1. Introduction
The subcutaneous space has many advantages as an alternative transplant site for pancreatic islets, because it is large enough to support a large number of islets, it is easily accessible, minimally invasive, and easy to monitor [
1] and/or the islet grafts can be removed if necessary [
2,
3]. However, the subcutaneous site is poorly vascularized and it must be modified before transplantation to support islet grafts [
4]. Various subcutaneous approaches have been developed for delivering grafts, including the use of devices [
5,
6], biomaterials [
7,
8,
9], endothelial cells [
10], and fibroblasts [
11]. Although all these approaches have improved islet engraftment to some degree, a large number of islets are still necessary to achieve normoglycemia.
To solve this situation, alternative strategies for subcutaneous islet transplantation using a two-step approach have been proposed, in which a favorable cavity for transplantation is first prepared using various materials, followed by islet transplantation into the preformed cavity [
12]. These approaches have several advantages: namely, new vessels for supporting islet grafts are induced in advance and foreign body responses to materials are reduced when the islets are transplanted. We recently reported that the number of new vessels in the subcutaneous space continuously increased until 6 weeks after the implantation of materials [
13]. We also demonstrated that inflammation in the subcutaneous space was extremely severe until 2 weeks after the implantation of the materials and that islet engraftment tended to be poor if the islets were transplanted during this period. These approaches efficiently deliver oxygen and nutrition to islet grafts and enable them to respond to hyperglycemia immediately, which is similar to the response when transplanted into the portal vein or the kidney subcapsular space [
14,
15].
We previously reported the efficacy of pretreatment with a recombinant peptide (RCP: alpha-1 sequence of recombinant collagen type I supplemented using a 12 RGD [Arg-Gly-Asp] motif 1 molecule) device to improve islet engraftment in subcutaneous transplantation [
16]. The main drawback of the RCP device is that it is barely absorbed in the body and it must be removed at the time of transplantation. In this process, substantial amounts of constructed vessels and the extracellular matrix (ECM) are destroyed. Therefore, we next focused on the gelatin hydrogel nonwoven fabric (GHNF), which is a biodegradable scaffold made from gelatin. GHNF was originally produced as a useful scaffold for various types of cell cultures [
17,
18]. It is known to degrade gradually and thereafter is absorbed in vivo and finally replaced by the host tissues; therefore, it can be an ideal pretreatment material for our purpose, since there is no need for removal. In fact, we could effectively restore diabetic mice with a marginal dose of subcutaneously transplanted islets [
13]. Moreover, the results of subcutaneous islet transplantation using GHNF combined with adipose tissue-derived stem cells were surprisingly superior to those of intraportal transplantation, which is the current standard procedure (manuscript submitted). However, these approaches have only been applied to a mouse model, and the efficacy of subcutaneous islet transplantation in animal models other than mice is still extremely low compared to intraportal transplantation [
19].
Therefore, in the present study, we adapted the GHNF pretreatment method for a rat model, in which GHNF sheets sandwiching a silicone spacer were implanted in the subcutaneous space of Lewis rats for three weeks (GHNF was absorbed more rapidly in a rat model), followed by islet transplantation into the space where the silicone spacer was removed. We investigated whether the preimplantation of GHNF could induce efficient new vessels and thereby improve subcutaneous islet engraftment, even in a rat model.
2. Materials and Methods
2.1. Animals
All animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health [
20]. All experimental protocols used in this study (protocol ID:2020 MdA-022) were approved by the Animal Experimental Committee of Tohoku University. Lewis rats were used as recipients and islet donors (8-10 weeks of age; Japan SLC Inc., Shizuoka, Japan). All surgical operations were performed under anesthesia, and efforts were made to minimize suffering.
2.2. The induction and diagnosis of diabetes in the recipients
Diabetes was induced by the intravenous injection of 70 mg/kg streptozotocin (STZ) (Sigma-Aldrich, Inc., MO, USA). Rats with non-fasting blood glucose levels ≥400 mg/dL on two consecutive measurements were considered to be diabetic. The serial blood glucose levels were determined, and recipients whose non-fasting blood glucose levels were <200 mg/dL on two consecutive measurements were considered to be cured.
2.3. Islet isolation
Islet isolation and culture were performed as previously described [
21]. Briefly, the bile duct was identified and clamped in the papilla of Vater. Two milliliters of cold Hank’s balanced salt solution (HBSS) containing 1 mg/mL collagenase (Sigma type V; Sigma Chemicals, St. Louis, MO, USA) was injected into the common bile duct, leading to the pancreas, under a stereomicroscope. The pancreas was removed and incubated in a water bath at 37°C for 12 min before digestion. The cell suspension was washed three times in cold HBSS and then centrifuged for 1 min. Density-gradient centrifugation was performed for 10 min using Histopaque-1119 (Sigma Diagnostics, St. Louis, MO, USA) and Lymphoprep™ (Axis-Shiled, Oslo, Norway) to isolate pancreatic islets. The islets were cultured overnight in Roswell Park Memorial Institute-1640 medium containing 5.5 mmol/L glucose and 10% fetal bovine serum at 37°C in 5% CO2 and humidified air before transplantation.
2.4. Preparation of a gelatin hydrogel nonwoven fabric and islet transplantation
A gelatin hydrogel nonwoven fabric (GHNF; NIKKE MEDICAL Co., Ltd., Osaka, Japan) was prepared using a previously reported method [
17] and processed into a circular sheet (22 mm diameter, 0.5 mm thickness). Two GHNF sheets sandwiching a silicone spacer (26 mm diameter, 0.5 mm thickness) were placed into the left dorsal subcutaneous space 3 weeks before islet transplantation (GHNF group), and a silicone spacer without GHNF was also placed in the same way (control group). The duration of pretreatment was determined according to a previous report in mice by comparison with Hematoxylin Eosin staining [
13]. GHNF and silicone spacers were implanted into healthy rats, which were injected with STZ 7 days before islet transplantation.
After the removal of the silicone spacer, 5,400 islet equivalents (IEQs) of syngeneic rat islets were transplanted into the pretreated space using a gastight syringe (Hamilton Co., Reno, NV, USA) in the GHNF and control groups [
22]. The recipients were followed by measuring the non-fasting blood glucose levels every 3-4 days throughout the study period (60 days after islet transplantation).
2.5. Intravenous glucose tolerance test
An intravenous glucose tolerance test (IVGTT) was performed 60-65 days after islet transplantation as described previously [
23]. In brief, recipients that had fasted for 12 h were intravenously infused with D-glucose (3.0 g/kg), and the blood glucose concentrations were determined before and at 5, 10, 20, 30, 60, 90, and 120 min after the injection of glucose. A blood glucose curve was generated, and the area under the curve (AUC) was used for comparison purposes.
2.6. Immunohistochemical analyses
Three weeks after the subcutaneous implantation of the GHNF sheets before islet transplantation, recipient tissue at the site of subcutaneous implantation was procured, fixed with 4% paraformaldehyde, and embedded in paraffin for immunohistochemical staining. Seven days after transplantation, the tissue at the site of subcutaneous islet transplantation was procured. Immunohistochemical staining was performed using anti-insulin (ab181547; Abcam, Cambridge, UK), anti-von Willebrand factor (vWF) (ab6994; Abcam), anti-collagen III (ab7778; Abcam), anti-collagen IV (ab6586; Abcam), and anti-laminin (ab11575; Abcam) antibodies. The EnVision+ System- HRP labelled polymer anti-rabbit (4003; DAKO, Glostrup, Denmark) was used as the secondary antibody. To evaluate neovascularization, vWF-positive vessels in the capsule touching the silicone spacer were counted [
24]. In collagen III [
25], collagen IV, and laminin staining, “positive” was defined as marked immunopositivity that was detectable in the fibrous capsule around the islets. More than six sections for each animal from each experimental group (GHNF; n=5, control; n=5) before islet transplantation and 10 sections after islet transplantation (GHNF; n=5, control; n=5) were evaluated by a pathologist using a blinded method.
2.7. Islet evaluation
Seven days after transplantation, the tissue at the site of subcutaneous islet transplantation was procured and stained with an anti-insulin antibody. All insulin-positive islets were evaluated in both groups. The area of β-cells was calculated using the ImageJ software program (National Institutes of Health, Maryland, USA) [
26], and the area with a threshold of 0-70 was calculated as the insulin-positive area.
2.8. Lectin angiography
Lectin angiography was performed in the GHNF (n=5) and control (n=4) groups three weeks after pretreatment [
27]. The amount of tomato lectin was determined according to a previous report [
28], but newly formed subcutaneous vessels could not be detected at this dose. Therefore, in this study, we used 3.75 mg/kg body weight of tomato lectin, which was three times larger than that used in the previous study. The rats were lightly sedated with isoflurane (Viatris Inc., Tokyo, Japan), and DyLight® 488 Lycopersican esculentum agglutinin (LEA, tomato lectin; Vector Labs, Burlingame, CA, USA) was injected intravascularly via the penile vein. Five minutes after tomato lectin injection, the animals were perfused through the heart using an electric pump connected to a 24-G needle set at a pumping rate of 5 ml/min. Na-PO4 buffered 4% paraformaldehyde was used as the perfusion fluid. Following perfusion fixation, tissues at the site of subcutaneous pretreatment were procured and placed in 4% paraformaldehyde and stored overnight in the dark at 4 °C. The following day, the tissues were washed twice with PBS and transferred to a solution of 10% sucrose in PBS. Six hours later, the tissues were transferred to 30% sucrose in PBS and stored in the dark at 4℃ for one day.
The tissues were cut using a microtome into about 50 μm thickness and then were mounted on slides, and coverslipped. Care was taken to protect the tissues from exposure to overhead room fluorescent light as much as possible. The sections were examined under a confocal microscope (LSM780; Carl-Zeiss., Oberkochen, Germany). The same controls for brightness and exposure times were used among the groups to maintain consistency. The images were imported into a Zeiss IMARIS (Carl-Zeiss, Germany) to perform vessel volumetry and analysis. The vascular volume in the subcutaneous capsules surrounding the silicone spacer or GHNF was calculated. To determine the density of blood vessels, the capsular volume was calculated and the vascular volume was divided by the vascular volume. The brightness value of the vascular area was defined as >20, and the minimum volume was defined as <5000 μm3 to extract artifacts.
2.9. Statistical analyses
All data are expressed as the mean ± standard error. All statistical analyses were performed using JMP Pro 16 software program (SAS Institute Inc., Cary, NC, USA). Changes in the blood glucose levels and IVGTT were analyzed using a mixed-effect model analysis. The area under the curve (AUC) of the IVGTT, number of vWF-positive vessels, vascular density, and area of β cells were analyzed by Student’s t-test between the groups. The immunopositivity rate in ECM staining was analyzed using Pearson’s chi-squared test. Kaplan-Meier curves were compared using a log-rank test. P values less than 0.05 were considered to indicate statistical significance.
3. Results
3.1. Time-course changes of GHNF absorption under the subcutaneous space
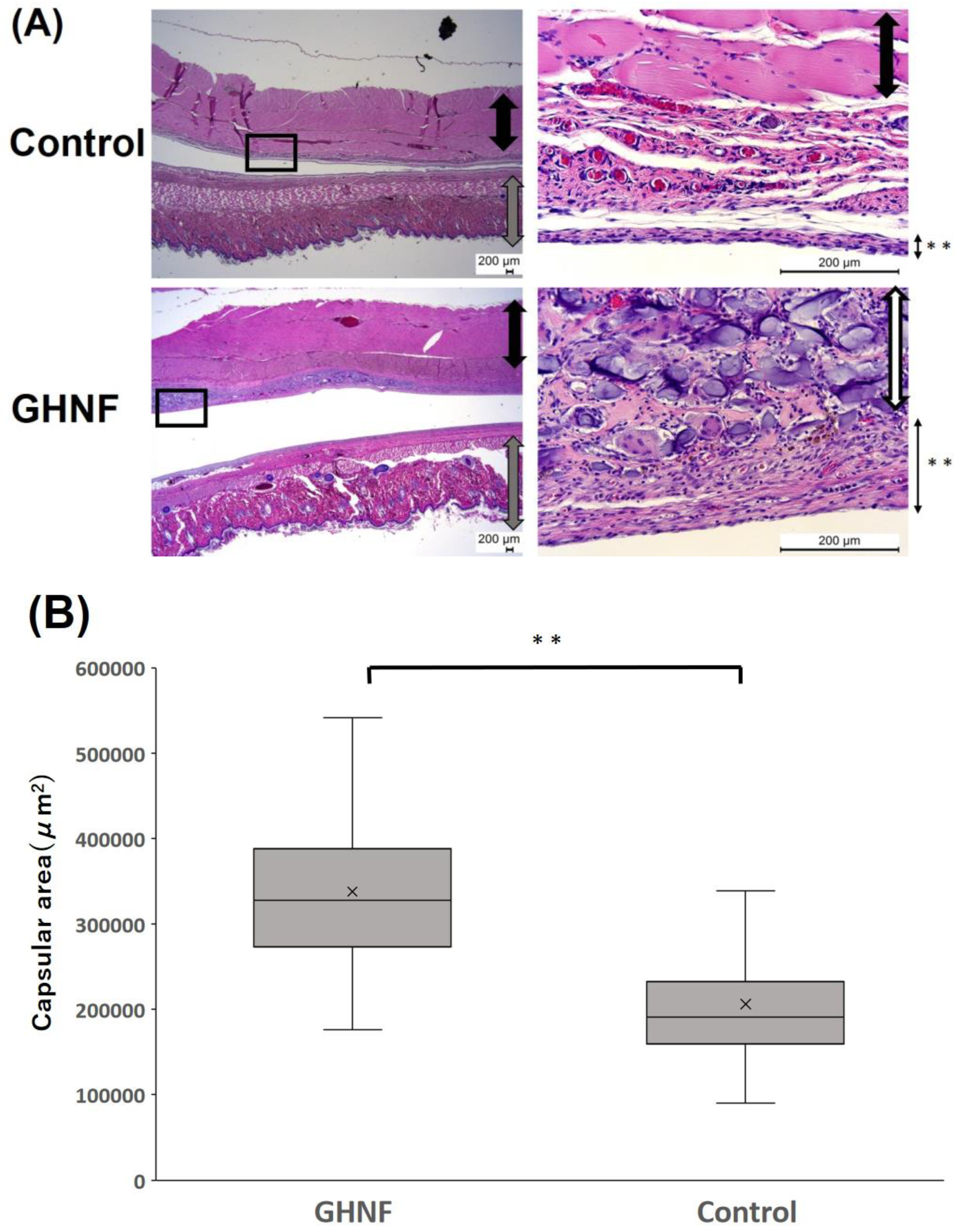
After pretreatment with GHNF and a silicone spacer, subcutaneous tissues, including GHNF, were removed and stained with hematoxylin and eosin. GHNF was absorbed, and its volume gradually decreased. At 3 weeks after implantation, the thickness of the capsule touching the silicone spacer in the GHNF group appeared to be thicker than that in the control group (
Figure 1A). The average area of the capsule in the GHNF group (observed at ×100 magnification) was significantly larger than that in the control group (GHNF:338,209±8,691 vs. control:206,453±7,472 μm2, p<0.01) (
Figure 1B).
3.2. The comparison of islet engraftment after marginal islet mass transplantation between the GHNF and control groups
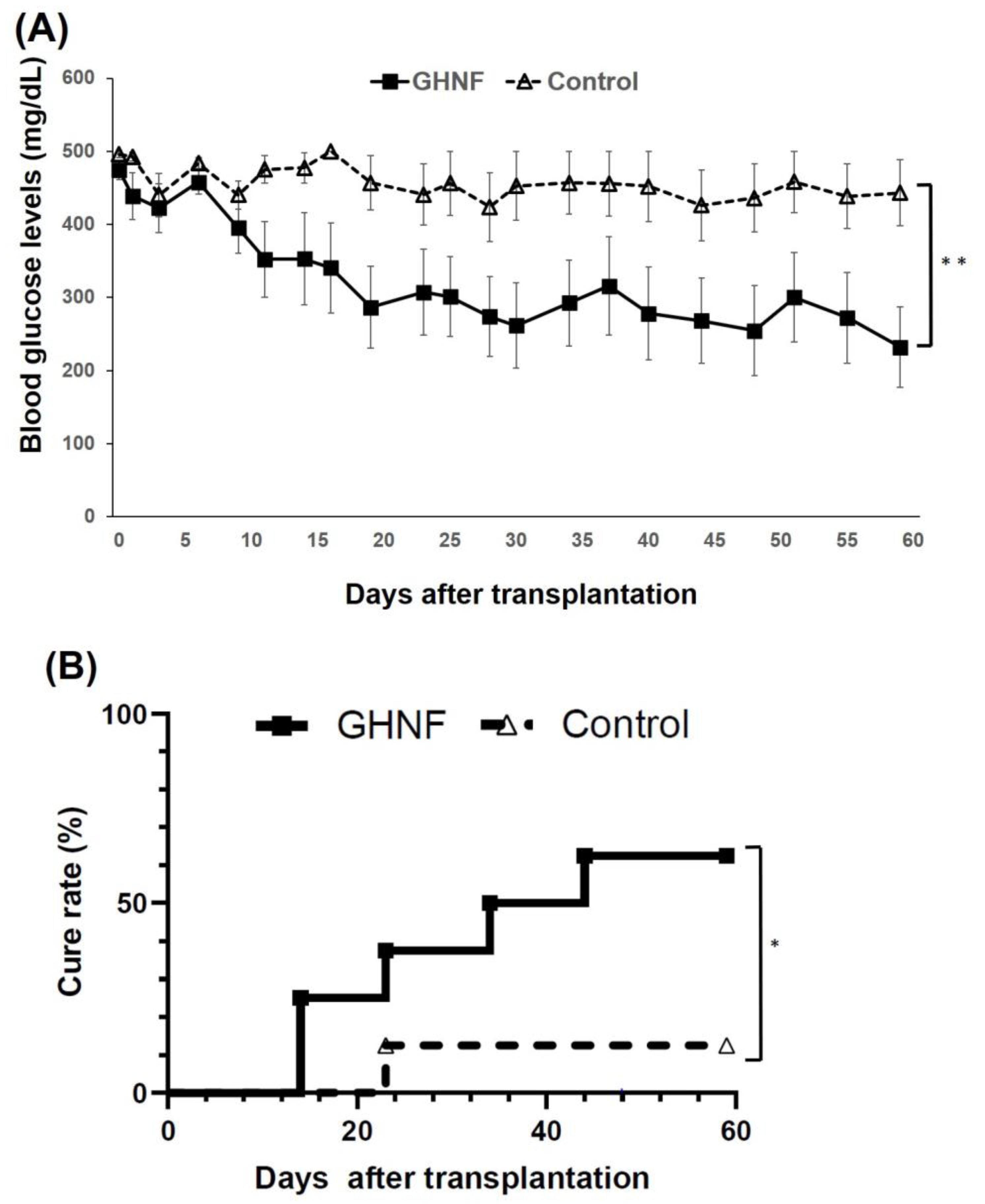
The blood glucose levels after islet transplantation in the GHNF group (n=8) were significantly higher than those in the control group (n=8) (p<0.01) (
Figure 2A). The cure rate of the diabetic rats 60 days after islet transplantation in the GHNF group was significantly higher than that in the control group (GHNF:62.5% [5/8] vs. control:12.5% [1/8], p<0.05) (
Figure 2B).
3.3. Intravenous glucose tolerance test
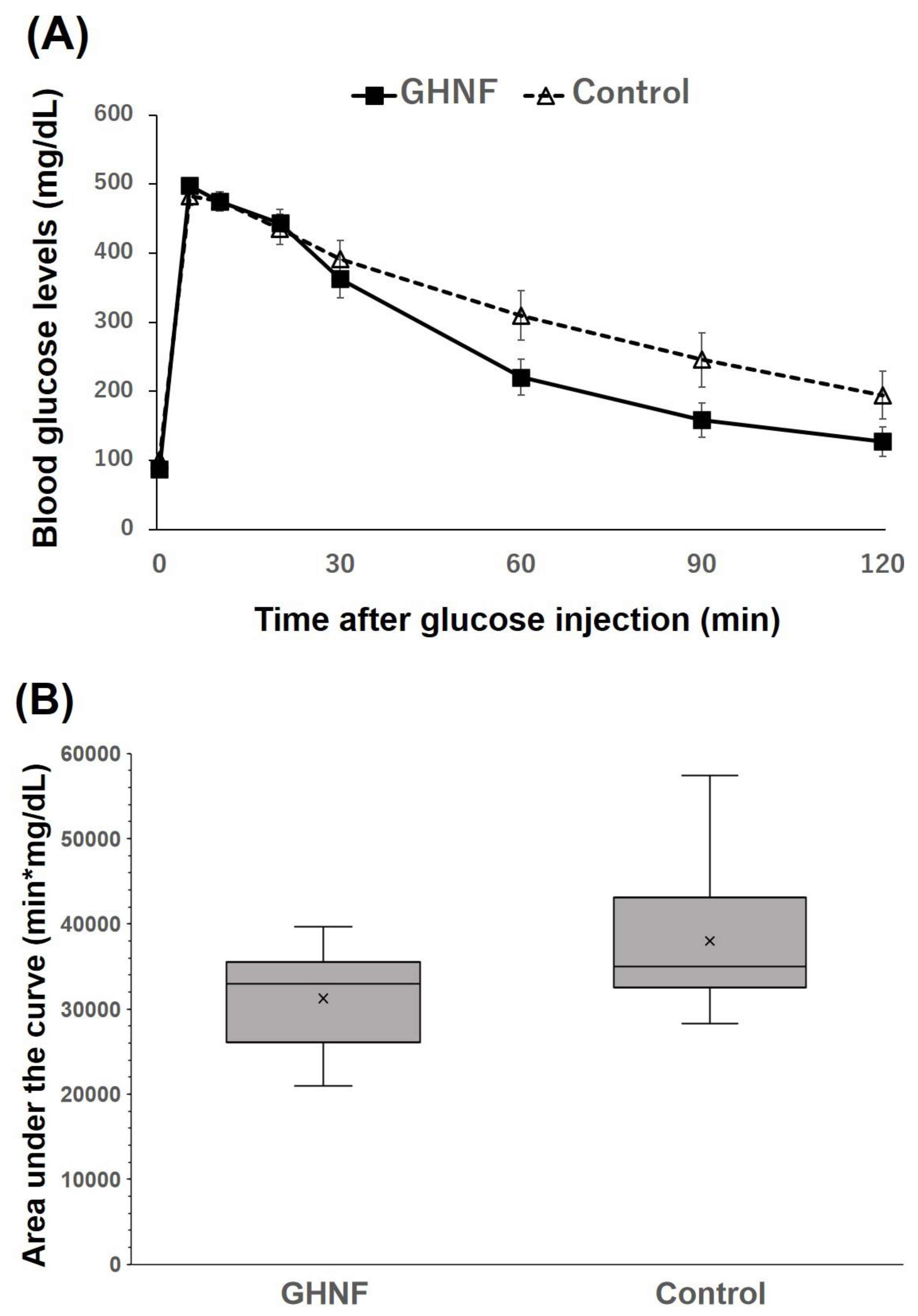
The blood glucose changes in the IVGTT did not differ between the two groups (p=0.98) (
Figure 3A). Although the difference was not statistically significant, the AUC in the GHNF group was lower than that in the control group (GHNF:31,257±2,159 vs. control:38,994±3,252, p=0.11) (
Figure 3B).
3.4. Immunohistochemical analyses
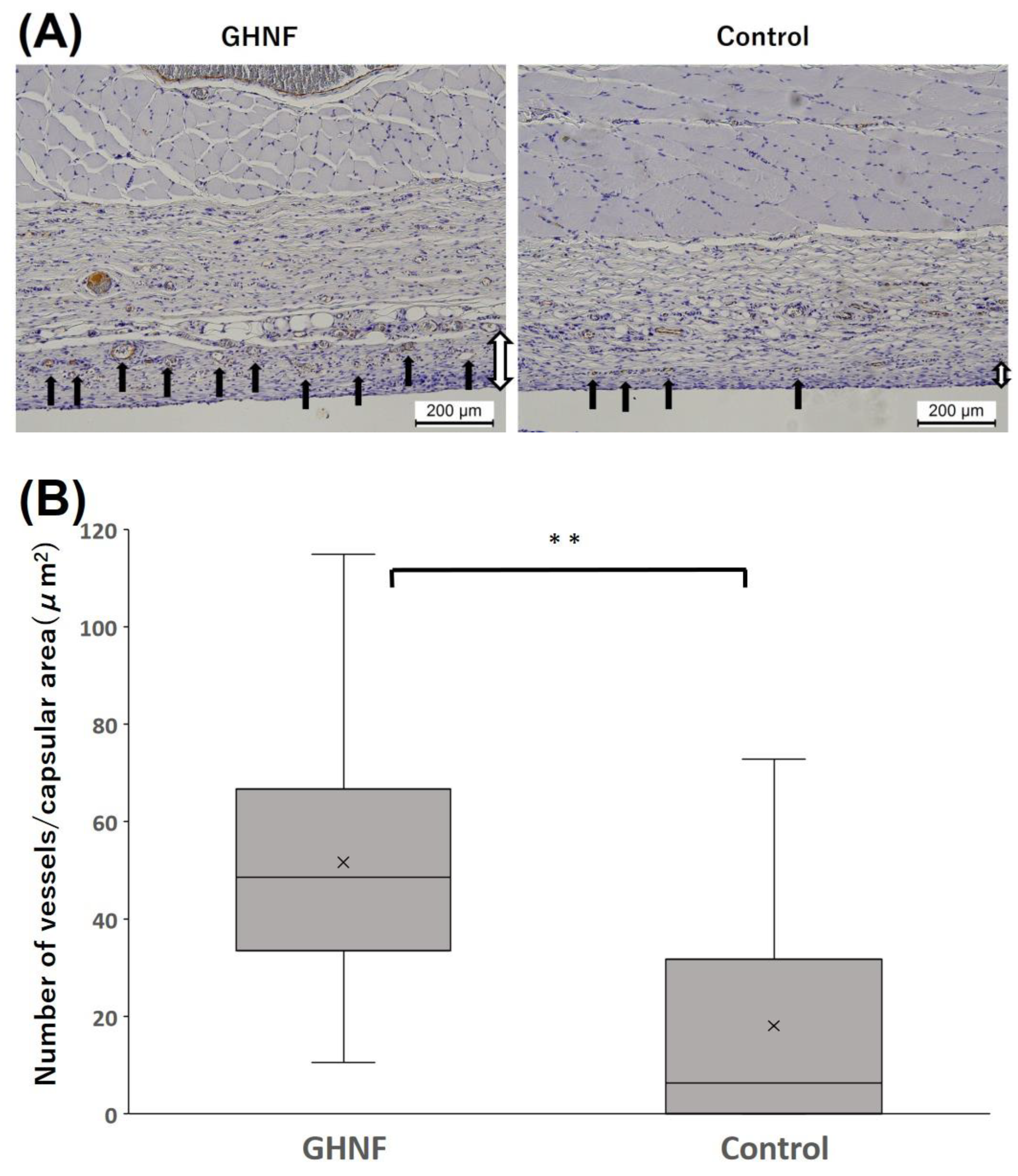
The number of vWF-positive vessels in the capsule touching the silicone spacer was counted and divided by the capsular area to examine neovascularization prior to islet transplantation (
Figure 4A). The number of vWF-positive vessels in the GHNF group was significantly higher than that in the control group (p<0.01) (
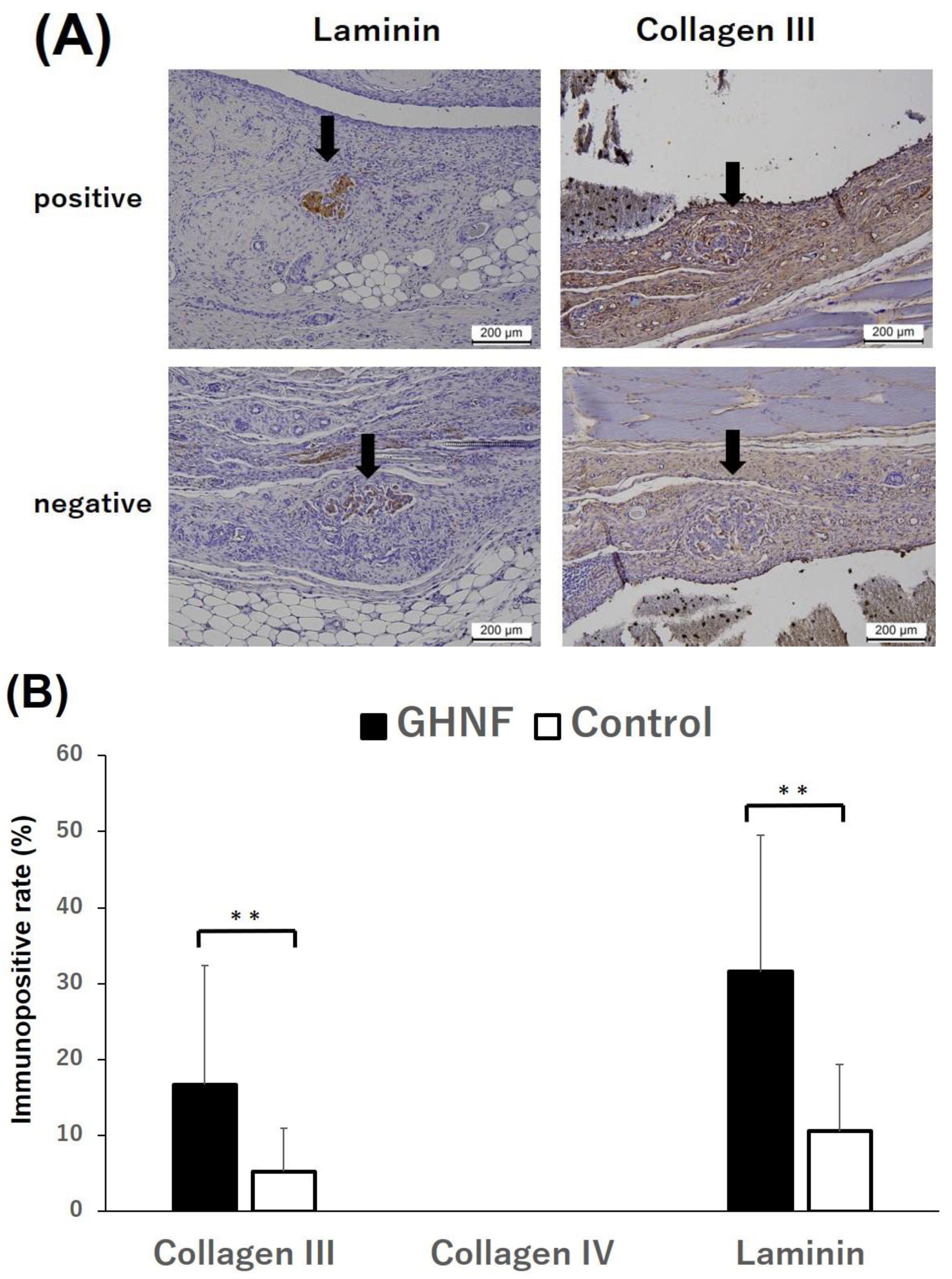
Figure 4B). To examine the effect of GHNF pretreatment on the ECM components in subcutaneous capsules, the rate of immunopositive sections in terms of collagen III, collagen IV, and laminin in the fibrous capsule around the islet grafts was evaluated (
Figure 5A). Although there was no collagen IV positivity in either group, the rates of collagen III and laminin positivity in the GHNF group were significantly higher than those in the control group (p<0.01) (
Figure 5B).
3.5. Islet evaluation
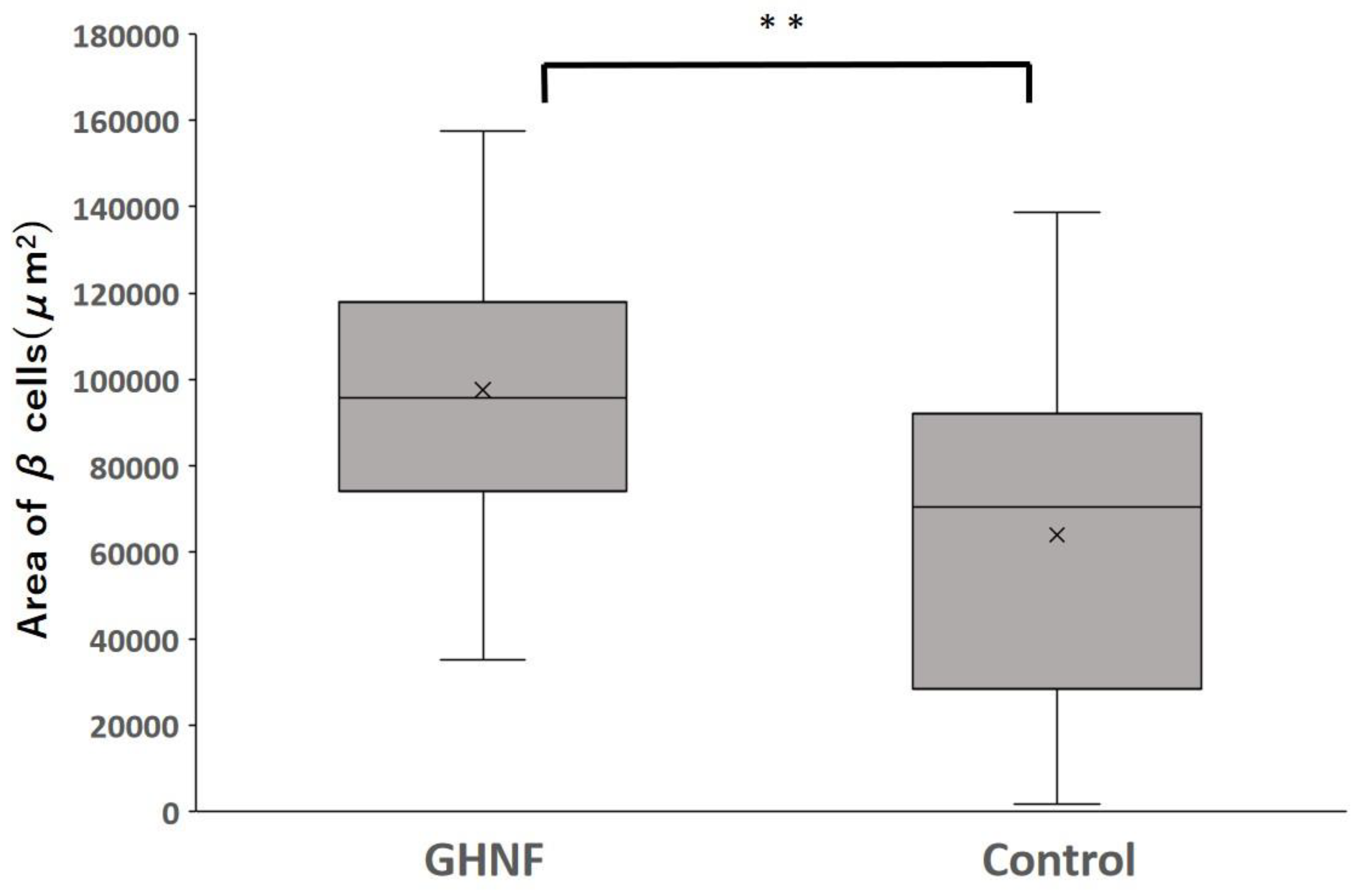
All insulin-positive islets in a pathological specimen obtained 1 week after islet transplantation were evaluated (GHNF, n=60; control, n=54). The average area of β cells in the GHNF group was significantly larger than that in the control group (GHNF:97,486±4,214 vs. control:64,022±4,954 μm2, respectively, p<0.01) (
Figure 6).
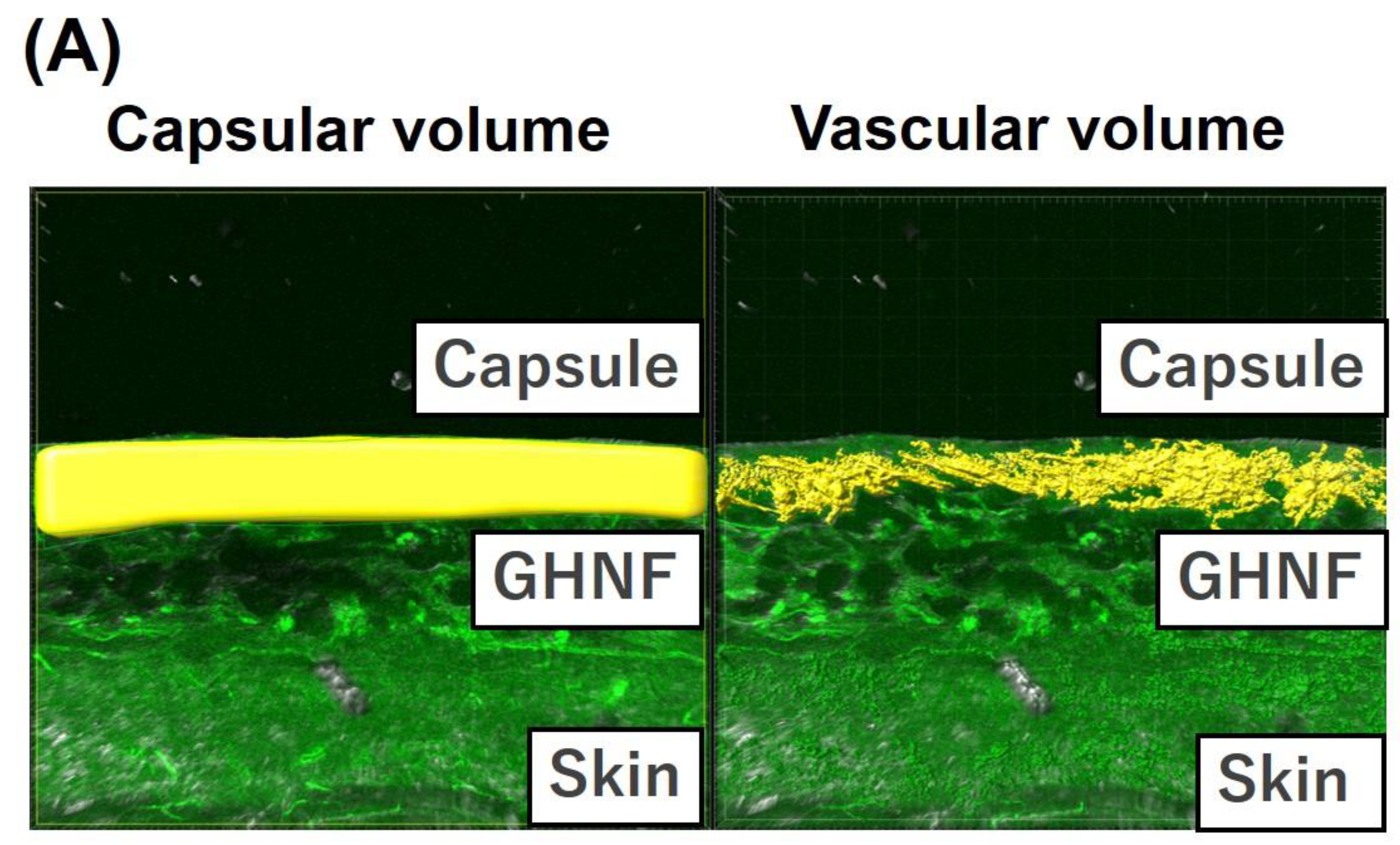
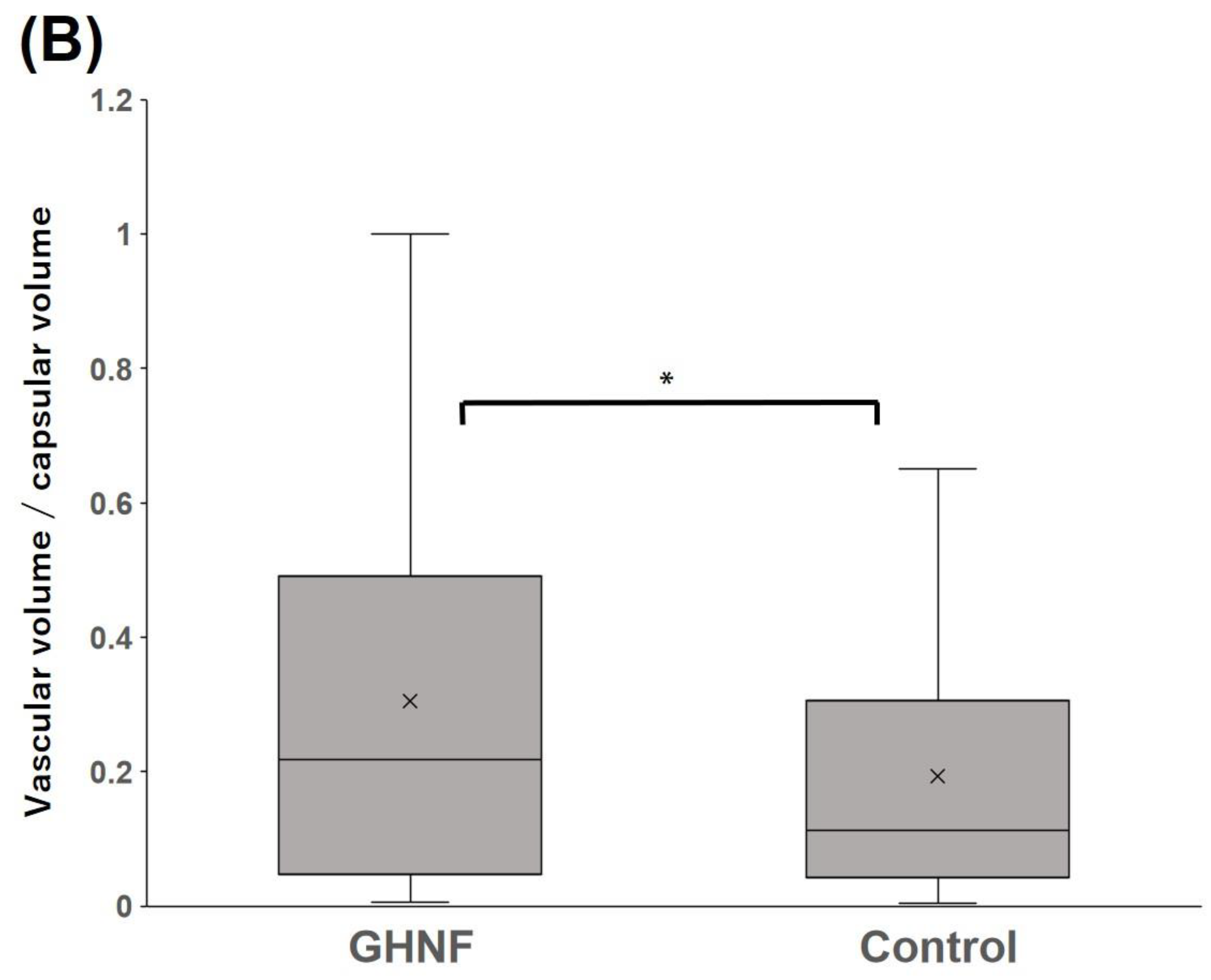
3.6. Quantification of the vascular volume using Lectin angiography
Lectin angiography revealed that the blood vessel density in the GHNF group was significantly higher than that in the control group (GHNF:0.30±0.032 vs. control:0.19±0.038, p<0.05) (
Figure 7A, B).
4. Discussion
The present study clearly showed that pretreatment with GHNF improved subcutaneous islet engraftment even in a rat model. Unlike in a mouse model, GHNF was completely absorbed over three weeks after implantation in a rat model. Three weeks after GHNF pretreatment, only a limited amount of GHNF remained in the subcutaneous capsules, but inflammation based on the foreign body responses to GHNF had already disappeared. In a rat model, GHNF preimplantation induced sufficient new vessels in the subcutaneous space compared to the control group (
Figure 4B, 7B). In addition, it enhanced ECM compensation surrounding the subcutaneously transplanted islets (
Figure 5B).
The effectiveness of subcutaneous islet transplantation is extremely low, most likely due to the low concentration of oxygen and/or nutrition, which is based on poor vascularization in the subcutaneous space [
29,
30]. Therefore, optimization of the subcutaneous environment prior to islet transplantation is logical and it should be useful for improving the outcomes of subcutaneous islet transplantation. In this process, the duration of pretreatment is of great importance for islet engraftment. Patikova et al. reported that the ideal timing of islet transplantation, when the capillary network and collagen synthesis are maximized, is important for islet engraftment [
31]. We also reported that the duration of GHNF pretreatment had a strong impact on the outcomes of subcutaneous islet transplantation and that six weeks was the best duration when neovascularization was maximized and inflammatory reactions were minimized in a mouse model [
13]. However, the extent of absorption of GHNF varies among animal species. In our preliminary studies, GHNF was completely absorbed 4 weeks after implantation in a rat model (data not shown). Therefore, we next compared the remnant volume of GHNF using HE staining in mouse and rat models. According to these evaluations, 3 weeks in a rat model, which corresponded to 6 weeks in a mouse model, was considered the best duration of pretreatment. In the present study, GHNF sheets were implanted in the subcutaneous space three weeks prior to islet transplantation, and islets were transplanted into the space where the silicone spacer was removed. If GHNF is applied to other species, including humans, observation of GHNF remnants by HE staining would thus be helpful in determining the ideal duration of pretreatment.
During islet isolation, ECM on the islet basement membrane is intensively digested by cell dissociation enzymes [
32,
33]. However, collagen III has been reported to improve islet function by protecting against apoptosis and increasing insulin secretion [
34]. Likewise, laminin is also an important ECM that comprises 80% of the islet basement membrane and accelerates β-cell survival and insulin secretion [
35,
36]. Therefore, the compensation of the crucial ECM around the islet capsule, which is lost during islet isolation, is necessary for islet survival and function [
15]. Considering the results of the present study that the immunopositive rate of laminin and collagen III in the GHNF group was significantly higher than that in the control group (
Figure 5B), the ECM compensation around islets may be one of the crucial mechanisms of the GHNF effects in a rat model.
GHNF has been reported to be absorbed in vivo and replaced by host cells and ECM [
18,
37]. We previously reported that M2 macrophages, which have anti-inflammatory properties [
38], accumulated between the gaps of GHNF [
13]. The concentration of insulin-like growth factor-2, which maintains islet viability and has anti-apoptotic effects on islets [
39,
40], in the subcutaneous space is high in the GHNF group in a mouse model [
15]. Although we did not evaluate these issues in the present study, these factors could be one possible explanation for the larger area of β cells observed in the GHNF group than in the control group (
Figure 6A).
Neovascularization in the subcutaneous space is undoubtedly an essential factor for obtaining a better and longer functioning of transplanted islets. The density of subcutaneous vessels was evaluated using vWF staining and lectin angiography. Lectin angiography can theoretically detect vessels less than 10 μm in diameter, and it enables three-dimensional evaluation of vessels in the microstructure [
41]. In the present study, vessels located in the capsule (with a thickness ranging from 10 to 100 μm), where the transplanted islets were directly attached, were evaluated. We recently reported that constructed vessels in the subcutaneous space could be clearly observed, and these results were consistent with those of vWF staining (manuscript submitted). In the present study, the subcutaneous vessel density in the GHNF group was significantly higher than that in the control group in both vWF staining and lectin angiography (
Figure 4B, 7B). From these results, the effectiveness of lectin angiography in a rat model was confirmed, and a high number of new vessels appeared to result in good outcomes in the GHNF group (
Figure 2B).
Although GHNF pretreatment was effective for improving the engraftment of subcutaneously transplanted islets in a rat model, substantial amounts of islets (5,400 IEQs), which corresponds to approximately four times higher doses than intraportal transplantation [
42] are still required to cure a diabetic recipient in this model. In contrast, in a mouse model, the outcome of subcutaneous islet transplantation was superior to that of intraportal islet transplantation [
15]. This discrepancy may suggest that neovascularization and/or ECM compensation are still insufficient; otherwise, crucial factors, rather than neovascularization and ECM compensation, may exist in a rat model [
43]. Hence, the combination of GHNF with adipose-derived stem cells (ADSCs), which secrete various growth factors and effectively induce neovascularization [
44,
45,
46], would be one option.
5. Conclusions
The present study showed that GHNF pretreatment effectively improved the outcomes of subcutaneous islet transplantation in a rat model. The main mechanisms of this beneficial effect may be the induction of sufficient new vessels in the subcutaneous capsules and compensation of the ECM surrounding the transplanted islets.
6. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Author Contributions
R.S., A.I., and M.G.conceived of and contributed to the design. of the project: R.S., T.I., N.K., H.M., Y.E.K., T.K., S.S., and K.T. participated in data collection, A.H., and Y.N. undertook the analysis, and T.K., M.U., and K.W. contributed to interpretation. Y.T. provided technological advice on gelatin hydrogel nonwoven fabrics. The manuscript was drafted by M.G., with all authors participating in its critical revision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Japanese Grant-in-Aid for Scientific Research (A) (Grant Number 18H04056) and Research (B) (Grant Number 22H03133) from the Japan Society for the Promotion of Science and by AMED under Grant Number JP19bm0404043.
Institutional Review Board Statement
All animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. All experimental protocols used in this study (protocol ID:2020 MdA-022) were approved by the Animal Experimental Committee of Tohoku University. All surgical operations were performed under anesthesia, and efforts were made to minimize suffering.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are included in this manuscript.
Acknowledgments
The authors thank Kozue Maya and Megumi Goto (Division of Transplantation and Regenerative Medicine, Tohoku University) for their valuable technical assistance. The authors also acknowledge the support of the Biomedical Research Core of Tohoku University, Graduate School of Medicine, and Tohoku Advanced Medical Research and Incubation Center (TAMRIC).
Conflicts of Interest
The authors declare no conflicts of interest in association with the present study, although this study was conducted according to a patent application agreement with NIKKE MEDICAL Co. Ltd.
References
- Sakata, N.; Goto, M.; Gumpei, Y.; et al. Intraoperative ultrasound examination is useful for monitoring transplanted islets: a case report. Islets 2012, 4, 339–342. [Google Scholar] [CrossRef]
- Rajab, A. Islet transplantation: alternative sites. Curr. Diabetes Rep. 2010, 10, 332–337. [Google Scholar] [CrossRef]
- Yasunami, Y.; Nakafusa, Y.; Nitta, N.; et al. A Novel Subcutaneous Site of Islet Transplantation Superior to the Liver. Transplantation 2018, 102, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M.J. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nature Biotechnology. 2015, 33, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, A.; Molano, R.D.; Ricordi, C.; et al. Reversal of Diabetes by Pancreatic Islet Transplantation into a Subcutaneous, Neovascularized Device. Transplantation 2006, 81, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ajima, K.; Tsuda, N.; Takaki, T.; et al. A porcine islet-encapsulation device that enables long-term discordant xenotransplantation in immunocompetent diabetic mice. Cell Rep. Methods 2023, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Mahou, R.; Zhang, D.K.Y.; Vlahos, A.E.; Sefton, M.V. Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space. Biomaterials 2017, 131, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chiu, A.; Wang, L.H.; et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 2019, 10, 1. [Google Scholar] [CrossRef]
- Nakafusa, Y.; Nitta, N.; Ishii, K.; et al. Acceptance of Murine Islet Allografts Without Immunosuppression in Inguinal Subcutaneous White Adipose Tissue Pretreated With, b.F.G.F. Diabetes 2022, 71, 1721–1734. [Google Scholar] [CrossRef]
- Vlahos, A.E.; Cober, N.; Sefton, M.V. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc. Natl. Acad. Sci. USA 2017, 114, 9337–9342. [Google Scholar] [CrossRef]
- Perez-Basterrechea, M.; Esteban, M.M.; Alvarez-Viejo, M.; et al. Fibroblasts accelerate islet revascularization and improve long-term graft survival in a mouse model of subcutaneous islet transplantation. PLOS ONE 2017, 12, e0180695. [Google Scholar] [CrossRef] [PubMed]
- Smink, A.M.; Li, S.; Hertsig, D.T.; et al. The Efficacy of a Prevascularized, Retrievable Poly(D,L,-lactide-co-ε-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 2017, 101, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Inagaki, A.; Nakamura, Y.; et al. Ideal Duration of Pretreatment Using a Gelatin Hydrogel Nonwoven Fabric Prior to Subcutaneous Islet Transplantation. Cell Transplant. 2023, 32, 09636897231186063. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Pawlick, R.; Gala-Lopez, B.; et al. Diabetes Is Reversed in a Murine Model by Marginal Mass Syngeneic Islet Transplantation Using a Subcutaneous Cell Pouch Device. Transplantation 2015, 99, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Kanai, N.; Inagaki, A.; Nakamura, Y.; et al. A gelatin hydrogel nonwoven fabric improves outcomes of subcutaneous islet transplantation. Sci. Rep. 2023, 13, 11968. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.S.; Inagaki, A.; Nakamura, Y.; et al. The Optimization of the Prevascularization Procedures for Improving Subcutaneous Islet Engraftment. Transplantation 2018, 102, 387–395. [Google Scholar] [CrossRef]
- Nakamura, K.; Saotome, T.; Shimada, N.; Matsuno, K.; Tabata, Y. A Gelatin Hydrogel Nonwoven Fabric Facilitates Metabolic Activity of Multilayered Cell Sheets. Tissue Eng. Part C Methods 2019, 25, 344–352. [Google Scholar] [CrossRef]
- Matsuno, K.; Saotome, T.; Shimada, N.; Nakamura, K.; Tabata, Y. Effect of cell seeding methods on the distribution of cells into the gelatin hydrogel nonwoven fabric. Regen. Ther. 2020, 14, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, A.E.; Talior-Volodarsky, I.; Kinney, S.M.; Sefton, M.V. A scalable device-less biomaterial approach for subcutaneous islet transplantation. Biomaterials 2021, 269, 120499. [Google Scholar] [CrossRef]
- Bayne, K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist 1996, 39, 199–208. [Google Scholar]
- Dendo, M.; Maeda, H.; Yamagata, Y.; et al. Synergistic Effect of Neutral Protease and Clostripain on Rat Pancreatic Islet Isolation. Transplantation 2015, 99, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Inagaki, A.; Fathi, I.; et al. Improvement of hepatocyte engraftment by co-transplantation with pancreatic islets in hepatocyte transplantation. J. Tissue Eng. Regen. Med. 2021, 15, 361–374. [Google Scholar] [CrossRef]
- Inagaki, A.; Imura, T.; Nakamura, Y.; Ohashi, K.; Goto, M. The Liver Surface Is an Attractive Transplant Site for Pancreatic Islet Transplantation. J. Clin. Med. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, T.; Inagaki, A.; Imura, T.; et al. A Novel Resting Strategy for Improving Islet Engraftment in the Liver. Transplantation 2014, 97, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fujio, A.; Murayama, K.; Yamagata, Y.; et al. Collagenase H is crucial for isolation of rat pancreatic islets. Cell Transplant. 2014, 23, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Robertson, R.T.; Levine, S.T.; Haynes, S.M.; et al. Use of labeled tomato lectin for imaging vasculature structures. Histochem. Cell Biol. 2015, 143, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nozawa-Inoue, K.; Harada, F.; Magara, J.; Ohazama, A.; Maeda, T. Contribution of synovial lining cells to synovial vascularization of the rat temporomandibular joint. J. Anat. 2016, 228, 520–529. [Google Scholar] [CrossRef]
- Mitsugashira, H.; Imura, T.; Inagaki, A.; et al. Development of a novel method for measuring tissue oxygen pressure to improve the hypoxic condition in subcutaneous islet transplantation. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Kawakami, Y.; Iwata, H.; Gu, Y.; et al. Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell Transplant. 2000, 9, 729–732. [Google Scholar] [CrossRef]
- Patikova, A.; Vojtiskova, A.; Fabryova, E.; et al. The Optimal Maturation of Subcutaneous Pouch Can Improve Pancreatic Islets Engraftment in Rat Model. Transplantation 2022, 106, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Vaughan, R.H.; Willcox, A.J.; et al. Key Matrix Proteins Within the Pancreatic Islet Basement Membrane Are Differentially Digested During Human Islet Isolation. Am. J. Transplant. 2017, 17, 451–461. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Murayama, K.; Fathi, I.; et al. Strategy towards tailored donor tissue-specific pancreatic islet isolation. PLoS One 2019, 14, e0216136. [Google Scholar] [CrossRef]
- Hughes, S.J.; Clark, A.; McShane, P.; Contractor, H.H.; Gray, D.W.; Johnson, P.R. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation 2006, 81, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, K.I.; Powers, A.C. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes Metab. 2017, 19 (Suppl. 1), 124–136. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Raghunath, M.; Whitelock, J.; Poole-Warren, L. Matrix components and scaffolds for sustained islet function. Tissue Eng. Part B Rev. 2011, 17, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Saotome, T.; Shimada, N.; Matsuno, K.; Nakamura, K.; Tabata, Y. Gelatin hydrogel nonwoven fabrics of a cell culture scaffold to formulate 3-dimensional cell constructs. Regen. Ther. 2021, 18, 418–429. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Mohanasundaram, D.; Kireta, S.; Jessup, C.F.; Drogemuller, C.J.; Coates, P.T. Insulin-Like growth factor-II (IGF-II) prevents proinflammatory cytokine-induced apoptosis and significantly improves islet survival after transplantation. Transplantation 2013, 95, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, R.; Dusseault, J.; Henley, N.; Rosenberg, L.; Hallé, J.P. Insulin-like growth factor II allows prolonged blood glucose normalization with a reduced islet cell mass transplantation. Endocrinology 2003, 144, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Adler, K.; Liu, X.; Sun, W.; Mullins, R.F.; Sohn, E.H. Visualization of mouse choroidal and retinal vasculature using fluorescent tomato lectin perfusion. Transl. Vis. Sci. Technol. 2020, 9, 1–1. [Google Scholar] [CrossRef]
- Tokodai, K.; Goto, M.; Inagaki, A.; et al. C5a-inhibitory peptide combined with gabexate mesilate prevents the instant blood-mediated inflammatory reaction in a rat model of islet transplantation. Transplant Proc. 2010, 42, 2102–2103. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Johansson, U.; Eich, T.M.; et al. Key factors for human islet isolation and clinical transplantation. Transpl. Proc. 2005, 37, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell. Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Yamana, H.; Inagaki, A.; Imura, T.; et al. Cotransplantation with Adipose Tissue-derived Stem Cells Improves Engraftment of Transplanted Hepatocytes. Transplantation 2022, 106, 1963–1973. [Google Scholar] [CrossRef]
Figure 1.
(A) Hematoxylin Eosin staining of the subcutaneous tissues at 3 weeks after implantation. GHNF was absorbed and its volume gradually decreased time-dependently. The pictures on the right represented the enlargement of the boxed area in the pictures on the left. Black arrow: muscles, Gray arrow: skin and subcutaneous tissues, White arrow: GHNF, Black arrow with * *: capsules. (B) The area of the capsule touching the silicone spacer in the GHNF group was larger than that of the control group.
Figure 1.
(A) Hematoxylin Eosin staining of the subcutaneous tissues at 3 weeks after implantation. GHNF was absorbed and its volume gradually decreased time-dependently. The pictures on the right represented the enlargement of the boxed area in the pictures on the left. Black arrow: muscles, Gray arrow: skin and subcutaneous tissues, White arrow: GHNF, Black arrow with * *: capsules. (B) The area of the capsule touching the silicone spacer in the GHNF group was larger than that of the control group.
Figure 2.
The outcomes of islet engraftment after marginal islet mass transplantation (5,400 IEQs). (A) The change of the blood glucose levels after islet transplantation. GHNF (filled square, n=8) group showed significantly better glucose changes than the control group (open triangle, n=8) (**, p<0.01). (B) The cure rate after islet transplantation in each group. The cure rate at 60 days after islet transplantation in the GHNF group (62.5%) was significantly higher than that in the control group (12.5%) (*; p<0.05).
Figure 2.
The outcomes of islet engraftment after marginal islet mass transplantation (5,400 IEQs). (A) The change of the blood glucose levels after islet transplantation. GHNF (filled square, n=8) group showed significantly better glucose changes than the control group (open triangle, n=8) (**, p<0.01). (B) The cure rate after islet transplantation in each group. The cure rate at 60 days after islet transplantation in the GHNF group (62.5%) was significantly higher than that in the control group (12.5%) (*; p<0.05).
Figure 3.
The glucose tolerance profiles of the GHNF and control groups. (A) Blood glucose changes in the intravenous glucose tolerance test (IVGTT) at approximately 60 days after islet transplantation. There was no significant difference in the IVGTT between the GHNF (filled square, n=8) and control (open triangle, n=8) groups. (B) The area under the curve (AUC) of the IVGTT in each group is shown. Although the difference did not reach statistical significance, the AUC of the GHNF group was lower than that of the control group (p=0.106).
Figure 3.
The glucose tolerance profiles of the GHNF and control groups. (A) Blood glucose changes in the intravenous glucose tolerance test (IVGTT) at approximately 60 days after islet transplantation. There was no significant difference in the IVGTT between the GHNF (filled square, n=8) and control (open triangle, n=8) groups. (B) The area under the curve (AUC) of the IVGTT in each group is shown. Although the difference did not reach statistical significance, the AUC of the GHNF group was lower than that of the control group (p=0.106).
Figure 4.
Immunohistochemical analyses of von Willebrand factor (vWF)-positive vessels. (A) Photomicrographs of vWF staining before islet transplantation. The vWF-positive vessels (black arrows) in the capsule around the GHNF were counted. Magnification: ×100. Calibration bars: 200 µm. (B) The number of vessels divided by the capsular area in each group. The number of vWF-positive vessels of the GHNF group was significantly higher than that of the control group (**, p<0.01). Black arrow: vWF-positive vessels, White arrow: capsules.
Figure 4.
Immunohistochemical analyses of von Willebrand factor (vWF)-positive vessels. (A) Photomicrographs of vWF staining before islet transplantation. The vWF-positive vessels (black arrows) in the capsule around the GHNF were counted. Magnification: ×100. Calibration bars: 200 µm. (B) The number of vessels divided by the capsular area in each group. The number of vWF-positive vessels of the GHNF group was significantly higher than that of the control group (**, p<0.01). Black arrow: vWF-positive vessels, White arrow: capsules.
Figure 5.
Immunohistochemical analyses of the extracellular matrix (ECM). (A) Representative photomicrographs of laminin and collagen III staining. Black arrows represent the transplanted islets. “Positive” for laminin, collagen III, and collagen IV indicates that distinct immunopositivity was detectable in the fibrous capsule around the islets. “Negative” indicates that immunopositivity was undetectable. Magnification: ×200. Calibration bars: 200 µm. (B) The rates of laminin and collagen III positivity in the GHNF (black box) and control (white box) groups. The positivity of collagen III and laminin in the GHNF group was significantly higher than that in the control group (**, p<0.01).
Figure 5.
Immunohistochemical analyses of the extracellular matrix (ECM). (A) Representative photomicrographs of laminin and collagen III staining. Black arrows represent the transplanted islets. “Positive” for laminin, collagen III, and collagen IV indicates that distinct immunopositivity was detectable in the fibrous capsule around the islets. “Negative” indicates that immunopositivity was undetectable. Magnification: ×200. Calibration bars: 200 µm. (B) The rates of laminin and collagen III positivity in the GHNF (black box) and control (white box) groups. The positivity of collagen III and laminin in the GHNF group was significantly higher than that in the control group (**, p<0.01).
Figure 6.
The average area of β cells in each group. The average area of β cells in the GHNF group was significantly larger than that in the control group (GHNF: 97,486±4,214 vs. control: 64,022±4,954 μm2, respectively, p<0.01).
Figure 6.
The average area of β cells in each group. The average area of β cells in the GHNF group was significantly larger than that in the control group (GHNF: 97,486±4,214 vs. control: 64,022±4,954 μm2, respectively, p<0.01).
Figure 7.
Quantification of the vascular volume determined using lectin angiography in the capsule at the transplant site of the GHNF and control groups. (A) Image of lectin angiography in the GHNF group. The volume of the capsules (left) and constructed vessels (right) (Shown as a yellow structure). (B) The vascular density in each group. The results represent the vascular volume (μm3)/capsular volume (μm3) and the density of the vessels in the GHNF group was significantly higher than that in the control group (*, p<0.05).
Figure 7.
Quantification of the vascular volume determined using lectin angiography in the capsule at the transplant site of the GHNF and control groups. (A) Image of lectin angiography in the GHNF group. The volume of the capsules (left) and constructed vessels (right) (Shown as a yellow structure). (B) The vascular density in each group. The results represent the vascular volume (μm3)/capsular volume (μm3) and the density of the vessels in the GHNF group was significantly higher than that in the control group (*, p<0.05).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).