Submitted:
10 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods of trichogenic SeNPs biosynthesis
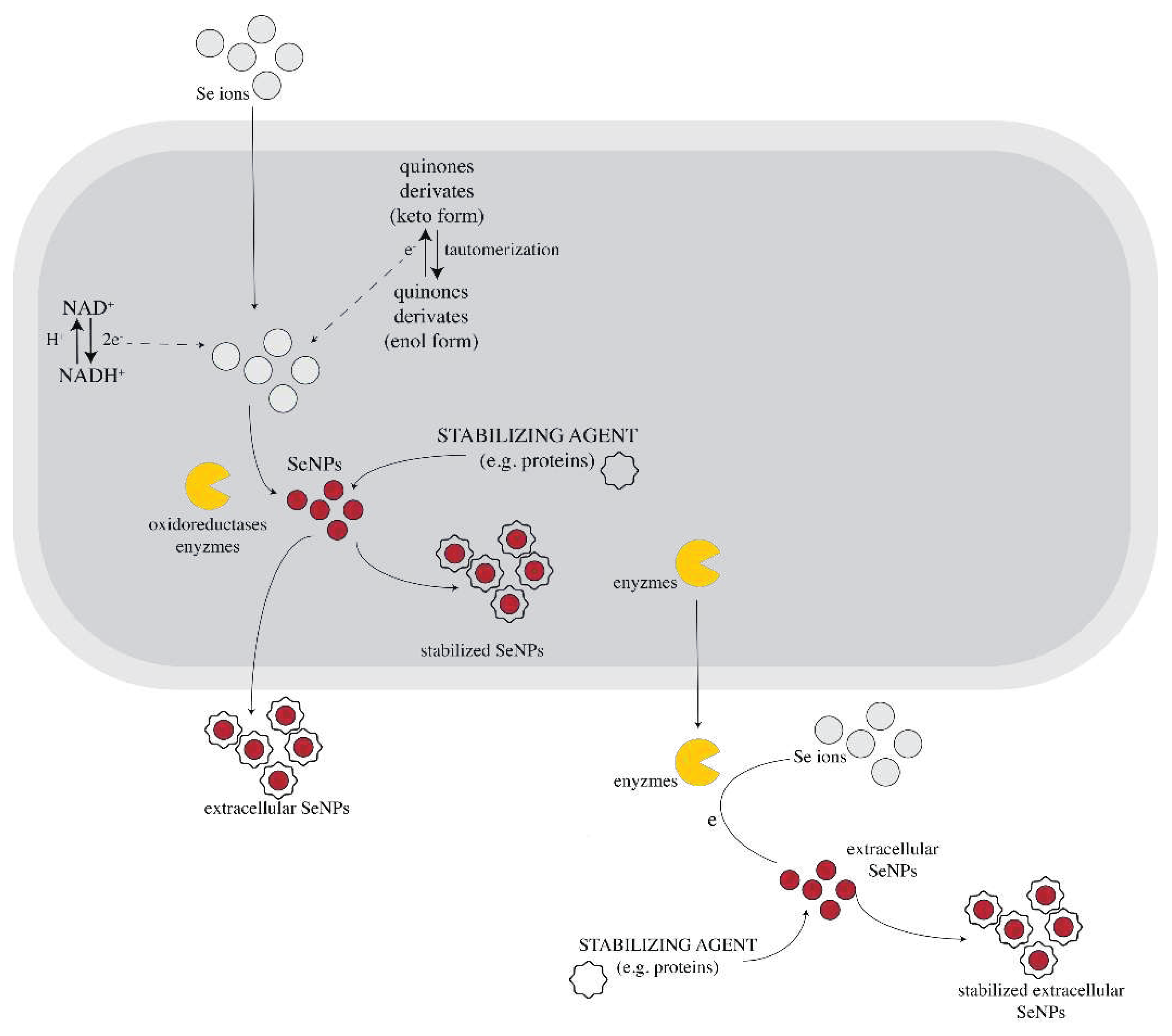
3. Mechanism of trichogenic SeNPs biosynthesis
4. Characterization and manipulation issues of SeNPs
5. Applications of trichogenic SeNPs in plant protection and as plant biostimulants
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of selenium nanoparticles in biomedical science. New Journal of Chemistry 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Xiao, D.; Li, T.; Huang, X.; Zhu, K.; Li, Z.; Dong, Y.; Wang, L.; Huang, J. Advances in the Study of Selenium-Enriched Probiotics: From the Inorganic Se into Se Nanoparticles. Molecular Nutrition & Food Research 2023, 2300432.
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21 st century: status and challenges for healthy human nutrition. Plant and Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Banerjee, M.; Chakravarty, D.; Kalwani, P.; Ballal, A. Voyage of selenium from environment to life: Beneficial or toxic? Journal of Biochemical and Molecular Toxicology 2022, 36, e23195. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS chemical biology 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Constantinescu-Aruxandei, D.; Frîncu, R.M.; Capră, L.; Oancea, F. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients 2018, 10, 1466. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Broadley, M.R. Foliar selenium biofortification of broccolini: effects on plant growth and mineral accumulation. Journal of Horticultural Science & Biotechnology 2022, 97, 730–738. [Google Scholar] [CrossRef]
- Liu, H.D.; Xiao, C.M.; Qiu, T.C.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.Y.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants-Basel 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Waraich, E.A.; Nawaz, F.; Ashraf, M.Y.; Khalid, M. Selenium (Se) improves drought tolerance in crop plants–a myth or fact? Journal of the Science of Food and Agriculture 2016, 96, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.H.; Wu, H.H.; Yuan, Q.H.; Wang, J.H.; Cui, J.; Lin, A.J. Effects of selenium fertilizer application and tomato varieties on tomato fruit quality: A meta-analysis. Scientia Horticulturae 2022, 304. [Google Scholar] [CrossRef]
- Dima, S.-O.; Neamțu, C.; Desliu-Avram, M.; Ghiurea, M.; Capra, L.; Radu, E.; Stoica, R.; Faraon, V.-A.; Zamfiropol-Cristea, V.; Constantinescu-Aruxandei, D. Plant Biostimulant Effects of Baker’s Yeast Vinasse and Selenium on Tomatoes through Foliar Fertilization. Agronomy 2020, 10, 133. [Google Scholar] [CrossRef]
- Dima, Ș.-O.; Constantinescu-Aruxandei, D.; Tritean, N.; Ghiurea, M.; Capră, L.; Nicolae, C.-A.; Faraon, V.; Neamțu, C.; Oancea, F. Spectroscopic Analyses Highlight Plant Biostimulant Effects of Baker’s Yeast Vinasse and Selenium on Cabbage through Foliar Fertilization. Plants 2023, 12, 3016. [Google Scholar] [PubMed]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. Journal of Biotechnology 2021, 325, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.S.; Srinivasan, S.; Muthuvel, A. Selenium nanomaterial is a promising nanotechnology for biomedical and environmental remediation: A detailed review. Biocatalysis and Agricultural Biotechnology 2023, 102766. [Google Scholar] [CrossRef]
- Manjunatha, C.; Rao, P.P.; Bhardwaj, P.; Raju, H.; Ranganath, D. New insight into the synthesis, morphological architectures and biomedical applications of elemental selenium nanostructures. Biomedical Materials 2021, 16. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. Journal of Trace Elements in Medicine and Biology 2021, 67. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, C.Q. New horizons for selenium in animal nutrition and functional foods. Animal Nutrition 2022, 11, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Cattaneo, D.; Abbate, R.; Manoni, M.; Ottoboni, M.; Luciano, A.; von Holst, C.; Pinotti, L. Advances in selenium supplementation: From selenium-enriched yeast to potential selenium-enriched insects, and selenium nanoparticles. Animal Nutrition 2023, 14, 193–203. [Google Scholar] [CrossRef]
- Parvin, S.; Khan, S.; Alam, P.; Khan, T.H.; Khataibeh, M.; Khan, M.A.; Samad, A.; Baker, A.; Mansoor, S. A Review on Potentialities of Selenium Nanoparticles and Its Application Using Air Borne Fungus. Applied Ecology and Environmental Sciences 2021, 9, 607–612. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Materials Advances 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: current status and future prospects. Applied microbiology and biotechnology 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Divya, M.; Durán-Lara, E.F.; Prasannakumar, M.; Vaseeharan, B. A Review on Biogenic Synthesis of Selenium Nanoparticles and Its Biological Applications. Journal of Inorganic and Organometallic Polymers and Materials 2022, 1–16. [Google Scholar] [CrossRef]
- Arunthirumeni, M.; Veerammal, V.; Shivakumar, M.S. Biocontrol efficacy of mycosynthesized selenium nanoparticle using Trichoderma sp. on insect pest Spodoptera litura. Journal of Cluster Science 2022, 33, 1645–1653. [Google Scholar] [CrossRef]
- Reddy, B.; Bandi, R. Synthesis of selenium nanoparticles by using microorganisms and agri-based products. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: 2022; pp. 655-683.
- Mates, I.; Antoniac, I.; Laslo, V.; Vicas, S.; Brocks, M.; Fritea, L.; Milea, C.; Mohan, A.; Cavalu, S. Selenium nanoparticles: Production, characterization and possible applications in biomedicine and food science. Sci. Bull. B Chem. Mater. Sci. UPB 2019, 81, 205–216. [Google Scholar]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial nano-factories: synthesis and biomedical applications. Frontiers in Chemistry 2021, 9, 626834. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. Journal of Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Scientific Reports 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Zielonka, A.; Klimek-Ochab, M. Fungal synthesis of size-defined nanoparticles. Advances in Natural Sciences: Nanoscience and Nanotechnology 2017, 8, 043001. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications; Elsevier: 2022.
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis and structural characterization of selenium nanoparticles using Gliocladium roseum. Journal of Cluster Science 2015, 26, 1473–1482. [Google Scholar] [CrossRef]
- Zare, B.; Babaie, S.; Setayesh, N.; Shahverdi, A.R. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomedicine journal 2013, 1, 13–19. [Google Scholar]
- Sarkar, J.; Dey, P.; Saha, S.; Acharya, K. Mycosynthesis of selenium nanoparticles. Micro & nano letters 2011, 6, 599–602. [Google Scholar]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microbial pathogenesis 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm Agrotis ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A State-of-the-Art Systemic Review on Selenium Nanoparticles: Mechanisms and Factors Influencing Biogenesis and Its Potential Applications. Biological Trace Element Research 2023, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Garza-García, J.J.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The role of selenium nanoparticles in agriculture and food technology. Biological Trace Element Research 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Abdelaziz, A.M.; Attia, M.S.; Salem, S.S. Selenium and Nano-Selenium-Mediated Biotic Stress Tolerance in Plants. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer International Publishing: Cham, 2022; pp. 209–226. [Google Scholar]
- Song, J.; Yu, S.; Yang, R.; Xiao, J.; Liu, J. Opportunities for the use of selenium nanoparticles in agriculture. NanoImpact 2023, 31, 100478. [Google Scholar] [CrossRef] [PubMed]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. International Journal of Molecular Sciences 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, G.E.; Adebayo-Tayo, B.C.; Oyinlola, K.A. Biological evaluation of extracellular mycosynthesized silver nanoparticles by Trichoderma asperellum. BioMetals 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Castro-Longoria, E. Biosynthesis of Metal-Based NanoparticlesNanoparticles by TrichodermaTrichodermaand Its Potential Applications. In Advances in Trichoderma Biology for Agricultural Applications, Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Springer International Publishing: Cham, 2022; pp. 433-463.
- Khabat, V.; Mansoori, G.A.; Karimi, S. Biosynthesis of silver nanoparticles by fungus Trichoderma Reesei. Insciences J 2011, 1, 65–79. [Google Scholar]
- Gharieb, M.; Wilkinson, S.; Gadd, G. Reduction of selenium oxyanions by unicellular, polymorphic and filamentous fungi: cellular location of reduced selenium and implications for tolerance. Journal of Industrial Microbiology 1995, 14, 300–311. [Google Scholar] [CrossRef]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Scientific Reports 2017, 7, 2612. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Liang, X.; Perez, M.A.M.-J.; Nwoko, K.C.; Egbers, P.; Feldmann, J.; Csetenyi, L.; Gadd, G.M. Fungal formation of selenium and tellurium nanoparticles. Applied microbiology and biotechnology 2019, 103, 7241–7259. [Google Scholar] [CrossRef] [PubMed]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Materials Chemistry and Physics 2020, 246, 122583. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.-i. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Zhang, X.; Park, S.; Wang, M.-H. Purinoceptor targeted cytotoxicity of adenosine triphosphate-conjugated biogenic selenium nanoparticles in human colon cancer cells. Pharmaceuticals 2022, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; De Britto, S.; Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. Journal of Biotechnology 2021, 325, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.Y. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nature Reviews Chemistry 2020, 4, 638–656. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Golkar-Narenji, A. Biogenesis of Selenium Nanoparticles Using Green Chemistry. Topics in Current Chemistry 2017, 375, 88. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green synthesis of selenium and tellurium nanoparticles: current trends, biological properties and biomedical applications. International Journal of Molecular Sciences 2021, 22, 989. [Google Scholar] [CrossRef]
- Tsivileva, O.; Pozdnyakov, A.; Ivanova, A. Polymer nanocomposites of selenium biofabricated using fungi. Molecules 2021, 26, 3657. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Ganesan, S.; Murugan, K.; Janjaroen, D. Recent breakthroughs set by fungal enzymes in the biosynthesis of nanoparticles. In Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications; Elsevier: 2023; pp. 131-162.
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. Journal of Nanobiotechnology 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Critical reviews in biotechnology 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: current status and future challenges. Journal of Nanostructure in Chemistry 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci 2012, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Lo, C.-T.; Chen, C.; Liu, M.-Y.; Chen, J.-H.; Peng, K.-C. Efficient isolation of anthraquinone-derivatives from Trichoderma harzianum ETS 323. Journal of Biochemical and Biophysical Methods 2007, 70, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Cervantes, A.; Ruiz Allica, J.; Calderón Celis, F.; Costa-Fernández, J.M.; Ruiz Encinar, J. The Potential of ICP-MS as a Complementary Tool in Nanoparticle–Protein Corona Analysis. Nanomaterials 2023, 13, 1132. [Google Scholar] [PubMed]
- Nwoko, K.C.; Liang, X.; Perez, M.A.M.J.; Krupp, E.; Gadd, G.M.; Feldmann, J. Characterisation of selenium and tellurium nanoparticles produced by Aureobasidium pullulans using a multi-method approach. Journal of Chromatography A 2021, 1642, 462022. [Google Scholar] [CrossRef]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results in Chemistry 2022, 100581. [Google Scholar] [CrossRef]
- Biswas, K.C.; Barton Ll Fau - Tsui, W.L.; Tsui Wl Fau - Shuman, K.; Shuman K Fau - Gillespie, J.; Gillespie J Fau - Eze, C.S.; Eze, C.S. A novel method for the measurement of elemental selenium produced by bacterial reduction of selenite.
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-assisted mycogenic selenium nanoparticles synthesized under gamma irradiation for robust reluctance of resistant urinary tract infection-causing pathogens. Biological trace element research 2020, 195, 323–342. [Google Scholar] [CrossRef]
- Hamley, I.W. Small-angle scattering: theory, instrumentation, data and applications John Wiley & Sons, Inc.,: 111 River Street, Hoboken, NJ 07030, USA, 2022; p. 278.
- Gommes, C.J.; Jaksch, S.; Frielinghaus, H. Small-angle scattering for beginners. Journal of Applied Crystallography 2021, 54, 1832–1843. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-ray Scattering for Nanoparticle Research. Chemical Reviews 2016, 116, 11128–11180. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Hubbell, H.H. Low Angle X-Ray Diffraction of Colloidal Gold and Carbon Black1a. Journal of the American Chemical Society 1951, 73, 1–7. [Google Scholar] [CrossRef]
- Gablech, E.; Fohlerova, Z.; Svec, K.; Zales, F.; Benada, O.; Kofronova, O.; Pekarkova, J.; Caha, O.; Gablech, I.; Gabriel, J.; et al. Selenium nanoparticles with boron salt-based compound act synergistically against the brown-rot Serpula lacrymans. International Biodeterioration & Biodegradation 2022, 169. [Google Scholar] [CrossRef]
- Apryatina, K.V.; Murach, E.I.; Amarantov, S.V.; Erlykina, E.I.; Veselov, V.S.; Smirnova, L.A. Synthesis of a Bioactive Composition of Chitosan-Selenium Nanoparticles. Applied Biochemistry and Microbiology 2022, 58, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Borgatta, J.R.; Lochbaum, C.A.; Elmer, W.H.; White, J.C.; Pedersen, J.A.; Hamers, R.J. Biomolecular corona formation on CuO nanoparticles in plant xylem fluid. Environmental Science: Nano 2021, 8, 1067–1080. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Cheng, H.; Zhan, X.; Xia, W. Synthesis, characterization, and anticancer activity of protamine sulfate stabilized selenium nanoparticles. Food Research International 2023, 164, 112435. [Google Scholar] [CrossRef] [PubMed]
- Dahman, Y. Chapter 5 - Nanoparticles**By Yaser Dahman, Hoda Javaheri, Jiafu Chen, and Basel Al-Chikh Sulaiman. In Nanotechnology and Functional Materials for Engineers, Dahman, Y., Ed.; Elsevier: 2017; pp. 93-119.
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? Journal of controlled release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valdespino, C.A.; Orrantia-Borunda, E. Trichoderma and nanotechnology in sustainable agriculture: a review. Frontiers in Fungal Biology 2021, 61. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mashwani, Z.U.; Mehak, A.; Fatima, N.; Sarwar, S.; Haq, E.U.; Yousaf, T. Green synthesis and characterization of selenium nanoparticles and its application in plant disease management: a review. Pakistan Journal of Phytopathology 2022, 34, 189–102. [Google Scholar] [CrossRef]
- Helmy, E.; Salah, R.; El-Shazly, M.M.; Alqhtani, A.; Pokoo-Aikins, A.; Yosri, M. Investigation of the Impact of Mycogenic Titanium and Selenium Nanoparticles on Fusarium Wilt Infection of Tomato Plant. Journal of Pure and Applied Microbiology 2023, 17. [Google Scholar] [CrossRef]
- Bărbieru, O.-G.; Dimitriu, L.; Călin, M.; Răut, I.; Constantinescu-Aruxandei, D.; Oancea, F. Plant Biostimulants Based on Selenium Nanoparticles Biosynthesized by Trichoderma Strains. Proceedings 2019, 29, 95. [Google Scholar]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Processing and Synthesis 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Dobias, J.; Suvorova, E.I.; Bernier-Latmani, R. Role of proteins in controlling selenium nanoparticle size. Nanotechnology 2011, 22, 195605. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Lens, P.N.L. Ecology and Biotechnology of Selenium-Respiring Bacteria. Microbiology and Molecular Biology Reviews 2015, 79, 61–80. [Google Scholar] [CrossRef]
| Trichoderma strain | Se precursor | Substrate concentration (mM) | Types of synthesis | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Trichoderma sp. | Na2SeO3 | 20 mM | in vitro (intracellular)* | 40-100 | [23] |
| T. reesei | Na2SeO3 | 1, 5, 10 mM | in vivo (intracellular, extracellular) |
- | [46] |
|
T. brevicompactum T. asperellum, T. atroviride, T. harzianum, T. virens, T. longibrachiatum |
Na2SeO3 | 25 mM | in vitro (intracellular, extracellular, cell walls) |
49- 312 | [47] |
|
T. harzianum (three strains), T. koningii, T. longibranchiatum, T. atroviride, T. asperellum, T. virens |
Na2SeO3 | 5 mM | in vitro (intracellular, extracellular) |
50-60 | [48] |
| T. harzianum | Na2SeO3 | 1 mM | in vivo (intracellular, extracellular) |
[49] | |
| Trichoderma sp. WL-Go | SeO2 | 2 mM | in vivo (intracellular, extracellular) |
147 | [50] |
| T. atroviride (Tri_AtJSB2) sp. | Na2SeO3 | 25 mM | in vitro (intracellular, extracellular, cell walls) |
60- 123 | [51] |
| T. harzianum | Na2SeO3 | 25 mM | in vitro (intracellular) | 26 | [52] |
| T. atroviride (Tri_AtJSB2) sp. | Na2SeO3 | 25 mM | in vitro (extracellular) | 60-123 (Z av. 98.5, ref. 32) | [53] |
| Trichoderma strain | Bio-corona composition | Zeta potential (mV) | Crystallinity | Methods | Ref. |
|---|---|---|---|---|---|
| Trichoderma sp. | apparently, proteins | - | crystalline | FTIR, XRD | [23] |
|
T. brevicompactum T. asperellum, T. atroviride, T. harzianum, T. virens, T. longibrachiatum |
proteins | −200 to + 11.8 | amorphous/ nano-crystalline | FTIR, XRD | [47] |
|
T. harzianum (three strains), T. koningii, T. longibranchiatum, T. atroviride, T. asperellum, T. virens |
Proteins, organic acids, amino acids, sugars, intermediates from the carbohydrate metabolism | - | amorphous | FTIR, LC-MS/MS, XRD |
[48] |
| T. harzianum | - | - | amorphous + Se oxide |
XRD | [49] |
| Trichoderma sp. WL-Go | proteins, sugars | -24.6 | crystalline | FTIR, zeta potential, XRD | [50] |
| T. atroviride (Tri_AtJSB2) sp. | proteins | −49.3 to −43.7 | crystalline | FTIR, zeta potential, XRD | [51] |
| T. harzianum | proteins, hydroxyl molecules |
−37.8 ± 0.36 | crystalline | FTIR, zeta potential, XRD | [52] |
| Trichoderma strain | Phytopathogen / pest | Plant | Effect | Ref. |
|---|---|---|---|---|
| Trichoderma sp. | Spodoptera litura | castor leaves | larvicidal and antifeedant activity | [23] |
|
T. brevicompactum, T. asperellum, T. atroviride, T. harzianum, T. virens T. longibrachiatum |
Sclerospora graminicola | pearl millet [Pennisetum glaucum (L.) R. Br.] | Sporulation and downy mildew inhibition | [47] |
|
T. harzianum (three strains), T. koningii, T. longibranchiatum, T. atroviride, T. asperellum, T. virens |
Fusarium verticillioide, Alternaria alternata, F. graminearum | - | Antifungal, functional biocontrol of mycotoxins | [48] |
| T. atroviride (Tri_AtJSB2) sp. | Phytophthora infestans, Pyricularia grisea, Colletotrichum capsica, Alternaria solani | one-month-old chili (Capsicum annuum L.) cultivar ArkaKhyati and tomato leaves | zoosporicidal and antifungal activities | [51] |
| T. atroviride (Tri_AtJSB2) sp. | Phytophthora infestans | tomato | priming effect, enhanced resistance to late blight disease | [53] |
| T. harzianum | F. oxysporum, Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus, Penicillium marnefeii, Candida albicans, Candida lipolytica, Salmonella typhimurium, Bacillus subtilis, Pseudomonas aeruginosa, Methicillin Resistant Staphylococcus aureus (MRSA), Staphylococcus aureus, Escherichia coli, Enterococcus faecalis | tomato | antimicrobial activity, reduction of Fusarium wilt infection | [81] |
| Trichoderma spp. | Fusarium sp. | Vigna radiata | Reduced toxicity, antifungal, biostimulant | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





