity in Human D1 Knock-In Mice without Causing Stereotypy or Tachyphylaxis. 2017, 360, 117–128.
- Mailman, R.B.; Yang, Y.; Huang, X. D.1.; not, D.2.; dopamine receptor activation dramatically improves MPTP-induced parkinsonism unresponsive to levodopa. Eur. J. Pharmacol. 2021, 892, 173760. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, S.H.; Hauser, R.A.; Pahwa, R.; Gray, D.; Duvvuri, S. Dopamine agonists in Parkinson’s disease: Impact of D1-like or D2-like dopamine receptor subtype selectivity and avenues for future treatment. Clin. Parkinsonism and Rel Disord. 2023, 9, 100212. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.; Liu, W.; Duvvuri, S.; Thayer, K.; Gray, D.L. Evaluation of D1/D5 partial agonist PF-06412562 in Parkinson’s disease following oral administration. Neurodegener. Dis. 2018, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Xuemei, H.; Lewis, M.M.; Van Scoy, L.J.; De Jesus, S.; Eslinger, P.J.; Arnold, A.C.; Miller, A.J.; et al. The D1/D5 Dopamine Partial Agonist PF-06412562 in Advanced-Stage Parkinson’s Disease: A Feasibility Study. J. Parkinson’s Dis. 2020, 10, 1515–1527. [Google Scholar]
- Pothos, E.N.; Przedborski, S.; Davila, V.; Schmitz, Y.; Sulzer, D. D2-Like Dopamine Autoreceptor Activation Reduces Quantal Size in PC12 Cells. J. Neurosci. 1998, 18, 5575–5585. [Google Scholar] [CrossRef]
- Cragg, S.J.; Greenfield, S.A. Differential Autoreceptor Control of Somatodendritic and Axon Terminal Dopamine Release in Substantia Nigra, Ventral Tegmental Area, and Striatum. J. Neurosci. 1997, 17, 5738–5746. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.; Ruffieux, A.; Aebischer, P. The activity of pars compacta neurons of the monkey substantia nigra in relation to motor activation. Exp. Brain Res. 1983, 51, 377–387. [Google Scholar] [CrossRef]
- da Silva, J.A.; Tecuapetla, F.; Paixão, V.; Costa, R.M. Dopamine neuron activity before action Initiation gates and invigorates future movements. Nature 2018, 554, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Coddington, L.T.; Dudman, J.T. Learning from Action: Reconsidering Movement Sinaling in Midbrain Dopamine Neuron Activity. Neuron 2019, 104, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; da Silva, J.A.; Costa, R.M. What, If, and When to Move: Basal Ganglia Circuits and Self-Paced Action Initiation. Ann. Rev. Neurosci. 2019, 42, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, F.; Shahabi, H.N.; Nissbrandt, H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003, 973, 81–91. [Google Scholar] [CrossRef]
- Hebert, M.A.; Gerhardt, G.A. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998, 797, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Yurek, D.M.; Hipkens, S.B.; Hebert, M.A.; Gash, D.M.; Gerhardt, G.A. Age-related decline in striatal dopamine release and motoric function in Brown Norway/Fischer 344 hybrid rats. Brain Res. 1998, 791, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, M.N.; Genc, O.; Bobela, W.; Mohanna, S.; Ardah, M.T.; El-Agnaf, O.M.; Cantoni, M.; Bensadoun, J.C.; Schneggenburger, R.; Knott, G.W.; Aebischer, P.; Schneider, B.L. Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathologica 2012, 123, 653–669. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Larson, G.A.; Swant, J.; Sen, N.; Javitch, J.A.; Zahniser, N.R.; De Felice, L.J.; Khoshbouei, H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 2009, 284, 2978–2989. [Google Scholar] [CrossRef]
- Kahlig, K.M.; Binda, F.; Khoshbouei, H.; Blakely, R.D.; McMahon, D.G.; Javitch, J.A.; Galli, A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. 2005, 102, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Rivière, G.J.; Byrnes, K.A.; Gentry, W.B.; Owens, S.M. Spontaneous Locomotor Activity and Pharmacokinetics of Intravenous Methamphetamine and Its Metabolite Amphetamine in the Rat. 1999, 291, 1220–1226.
- Laruelle, M.; Abi-Dargham, A.; van Dyck, C.H.; Rosenblatt, W.; Zea-Ponce, Y.; Zoghbi, S.S.; Baldwin, R.M.; Charney, D.S.; Hoffer, P.B.; Kung, H.F.; Innis, R.B. SPECT Imaging of Striatal Dopamine Release after Amphetamine Challenge. J. Nuc Med. 1995, 36, 1182–1190. [Google Scholar]
- Hall, D.A.; Stanis, J.J.; Avila, H.M.; Gulley, J.M. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology 2008, 195, 469–478. [Google Scholar] [CrossRef]
- Ciliax, B.J.; Heilman, C.; Demchyshyn, L.L.; Pristupa, Z.B.; Ince, E.; Hersch, S.M.; Niznik, H.B.; Levey, A.I. The dopamine transporter: immunochemical characterization and localization in brain. 1995, 15, 1714–1723.
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The Dopamine Transporter Is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci. 1996, 16, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, P489–P496. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.F.; Lupica, C.R.; Gerhardt, G.A. Dopamine transporter activity in the substantia nigra and striatum assessed by high-speed chronoamperometric recordings in brain slices. J. Pharmacol. Exp. Ther. 1998, 287, 487–496. [Google Scholar] [PubMed]
- Ford, C.P.; Gantz, S.C.; Phillips, P.E.M.; Williams, J.T. Control of extracellular dopamine at dendrite and axon terminals. J. Neurosci. 2010, 30, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.J.; Rice, M.E.; Greenfield, S.A. Heterogeneity of Electrically Evoked Dopamine Release and Reuptake in Substantia Nigra, Ventral Tegmental Area, and Striatum. J. Neurophysiol. 1997, 77, 863–873. [Google Scholar] [CrossRef]
- Ma, S.Y.; Ciliax, B.J.; Stebbins, G.; Jaffar, S.; Joyvce, J.N.; Cochran, E.J.; Kordower, J.H.; Mash, D.C.; Levey, A.I.; Mufson, E.J. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J. Comp. Neurol. 1999, 409, 25–37. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Apparsundaram, S.; Gerhardt, G.A. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol. Aging 2003, 24, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. npj Parkinson’s Dis. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Calipari, E.S.; Jones, S.R. Regulation of tyrosine hydroxylase expression and phosphorylation in dopamine transporter-deficient mice. ACS Chem. Neurosci. 2016, 7, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Morón, J.A.; Brockiington, A.; Wise Rocha, B.A.; Hope, B.T. Dopamine Uptake through the Norepinephrine Transporter in Brain Regions with Low Levels of the Dopamine Transporter: Evidence from Knock-Out Mouse Lines. J. Neurosci. 2002, 22, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Chotibut, T.; Apple, S.M.; Jefferis, R.; Salvatore, M.F. Dopamine Transporter Loss in 6-OHDA Parkinson’s Model Is Unmet by Parallel Reduction in Dopamine Uptake. PLoS ONE 2012, 7, e52322. [Google Scholar] [CrossRef]
- Chotibut, T.; Fields, V.; Salvatore, M.F. Norepinephrine transporter inhibition with desipramine exacerbates L-DOPA-induced dyskinesia: role for synaptic dopamine regulation in denervated nigrostriatal terminals. Mol. Pharmacol. 2014, 86, 675–685. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Spielewoy, C.; Roubert, C.; Hamon, M.; Nosten-Bertrand, M.; Betancur, C.; Giros, B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 2000, 11, 279–290. [Google Scholar] [CrossRef]
- Stanford, J.A.; Vorontsova, E.; Surgener, S.P.; Gerhardt, G.A.; Fowler, S.C. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: exacerbation by nomifensine. Neurosci. Lett. 2002, 333, 195–198. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Kasanga, E.A.; Kelly, D.P.; Venable, K.E.; McInnis, T.R.; et al. Modulation of nigral dopamine signaling mitigates parkinsonian signs of aging: evidence from intervention with caloric restriction or inhibition of dopamine uptake. GeroScience 2023, 45, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.; Damsma, G.; Fibiger, H. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J. Neurosci. 1991, 11, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A.; Cheramy, A.; Glowinski, J. Nigral and striatal dopamine release under sensory stimuli. Nature 1977, 269, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Coddington, L.T.; Dudman, J.T. The timing of action determines reward prediction signals in identified midbrain dopamine neurons. Nat. Neurosci. 2018, 21, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; McInnnis, T.R.; Cantu, M.A.; Apple, D.M.; Pruett, B.S. Tyrosine Hydroxylase Inhibition in Substantia Nigra Decreases Movement Frequency. Mol. Neurobiol. 2019, 56, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Terrebonne, J.; Cantu, M.A.; McInnis, T.; Venable, K.; Kelley, P.; Kasanga, E.A.; Latimer, B.; Owens, C.L.; et al. Dissociation of striatal dopamine and tyrosine hydroxylase expression from aging-related motor decline: evidence from calorie restriction intervention. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.R.; Nissbrandt, H.; Bergquist, F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur. J. Neurosci. 2006, 24, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Pruett, B.S.; Spann, S.L.; Dempsey, C. Aging Reveals a Role for Nigral Tyrosine Hydroxylase ser31 Phosphorylation in Locomotor Activity Generation. PLoS ONE 2009, 4, e8466. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Garcia-Espana, A.; Goldstein, M.; Deutch, A.Y.; Haycock, J.W. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: effects of acute haloperidol and related compounds. J. Neurochem. 2000; 75, 225–232. [Google Scholar]
- Salvatore, M.F.; Pruett, B.S.; Dempsey, C.; Fields, V. Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area. J Vis Exp. 2012, 66, 4171. [Google Scholar] [CrossRef]
- Emborg, M.E.; Ma, S.Y.; Mufson, E.J.; Levey, A.I.; Taylor, M.D.; Brown, W.D.; Holden, J.E.; Kordower, J.H. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J. Comp. Neurol. 1998, 401, 253–265. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef]
- Chu, Y.; Hirst, W.D.; Federoff, H.J.; Harms, A.S.; Stoessl, A.J.; Kordower, J.H. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. 2023; awad388. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovich, H.; Abbott, R.D.; Nelson, J.; Markesbery, W.; Davis, D.; Hardman, J.; Launer, L.; Masaki, K.; Tanner, C.M.; White, L.R. Parkinsonian signs substantia nigra neuron density in descendent elders without, P.D. Ann. Neurol. 2004, 56, 532–529. [Google Scholar] [CrossRef]
- Buchman, A.S.; Shulman, J.M.; Nag, S.; Leurgans, S.E.; Arnold, S.E.; Morris, M.C.; Schneider, J.A.; Bennett, D.A. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann. Neurol. 2012, 71, 258–266. [Google Scholar] [CrossRef]
- Wolf, M.E.; LeWitt, P.A.; Bannon, M.J.; Dragovic, L.J.; Kapatos, G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J. Neurochem. 1991, 56, 1191–1200. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Rajput, A.; Deck, J.H.N.; Hornykiewicz, O. Aging produces a specific pattern of striatal dopamine loss: Implications for the etiology of idiopathic Parkinson's disease. J Neurochem 1992, 58, 642–648. [Google Scholar] [CrossRef]
- Haycock, J.W.; Becker, L.; Ang, L.; Yoshiaki, F.; Hornykiewicz, O.; Kish, S.J. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 2003, 87, 574–585. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Waymire, J.C.; Haycock, J.W. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J. Neurochem. 2001, 79, 349–360. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Cass, W.A.; Yi, A.; Zhang, Z.; Gash, D.M. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J. Neurochem. 2002, 80, 168–177. [Google Scholar] [CrossRef]
- Irwin, I.; DeLanney, L.E.; McNeill, T.; Chan, P.; Forno, L.S.; et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration 1994, 3, 251–265. [Google Scholar]
- Siddiqi, Z.; Kemper, T.L.; Killiany, R. Age-related Neuronal Loss from the Substantia Nigra-Pars Compacta and Ventral Tegmental Area of the Rhesus Monkey. J. Neuropath Exp. Neurol. 1999, 58, 959–971. [Google Scholar] [CrossRef]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurological Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D. Parkinson’s disease. Lancet 1990, 335, 948–952. [Google Scholar] [CrossRef]
- Collier, T.J.; Lipton, J.; Daley, B.F.; Palfi, S.; Chu, Y.; Sortwell, C.; Bakay, R.A.; Sladek, J.R.; Kordower, J.H. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: Diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007, 26, 56–65. [Google Scholar] [CrossRef]
- Pifl, C.; Hornykiewicz, O. Dopamine turnover is upregulated in the caudate/putamen of asymptomatic MPTP-treated rhesus monkeys. Neurochem. Int. 2006, 49, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, M.J. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol. Dis. 1997, 4, 247–253. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Lopez-Gonzalez del Rey, N.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson's disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef]
- Sarre, S.; Yuan, H.; Jonkers, N.; Van Hemelrijck, A.; Ebinger, G.; Michotte, Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004, 90, 29–39. [Google Scholar] [CrossRef]
- 174. Suhara, T.; Fukuda, H.; Inoue, O.; Itoh, T.; Suzuki, K.; Yamasaki, T.; et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology. 1991, 103, 41–45. [Google Scholar] [CrossRef]
- Kaasinen, V.; Vahlberg, T.; Stoessl, J.A.; Strafella, A.P.; Antonini, A. Dopamine receptors in Parkinson’s disease: A meta-analysis of imaging studies. Mov. Disord. 2021, 36, 1781–1791. [Google Scholar] [CrossRef]
- Shui, H.A.; Peng, Y.I.; Wu, R.M.; Tsai, Y.F. Evaluation of l-DOPA biotransformation during repeated l-DOPA infusion into the striatum in freely-moving young and old rats. Dev. Brain Res. 2000, 121, 123–131. [Google Scholar] [CrossRef]
- Orosz, D.; Bennett, J.P. Simultaneous microdialysis in striatum and substantia nigra suggests that the nigra is a major site of action of l-dihydroxyphenylalanine in the “Hemiparkinsonian” rat. Exp. Neurol. 1992, 115, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Sarre, S.; Herregodts, P.; Deleu, D.; Devrieze, A.; De Klippel, N.; Ebinger, G.; Michotte, Y. Biotransformation of L-DOPA in striatum and substantia nigra of rats with a unilateral, nigrostriatal lesion: a microdialysis study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1992, 346, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Grondin, R.; Zhang, Z.; Yi, A.; Cass, W.A.; Maswood, N.; Andersen, A.H.; Elsberry, D.D.; Klein, M.C.; Gerhardt, G.A.; Gash, D.M. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain 2002, 125, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gash, D.M.; Smith, C.D.; Gerhardt, G.A.; Kryscio, R.; Chebrolu, H.; Walton, A.; Wagner, R.; Young, A.B. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 2012, 106, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Pavese, N.; Javed, S.; Hotton, G.R.; Brooks, D.J.; Gill, S.S. Benefits of putaminal GDNF infusion in Parkinson disease are maintained after GDNF cessation. Neurology 2013, 81, 13. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gerhardt, G.A.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputamenal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005, 102, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, E.A.; Owens, C.L.; Cantu, M.A.; Richard, A.D.; Davis, R.W.; McDivitt, L.M.; Blancher, B.; Pruett, B.S.; Tan, C.; Gajewski, A.; Manfredsson, F.P.; Nejtek, V.A.; Salvatore, M.F. GFR-α1 Expression in Substantia Nigra Increases Bilaterally Following Unilateral Striatal GDNF in Aged Rats and Attenuates Nigral Tyrosine Hydroxylase Loss Following 6-OHDA Nigrostriatal Lesion. ACS Chem. Neurosci. 2019, 10, 4237–4249. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Bjorklund, A.; Gash, D.M.; Whone, A.; Van Laar, A.; Kordower, J.H.; Bankiewicz, K.; Kieburtz, K.; Saarma, M.; Booms, S.; Huttunen, H.; et al. GDNF and Parkinson’s Disease: Where Next? A Summary from a Recent Workshop. J. Parkinsons Dis. 2020, 10, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Tomac, A.; Widenfalk, J.; Lin, L.F. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc. Natl. Acad. Sci. USA 1995, 92, 8274–8278. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors- relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef]
- Leitner, M.L.; Molliver, D.C.; Osborne, P.A.; Vejsada, R.; Golden, J.P.; Lampe, P.A.; Kato, A.C.; Milbrandt, J.; Johnson, E.M. Analysis of the retrograde transport of glial cell line-derived neurotrophic factor (GDNF), Neurturin, and Persephin suggests that in vivo signaling for the GDNF family is GFRα coreceptor-specific. J. Neurosci. 1999, 19, 9322–9331. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Ai, Y.; Fischer, B.; Zhang, A.M.; Grondin, R.C.; Zhang, Z.; Gerhardt, G.A.; Gash, D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006, 202, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Geffen, L.B.; Jessell, T.M.; Cuello, A.C.; Iversen, L.L. Release of dopamine from dendrites in rat substantia nigra. Nature 1976, 260, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Cheramy, A.; Leviel, V.; Glowinski, J. Dendritic release of dopamine in the substantia nigra. Nature 1981, 289, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, B.L.; Walters, J.R. Dopamine modulation of the effects of gamma-aminobutyric acid on substantia nigra pars reticulata neurons. Science 1983, 220, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, A.; Schultz, W. Dopaminergic activation of reticulate neurons in the substantia nigra. Nature 1980, 285, 240–241. [Google Scholar] [CrossRef]
- atuszewich, L.; Yamamoto, B.K. Modulation of GABA release by dopamine in the substantia nigra. Synapse 1999, 32, 29–36. [Google Scholar] [CrossRef]
- Lahiri, A.K.; Bevan, M.D. Dopaminergic transmission rapidly and persistently enhances excitability of D1 receptor-expressing striatal projection neurons. Neuron 2020, 106, 288–290. [Google Scholar] [CrossRef]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Aging and Parkinson’s disease: different sides of the same coin? Mov. Disord. 2017, 32, 983–990. [Google Scholar] [CrossRef]
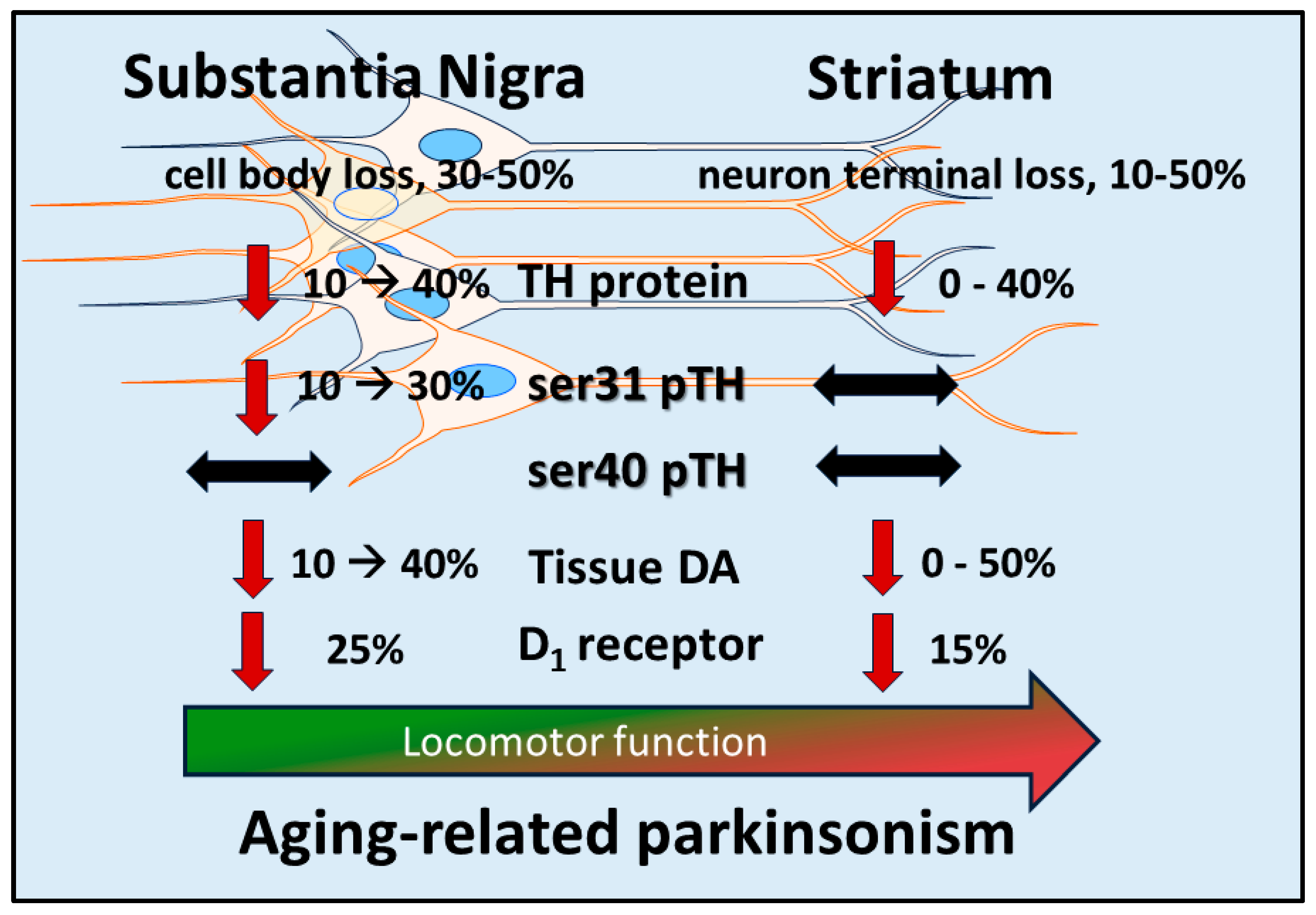
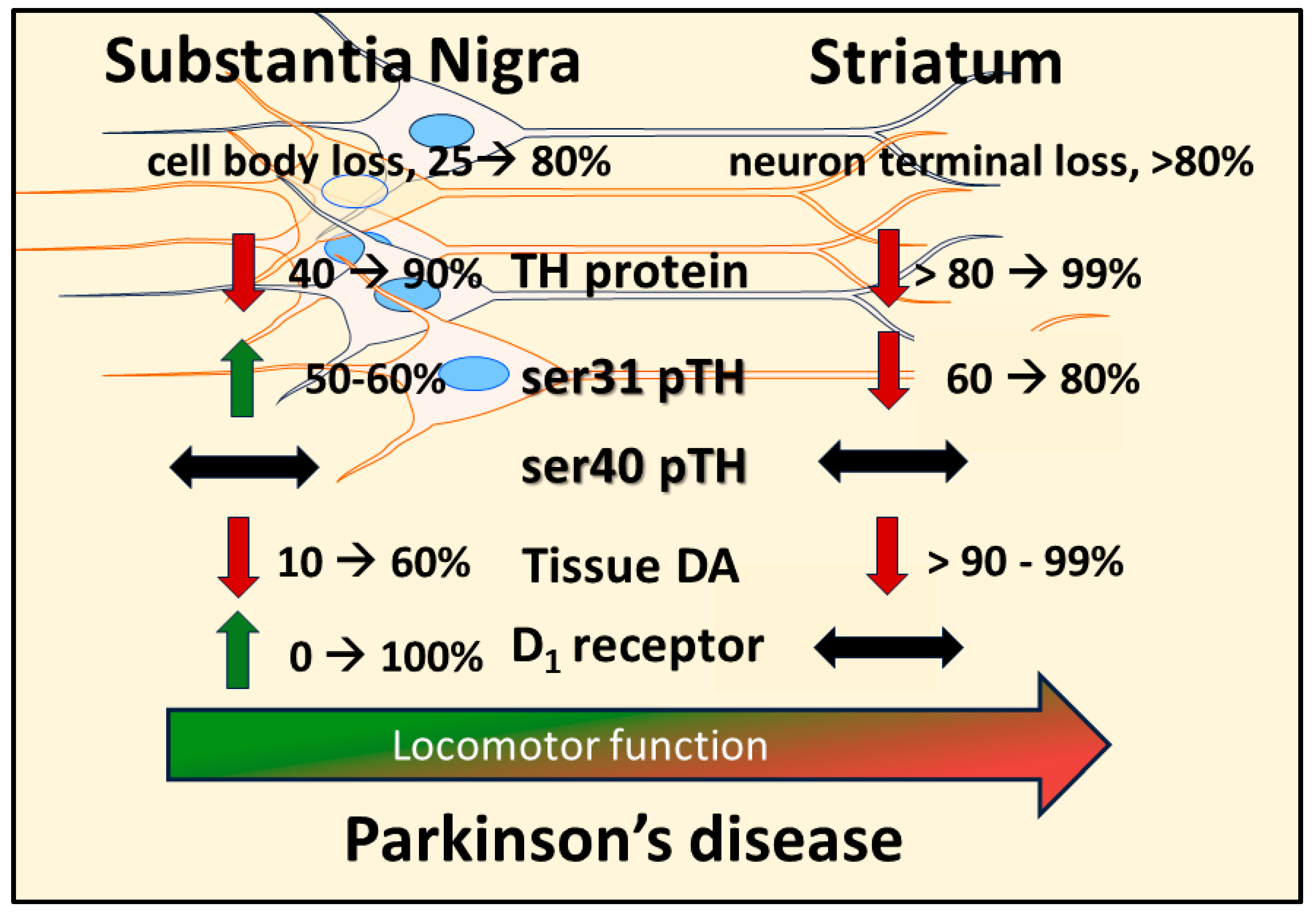
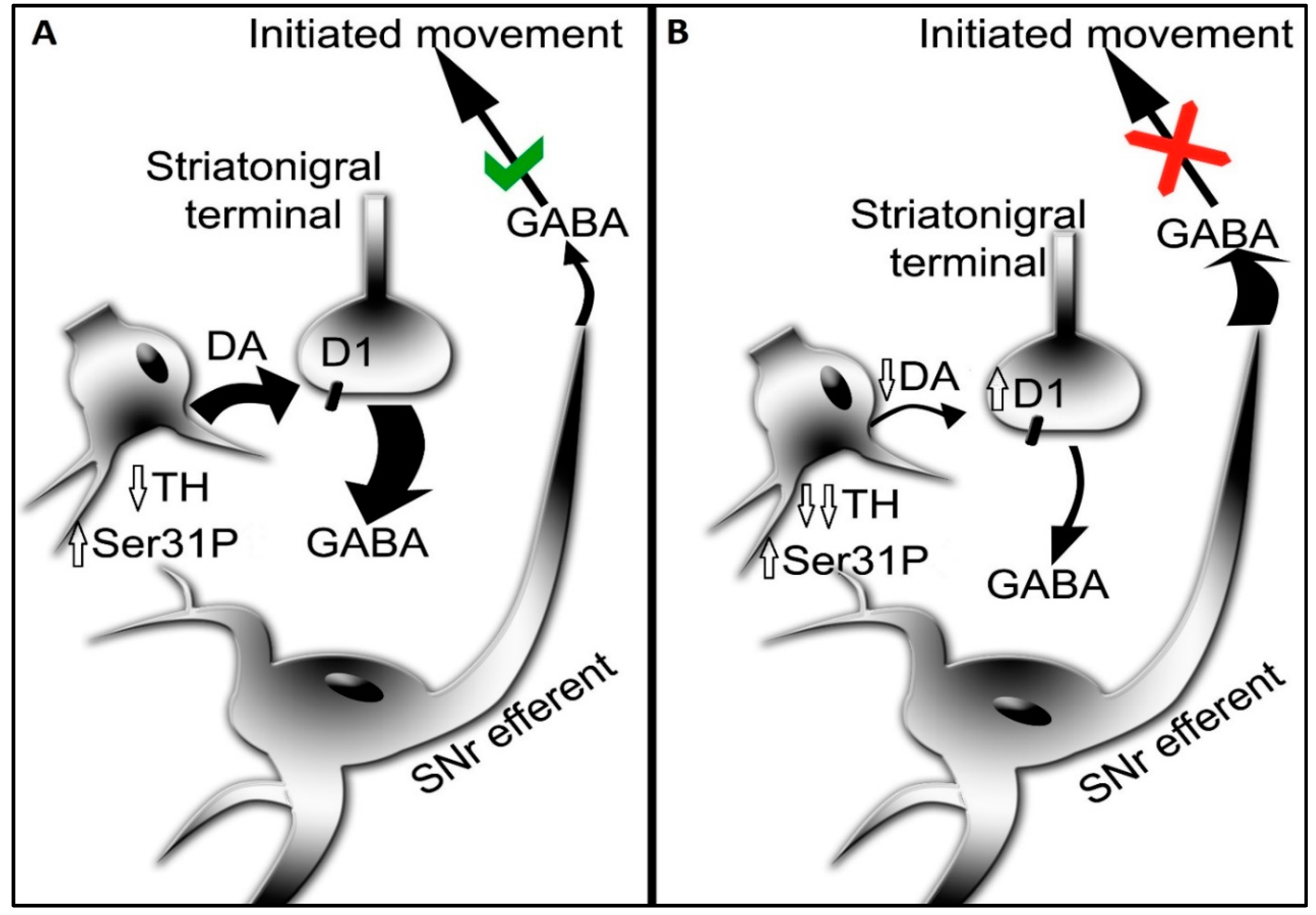
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




