Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Results
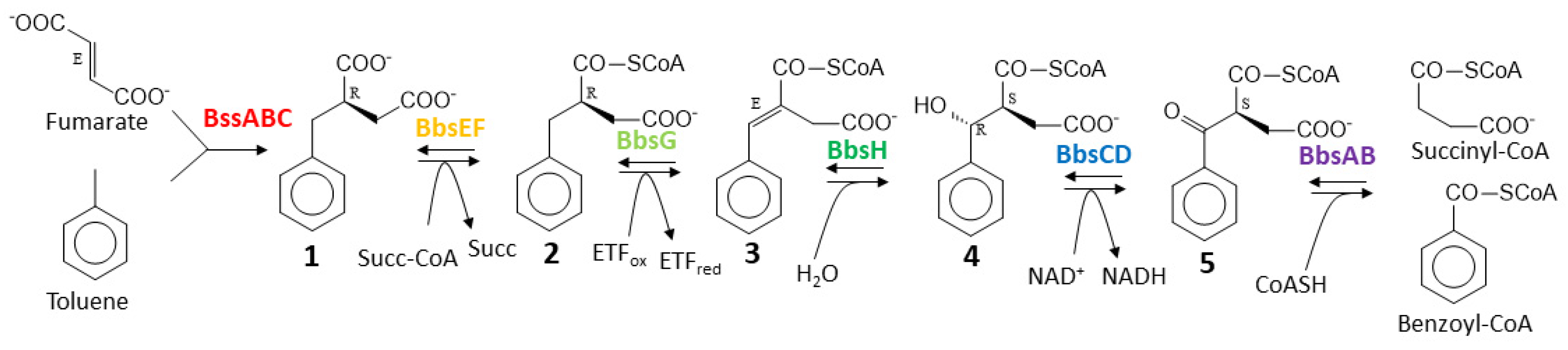
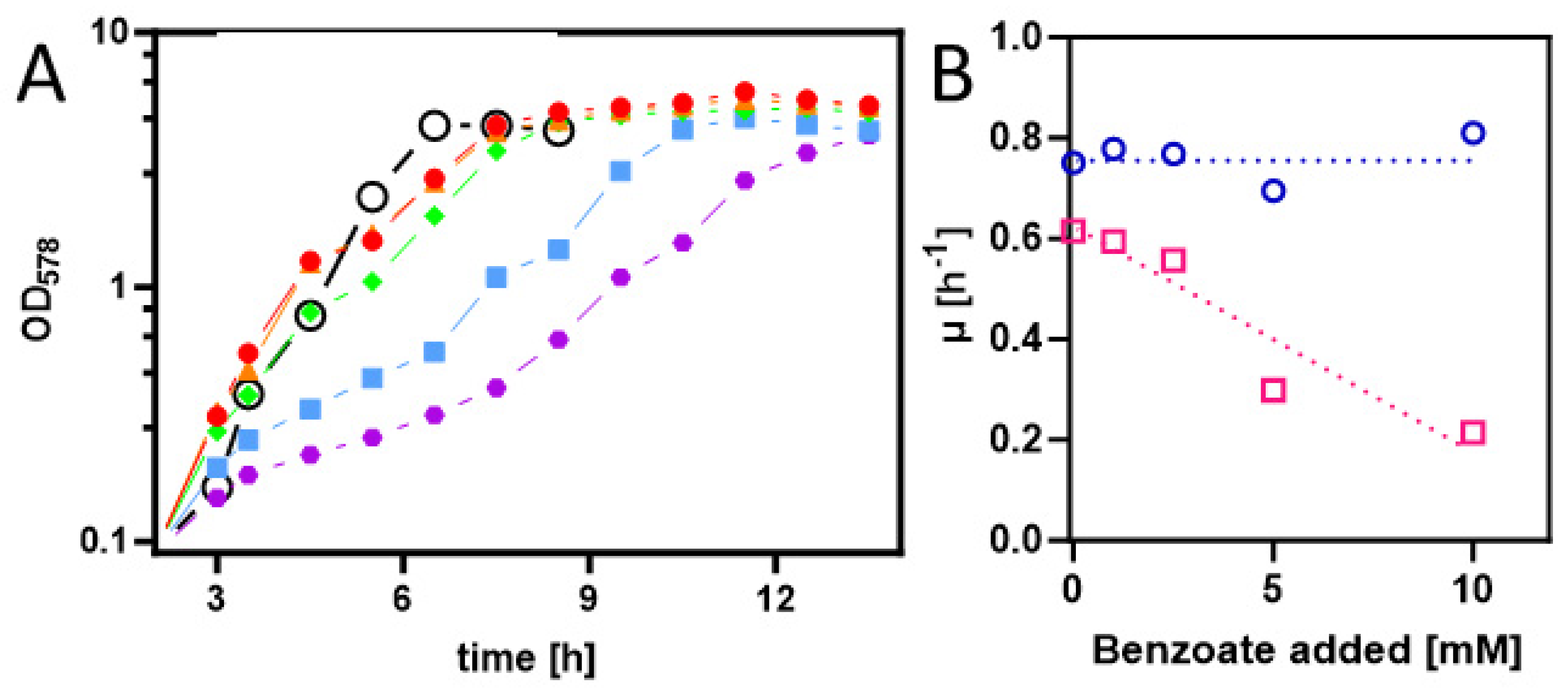
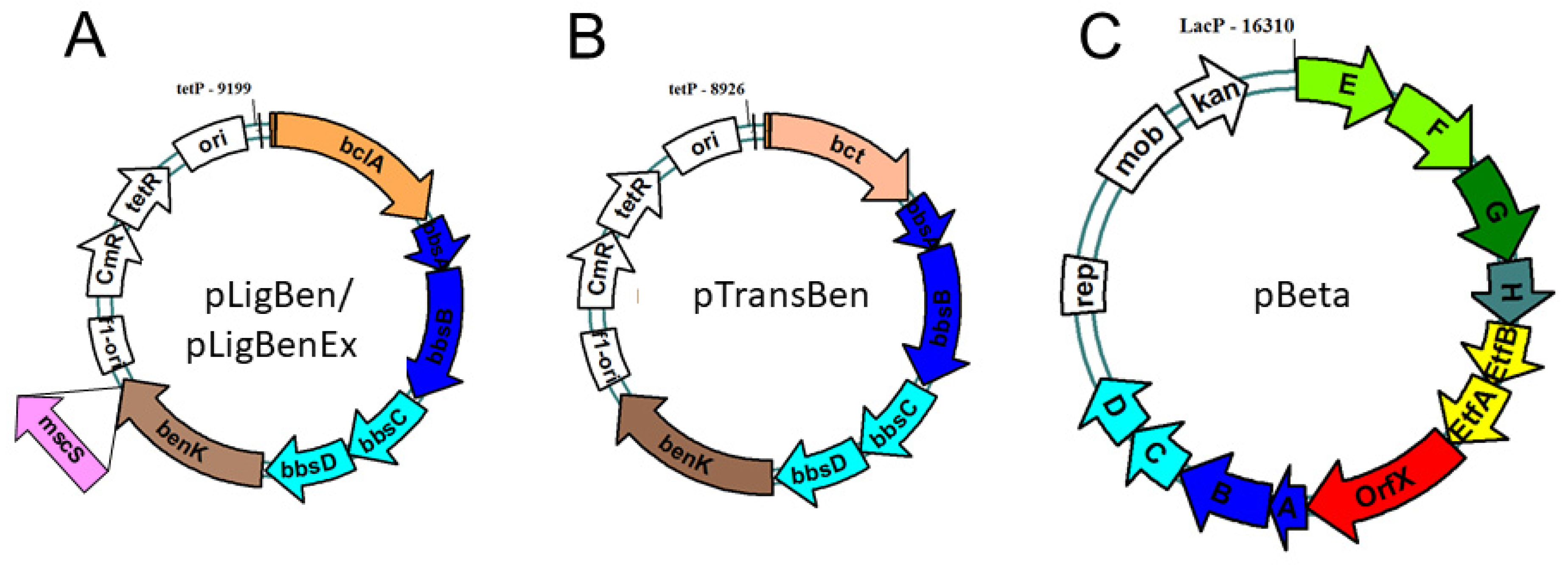
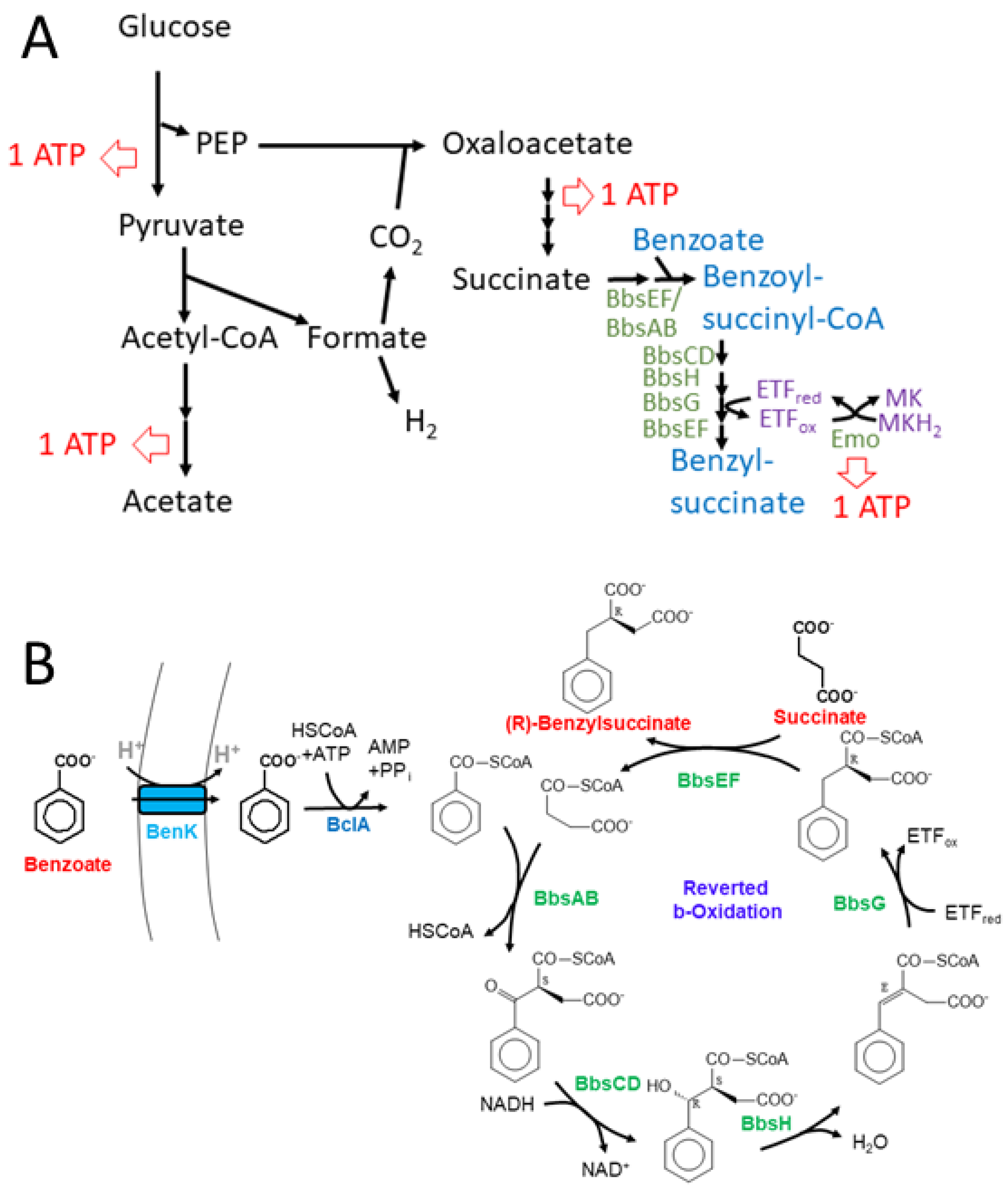
2.1. Biosynthetic modules for benzoyl-CoA synthesis
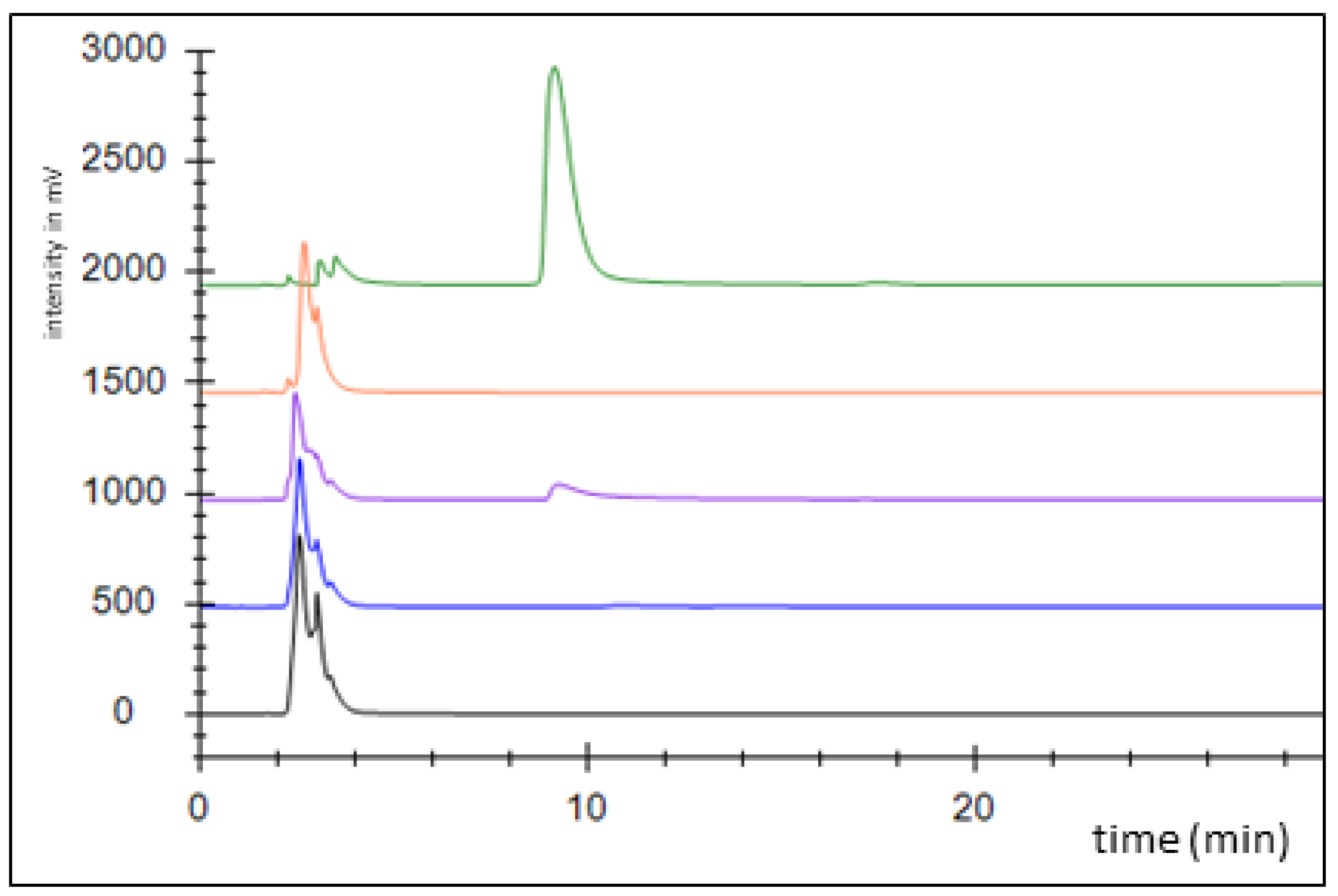
2.2. Benzylsuccinate synthetic module
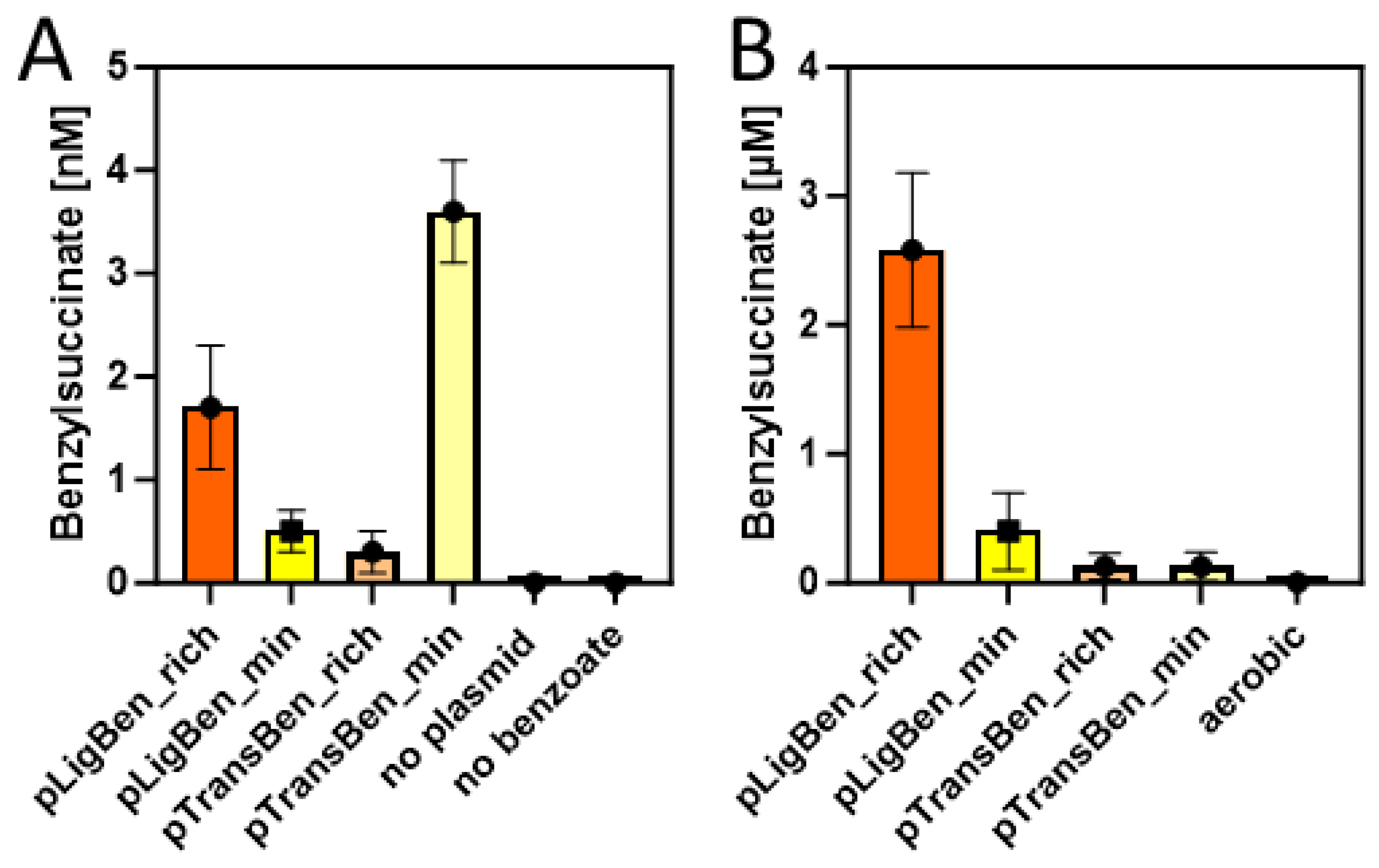
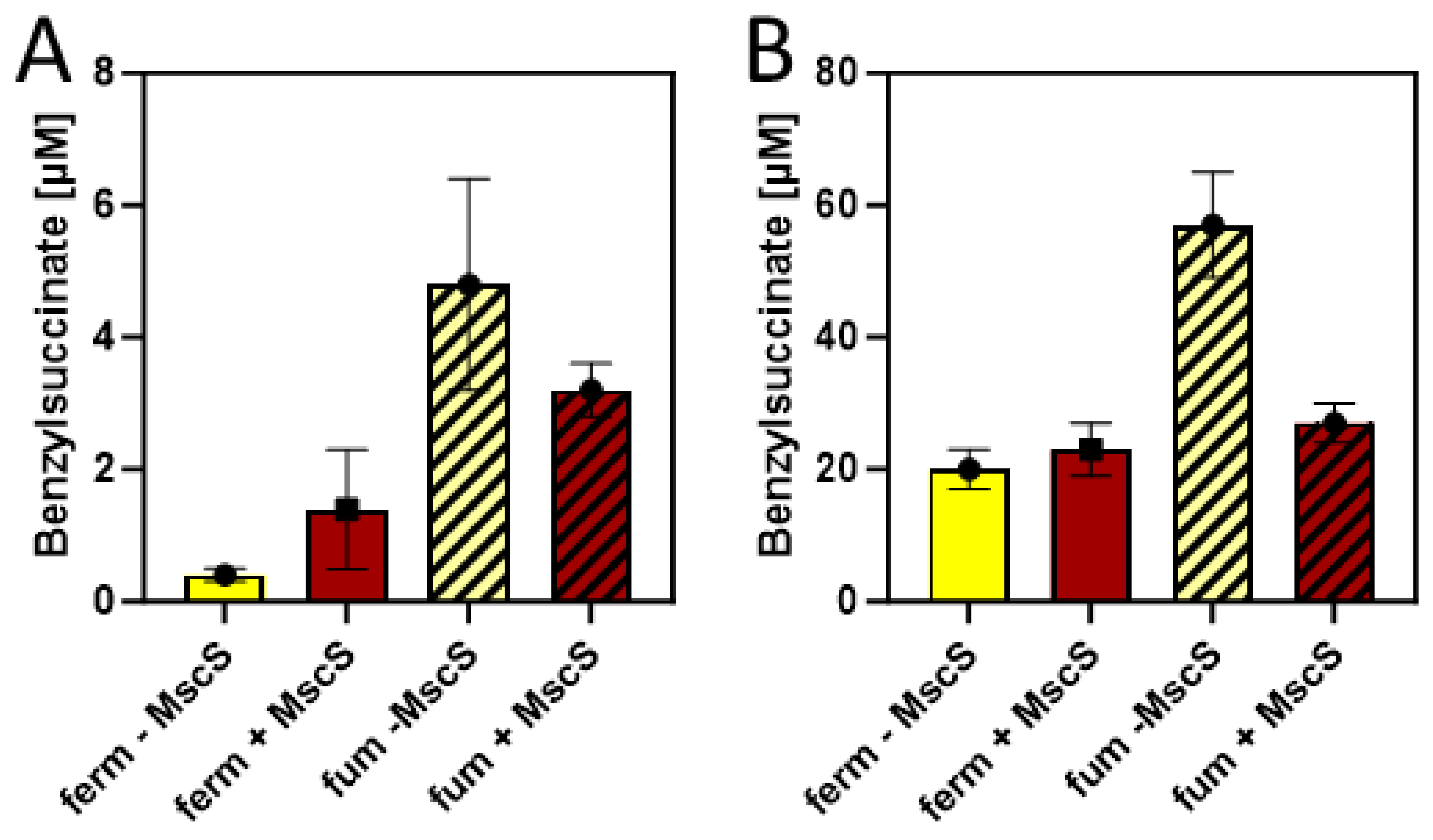
2.3. Benzylsuccinate production assays
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
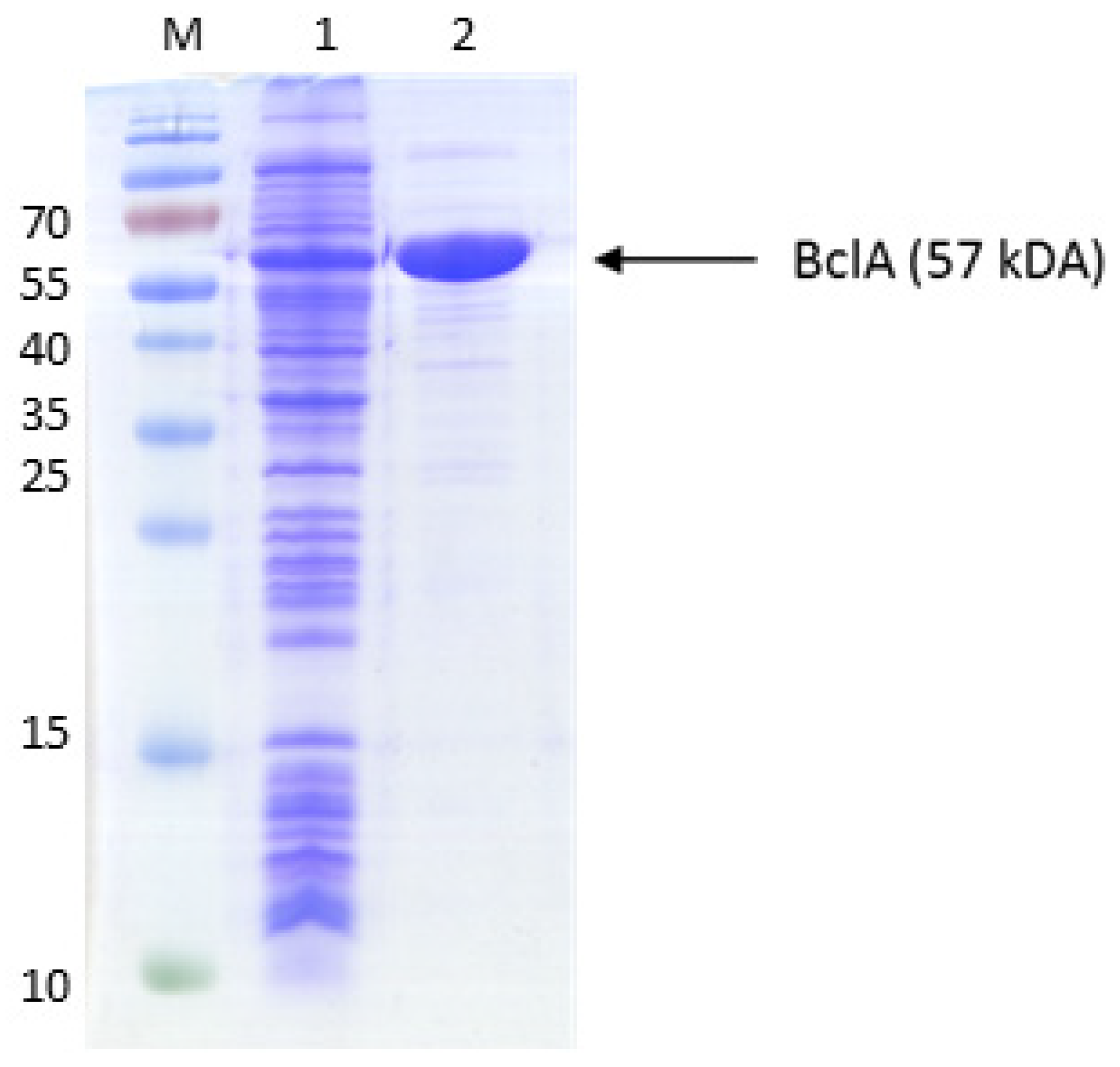
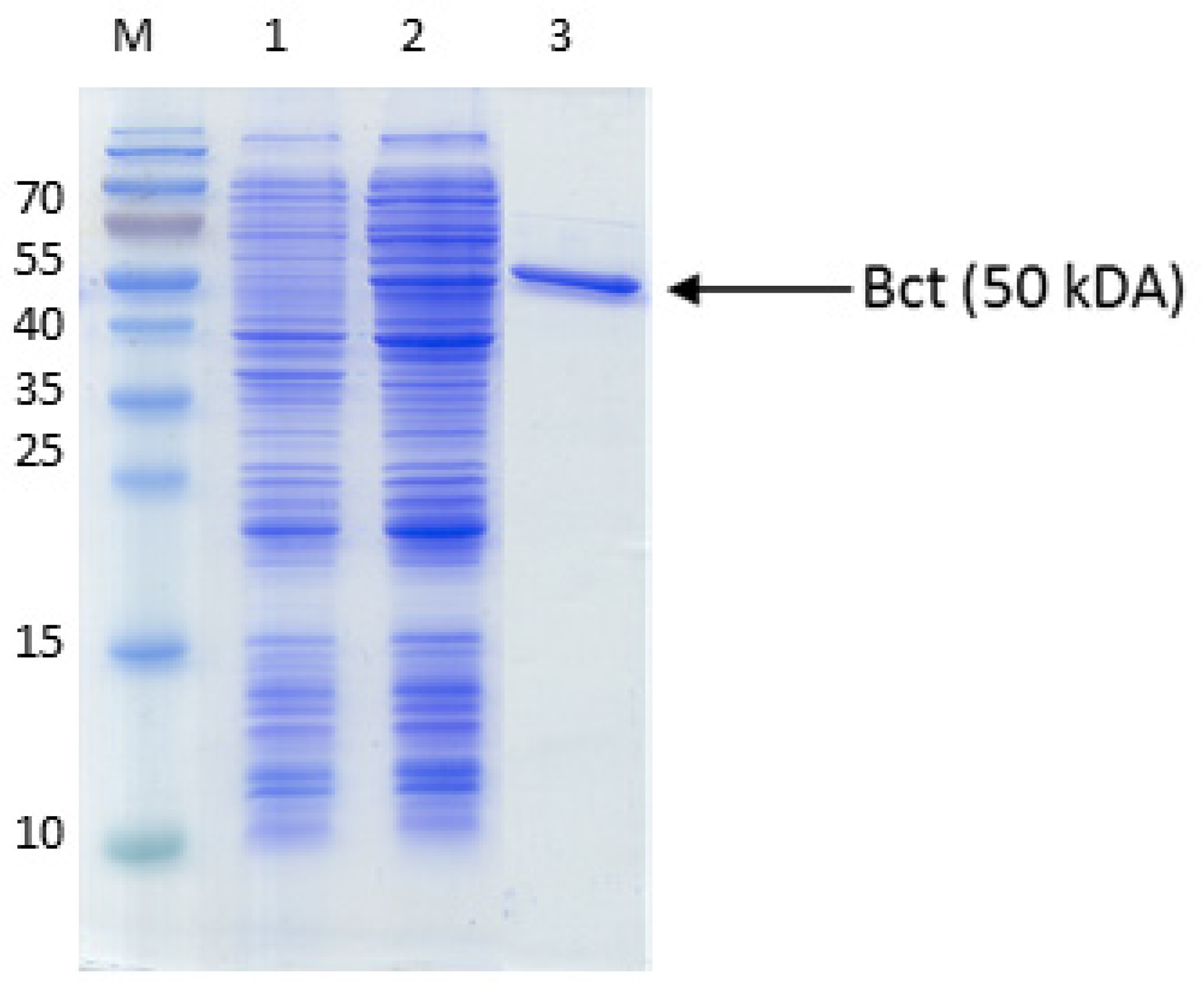
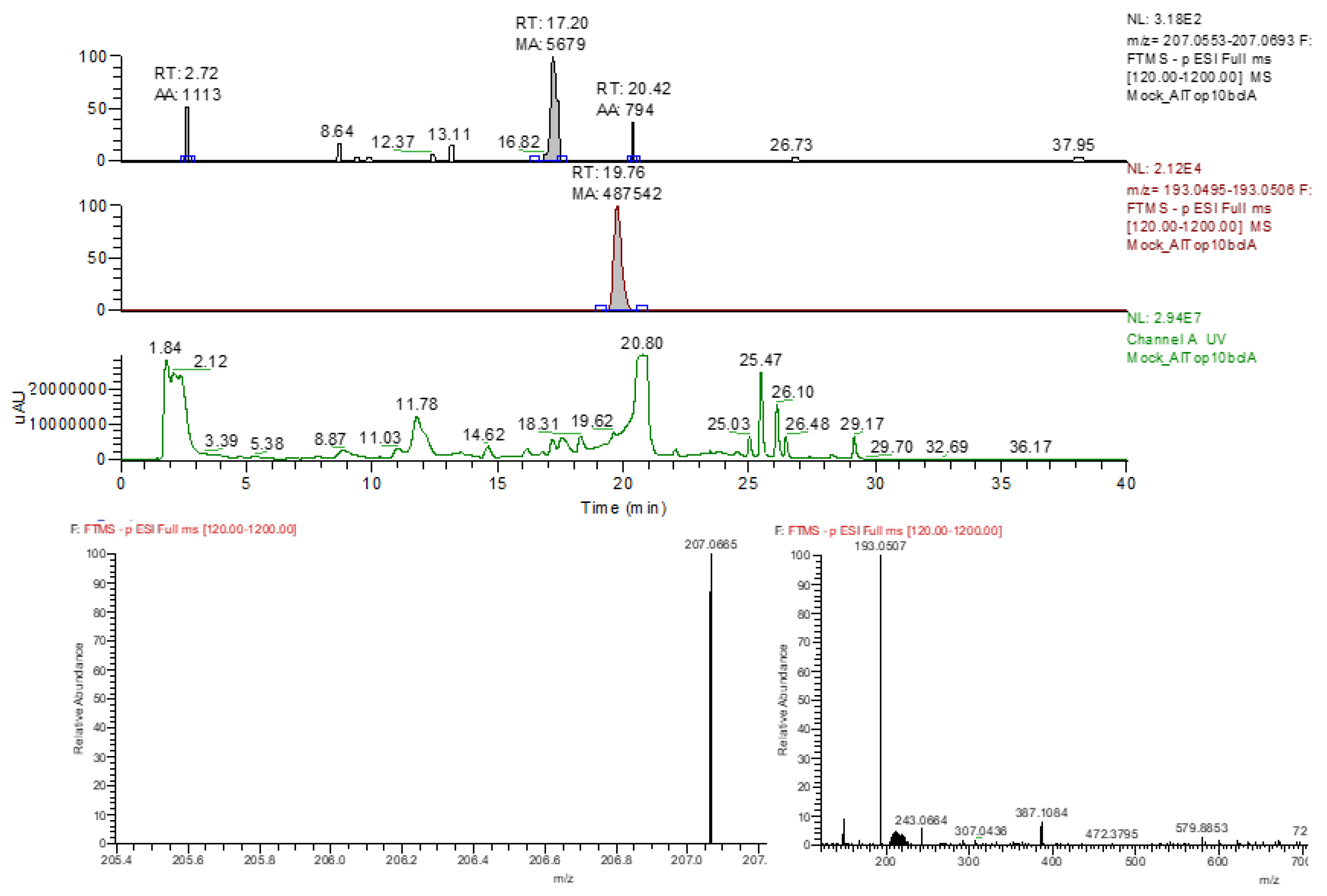
| Primer | amplicon | sequence |
|---|---|---|
| bclA_LguI for | bclA gene | AAGCTCTTCAATGCACACGCTCAGCGCGG |
| bclA_LguI rev | bclA gene | AAGCTCTTCACCCACGCCCGGCACCCTGC |
| benK_LguI for | benK gene | AAGCTCTTCAATGCGAAAAATCGATGTTCACGAGTTG |
| benK_LguI rev | benK gene | AAGCTCTTCACCCTCGGCGCGATGAACTGG |
| Mscs LguI for | mcsS gene | AAGCTCTTCAATGGAAGATTTGAATGTTGTC |
| Mscs LguI rev | mcsS gene | AAGCTCTTCACCCCGCAGCTTTGTCTTCT |
| Gmet_bct s Lgu | bct gene | AAGCTCTTCAATGAACGTCGTCACAC |
| Gmet_bct as LguI | bct gene | AAGCTCTTCACCCTGTTCAGATTCTGTTCATTCTC |
| Gmet_bbsA | bbsAB or bbsABCD | AAGCTCTTCAATGGCAAAGGAAGAGGTTAAG |
| Gmet_bbsB_as | bbsAB genes | AAGCTCTTCACCCCCACCCCTTTACCAGC |
| Gmet_bbsC_ | bbsCD genes | AAGCTCTTCAATGAAAGACAGAACAGCAC |
| Gmet_bbsD_as | bbsCD or bbsABCD | AAGCTCTTCACCCCCCTGCAAACAGACTTC |
| bbsGmet for | bbs operon | ATGAGTAATACCGGATCGTTATCATATC |
| bbsGmet rev | bbs operon | TCACCCTGCAAACAGACTTCT |
| pBBR for | plasmid pBBR2 | ATCGAATTCCTGCAGCC |
| pBBR rev | plasmid pBBR2 | ATCAAGCTTATCGATACCGTC |
References
- Bullock, T.L.; Branchaud, B.; Remington, S.J. Structure of the Complex of L-Benzylsuccinate with Wheat Serine Carboxypeptidase II at 2.0-Å Resolution. Biochemistry 1994, 33, 11127–11134. [CrossRef]
- Byers, L.D.; Wolfenden, R. Binding of the By-Product Analog Benzylsuccinic Acid by Carboxypeptidase A. Biochemistry 1973, 12, 2070–2078. [CrossRef]
- Biegert, T.; Fuchs, G.; Heider, J. Evidence That Anaerobic Oxidation of Toluene in the Denitrifying Bacterium Thauera Aromatica Is Initiated by Formation of Benzylsuccinate from Toluene and Fumarate. Eur. J. Biochem. 1996, 238, 661–668. [CrossRef]
- Heider, J.; Spormann, A.M.; Beller, H.R.; Widdel, F. Anaerobic Bacterial Metabolism of Hydrocarbons. FEMS Microbiol. Rev. 1998, 22, 459–473. [CrossRef]
- Beller, H.R.; Spormann, A.M. Anaerobic Activation of Toluene and o-Xylene by Addition to Fumarate in Denitrifying Strain T. J. Bacteriol. 1997, 179, 670–676. [CrossRef]
- Reusser, D.E.; Field, J.A. Determination of Benzylsuccinic Acid in Gasoline-Contaminated Groundwater by Solid-Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2002, 953, 215–225. [CrossRef]
- Alumbaugh, R.E.; Gieg, L.M.; Field, J.A. Determination of Alkylbenzene Metabolites in Groundwater by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2004, 1042, 89–97. [CrossRef]
- Jobelius, C.; Ruth, B.; Griebler, C.; Meckenstock, R.U.; Hollender, J.; Reineke, A.; Frimmel, F.H.; Zwiener, C. Metabolites Indicate Hot Spots of Biodegradation and Biogeochemical Gradients in a High-Resolution Monitoring Well. Environ. Sci. Technol. 2011, 45, 474–481. [CrossRef]
- Migaud, M.E.; Chee-Sanford, J.C.; Tiedje, J.M.; Frost, J.W. Benzylfumaric, Benzylmaleic, and Z- and E-Phenylitaconic Acids: Synthesis, Characterization, and Correlation with a Metabolite Generated by Azoarcus Tolulyticus Tol-4 during Anaerobic Toluene Degradation. Appl. Environ. Microbiol. 1996, 62, 974–978. [CrossRef]
- Phillippe, H.M.; Wargo, K.A. Mitiglinide: A Novel Agent for the Treatment of Type 2 Diabetes Mellitus. Ann. Pharmacother. 2010, 44, 1615–1623. [CrossRef]
- Xu, J.; Guo, B.H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1162. [CrossRef]
- Heider, J.; Szaleniec, M.; Martins, B.M.; Seyhan, D.; Buckel, W.; Golding, B.T. Structure and Function of Benzylsuccinate Synthase and Related Fumarate-Adding Glycyl Radical Enzymes. J. Mol. Microbiol. Biotechnol. 2016, 26, 29–44. [CrossRef]
- Leutwein, C.; Heider, J. Anaerobic Toluene-Catabolic Pathway in Denitrifying Thauera Aromatica : Activation and β-Oxidation of the First Intermediate, (R)-(+)-Benzylsuccinate. Microbiology 1999, 145, 3265–3271. [CrossRef]
- Beller, H.R.; Spormann, A.M. Analysis of the Novel Benzylsuccinate Synthase Reaction for Anaerobic Toluene Activation Based on Structural Studies of the Product. J. Bacteriol. 1998, 180, 5454–5457. [CrossRef]
- Leuthner, B.; Leutwein, C.; Schulz, H.; North, P.; Haehnel, W.; Schiltz, E.; Schägger, H.; Heider, J. Biochemical and Genetic Characterization of Benzylsuccinate Synthase from Thauera Aromatica: A New Glycyl Radical Enzyme Catalysing the First Step in Anaerobic Toluene Metabolism. Mol. Microbiol. 1998, 28, 615–628. [CrossRef]
- Beller, H.R.; Spormann, A.M. Substrate Range of Benzylsuccinate Synthase from Azoarcus Sp. Strain T. FEMS Microbiol. Lett. 1999, 178, 147–153. [CrossRef]
- Qiao, C.; Marsh, E.N.G. Mechanism of Benzylsuccinate Synthase: Stereochemistry of Toluene Addition to Fumarate and Maleate. J. Am. Chem. Soc. 2005, 127, 8608–8609. [CrossRef]
- Seyhan, D.; Friedrich, P.; Szaleniec, M.; Hilberg, M.; Buckel, W.; Golding, B.T.; Heider, J. Elucidating the Stereochemistry of Enzymatic Benzylsuccinate Synthesis with Chirally Labeled Toluene. Angew. Chemie - Int. Ed. 2016, 55, 11664–11667. [CrossRef]
- Szaleniec, M.; Heider, J. Modeling of the Reaction Mechanism of Enzymatic Radical C–C Coupling by Benzylsuccinate Synthase. Int. J. Mol. Sci. 2016, 17, 514. [CrossRef]
- Funk, M.A.; Judd, E.T.; Marsh, E.N.G.; Elliott, S.J.; Drennan, C.L. Structures of Benzylsuccinate Synthase Elucidate Roles of Accessory Subunits in Glycyl Radical Enzyme Activation and Activity. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 10161–10166. [CrossRef]
- Funk, M.A.; Marsh, E.N.G.; Drennan, C.L. Substrate-Bound Structures of Benzylsuccinate Synthase Reveal How Toluene Is Activated in Anaerobic Hydrocarbon Degradation. J. Biol. Chem. 2015, 290, 22398–22408. [CrossRef]
- Leuthner, B.; Heider, J. Anaerobic Toluene Catabolism of Thauera Aromatica: The Bbs Operon Codes for Enzymes of β Oxidation of the Intermediate Benzylsuccinate. J. Bacteriol. 2000, 182, 272–277. [CrossRef]
- Leutwein, C.; Heider, J. Succinyl-CoA:(R)-Benzylsuccinate CoA-Transferase: An Enzyme of the Anaerobic Toluene Catabolic Pathway in Denitrifying Bacteria. J. Bacteriol. 2001, 183, 4288–4295. [CrossRef]
- Leutwein, C.; Heider, J. (R)-Benzylsuccinyl-CoA Dehydrogenase of Thauera Aromatica, an Enzyme of the Anaerobic Toluene Catabolic Pathway. Arch. Microbiol. 2002, 178, 517–524. [CrossRef]
- von Horsten, S.; Lippert, M.-L.; Geisselbrecht, Y.; Schühle, K.; Schall, I.; Essen, L.O.; Heider, J. Inactive Pseudoenzyme Subunits in Heterotetrameric BbsCD, a Novel Short-Chain Alcohol Dehydrogenase Involved in Anaerobic Toluene Degradation. FEBS J. 2021, 1023–1042. [CrossRef]
- Weidenweber, S.; Schühle, K.; Lippert, M.L.; Mock, J.; Seubert, A.; Demmer, U.; Ermler, U.; Heider, J. Finis Tolueni: A New Type of Thiolase with an Integrated Zn-Finger Subunit Catalyzes the Final Step of Anaerobic Toluene Metabolism. FEBS J. 2022, 289, 5599–5616. [CrossRef]
- Boll, M.; Kung, J.W.; Ermler, U.; Martins, B.M.; Buckel, W. Fermentative Cyclohexane Carboxylate Formation in Syntrophus Aciditrophicus. J. Mol. Microbiol. Biotechnol. 2016, 26, 165–179. [CrossRef]
- Boll, M.; Estelmann, S.; Heider, J. Anaerobic Degradation of Hydrocarbons: Mechanisms of Hydrocarbon Activation in the Absence of Oxygen. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; 2020.
- Himo, F. Catalytic Mechanism of Benzylsuccinate Synthase, a Theoretical Study. J. Phys. Chem. B 2002, 106, 7688–7692. [CrossRef]
- Rabus, R.; Kube, M.; Heider, J.; Beck, A.; Heitmann, K.; Widdel, F.; Reinhardt, R. The Genome Sequence of an Anaerobic Aromatic-Degrading Denitrifying Bacterium, Strain EbN1. Arch. Microbiol. 2005, 183, 27–36. [CrossRef]
- Kim, J.; Darley, D.; Selmer, T.; Buckel, W. Characterization of (R)-2-Hydroxyisocaproate Dehydrogenase and a Family III Coenzyme A Transferase Involved in Reduction of L-Leucine to Isocaproate by Clostridium Difficile. Appl. Environ. Microbiol. 2006, 72, 6062–6069. [CrossRef]
- Salii, I.; Szaleniec, M.; Zein, A.A.; Seyhan, D.; Sekuła, A.; Schühle, K.; Kaplieva-Dudek, I.; Linne, U.; Meckenstock, R.U.; Heider, J. Determinants for Substrate Recognition in the Glycyl Radical Enzyme Benzylsuccinate Synthase Revealed by Targeted Mutagenesis. ACS Catal. 2021, 11, 3361–3370. [CrossRef]
- Schühle, K.; Gescher, J.; Feil, U.; Paul, M.; Jahn, M.; Schägger, H.; Fuchs, G. Benzoate-Coenzyme a Ligase from Thauera Aromatica: An Enzyme Acting in Anaerobic and Aerobic Pathways. J. Bacteriol. 2003, 185, 4920–4929. [CrossRef]
- Heider, J.; Boll, M.; Breese, K.; Breinig, S.; Ebenau-Jehle, C.; Feil, U.; Gad’on, N.; Laempe, D.; Leuthner, B.; Mohamed, M.E.S.; et al. Differential Induction of Enzymes Involved in Anaerobic Metabolism of Aromatic Compounds in the Denitrifying Bacterium Thauera Aromatica. Arch. Microbiol. 1998, 170, 120–131. [CrossRef]
- Oberender, J.; Kung, J.W.; Seifert, J.; von Bergen, M.; Boll, M. Identification and Characterization of a Succinyl-Coenzyme A (CoA): Benzoate CoA Transferase in Geobacter Metallireducens. J. Bacteriol. 2012, 194, 2501–2508. [CrossRef]
- Karapetyan, L.; Pinske, C.; Sawers, G.; Trchounian, A.; Trchounian, K. Influence of C4-Dcu Transporters on Hydrogenase and Formate Dehydrogenase Activities in Stationary Phase-Grown Fermenting Escherichia Coli. IUBMB Life 2020, 72, 1680–1685. [CrossRef]
- Unden, G.; Strecker, A.; Kleefeld, A.; Kim, O. Bin C 4 -Dicarboxylate Utilization in Aerobic and Anaerobic Growth . EcoSal Plus 2016, 7. [CrossRef]
- Schubert, C.; Unden, G. C4-Dicarboxylates as Growth Substrates and Signaling Molecules for Commensal and Pathogenic Enteric Bacteria in Mammalian Intestine. J. Bacteriol. 2022, 204, e0054521. [CrossRef]
- Aklujkar, M.; Krushkal, J.; Dibartolo, G.; Lapidus, A.; Land, M.L.; Lovley, D.R. The Genome Sequence of Geobacter Metallireducens: Features of Metabolism, Physiology and Regulation Common and Dissimilar to Geobacter Sulfurreducens. BMC Microbiol. 2009, 9, 109. [CrossRef]
- Vogt, M.S.; Schühle, K.; Kölzer, S.; Peschke, P.; Chowdhury, N.P.; Kleinsorge, D.; Buckel, W.; Essen, L.O.; Heider, J. Structural and Functional Characterization of an Electron Transfer Flavoprotein Involved in Toluene Degradation in Strictly Anaerobic Bacteria. J. Bacteriol. 2019, 201, e00326-19. [CrossRef]
- Agne, M.; Estelmann, S.; Seelmann, C.S.; Kung, J.; Wilkens, D.; Koch, H.G.; van der Does, C.; Albers, S. V.; von Ballmoos, C.; Simon, J.; et al. The Missing Enzymatic Link in Syntrophic Methane Formation from Fatty Acids. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2111682118. [CrossRef]
- Kovach, M.E.; Phillips, R.W.; Elzer, P.H.; Roop, R.M.; Peterson, K.M. PBBR1MCS: A Broad-Host-Range Cloning Vector. Biotechniques 1994, 16, 800–802.
- Becker, M.; Börngen, K.; Nomura, T.; Battle, A.R.; Marin, K.; Martinac, B.; Krämer, R. Glutamate Efflux Mediated by Corynebacterium Glutamicum MscCG, Escherichia Coli MscS, and Their Derivatives. Biochim. Biophys. Acta - Biomembr. 2013, 1828, 1230–1240. [CrossRef]
- Agne, M.; Seelmann, C.S.; Estelmann, S.; Appel, L.; Kung, J.W.; Boll, M. A Di-Heme FeS Oxidoreductase Involved in Syntrophic Methane Formation and Enoyl-CoA Respiration via Formate Cycling. Biochim. Biophys. Acta - Bioenerg. 2022, 1863, e0374021. [CrossRef]
- Unden, G.; Bongaerts, J. Alternative Respiratory Pathways of Escherichia Coli: Energetics and Transcriptional Regulation in Response to Electron Acceptors. Biochim. Biophys. Acta - Bioenerg. 1997, 1320, 217–234.
- Unden, G.; Kleefeld, A. C 4 -Dicarboxylate Degradation in Aerobic and Anaerobic Growth . EcoSal Plus 2004, 1. [CrossRef]
- Liu, B.; Raeth, T.; Beuerle, T.; Beerhues, L. Biphenyl Synthase, a Novel Type III Polyketide Synthase. Planta 2007, 225, 1495–1503. [CrossRef]
- Busnena, B.A.; Beerhues, L.; Liu, B. Biphenyls and Dibenzofurans of the Rosaceous Subtribe Malinae and Their Role as Phytoalexins. Planta 2023, 258, 78. [CrossRef]
- Janausch, I.G.; Zientz, E.; Tran, Q.H.; Kröger, A.; Unden, G. C4-Dicarboxylate Carriers and Sensors in Bacteria. Biochim. Biophys. Acta - Bioenerg. 2002, 1553, 39–56. [CrossRef]
- Trautwein, K.; Kühner, S.; Wöhlbrand, L.; Halder, T.; Kuchta, K.; Steinbüchel, A.; Rabus, R. Solvent Stress Response of the Denitrifying Bacterium “Aromatoleum Aromaticum” Strain EbN1. Appl. Environ. Microbiol. 2008, 74, 2267–2274. [CrossRef]
- Lu, S.; Eiteman, M.A.; Altman, E. Effect of CO2 on Succinate Production in Dual-Phase Escherichia Coli Fermentations. J. Biotechnol. 2009, 143, 213–223. [CrossRef]
- D’ambrosio, S.; Alfano, A.; Cimini, D. Production of Succinic Acid From Basfia Succiniciproducens. Front. Chem. Eng. 2021, 3, 785691. [CrossRef]
- Thimann, K. V. The Life of Bacteria; Macmilllan: New York, NY, 1955; ISBN 978-0024200808.
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy Conservation in Chemotrophic Anaerobic Bacteria. Bacteriol. Rev. 1977, 41, 100–180. [CrossRef]
- Pinske, C.; Jaroschinsky, M.; Linek, S.; Kelly, C.L.; Sargent, F.; Sawers, R.G. Physiology and Bioenergetics of [NiFe]-Hydrogenase 2-Catalyzed H2-Consuming and H2-Producing Reactions in Escherichia Coli. J. Bacteriol. 2015, 197, 296–306. [CrossRef]
- Sawers, R.G.; Jamieson, D.J.; Higgins, C.F.; Boxer, D.H. Characterization and Physiological Roles of Membrane-Bound Hydrogenase Isoenzymes from Salmonella Typhimurium. J. Bacteriol. 1986, 168, 398–404. [CrossRef]
- Sawers, G.; Heider, J.; Zehelein, E.; Bock, A. Expression and Operon Structure of the Sel Genes of Escherichia Coli and Identification of a Third Selenium-Containing Formate Dehydrogenase Isoenzyme. J. Bacteriol. 1991, 173, 4983–4993. [CrossRef]
- Benoit, S.L.; Maier, R.J.; Sawers, R.G.; Greening, C. Molecular Hydrogen Metabolism: A Widespread Trait of Pathogenic Bacteria and Protists. Microbiol. Mol. Biol. Rev. 2020, 84, e00092-1. [CrossRef]
- Selesi, D.; Jehmlich, N.; Von Bergen, M.; Schmidt, F.; Rattei, T.; Tischler, P.; Lueders, T.; Meckenstock, R.U. Combined Genomic and Proteomic Approaches Identify Gene Clusters Involved in Anaerobic 2-Methylnaphthalene Degradation in the Sulfate-Reducing Enrichment Culture N47. J. Bacteriol. 2010, 192, 295–306. [CrossRef]
- Kallscheuer, N.; Polen, T.; Bott, M.; Marienhagen, J. Reversal of β-Oxidative Pathways for the Microbial Production of Chemicals and Polymer Building Blocks. Metab. Eng. 2017, 42, 33–42. [CrossRef]
- Miller, J.H. A Short Course in Bacterial Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1992; ISBN 0-87969-349-5.
- Studier, F.W. Protein Production by Auto-Induction in High Density Shaking Cultures. Protein Expr. Purif. 2005, 41, 207–234. [CrossRef]
- Begg, Y.A.; Whyte, J.N.; Haddock, B.A. The Identification of Mutants of Escherichia Coli Deficient in Formate Dehydrogenase and Nitrate Reductase Activities Using Dye Indicator Plates. FEMS Microbiol. Lett. 1977, 2, 47–50. [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid Isolation of High Molecular Weight Plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [CrossRef]
- Sambrook J.; E.F., F.; Maniatis T Molecular Cloning: A Laboratory Manual, 2nd Ed., Vols. 1, 2 and 3; old Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 1989; ISBN 0-87969-309-6.
- Inoue, H.; Nojima, H.; Okayama, H. High Efficiency Transformation of Escherichia Coli with Plasmids. Gene 1990, 96, 23–28. [CrossRef]
- Ziegler, K.; Buder, R.; Winter, J.; Fuchs, G. Activation of Aromatic Acids and Aerobic 2-Aminobenzoate Metabolism in a Denitrifying Pseudomonas Strain. Arch. Microbiol. 1989, 151, 171–176. [CrossRef]
- Djurdjevic, I.; Zelder, O.; Buckel, W. Production of Glutaconic Acid in a Recombinant Escherichia Coli Strain. Appl. Environ. Microbiol. 2011, 77, 320–322. [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [CrossRef]
- Howland, J.L. Current Protocols in Protein Science. Biochem. Educ. 1996, 24. [CrossRef]
| Purification step | Volume (ml) | Protein (mg) | Activity (U) | Specific activity (U mg-1) |
Yield | Enrichment |
|---|---|---|---|---|---|---|
| Cell extract | 12 | 365 | 2412 | 6.6 | 100% | 1 |
| (NH4)2SO4-precipitation (33-60 % saturation) | 2.5 | 80 | 1593 | 20 | 66% | 3 |
| PD10 fraction | 2.0 | 58 | 1124 | 19 | 47% | 3 |
| UnoQ fraction | 3.0 | 2.6 | 274 | 107 | 11% | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





