Submitted:
11 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biology of adipose tissue
3. Biochemical aspects of fructose
4. Sources and consumption of fructose
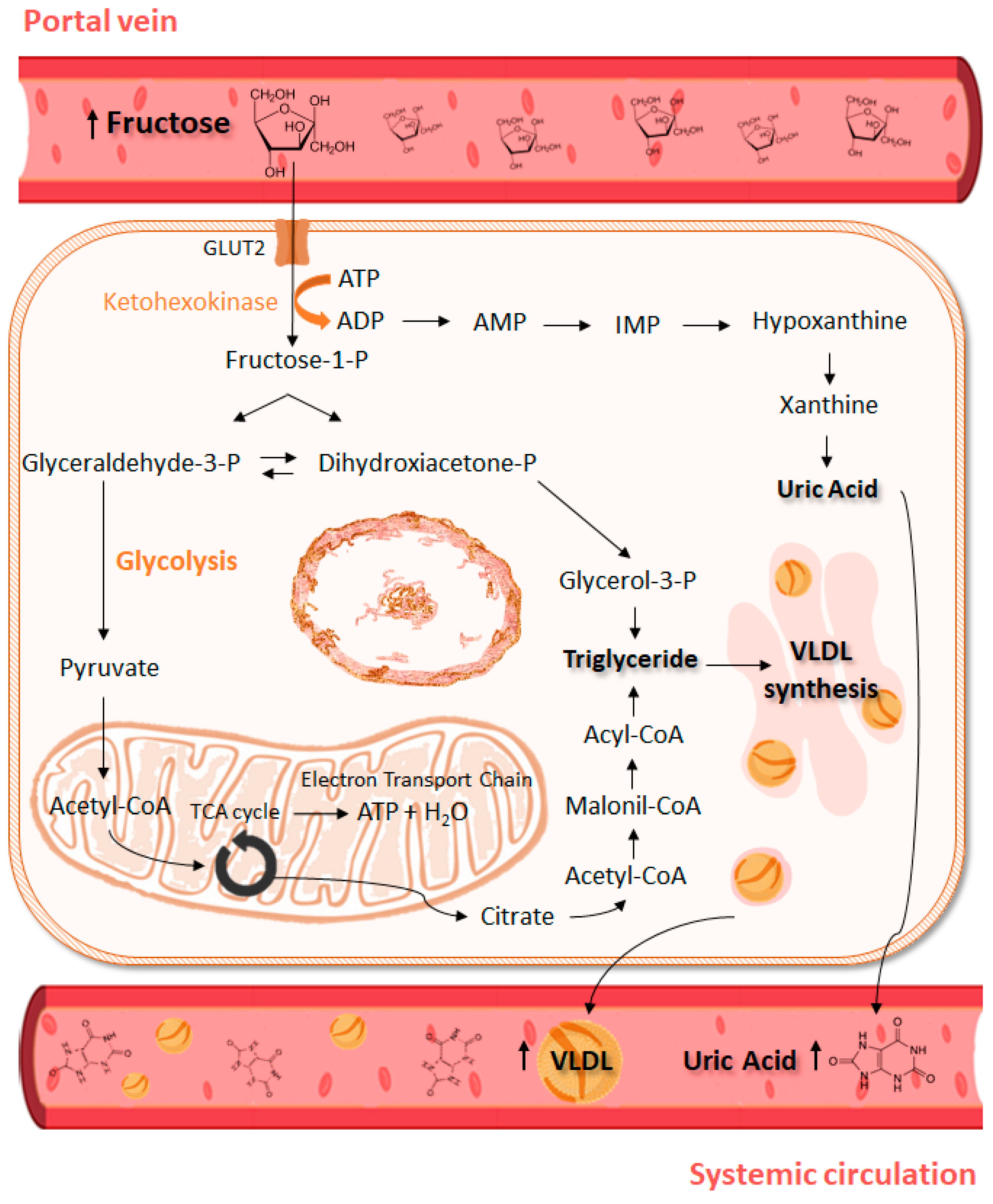
5. Excessive fructose intake and its metabolic implications
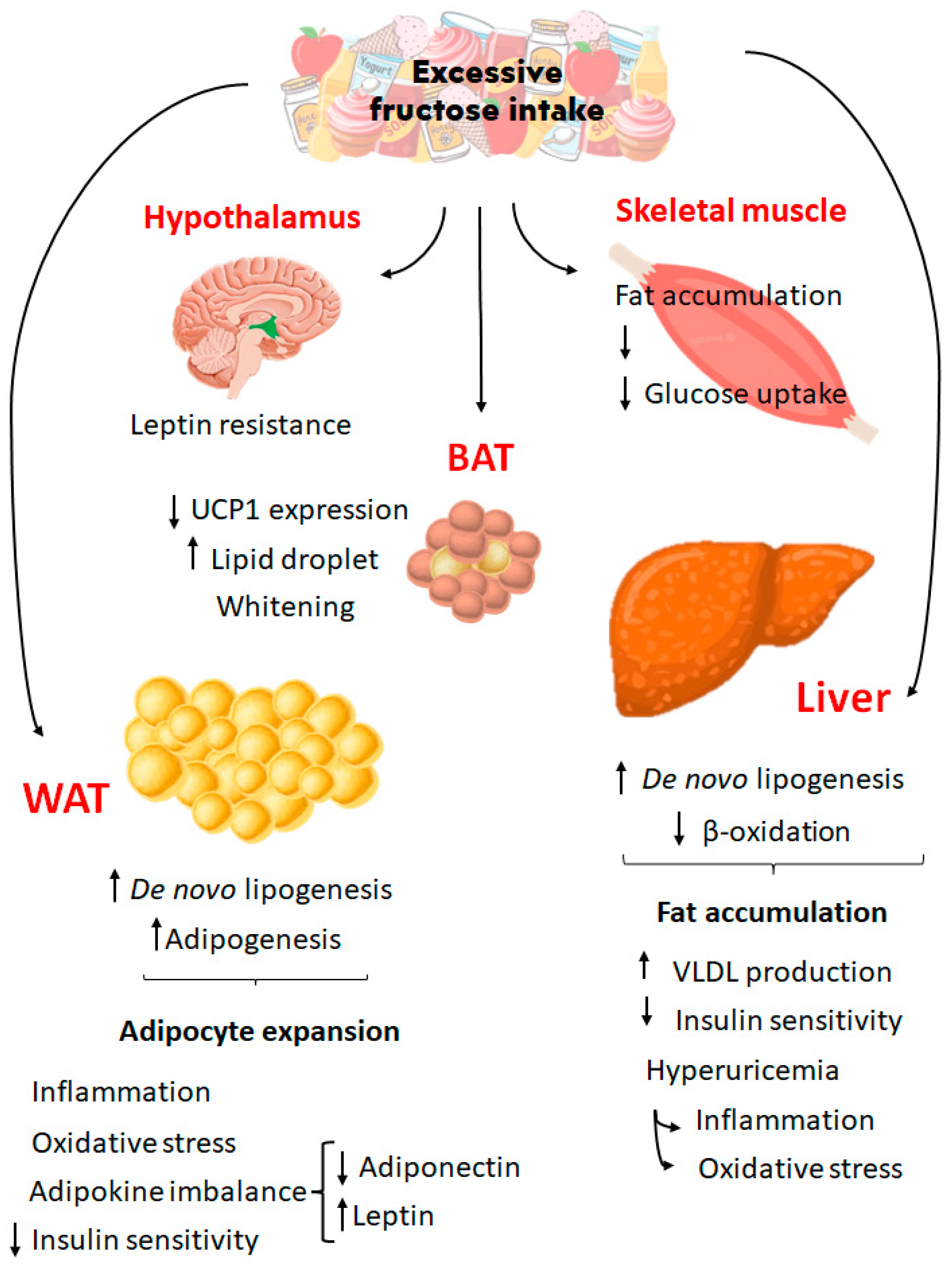
6. Fructose and obesity: what happens with adipose tissue
7. The multiple causes of childhood obesity
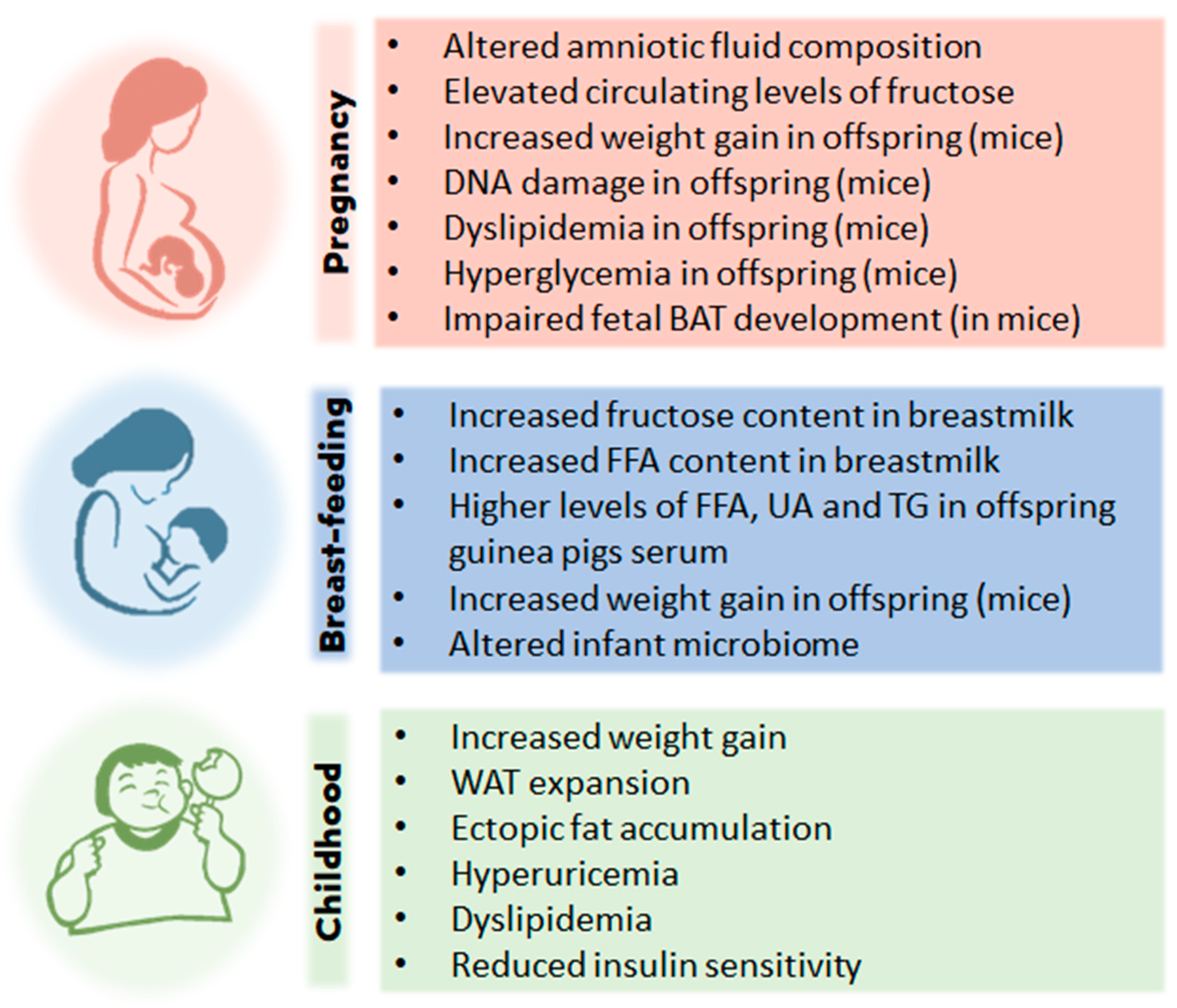
8. Fructose and childhood obesity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apperley LJ, Blackburn J, Erlandson-Parry K, Gait L, Laing P, Senniappan S. Childhood obesity: A review of current and future management options. Clin Endocrinol. 2022, 96, 288–301. [CrossRef]
- Thomas-Eapen N. Childhood Obesity. Prim Care - Clin Off Pract. 2021, 48, 505–15. [CrossRef]
- Pandita A, Sharma D, Pandita D, Pawar S, Tariq M, Kaul A. Childhood obesity: Prevention is better than cure. Diabetes, Metab Syndr Obes. 2016, 9, 83–9. [CrossRef]
- Sahoo K, Sahoo B, Choudhury A, Sofi N, Kumar R, Bhadoria A. Childhood obesity: causes and consequences. J Fam Med Prim Care. 2015, 4, 187-192. [CrossRef]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019,15, 288–98. [CrossRef]
- Lin X, Li H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front Endocrinol. 2021, 12, 1–9. [CrossRef]
- World Obesity Federation. World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 27 May 2023).
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease1-3. Am J Clin Nutr. 2007, 86, 899–906. [CrossRef]
- Pereira RM, Botezelli JD, da Cruz Rodrigues KC, Mekary RA, Cintra DE, Pauli JR, et al. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients. 2017, 9, 1–21. [CrossRef]
- Helsley RN, Moreau F, Gupta MK, Radulescu A, Debosch B, Softic S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr Diab Rep. 2020, 20, 1–16. [CrossRef]
- Czerwonogrodzka-Senczyna A, Rumińska M, Majcher A, Credo D, Jeznach-Steinhagen A, Pyrżak B. Fructose Consumption and Lipid Metabolism in Obese Children and Adolescents. Adv Exp Med Biol. 2019, 1153, 91–100. [CrossRef]
- Johnson RJ, Sánchez-lozada LG, Andrews P, Lanaspa MA. Perspective : A Historical and Scienti fi c Perspective of Sugar and Its Relation with Obesity and Diabetes. Adv Nutr. 2017, 8, 412–22. [CrossRef]
- Kwok KH, Lam KS, XU A. Heterogeneity of white adipose tim ssue: molecular basis and clinical implications. Exp Mol Med. 2016, 48, e215. [CrossRef]
- Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018, 221, jeb162958. [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004, 84, 277-359. [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends in Endocrinol Metabol. 2018, 29, 191-200. [CrossRef]
- Clemente-Suárez, V. J. et al. The role of adipokines in health and disease. Biomedicines, 2023, 11, 1290. [CrossRef]
- Song Z, Xiaoli AM, Yang F. Regulation and metabolic significance of De Novo lipogenesis in adipose tissues. Nutrients. 2018, 10,1383. [CrossRef]
- Poissonnet, C. M., Burdi, A. R. & Garn, S. M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11,1. [CrossRef]
- Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev Rep. 2012, 8, 55–66. [CrossRef]
- Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019, 20, 242–58. [CrossRef]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011, 12, 722–34. [CrossRef]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 1979, 63, 239-46. [CrossRef]
- Cameron M, Demerath EW. Critical Periods in Human Growth and Their Relationship to Diseases of Aging. Am J Phys Anthropol. 2002, 45, 159–184. [CrossRef]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, N€aslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783-7. [CrossRef]
- Jeffery E, Church CD, Holtrup B, Colman L & Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 2015, 17, 376–385. [CrossRef]
- Schwenk RW, Holloway GP, Luiken JJFP, Bonen A, Glatz JFC. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins leukot essent fatty acids, 2010, 82, 4-6, 49-54. [CrossRef]
- Bódis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Europ J Clin Invest. 2018, 48, 13017. [CrossRef]
- Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [CrossRef]
- Hirsch J, Farquhar JW, Ahrens Jr EH, Peterson ML, Stoffel W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr. 1960, 8, 499-511. [CrossRef]
- Schaefer-Graf UM, Meitzner K, Ortega-Senovilla H, Graf K, Vetter K, Abou-Dakn M, Herrera E. Differences in the implications of maternal lipids on fetal metabolism and growth between gestacional diabetes mellitus and control pregnancies. Diabet Med. 2011, 28, 1053-9. [CrossRef]
- Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014, 27, 63–93. [CrossRef]
- Bolsoni-Lopes A., Alonso-Vale MI. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [CrossRef]
- Desoye G, Herrera E. Adipose tissue development and lipid metabolism in the human fetus: The 2020 perspective focusing on maternal diabetes and obesity. Prog Lipid Res. 2021, 81, 101082. [CrossRef]
- Marcus C, Ehren H, Bolme P, Arner P. Regulation of lipolysis during the neonatal period: importance of thyrotropin. J. Clin. Invest., 1988, 82. 1793-7. [CrossRef]
- Barreiros RC, Bossolan G, Trindade CEP. Fructose in humans: Metabolic effects, clinical utilization, and associated inherent errors. Rev Nutr. 2005, 18, 377–89. [CrossRef]
- Herman MA, Birnbaum MJ. Molecular aspects of fructose metabolism and metabolic disease. Cell metabolism. 2021, 7, 33, 2329-54. [CrossRef]
- Hallfrisch J. Metabolic effects of dietary fructose.FASEB J. 1990, 4, 2652-60. [CrossRef]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994, 725, 713–25. [CrossRef]
- Mueckler M, Thorens B. Molecular Aspects of Medicine The SLC2 ( GLUT ) family of membrane transporters. Mol Aspects Med. 2013, 34, 121–38. [CrossRef]
- Truswell S, Thorburn AW. Incomplete absorption of pure fructose in healthy subjects. AM J Clin Nutr. 1988, 48, 1424-1430. [CrossRef]
- Ferraris RP, Choe J, Patel CR. Intestinal Absorption of Fructose. Annu Rev Nutr. 2018, 38, 41–67. [CrossRef]
- Hannou SA, Mckeown NM, Herman MA, Hannou SA, Haslam DE, Mckeown NM, et al. Fructose metabolism and metabolic disease Fructose metabolism and metabolic disease. J Clin Invest. 2018, 128, 545–55. [CrossRef]
- Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme, J. Histochem. Cytochem, 2009, 57, 763–774. [CrossRef]
- Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, Liu W, Tesz GJ, Birnbaum MJ, Rabinowitz JD. The small intestine converts dietary fructose intoglucose and organic acids, Cell Metabol. 2019, 27, 351–361. [CrossRef]
- Bidwell AJ, Chronic fructose ingestion as a major health concern: is a sedentary lifestyle making it worse? A Review, Nutrients, 2017, 9. [CrossRef]
- Duro D, Rising R, Cedillo M, Lifshitz F. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy, Pediatrics, 2002, 109, 797–805. [CrossRef]
- Gaby AR. Adverse effects of dietary fructose, Alternative Med. Rev., 2005, 10, 294–306.
- Semnani-Azad Z, Khan TA, Mejia SB, de Souza RJ, Leiter LA, Kendall CW, Hanley AJ, Sievenpiper JL. Association of major food sources of fructose-containing sugars with incident metabolic syndrome: a systematic review and meta-analysis. JAMA Netw open, 2020, 3, e209993. [CrossRef]
- Merino B, Fernández-Díaz CM, Cózar-Castellano I, Perdomo G. Intestinal fructose and glucose metabolism in health and disease. Nutrients. 2019, 12, 94. [CrossRef]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N. D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; Mihatsch, W.; Molgaard, C.; Vora, R.; Fewtrell, M. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65 , 681–696. [CrossRef]
- World Health Organization. Sugars intake for adults and children. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 02 May 2023).
- UK’s Scientific Advisory Committee on Nutrition. Carbohydrates and health. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 02 May 2023).
- Young J, Scott S, Clark L, Lodge JK. Associations between free sugar intake and markers of health in the UK population: an analysis of the National Diet and Nutrition Survey rolling programme. Br J Nutr, 2022, 128, 225-36. [CrossRef]
- Kmietowicz Z. Countries that use large amounts of high fructose corn syrup have higher rates of type 2 diabetes BMJ 2012, 345, 7994. [CrossRef]
- Bragança, M.L.B.M.; Bogea, E.G.; de Almeida Fonseca Viola, P.C.; dos Santos Vaz, J.; Confortin, S.C.; Menezes, A.M.B.; Gonçalves, H.; Bettiol, H.; Barbieri, M.A.; Cardoso, V.C.; da Silva, A.A.M. High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium. Nutrients 2023, 15, 324. [CrossRef]
- Colchero MA, Guerrero-Lopez CM, Molina M, et al. Beverages sales in Mexico before and after Implementation of a sugar sweetened beverage tax. PLoS One. 2016, 11, e0163463. [CrossRef]
- Goodman S, Vanderlee L, Jones A, White C, Hammond D. Perceived healthiness of sweeteners among young adults in Canada. Can J Diet Pract Res. 2021, 82, 90–4. [CrossRef]
- Hock K, Acton RB, Jáuregui A, Vanderlee L, White CM, Hammond D. Experimental study of front-of-package nutrition labels’ efficacy on perceived healthfulness of sugar-sweetened beverages among youth in six countries. Prev Med Reports. 2021, 24, 101577. [CrossRef]
- Mantzari E, Vasiljevic M, Turney I, Pilling M, Marteau T. Impact of warning labels on sugar-sweetened beverages on parental selection: An online experimental study. Prev Med Reports.2018, 259–67. [CrossRef]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease1-3. Am J Clin Nutr. 2007, 86, 899–906. [CrossRef]
- Taskinen MR, Packard CJ, Borén J. Dietary fructose and the metabolic syndrome. Nutrients. 2019, 11, 1–16. [CrossRef]
- Klein AV, Kiat H. The mechanisms underlying fructose-induced hypertension: A review. J Hypertens. 2015, 33, 912–20. [CrossRef]
- Ichigo Y, Takeshita A, Hibino M, Nakagawa T, Hayakawa T, Patel D, et al. High-fructose diet-induced hypertriglyceridemia is associated with enhanced hepatic expression of ACAT2 in Rats. Physiol Res. 2019, 68, 1021–6. [CrossRef]
- DiStefano JK. Fructose-mediated effects on gene expression and epigenetic mechanisms associated with NAFLD pathogenesis. cell mol life sci. 2020, 77, 2079–90. [CrossRef]
- Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018, 128, 545–55. [CrossRef]
- Muriel P, López-sánchez P, Ramos-tovar E. Fructose and the liver. Int J Mol Sci. 2021, 22, 6969. [CrossRef]
- Lê KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009, 89, 1760–5. [CrossRef]
- Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab. 2005, 2, 1–14. [CrossRef]
- Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular Mechanisms of Insulin Resistance in Rats with Fructose-Induced Hypertension. Am J Hypertens. 2003, 16, 973–8. [CrossRef]
- Ueno M, Bezerra RMN, Silva MS, Tavares DQ, Carvalho CR, Saad MJA. A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats. Brazilian J Med Biol Res. 2000, 33, 1421–7. [CrossRef]
- Russo E, Leoncini G, Esposito P, Garibotto G, Pontremoli R, Viazzi F. Fructose and uric acid: Major mediators of cardiovascular disease risk starting at pediatric age. Int J Mol Sci. 2020, 21, 1–13. [CrossRef]
- Spiga R, Marini MA, Mancuso E, Di Fatta C, Fuoco A, Perticone F, et al. Uric Acid Is Associated with Inflammatory Biomarkers and Induces Inflammation Via Activating the NF-κB Signaling Pathway in HepG2 Cells. Arterioscler Thromb Vasc Biol. 2017, 37, 1241–9. [CrossRef]
- Wang Y, Qi W, Song G, Pang S, Peng Z, Li Y, et al. High-fructose diet increases inflammatory cytokines and alters gut microbiota composition in rats. Mediators Inflamm. 2020, 2020, 6672636. [CrossRef]
- Hernández-Díazcouder A, Romero-Nava R, Carbó R, Sánchez-Lozada LG, Sánchez-Muñoz F. High fructose intake and adipogenesis. Int J Mol Sci. 2019, 20, 2787. [CrossRef]
- Jürgens H, Haass W, Castañeda TR, Schürmann A, Koebnick C, Dombrowski F, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005, 13, 1145–56. [CrossRef]
- Pektas MB, Koca HB, Sadi G, Akar F. Dietary Fructose Activates Insulin Signaling and Inflammation in Adipose Tissue: Modulatory Role of Resveratrol. Biomed Res Int. 2016, 2016, 8014252. [CrossRef]
- Yahia H, Hassan A, El-Ansary MR, Al-Shorbagy MY, El-Yamany MF. IL-6/STAT3 and adipokine modulation using tocilizumab in rats with fructose-induced metabolic syndrome. Naunyn Schmiedebergs Arch Pharmacol. 2020, 393, 2279–2292. [CrossRef]
- Miranda CS, Silva-Veiga FM, Santana-Oliveira DA, Fernandes-da-Silva A, Brito GC, Martins FF, Souza-Mello V. Chronic Excessive Fructose Intake Maximizes Brown Adipocyte Whitening but Causes Similar White Adipocyte Hypertrophy Than a High-Fat Diet in C57BL/6 Mice. J Am Nutr Assoc. 2023, 42, 435-444. [CrossRef]
- Santos MP, Cauduro LFR, Ferreira MM, Martucci LF, Vecchiatto B, Vilas-boas EA, et al. Effect of Low-Dose Progesterone on Glycemic Metabolism , Morphology and Function of Adipose Tissue and Pancreatic Islets in Diet-Induced Obese Female Mice. 2023, 28, 312. [CrossRef]
- Crescenzo R, Bianco F, Coppola P, Mazzoli A, Valiante S, Liverini G, et al. Adipose tissue remodeling in rats exhibiting fructose-induced obesity. Eur J Nutr. 2014, 53, 413–9. [CrossRef]
- Zubiría MG, Alzamendi A, Moreno G, Rey MA, Spinedi E, Giovambattista A. Long-term fructose intake increases adipogenic potential: Evidence of direct effects of fructose on adipocyte precursor cells. Nutrients. 2016, 8, 198. [CrossRef]
- London E, Castonguay TW. High fructose diets increase 11β-hydroxysteroid dehydrogenase type 1 in liver and visceral adipose in rats within 24-h exposure. Obesity. 2011, 19, 925–32. [CrossRef]
- Legeza B, Balázs Z, Odermatt A. Fructose promotes the differentiation of 3T3-L1 adipocytes and accelerates lipid metabolism. FEBS Lett. 2014, 588, 490–6. [CrossRef]
- Prince PD, Santander YA, Gerez EM, Höcht C, Polizio AH, Mayer MA, et al. Fructose increases corticosterone production in association with NADPH metabolism alterations in rat epididymal white adipose tissue. J Nutr Biochem. 2017, 46, 109–16. [CrossRef]
- Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta - Mol Basis Dis. 2014, 1842, 473–81. [CrossRef]
- Park Y-K, Ge K. Glucocorticoid Receptor Accelerates, but Is Dispensable for, Adipogenesis. Mol Cell Biol. 2017, 37, e00260-16. [CrossRef]
- Du L, Heaney AP. Regulation of adipose differentiation by fructose and GluT5. Mol Endocrinol. 2012, 26, 1773–82. [CrossRef]
- Meneses MJ, Sousa-Lima I, Jarak I, Raposo JF, Alves MG, Macedo MP. Distinct impacts of fat and fructose on the liver, muscle, and adipose tissue metabolome: An integrated view. Front Endocrinol (Lausanne). 2022, 13, 898471. [CrossRef]
- Li J xiu, Ke D zhi, Yao L, Wang S, Ma P, Liu L, et al. Response of genes involved in lipid metabolism in rat epididymal white adipose tissue to different fasting conditions after long-term fructose consumption. Biochem Biophys Res Commun. 2017, 484, 336–41. [CrossRef]
- Mazzoli A, Porzio A Di, Gatto C, Crescenzo R, Nazzaro M, Spagnuolo MS, et al. Skeletal muscle insulin resistance and adipose tissue hypertrophy persist beyond the reshaping of gut microbiota in young rats fed a fructose-rich diet. J Nutr Biochem . 2023, 113, 109247. [CrossRef]
- Kovačević S, Brkljačić J, Vojnović Milutinović D, Gligorovska L, Bursać B, Elaković I, et al. Fructose Induces Visceral Adipose Tissue Inflammation and Insulin Resistance Even Without Development of Obesity in Adult Female but Not in Male Rats. Front Nutr. 2021, 8, 1–18. [CrossRef]
- Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011, 60, 1258–69. [CrossRef]
- Singh S, Sharma A, Guru B, Ahmad S, Gulzar F, Kumar P, et al. Fructose-mediated NLRP3 activation induces inflammation and lipogenesis in adipose tissue. J Nutr Biochem. 2022, 107, 109080. [CrossRef]
- Kuzma JN, Cromer G, Hagman DK, Breymeyer KL, Roth CL, Foster-Schubert KE, et al. No differential effect of beverages sweetened with fructose, high-fructose corn syrup, or glucose on systemic or adipose tissue inflammation in normal-weight to obese adults: A randomized controlled trial. Am J Clin Nutr. 2016, 104, 306–14. [CrossRef]
- Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab Syndr Relat Disord. 2015, 13, 423–44. [CrossRef]
- Bratoeva K, Radanova M, Merdzhanova A, Donev I. Protective role of S-Adenosylmethionine against fructose-induced oxidative damage in obesity. J Mind Med Sci. 2017, 4, 163–71. [CrossRef]
- Araoye E, Ckless K. Effects of High Fructose/Glucose on Nlrp3/Il1β Inflammatory Pathway. J Young Investig. 2016, 31, 25–30. [CrossRef]
- Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, Checherita IA. Uric Acid and Oxidative Stress—Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int J Mol Sci. 2022, 23, 3188. [CrossRef]
- Baiċc J, Bjelakoviċ G, Pavloviċ D, Kocić G, Jevtoviċ T, Stojanoviċ I, et al. Glucocorticoids and Oxidative Stress. J Basic Clin Physiol Pharmacol. 2007, 18, 115–28. [CrossRef]
- Imhoff BR, Hansen JM. Extracellular redox environments regulate adipocyte differentiation. Differentiation. 2010, 80, 31–9. [CrossRef]
- Han J, Choi HY, Dayem AA, Kim K, Yang G, Won J, et al. Regulation of Adipogenesis Through Differential Modulation of ROS and Kinase Signaling Pathways by 3,4′-Dihydroxyflavone Treatment. J Cell Biochem. 2017, 118, 1065–77. [CrossRef]
- Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci. 2020, 21, 3570. [CrossRef]
- Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci. 2021, 135, 731–52. [CrossRef]
- Maslov LN, Naryzhnaya N V., Boshchenko AA, Popov S V., Ivanov V V., Oeltgen PR. Is oxidative stress of adipocytes a cause or a consequence of the metabolic syndrome? J Clin Transl Endocrinol. 2019, 15, 1–5. [CrossRef]
- Rodrigues DF, Henriques MC do C, Oliveira MC, Menezes-Garcia Z, Marques PE, Souza D da G, et al. Acute intake of a high-fructose diet alters the balance of adipokine concentrations and induces neutrophil influx in the liver. J Nutr Biochem . 2014, 25, 388–94. [CrossRef]
- Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020, 7, 22. [CrossRef]
- Mendoza-herrera K, Florio AA, Moore M, Marrero A, Tamez M, Bhupathiraju SN, et al. The Leptin System and Diet : A Mini Review of the Current Evidence. 2021, 12, 1–12. [CrossRef]
- Jian-mei LI, Chuang W, Qing-hua HU, Ling-dong K. Fructose Induced Leptin Dysfunction and Improvement by Quercetin and Rutin in Rats. Chinese J Nat Med. 2008, 6, 466-473. [CrossRef]
- Shapiro A, Tu N, Gao Y, Cheng K, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr. 2011, 106, 390–7. [CrossRef]
- Shapiro A, Mu W, Roncal C, Cheng K, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. 2008, 32610, 1370–5. [CrossRef]
- Äijälä M,Malo E, Ukkola O, Bloigu R, Lehenkari P, Autio-harmainen H, Santaniemi M, et al. Long-term fructose feeding changes the expression of leptin receptors and autophagy genes in the adipose tissue and liver of male rats : a possible link to elevated triglycerides. 2013, 8, 623-35. [CrossRef]
- Haring SJ, Harris RBS. The relation between dietary fructose , dietary fat and leptin responsiveness in rats. Physiol Behav. 2011, 104, 914–22. [CrossRef]
- Miranda CS, Silva-Veiga F, Martins FF, Rachid TL, Mandarim-De-Lacerda CA, Souza-Mello V. PPAR-α activation counters brown adipose tissue whitening: a comparative study between high-fat– and high-fructose–fed mice. Nutrition. 2020, 78, 110791. [CrossRef]
- Machado TQ, Pereira-Silva DC, Goncalves LF, Fernandes-Santos C. Brown Adipose Tissue Remodeling Precedes Cardiometabolic Abnormalities Independent of Overweight in Fructose-Fed Mice. Integr Diabetes Cardiovasc Dis. 2019, 3, 72–82. [CrossRef]
- Richard G, Blondin DP, Syed SA, Rossi L, Fontes ME, Fortin M, et al. High-fructose feeding suppresses cold-stimulated brown adipose tissue glucose uptake independently of changes in thermogenesis and the gut microbiome. Cell Reports Med. 2022, 3, 100742. [CrossRef]
- Berger PK, Plows JF, Jones RB, et al. Associations of maternal fructose and sugar-sweetened beverage and juice intake during lactation with infant neurodevelopmental outcomes at 24 months. Am J Clin Nutr. 2020, 112, 1516-1522. [CrossRef]
- Larqué E, Labayen I, Flodmark CE, Lissau I, Czernin S, Moreno LA, Pietrobelli A, Widhalm K. From conception to infancy — early risk factors for childhood obesity. In Nature Rev Endocrinol. 2019, 15, 456-478. [CrossRef]
- Shaban Mohamed, M. A., AbouKhatwa, M. M., Saifullah, A. A., Hareez Syahmi, M., Mosaad, M., Elrggal, M. E., Dehele, I. S., Elnaem, M. H. Risk Factors, Clinical Consequences, Prevention, and Treatment of Childhood Obesity. In Children, 2022, 9. [CrossRef]
- Drozdz D., Alvarez-Pitti J., Wójcik M., Borghi C., Gabbianelli R., Mazur A., Herceg-čavrak V., Lopez-Valcarcel BG., Brzeziński M., Lurbe E., Wühl E. Obesity and cardiometabolic risk factors: From childhood to adulthood. In Nutrients. 2021, 13. [CrossRef]
- Avelar Rodriguez D., Toro Monjaraz EM., Ignorosa Arellano KR., Ramirez Mayans J. Childhood obesity in Mexico: Social determinants of health and other risk factors. BMJ Case Rep. 2018, bcr2017223862. [CrossRef]
- Lee EY., Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018, 12, 658–666. [CrossRef]
- Williams CB., MacKenzie KC., Gahagan,S. The effect of maternal obesity on the offspring. Clin Obstet Gynecol. 2014, 57, 508–515. [CrossRef]
- Lakshman R., Elks CE., Ong KK. Childhood obesity. Circulation, 2012, 126, 1770–1779. [CrossRef]
- Mahumud RA., Sahle BW., Owusu-Addo E. et al. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int J Obes. 2021, 45, 2404–2418. [CrossRef]
- Mittal, M., Jain, V. Management of Obesity and Its Complications in Children and Adolescents. In Indian Journal of Pediatrics, 2021, 88, 1222–1234. [CrossRef]
- Hemmingsson E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. In Curr Obes Reps, 2018, 7, 204–209. [CrossRef]
- Kostovski M., Tasic V., Laban N., Polenakovic M., Danilovski D., Gucev, Z. Obesity in childhood and adolescence, genetic factors. Pril (Makedonska Akademija Na Naukite i Umetnostite. Oddelenie Za Medicinski Nauki). 2017, 34, 85–89. [CrossRef]
- Holmberg NG, Kaplan B, Karvonen MJ, Lind J, Malm. M. Permeability of Human Placenta to Glucose, Fructose, and Xylose. Acta Physiol Scand. 1956, 36, 291–9. [CrossRef]
- Lintao RCV, Kammala AK, Vora N, Yaklic JL, Menon R. Fetal membranes exhibit similar nutrient transporter expression profiles to the placenta. Placenta. 2023, 135, 33–42. [CrossRef]
- Magenis ML, Damiani AP, de Bem Silveira G, Dagostin LS, de Marcos PS, de Souza E, de Roch Casagrande L, Longaretti LM, Silveira PC, de Andrade VM. Metabolic programming in offspring of mice fed fructose during pregnancy and lactation. Journal of Developmental Origins of Health and Disease. 2022, 13, 441-54. [CrossRef]
- Koo S, Kim M, Cho HM, Kim I. Maternal high-fructose intake during pregnancy and lactation induces metabolic syndrome in adult offspring. Nutrition Research and Practice. 2021, 15, 160-72. [CrossRef]
- Jia G, Hill MA, Sowers JR. Maternal exposure to high fructose and offspring health. Hypertension. 2019, 74, 499-501. [CrossRef]
- Wang P, Wu T, Fu Q, Liao Q, Li Y, Huang T, et al. Maternal High-Fructose Intake Activates Myogenic Program in Fetal Brown Fat and Predisposes Offspring to Diet-Induced Metabolic Dysfunctions in Adulthood. 2022, 9, 848983. [CrossRef]
- Englund-Ögge L, Brantsæter AL, Haugen M, Sengpiel V, Khatibi A, Myhre R, Myking S, Meltzer HM, Kacerovsky M, Nilsen RM, Jacobsson B. Association between intake of artificially sweetened and sugar-sweetened beverages and preterm delivery: a large prospective cohort study. The American journal of clinical nutrition. 2012, 96, 552-9. [CrossRef]
- Zhang H, Li X, Niu Y, et al. Fasting serum fructose is associated with risk of gestational diabetes mellitus. Bmc pregnancy and childbirth. 2022, 22, 446. [CrossRef]
- Wright LS, Rifas-Shiman SL, Oken E, Litonjua AA, Gold DR. Prenatal and Early Life Fructose, Fructose-Containing Beverages, and Mid childhood Asthma. Ann Am Thorac Soc. 2018, 15, 217-224. [CrossRef]
- Cohen JFW, Rifas-Shiman SL, Young J, Oken E. Associations of prenatal and child sugar intake with child cognition. Am J Prev Med. 2018, 54, 727-735. [CrossRef]
- Koski KG, Fergusson MA. Amniotic fluid composition responds to changes in maternal dietary carbohydrate and is related to metabolic status in term fetal rats. J Nutr. 1992, 122, 385–92. [CrossRef]
- Berger PK, Fields DA, Demerath EW, Fujiwara H, Goran MI. High-fructose corn syrup-sweetened beverage intake increases 5-hour breast milk fructose concentrations in lactating women. Nutrients. 2018, 10, 669. [CrossRef]
- Smith EVL, Dyson RM, Berry MJ, Gray C. Fructose Consumption During Pregnancy Influences Milk Lipid Composition and Offspring Lipid Profiles in Guinea Pigs. Front Endocrinol (Lausanne). 2020, 11, 550. [CrossRef]
- Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients. 2017, 9, 146. [CrossRef]
- Berger PK, Plows JF, Jones RB, et al. Associations of maternal fructose and sugar-sweetened beverage and juice intake during lactation with infant neurodevelopmental outcomes at 24 months. Am J Clin Nutr. 2020, 112, 1516-1522. [CrossRef]
- Jones RB, Berger PK, Plows JF, Alderete TL, Millstein J, Fogel J, et al. Lactose-reduced infant formula with added corn syrup solids is associated with a distinct gut microbiota in Hispanic infants. Gut Microbes. 2020, 12. [CrossRef]
- Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 2012, 22, 1147–62. [CrossRef]
- Giussani M, Lieti G, Orlando A, Parati G, Genovesi S. Fructose Intake, Hypertension and Cardiometabolic Risk Factors in Children and Adolescents: From Pathophysiology to Clinical Aspects. A Narrative Review. Front Med. 2022, 9, 1–19. [CrossRef]
- Febbraio MA, Karin M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–28. [CrossRef]
- Ranjit N, Evans MH, Byrd-Williams C, Evans AE, Hoelscher DM. Dietary and activity correlates of sugar-sweetened beverage consumption among adolescents. Pediatrics. 2010, 126, e754-e761. [CrossRef]
- Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004, 12, 778–88. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




