Submitted:
07 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Dataset
2.2. Random Forest Regression
2.3. Model Validation by Power-Law Reaction Kinetics
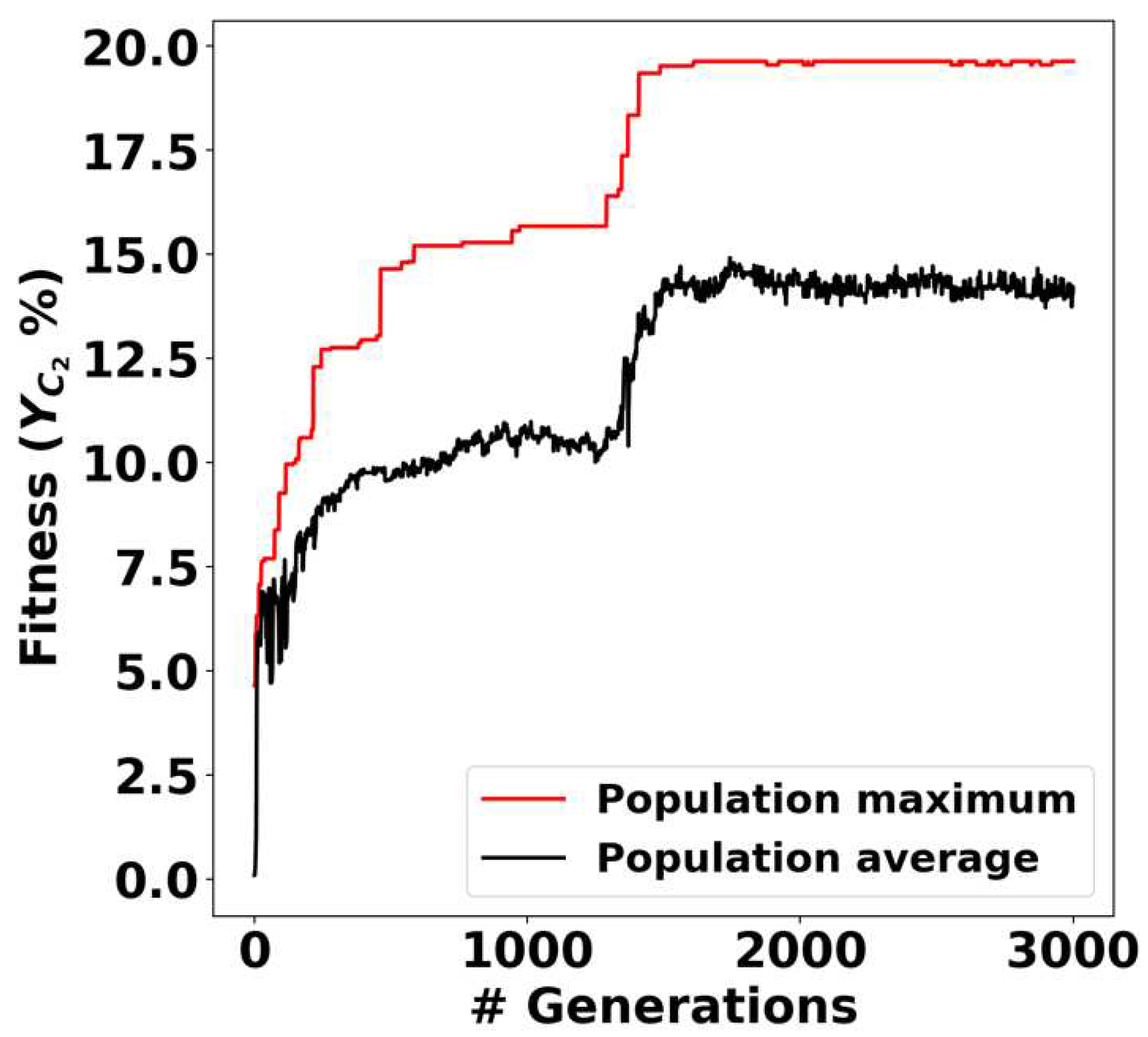
2.4. Genetic Algorithm for Multi-Objective Optimization
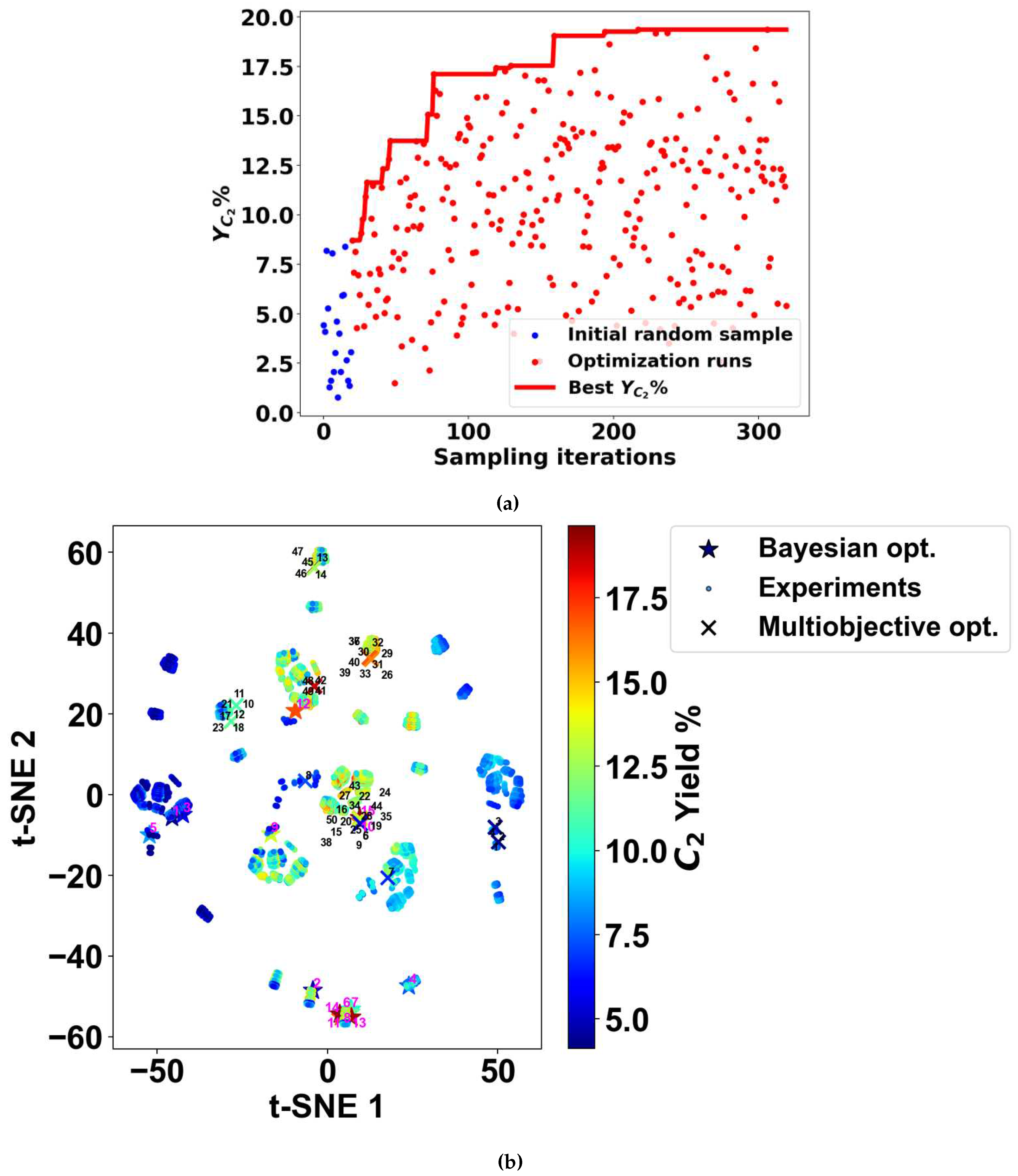
2.5. Bayesian Optimization for Adaptive Experimentation
3. Results and Discussion
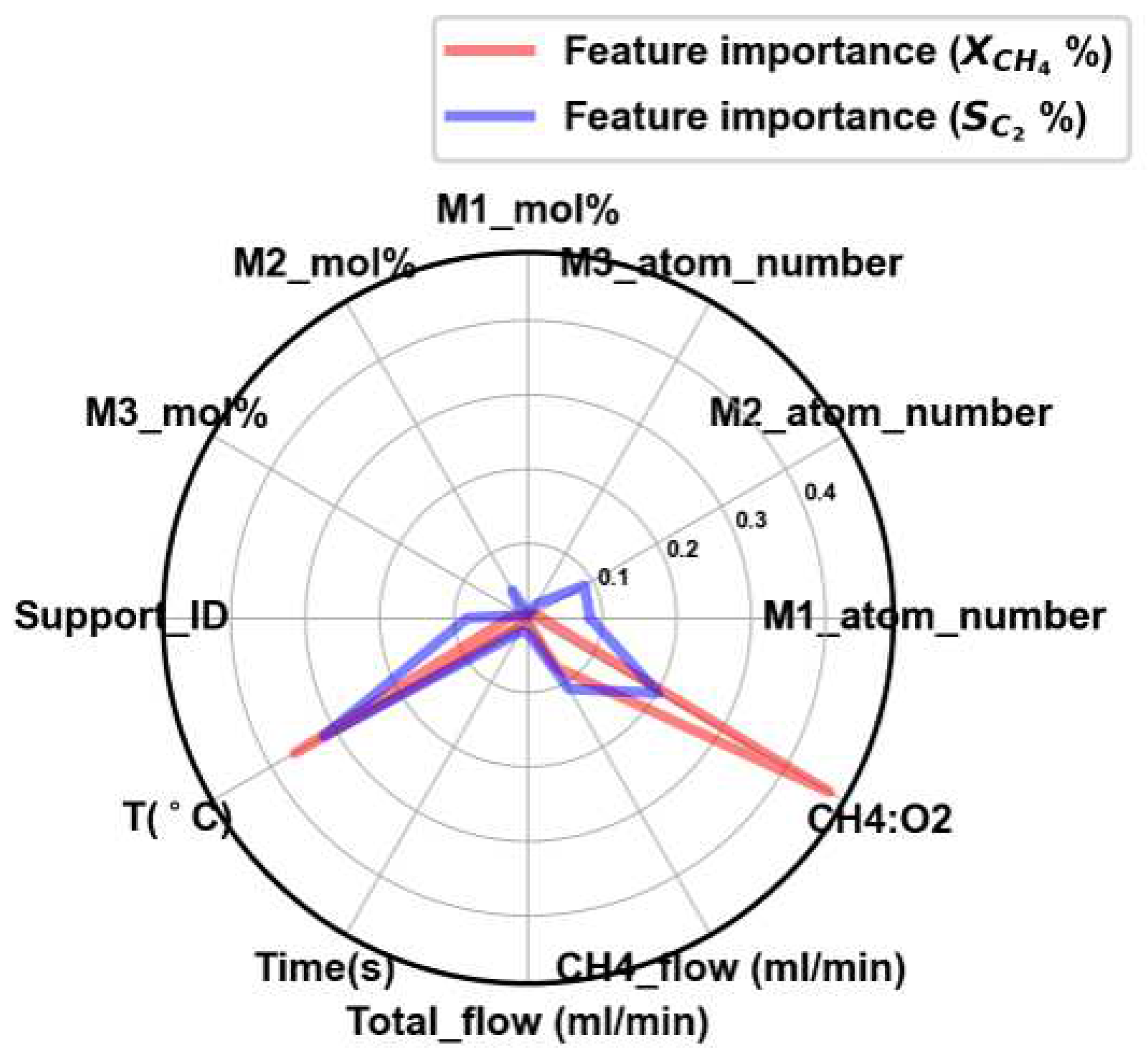
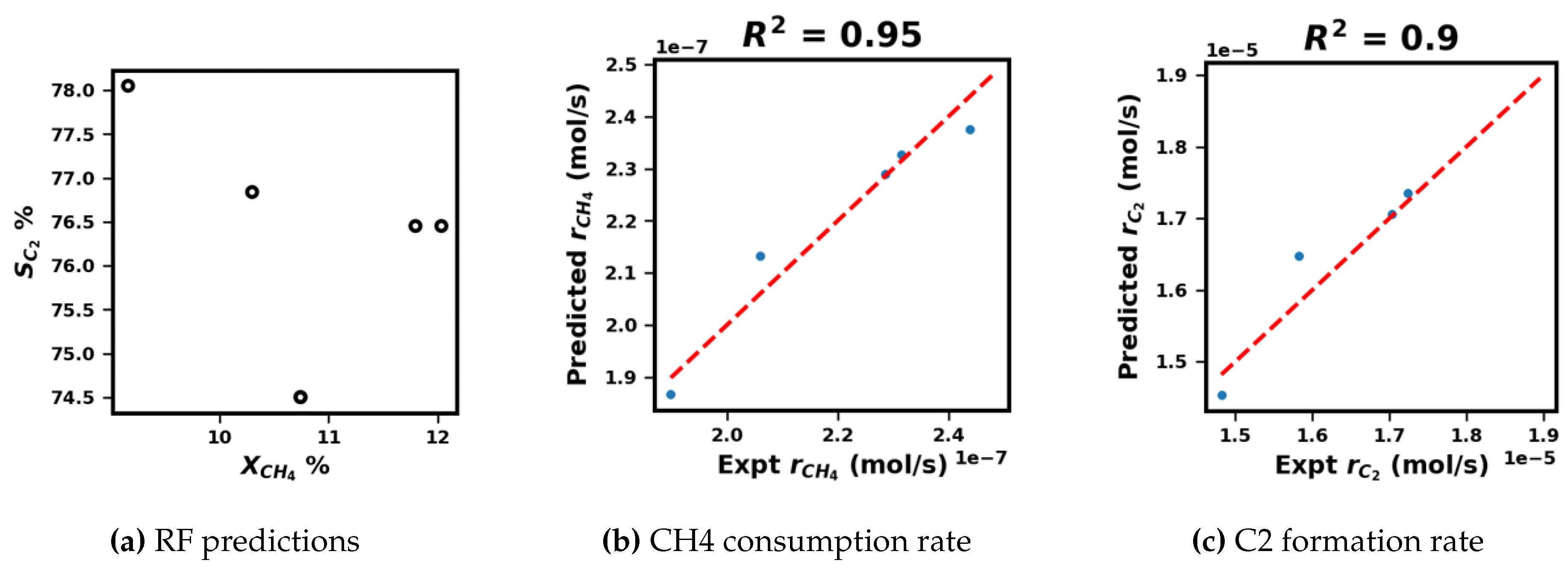
3.1. Assessment and Validation of Random Forest Regression via Kinetic Parameter Estimation
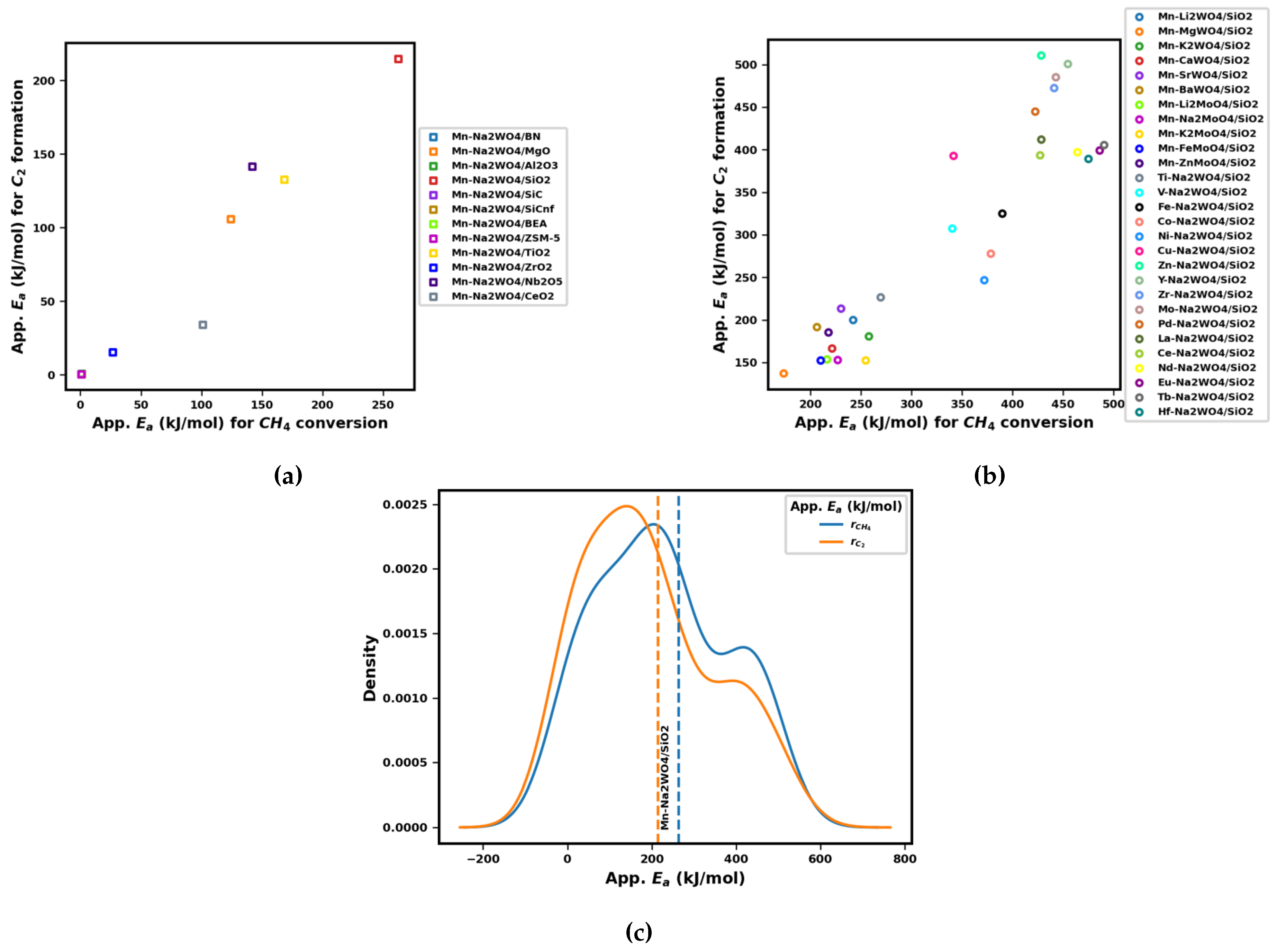
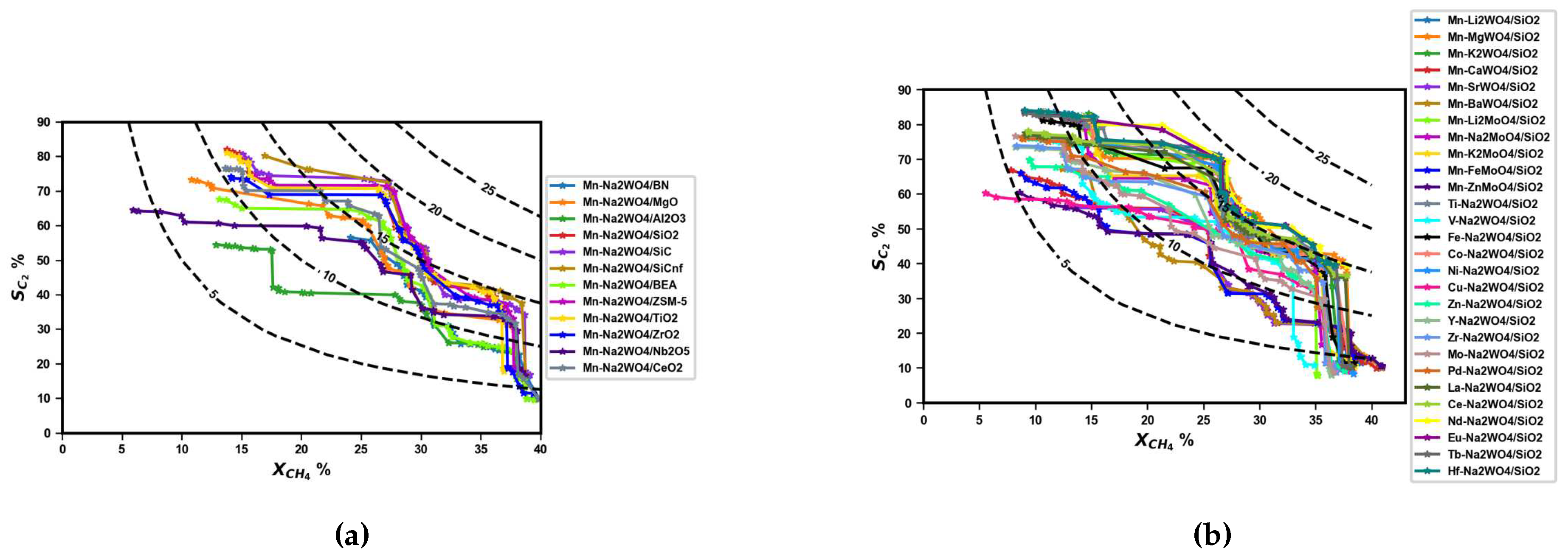
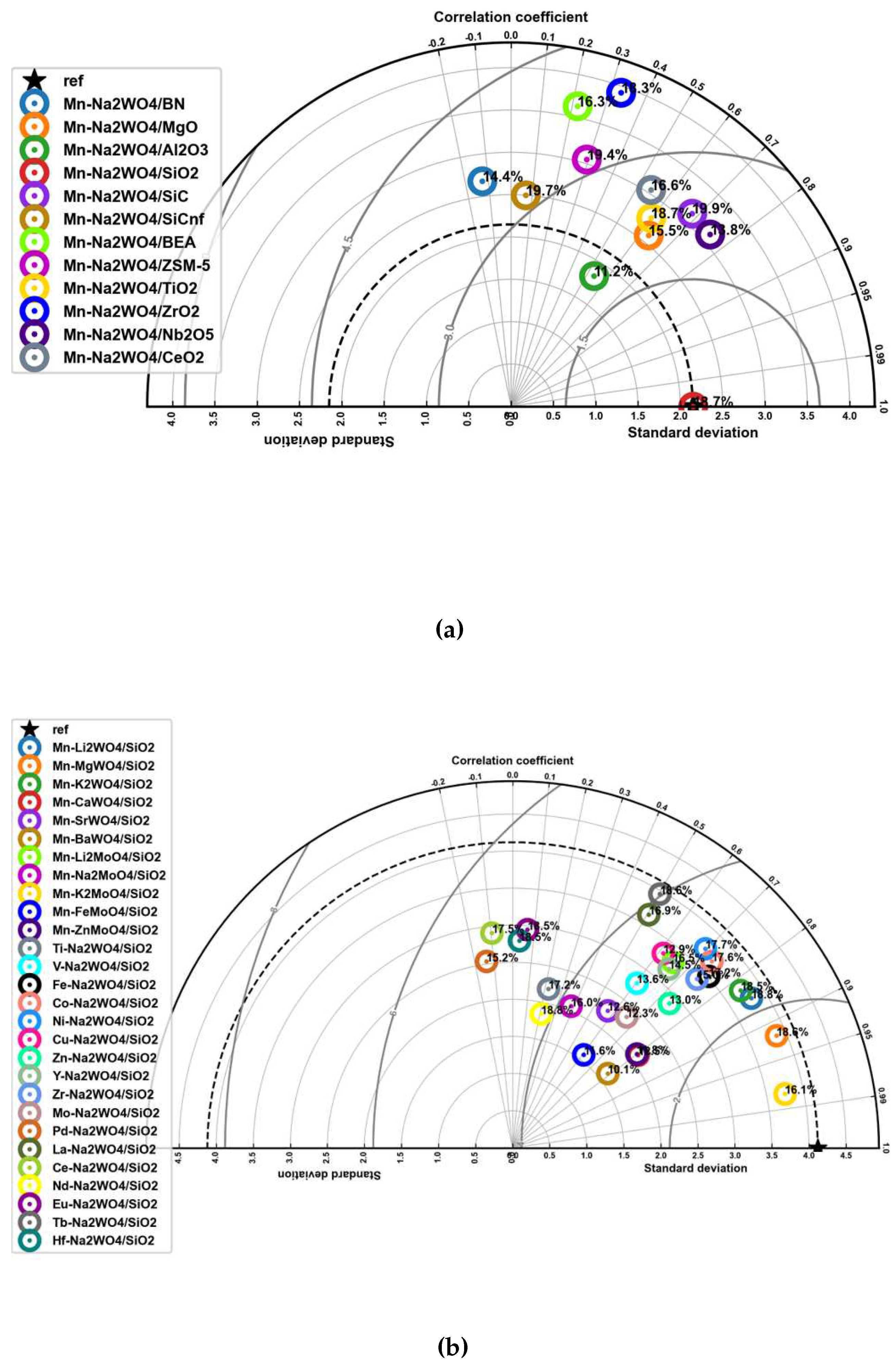
3.2. Performance Curves for Catalyst Screening
3.3. Proposed Candidates across Combinations of Catalysts and Operating Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Assessing the Random Forest Regression Model
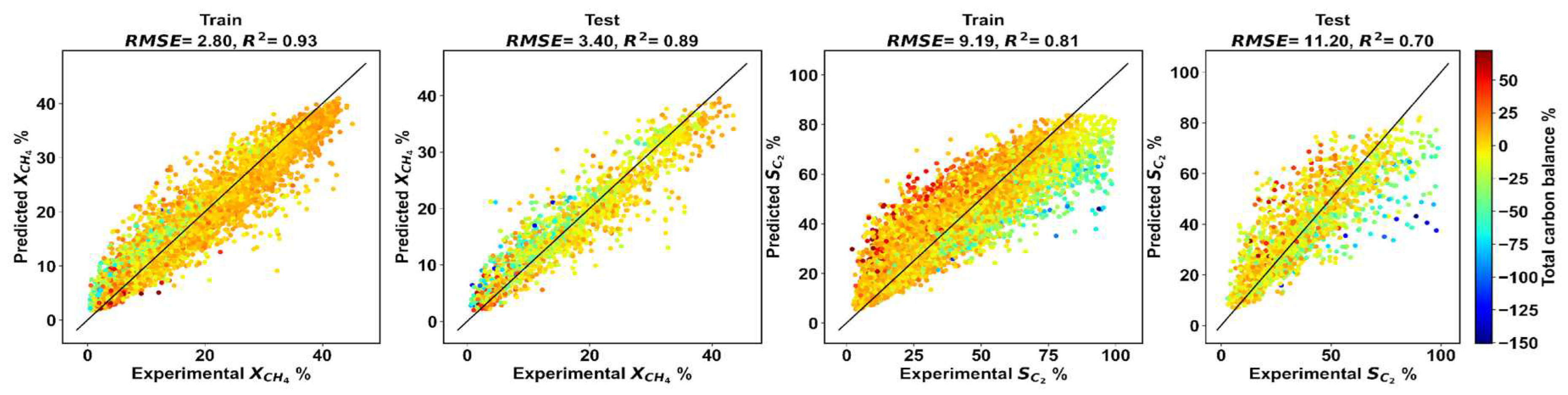
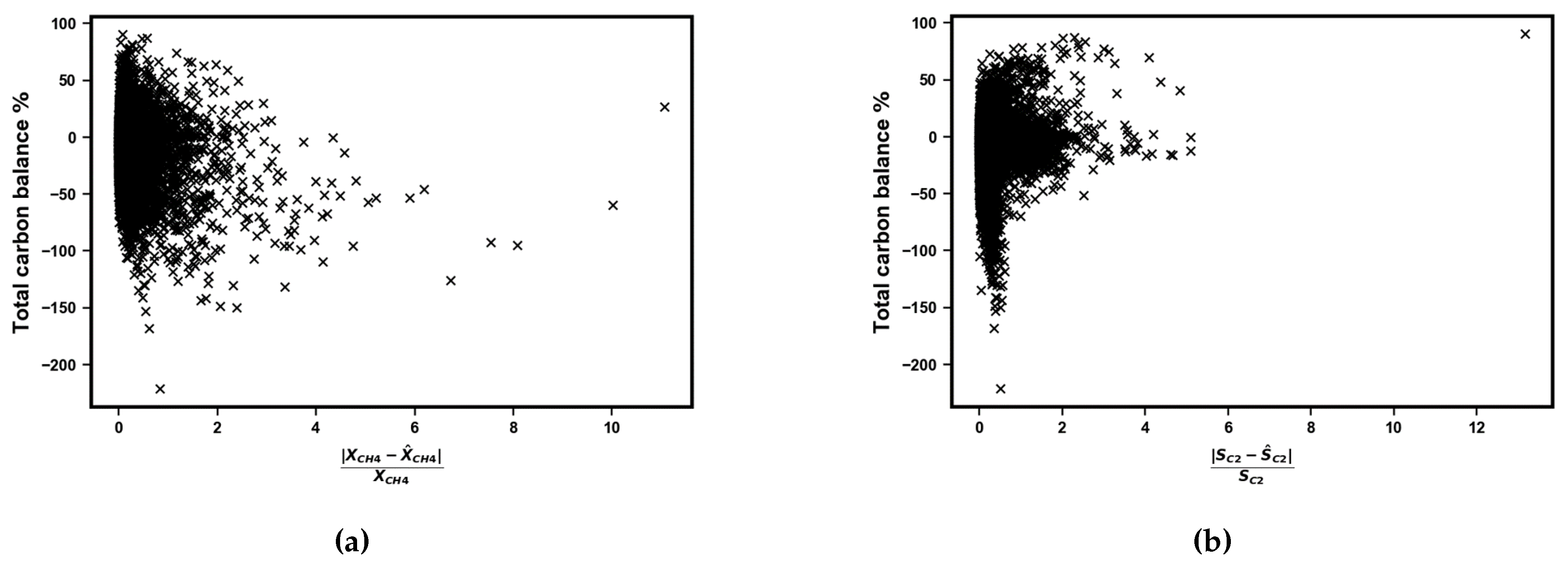
Appendix A.2. Tabulation of LHS Sample Points
| T(°C) | time(s) | (ml/min) | (ml/min) | CH4:O2 (mol:mol) |
|---|---|---|---|---|
| 756.17 | 0.60 | 21.62 | 10.92 | 15.41 |
| 747.83 | 0.41 | 21.04 | 10.42 | 16.67 |
| 751.17 | 0.53 | 20.88 | 10.08 | 12.74 |
| 752.83 | 0.47 | 21.63 | 10.75 | 12.29 |
| 749.50 | 0.72 | 21.13 | 10.58 | 19.54 |
| 754.50 | 0.66 | 21.21 | 10.25 | 10.70 |
Appendix A.3. Fits and Orders of Power-Law Kinetic Parameter Estimation
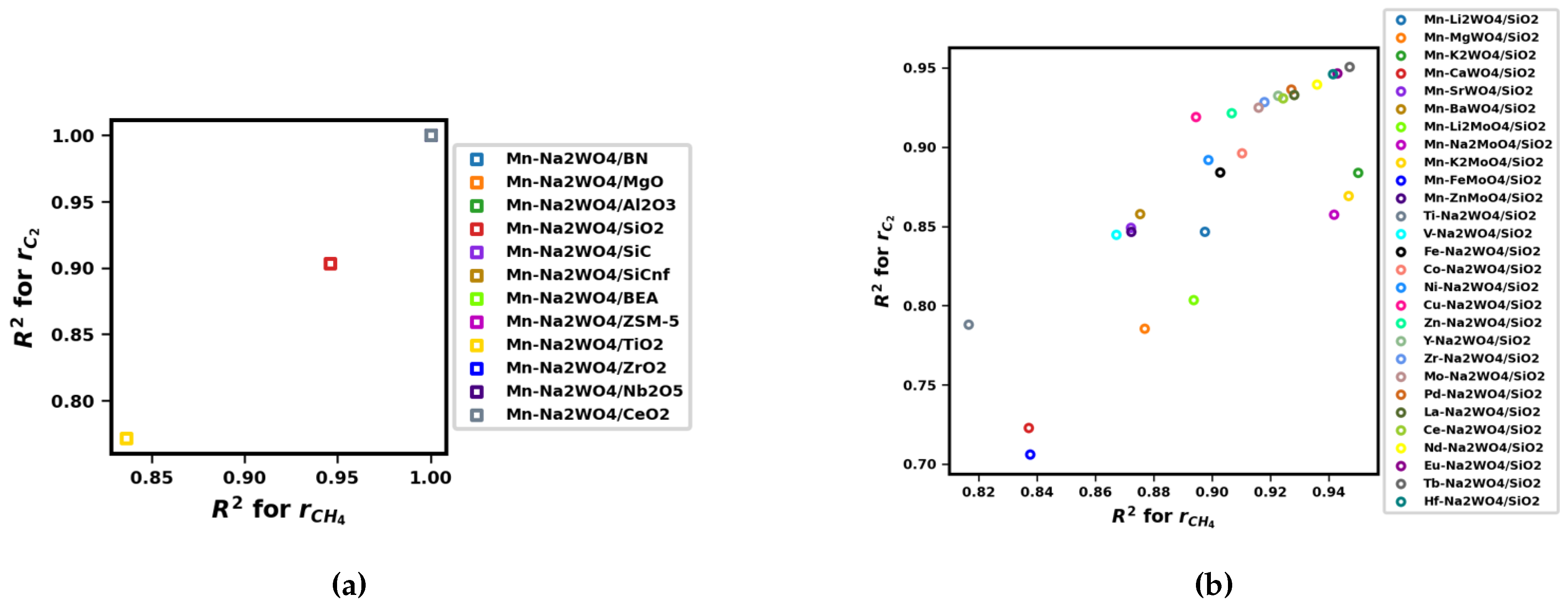
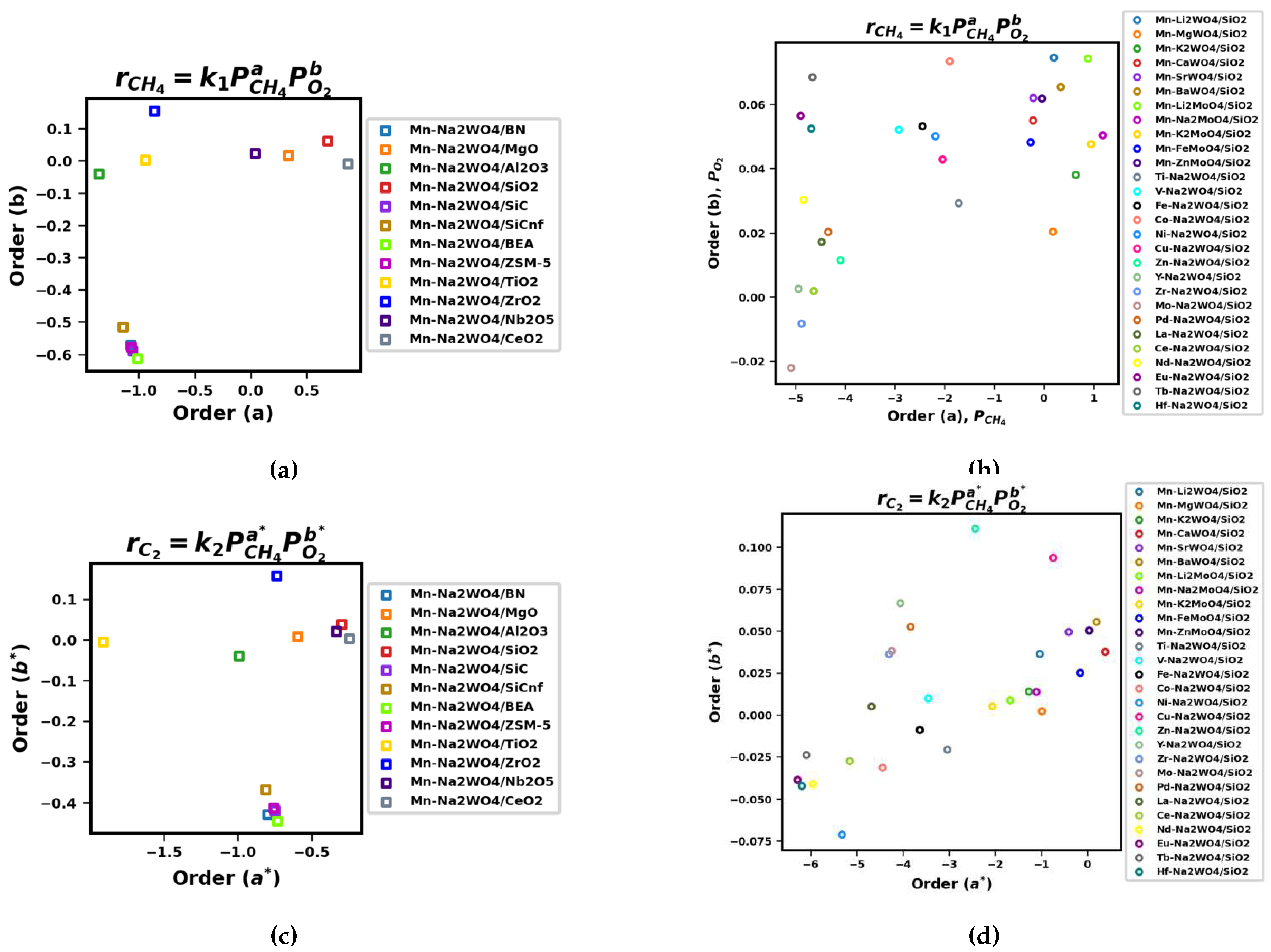
Appendix B
| Catalyst | Experimental conditions | S-X performance curve conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T(°C) | time(s) | (ml/min) | (ml/min) | CH4:O2 (mol:mol) | max | TCB% | T(°C) | time(s) | (ml/min) | (ml/min) | CH4:O2 (mol:mol) | max | improvement% | |
| Mn-Na2WO4/BN | 800.00 | 0.50 | 15.00 | 9.60 | 3.00 | 7.75 | -42.85 | 787.33 | 0.71 | 12.37 | 2.05 | 5.26 | 14.36 | 85.30 |
| Mn-Na2WO4/MgO | 800.00 | 0.50 | 15.00 | 3.40 | 3.00 | 9.32 | 6.05 | 812.79 | 0.46 | 15.67 | 2.10 | 5.61 | 15.47 | 66.02 |
| Mn-Na2WO4/Al2O3 | 750.00 | 0.38 | 20.00 | 12.80 | 3.00 | 8.08 | -15.04 | 822.76 | 0.40 | 18.44 | 2.15 | 3.19 | 11.21 | 38.77 |
| Mn-Na2WO4/SiO2 | 800.00 | 0.50 | 15.00 | 3.00 | 2.00 | 21.03 | -0.71 | 788.05 | 0.53 | 13.95 | 2.10 | 5.39 | 18.72 | -10.99 |
| Mn-Na2WO4/SiC | 800.00 | 0.50 | 15.00 | 3.40 | 3.00 | 19.59 | 2.06 | 808.30 | 0.59 | 16.55 | 2.06 | 5.66 | 19.90 | 1.59 |
| Mn-Na2WO4/SiCnf | 800.00 | 0.38 | 20.00 | 4.00 | 2.00 | 19.15 | -1.83 | 812.97 | 0.59 | 14.74 | 2.04 | 5.79 | 19.69 | 2.80 |
| Mn-Na2WO4/BEA | 800.00 | 0.38 | 20.00 | 4.50 | 3.00 | 15.56 | -0.77 | 792.61 | 0.50 | 12.74 | 2.04 | 5.23 | 16.33 | 4.93 |
| Mn-Na2WO4/ZSM-5 | 800.00 | 0.38 | 20.00 | 4.50 | 3.00 | 19.90 | -1.94 | 817.63 | 0.67 | 12.58 | 2.09 | 5.81 | 19.36 | -2.71 |
| Mn-Na2WO4/TiO2 | 750.00 | 0.38 | 20.00 | 4.00 | 2.00 | 18.29 | 5.69 | 821.57 | 0.57 | 14.80 | 2.11 | 5.31 | 18.71 | 2.29 |
| Mn-Na2WO4/ZrO2 | 800.00 | 0.38 | 20.00 | 4.80 | 4.00 | 11.21 | -3.64 | 793.97 | 0.60 | 14.02 | 2.05 | 5.26 | 18.28 | 63.11 |
| Mn-Na2WO4/Nb2O5 | 800.00 | 0.38 | 20.00 | 12.80 | 3.00 | 8.25 | -11.21 | 813.40 | 0.62 | 17.65 | 2.11 | 5.86 | 13.81 | 67.44 |
| Mn-Na2WO4/CeO2 | 775.00 | 0.75 | 10.00 | 2.00 | 2.00 | 18.04 | 0.23 | 819.77 | 0.55 | 18.39 | 2.15 | 5.94 | 16.62 | -7.86 |
| Catalyst | Experimental conditions | S-X performance curve conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T(°C) | time(s) | (ml/min) | (ml/min) | CH4:O2 (mol:mol) | max | TCB% | T(°C) | time(s) | (ml/min) | (ml/min) | CH4:O2 (mol:mol) | max | improvement% | |
| Mn-Li2WO4/SiO2 | 800.00 | 0.50 | 15.00 | 3.00 | 2.00 | 18.81 | 9.29 | 793.70 | 0.47 | 13.26 | 2.00 | 5.94 | 18.77 | -0.21 |
| Mn-MgWO4/SiO2 | 775.00 | 0.50 | 15.00 | 3.00 | 2.00 | 16.08 | 5.92 | 805.43 | 0.45 | 13.66 | 2.09 | 5.87 | 18.59 | 15.59 |
| Mn-K2WO4/SiO2 | 775.00 | 0.75 | 10.00 | 2.00 | 2.00 | 18.55 | 3.12 | 820.03 | 0.61 | 17.14 | 2.12 | 5.28 | 18.47 | -0.45 |
| Mn-CaWO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.80 | 4.00 | 8.51 | 10.87 | 870.22 | 0.39 | 17.95 | 2.02 | 5.08 | 12.55 | 47.46 |
| Mn-SrWO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.80 | 4.00 | 10.65 | 12.74 | 833.07 | 0.39 | 18.57 | 2.06 | 5.77 | 12.61 | 18.40 |
| Mn-BaWO4/SiO2 | 850.00 | 0.38 | 20.00 | 5.10 | 6.00 | 10.17 | 13.48 | 788.44 | 0.52 | 19.85 | 12.02 | 4.84 | 10.05 | -1.16 |
| Mn-Li2MoO4/SiO2 | 800.00 | 0.38 | 20.00 | 4.00 | 2.00 | 14.00 | 7.74 | 769.54 | 0.63 | 11.26 | 2.13 | 5.98 | 16.45 | 17.53 |
| Mn-Na2MoO4/SiO2 | 775.00 | 0.50 | 15.00 | 3.00 | 2.00 | 15.43 | -0.58 | 798.53 | 0.54 | 17.36 | 2.14 | 5.02 | 16.01 | 3.74 |
| Mn-K2MoO4/SiO2 | 800.00 | 0.38 | 20.00 | 4.50 | 3.00 | 16.60 | -6.61 | 814.59 | 0.47 | 12.99 | 2.03 | 5.06 | 16.13 | -2.84 |
| Mn-FeMoO4/SiO2 | 850.00 | 0.38 | 20.00 | 5.10 | 6.00 | 12.57 | 7.69 | 840.37 | 0.44 | 17.54 | 2.02 | 5.05 | 11.63 | -7.45 |
| Mn-ZnMoO4/SiO2 | 850.00 | 0.50 | 15.00 | 3.90 | 6.00 | 12.96 | 15.70 | 856.03 | 0.41 | 19.40 | 2.06 | 5.38 | 11.78 | -9.13 |
| Ti-Na2WO4/SiO2 | 800.00 | 0.75 | 10.00 | 2.00 | 2.00 | 20.23 | 9.12 | 800.11 | 0.71 | 12.22 | 2.10 | 5.11 | 17.21 | -14.95 |
| V-Na2WO4/SiO2 | 775.00 | 0.50 | 15.00 | 6.00 | 2.00 | 8.58 | -4.08 | 812.24 | 0.40 | 19.94 | 2.09 | 3.24 | 13.59 | 58.34 |
| Fe-Na2WO4/SiO2 | 800.00 | 0.75 | 10.00 | 2.00 | 2.00 | 15.24 | 5.16 | 812.21 | 0.49 | 16.08 | 2.05 | 5.31 | 17.16 | 12.59 |
| Co-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.50 | 3.00 | 16.14 | 7.41 | 823.83 | 0.51 | 14.33 | 2.14 | 5.90 | 17.64 | 9.32 |
| Ni-Na2WO4/SiO2 | 800.00 | 0.50 | 15.00 | 3.00 | 2.00 | 17.66 | 8.01 | 806.45 | 0.47 | 12.85 | 2.06 | 5.64 | 17.74 | 0.47 |
| Cu-Na2WO4/SiO2 | 800.00 | 0.38 | 20.00 | 8.00 | 2.00 | 9.11 | -5.59 | 796.12 | 0.40 | 17.58 | 2.02 | 2.57 | 12.91 | 41.73 |
| Zn-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.00 | 2.00 | 12.62 | 7.19 | 788.46 | 0.40 | 17.83 | 2.15 | 2.00 | 13.01 | 3.10 |
| Y-Na2WO4/SiO2 | 850.00 | 0.50 | 15.00 | 3.40 | 3.00 | 12.56 | -3.45 | 801.48 | 0.68 | 11.28 | 2.04 | 5.18 | 14.50 | 15.41 |
| Zr-Na2WO4/SiO2 | 800.00 | 0.75 | 10.00 | 2.00 | 2.00 | 13.86 | 2.69 | 811.17 | 0.66 | 12.01 | 2.09 | 5.32 | 14.99 | 8.14 |
| Mo-Na2WO4/SiO2 | 800.00 | 0.50 | 15.00 | 3.00 | 2.00 | 11.01 | 13.88 | 756.00 | 0.46 | 14.00 | 8.12 | 2.07 | 12.25 | 11.27 |
| Pd-Na2WO4/SiO2 | 800.00 | 0.75 | 10.00 | 2.00 | 2.00 | 15.45 | -2.82 | 794.41 | 0.73 | 10.61 | 2.15 | 5.16 | 15.20 | -1.64 |
| La-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.50 | 3.00 | 15.43 | 9.34 | 790.40 | 0.65 | 10.89 | 2.01 | 5.92 | 16.90 | 9.50 |
| Ce-Na2WO4/SiO2 | 800.00 | 0.75 | 10.00 | 2.00 | 2.00 | 16.75 | 2.48 | 815.08 | 0.69 | 11.59 | 2.06 | 5.55 | 17.49 | 4.39 |
| Nd-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.50 | 3.00 | 15.88 | 9.43 | 797.14 | 0.65 | 10.71 | 2.02 | 5.83 | 18.77 | 18.17 |
| Eu-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.00 | 2.00 | 16.09 | 8.48 | 788.82 | 0.75 | 11.02 | 2.15 | 5.51 | 18.46 | 14.71 |
| Tb-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.50 | 3.00 | 15.84 | 4.96 | 789.10 | 0.64 | 12.31 | 2.13 | 5.92 | 18.62 | 17.57 |
| Hf-Na2WO4/SiO2 | 850.00 | 0.38 | 20.00 | 4.00 | 2.00 | 16.01 | 4.52 | 824.64 | 0.70 | 10.26 | 2.10 | 5.57 | 18.54 | 15.79 |
Appendix C
References
- Zhu, Z.; Guo, W.; Zhang, Y.; Pan, C.; Xu, J.; Zhu, Y.; Lou, Y. Research progress on methane conversion coupling photocatalysis and thermocatalysis. Carbon Energy 2021, 3, 519–540. [Google Scholar] [CrossRef]
- Weber, J.M.; Guo, Z.; Zhang, C.; Schweidtmann, A.M.; Lapkin, A.A. Chemical data intelligence for sustainable chemistry, 2021. [CrossRef]
- Takahashi, K.; Tanaka, Y. Materials informatics: A journey towards material design and synthesis, 2016. [CrossRef]
- Takahashi, K.; Miyazato, I.; Nishimura, S.; Ohyama, J. Unveiling Hidden Catalysts for the Oxidative Coupling of Methane based on Combining Machine Learning with Literature Data. ChemCatChem 2018, 10, 3223–3228. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Nhat, T.T.P.; Takimoto, K.; Thakur, A.; Nishimura, S.; Ohyama, J.; Miyazato, I.; Takahashi, L.; Fujima, J.; Takahashi, K.; Taniike, T. High-Throughput Experimentation and Catalyst Informatics for Oxidative Coupling of Methane. ACS Catalysis 2020, 10, 921–932. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, L.; Le, S.D.; Kinoshita, T.; Nishimura, S.; Ohyama, J. Synthesis of Heterogeneous Catalysts in Catalyst Informatics to Bridge Experiment and High-Throughput Calculation. Journal of the American Chemical Society 2022, 144, 15735–15744. [Google Scholar] [CrossRef]
- Fujima, J.; Tanaka, Y.; Miyazato, I.; Takahashi, L.; Takahashi, K. Catalyst Acquisition by Data Science (CADS): A web-based catalyst informatics platform for discovering catalysts. Reaction Chemistry and Engineering 2020, 5, 903–911. [Google Scholar] [CrossRef]
- Ishioka, S.; Fujiwara, A.; Nakanowatari, S.; Takahashi, L.; Taniike, T.; Takahashi, K. Designing Catalyst Descriptors for Machine Learning in Oxidative Coupling of Methane. ACS Catalysis 2022, 12, 11541–11546. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Esterhuizen, J.; Liu, J.X.; Bartel, C.J.; Sutton, C. Machine learning for heterogeneous catalyst design and discovery. AIChE Journal 2018, 64, 2311–2323. [Google Scholar] [CrossRef]
- Tamtaji, M.; Gao, H.; Hossain, M.D.; Galligan, P.R.; Wong, H.; Liu, Z.; Liu, H.; Cai, Y.; Goddard, W.A.; Luo, Z. Machine learning for design principles for single atom catalysts towards electrochemical reactions. J. Mater. Chem. A 2022, 10, 15309–15331. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, L.; Nguyen, T.N.; Thakur, A.; Taniike, T. Multidimensional Classification of Catalysts in Oxidative Coupling of Methane through Machine Learning and High-Throughput Data. Journal of Physical Chemistry Letters 2020, 11, 6819–6826. [Google Scholar] [CrossRef]
- Ramprasad, R.; Batra, R.; Pilania, G.; Mannodi-Kanakkithodi, A.; Kim, C. Machine learning in materials informatics: Recent applications and prospects, 2017, [1707. 0 7294. [CrossRef]
- Zhang, N.; Yang, B.; Liu, K.; Li, H.; Chen, G.; Qiu, X.; Li, W.; Hu, J.; Fu, J.; Jiang, Y.; Liu, M.; Ye, J. Machine Learning in Screening High Performance Electrocatalysts for CO2 Reduction, 2021. [CrossRef]
- Mai, H.; Le, T.C.; Chen, D.; Winkler, D.A.; Caruso, R.A. Machine Learning for Electrocatalyst and Photocatalyst Design and Discovery, 2022. [CrossRef]
- Masood, H.; Toe, C.Y.; Teoh, W.Y.; Sethu, V.; Amal, R. Machine Learning for Accelerated Discovery of Solar Photocatalysts, 2019. [CrossRef]
- Li, Z.; Achenie, L.E.; Xin, H. An Adaptive Machine Learning Strategy for Accelerating Discovery of Perovskite Electrocatalysts. ACS Catalysis 2020, 10, 4377–4384. [Google Scholar] [CrossRef]
- Toyao, T.; Maeno, Z.; Takakusagi, S.; Kamachi, T.; Takigawa, I.; Shimizu, K.I. Machine Learning for Catalysis Informatics: Recent Applications and Prospects, 2020. [CrossRef]
- Chen, Y.Y.; Ross Kunz, M.; He, X.; Fushimi, R. Recent progress toward catalyst properties, performance, and prediction with data-driven methods, 2022. [CrossRef]
- Moses, O.A.; Chen, W.; Adam, M.L.; Wang, Z.; Liu, K.; Shao, J.; Li, Z.; Li, W.; Wang, C.; Zhao, H.; Pang, C.H.; Yin, Z.; Yu, X. Integration of data-intensive, machine learning and robotic experimental approaches for accelerated discovery of catalysts in renewable energy-related reactions, 2021. [CrossRef]
- Nishimura, S.; Li, X.; Ohyama, J.; Takahashi, K. Leveraging machine learning engineering to uncover insights into heterogeneous catalyst design for oxidative coupling of methane. Catalysis Science & Technology 2023. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohyama, J.; Nishimura, S.; Fujima, J.; Takahashi, L.; Uno, T.; Taniike, T. Catalysts informatics: paradigm shift towards data-driven catalyst design. Chemical Communications 2023, 59, 2222–2238. [Google Scholar] [CrossRef]
- Chen, K.; Tian, H.; Li, B.; Rangarajan, S. A chemistry-inspired neural network kinetic model for oxidative coupling of methane from high-throughput data. AIChE Journal 2022, 1–11. [Google Scholar] [CrossRef]
- Catalyst Acquisition by Data Science (CADS) homepage.
- Nguyen, T.N.; Nakanowatari, S.; Tran, T.P.N.; Thakur, A.; Takahashi, L.; Takahashi, K.; Taniike, T. Learning Catalyst Design Based on Bias-Free Data Set for Oxidative Coupling of Methane. ACS Catalysis 2021, 11, 1797–1809. [Google Scholar] [CrossRef]
- Segal, M.R. Machine learning benchmarks and random forest regression 2004.
- Ziu, K.; Solozabal, R.; Rangarajan, S.; Takáč, M. A deep neural network for oxidative coupling of methane trained on high-throughput experimental data. Journal of Physics: Energy 2022, 5, 014009. [Google Scholar] [CrossRef]
- Daneshpayeh, M.; Khodadadi, A.; Mostoufi, N.; Mortazavi, Y.; Sotudeh-Gharebagh, R.; Talebizadeh, A. Kinetic modeling of oxidative coupling of methane over Mn/Na2WO4/SiO2 catalyst. Fuel Processing Technology 2009, 90, 403–410. [Google Scholar] [CrossRef]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Transactions on Evolutionary Computation 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Fortin, F.A.; De Rainville, F.M.; Gardner, M.A.; Parizeau, M.; Gagné, C. DEAP: Evolutionary Algorithms Made Easy. Journal of Machine Learning Research 2012, 13, 2171–2175. [Google Scholar]
- Dewancker, I.; McCourt, M.; Clark, S. Bayesian: Optimization for Machine Learning: A Practical Guidebook. 2016; arXiv:cs.LG/1612.04858]. [Google Scholar]
- Xu, P.; Ji, X.; Li, M.; Lu, W. Small data machine learning in materials science. npj Computational Materials 2023, 9, 42. [Google Scholar] [CrossRef]
- Grömping, U. Variable Importance Assessment in Regression: Linear Regression versus Random Forest. The American Statistician 2009, 63, 308–319. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Baltrusaitis, J.; Wachs, I.E. Oxidative Coupling of Methane (OCM) by SiO2-Supported Tungsten Oxide Catalysts Promoted with Mn and Na. ACS Catalysis 2019, 9, 5912–5928. [Google Scholar] [CrossRef]
- Hu, L.; Pinto, D.; Urakawa, A. Catalytic Oxidative Coupling of Methane: Heterogeneous or Homogeneous Reaction? ACS Sustainable Chemistry & Engineering 2023, 11, 10835–10844. [Google Scholar] [CrossRef]
- Zavyalova, U.; Holena, M.; Schlögl, R.; Baerns, M. Statistical Analysis of Past Catalytic Data on Oxidative Methane Coupling for New Insights into the Composition of High-Performance Catalysts. ChemCatChem 2011, 3, 1935–1947. [Google Scholar] [CrossRef]
- Ortiz-Bravo, C.A.; Chagas, C.A.; Toniolo, F.S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies. Journal of Natural Gas Science and Engineering 2021, 96, 104254. [Google Scholar] [CrossRef]
- Amenomiya, Y.; Birss, V.I.; Goledzinowski, M.; Galuszka, J.; Sanger, A.R. Conversion of methane by oxidative coupling. Catalysis Reviews—Science and Engineering 1990, 32, 163–227. [Google Scholar] [CrossRef]
- Yildiz, M.; Simon, U.; Otremba, T.; Aksu, Y.; Kailasam, K.; Thomas, A.; Schomäcker, R.; Arndt, S. Support material variation for the MnxOy-Na2WO4/SiO2 catalyst. Catalysis Today 2014, 228, 5–14, Natural Gas Conversion the Status and Potentials in the Light of NGCS-10. [Google Scholar] [CrossRef]
- fu Ji, S.; cun Xiao, T.; ben Li, S.; zhi Xu, C.; ling Hou, R.; Coleman, K.S.; Green, M.L. The relationship between the structure and the performance of Na-W-Mn/SiO2 catalysts for the oxidative coupling of methane. Applied Catalysis A: General 2002, 225, 271–284. [Google Scholar] [CrossRef]
- Aireddy, D.R.; Roy, A.; Cullen, D.A.; Ding, K. TiOx-supported Na-Mn-W oxides for the oxidative coupling of methane. Catalysis Today 2023, 416, 113977, SI:Natural gas catalysis. [Google Scholar] [CrossRef]
- Gu, S.; Kang, J.; Lee, T.; Shim, J.; Choi, J.W.; Suh, D.J.; Lee, H.; Yoo, C.; Baik, H.; Choi, J.; Ha, J.M. Na2WO4/Mn supported on all-silica delaminated zeolite for the optimal oxidative coupling of methane via the effective stabilization of tetrahedral WO4: Elucidating effects of support precursors with different crystal structures, Al-addition, and morphologies. Chemical Engineering Journal 2023, 457, 141057. [Google Scholar] [CrossRef]
- Hayek, N.S.; Lucas, N.S.; Warwar Damouny, C.; Gazit, O.M. Critical Surface Parameters for the Oxidative Coupling of Methane over the Mn–Na–W/SiO2 Catalyst. ACS Applied Materials & Interfaces 2017, 9, 40404–40411. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Applied Catalysis A: General 2012, 425-426, 53–61. [Google Scholar] [CrossRef]
| Catalyst | M1 atom | M2 atom | M3 atom | M1 mol% | M2 mol% | M3 mol% | Support ID | T(°C) | time(s) | (ml/min) | (ml/min) | % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn-Na2WO4/CeO2 | 25 | 11 | 74 | 40.00 | 40.00 | 20.00 | 5 | 700.00 | 0.75 | 10.00 | 2.00 | 2.00 | 8.71 | |
| Mn-Li2MoO4/SiO2 | 25 | 3 | 42 | 40.00 | 40.00 | 20.00 | 11 | 775.00 | 0.75 | 10.00 | 7.30 | 6.00 | 9.07 | |
| Ti-Na2WO4/SiO2 | 22 | 11 | 74 | 40.00 | 40.00 | 20.00 | 11 | 700.00 | 0.75 | 10.00 | 2.40 | 4.00 | 9.78 | |
| Mn-FeMoO4/SiO2 | 25 | 26 | 42 | 40.00 | 40.00 | 20.00 | 11 | 850.00 | 0.38 | 20.00 | 4.50 | 3.00 | 10.92 | |
| Mn-CaWO4/SiO2 | 25 | 20 | 74 | 40.00 | 40.00 | 20.00 | 11 | 700.00 | 0.38 | 20.00 | 4.50 | 3.00 | 11.63 | |
| Fe-Li2MoO4/Nb2O5 | 26 | 3 | 42 | 44.81 | 27.25 | 26.48 | 8 | 815.49 | 0.65 | 15.76 | 11.26 | 2.69 | 12.32 | |
| Mo-Li2MoO4/ZrO2 | 42 | 3 | 42 | 44.24 | 27.44 | 26.84 | 13 | 821.44 | 0.49 | 16.37 | 6.30 | 5.73 | 12.80 | |
| Mo-Na2MoO4/ZrO2 | 42 | 11 | 42 | 44.02 | 27.77 | 26.37 | 13 | 799.38 | 0.70 | 10.16 | 3.62 | 5.59 | 13.73 | |
| Mn-CaWO4/TiO2 | 25 | 20 | 74 | 44.17 | 27.01 | 26.96 | 12 | 726.27 | 0.38 | 15.31 | 12.00 | 4.60 | 15.07 | |
| Cu-K2WO4/SiO2 | 29 | 19 | 74 | 44.49 | 27.94 | 26.83 | 11 | 823.97 | 0.74 | 17.49 | 3.30 | 4.81 | 17.11 | |
| Ti-K2MoO4/SiCnf | 22 | 19 | 42 | 44.44 | 27.54 | 26.10 | 10 | 799.36 | 0.49 | 15.55 | 2.11 | 4.26 | 17.42 | |
| V-K2WO4/CeO2 | 23 | 19 | 74 | 44.98 | 27.74 | 26.68 | 5 | 784.46 | 0.50 | 12.53 | 2.12 | 5.73 | 17.53 | |
| Mn-K2MoO4/SiCnf | 25 | 19 | 42 | 44.93 | 27.70 | 26.99 | 10 | 818.22 | 0.73 | 15.25 | 2.10 | 5.94 | 19.04 | |
| Ti-MgMoO4/ZSM-5 | 22 | 12 | 42 | 44.11 | 27.03 | 26.21 | 14 | 790.11 | 0.50 | 17.69 | 2.20 | 2.25 | 19.25 | |
| Mn-Li2WO4/SiO2 | 25 | 3 | 74 | 44.87 | 27.80 | 26.99 | 11 | 804.92 | 0.51 | 18.37 | 2.01 | 5.48 | 19.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





