Submitted:
01 December 2023
Posted:
04 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
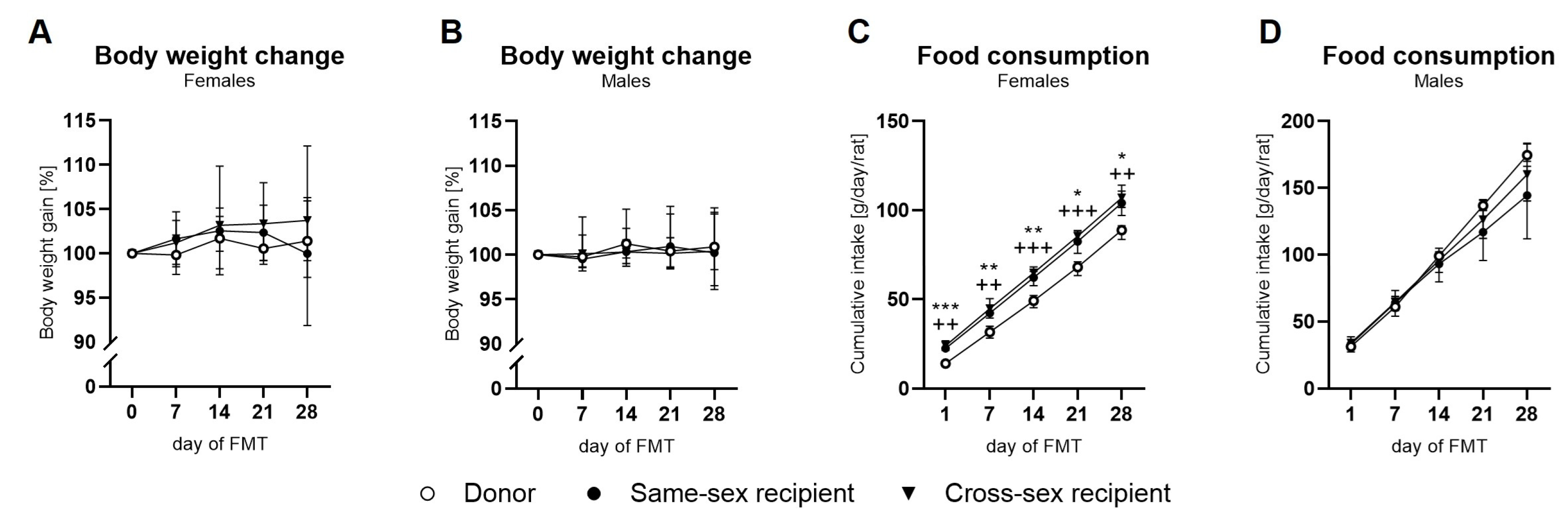
2.1. Body Weight and Food Intake
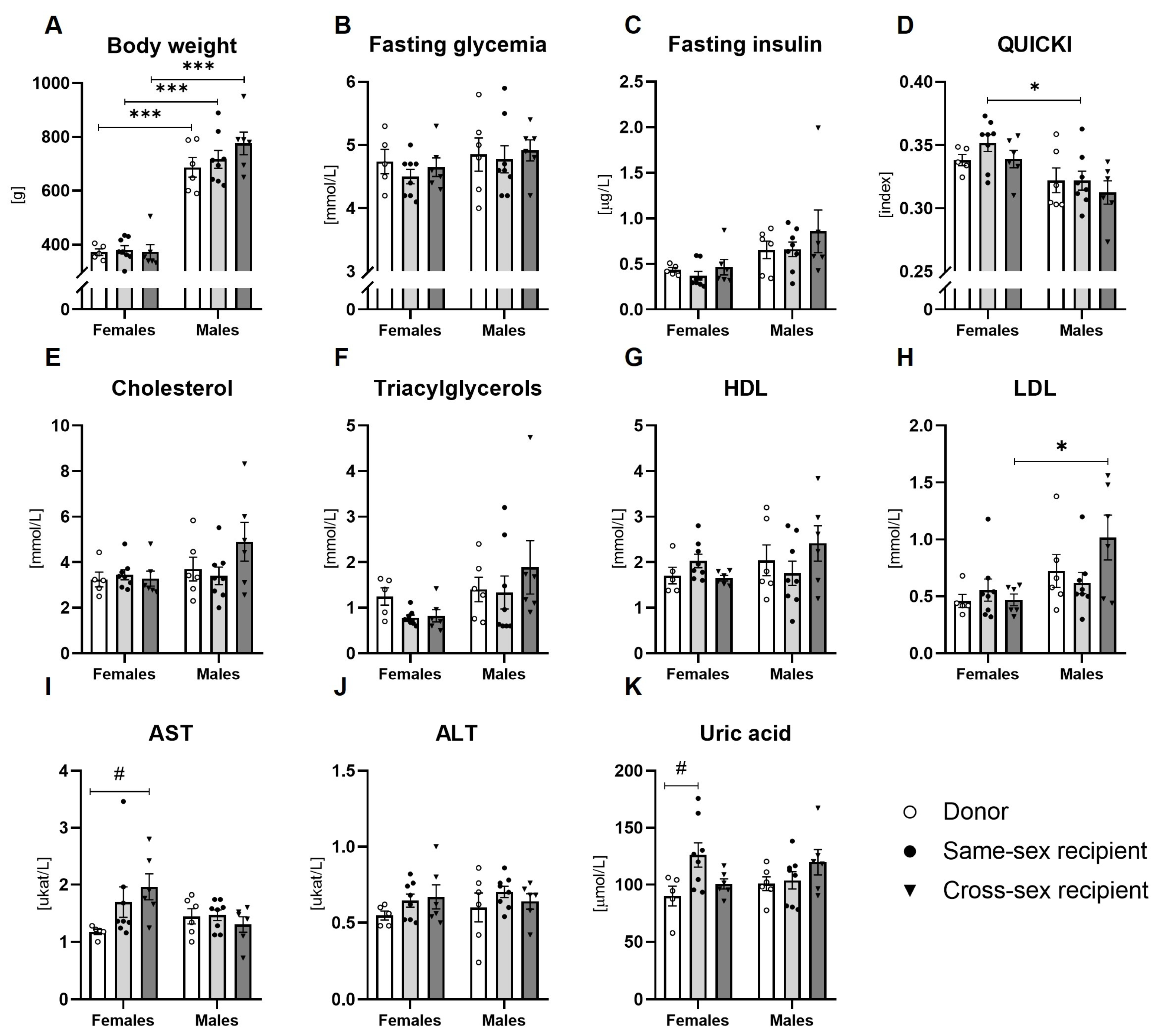
2.2. Metabolic Parameters
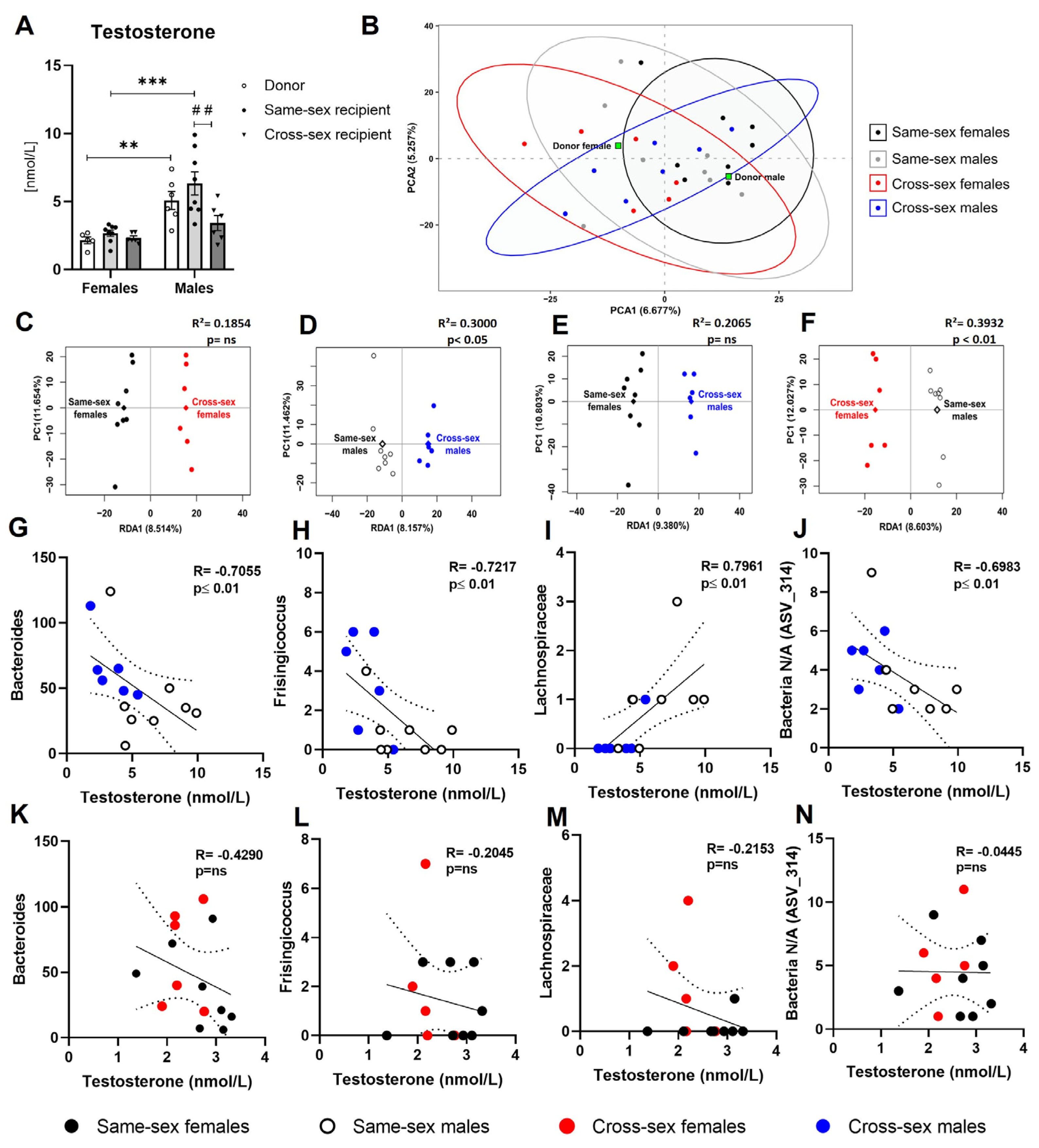
2.3. Testosterone
2.4. Gut Microbiome
3. Discussion
4. Materials and Methods
4.1. Animals
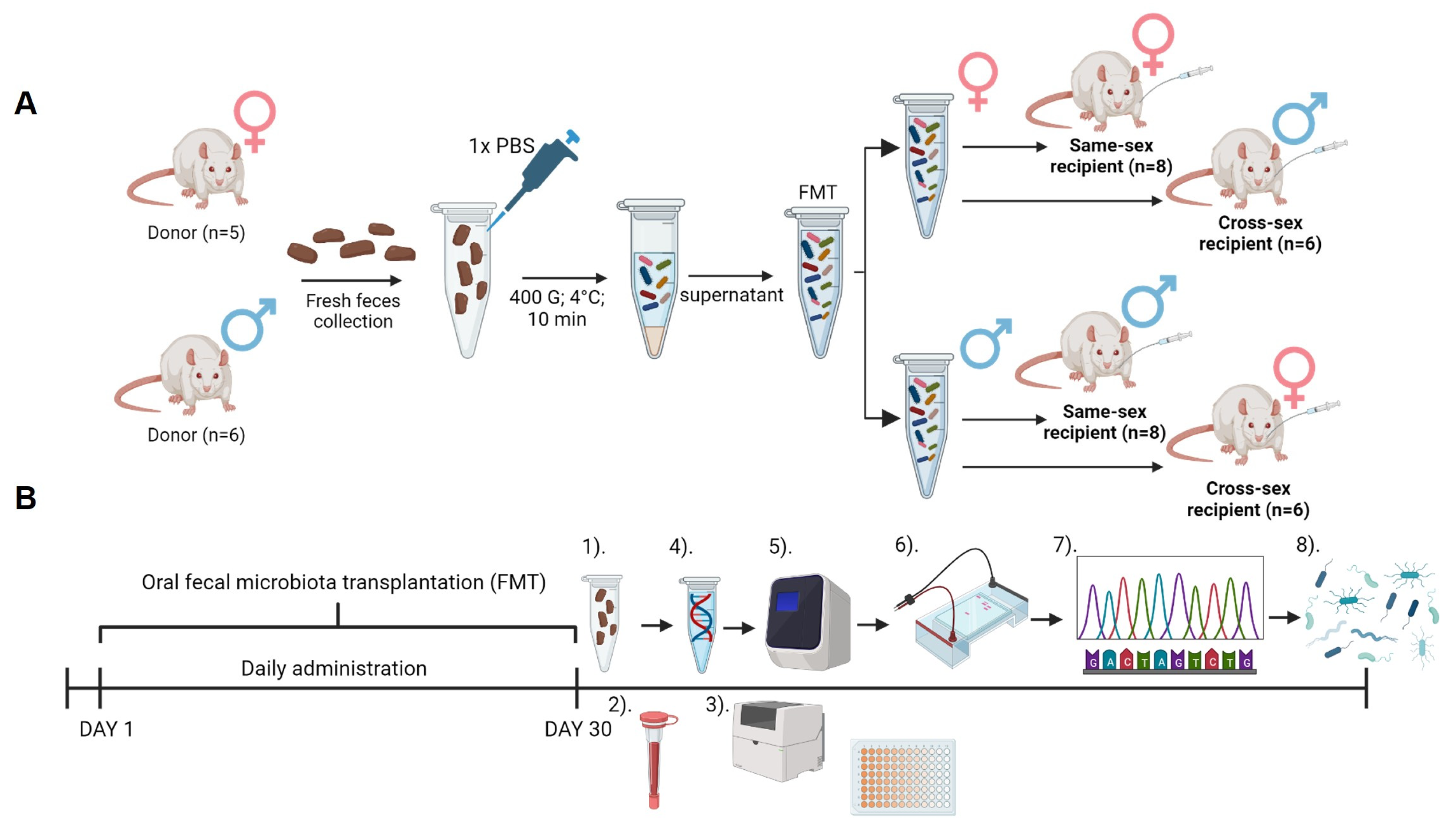
4.2. Experimental Groups
4.3. Fecal Microbiota Transplantation and Recipient Sample Collection
4.4. Blood Analysis
4.5. Microbiome Analysis
4.6. Data Processing and Results Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Apovian, C.M. Obesity: definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–185. [Google Scholar] [PubMed]
- Jaacks, L.M.; Siegel, K.R.; Gujral, U.P.; Narayan, K.M.V. Type 2 diabetes: A 21st century epidemic. Best. Pract. Res. Cl. En. 2016, 30, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex. Differ. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.Y.; Lee, M.S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Tang, X.; Zhang, L.; Wang, L.; He, Y.; Jin, T.; Yuan, D. Age- and sex-related differences in body composition in healthy subjects aged 18 to 82 years. Medicine 2018, 97, e11152. [Google Scholar] [CrossRef]
- Wells, J.C.K. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Mora-Ortiz, M.; Tena-Sempere, M.; Lopez-Miranda, J.; Camargo, A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biology of Sex. Differences 2023, 14. [Google Scholar]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- van Londen, G.J.; Levy, M.E.; Perera, S.; Nelson, J.B.; Greenspan, S.L. Body composition changes during androgen deprivation therapy for prostate cancer: A 2-year prospective study. Crit. Rev. Oncol. Hemat 2008, 68, 172–177. [Google Scholar] [CrossRef]
- Allan, C.A.; Strauss, B.J.; Burger, H.G.; Forbes, E.A.; McLachlan, R.I. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J. Clin. Endocrinol. Metab. 2008, 93, 139–146. [Google Scholar] [CrossRef]
- Chi, J.T.; Lin, P.H.; Tolstikov, V.; Oyekunle, T.; Chen, E.Y.; Bussberg, V.; Greenwood, B.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A.; Freedland, S.J. Metabolomic effects of androgen deprivation therapy treatment for prostate cancer. Cancer Med. 2020, 9, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, M.; van der Poorten, D. The gut microbiome. Aust. Fam. Physician 2017, 46, 206–211. [Google Scholar] [PubMed]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Gao, A.B.; Su, J.L.; Liu, R.X.; Zhao, S.Q.; Li, W.; Xu, X.Q.; Li, D.J.; Shi, J.; Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12. [Google Scholar]
- Shobeiri, P.; Kalantari, A.; Teixeira, A.L.; Rezaei, N. Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol. Sex. Differ. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ko, Y.; Kwak, C.; Yim, E.S. Gender differences in metabolic syndrome components among the Korean 66-year-old population with metabolic syndrome. Bmc Geriatr. 2016, 16. [Google Scholar]
- Wang, W.Y.; Li, C.H.; Wu, Y.S.; Chien, W.C.; Wang, K.Y.; Tzeng, W.C. Gender Differences in the Prevalence of Metabolic Syndrome Among Taiwanese Air Force Personnel A Population-Based Study. J. Cardiovasc. Nurs. 2020, 35, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Chen, Z.P.; Baudier, R.L.; Krousel-Wood, M.; Anderson, A.H.; Fonseca, V.A.; Mauvais-Jarvis, F. Sex Differences in the Progression of Metabolic Risk Factors in Diabetes Development. Jama Netw. Open 2022, 5. [Google Scholar]
- Ramezankhani, A.; Azizi, F.; Hadaegh, F. Gender differences in changes in metabolic syndrome status and its components and risk of cardiovascular disease: a longitudinal cohort study. Cardiovasc. Diabetol. 2022, 21. [Google Scholar]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10. [Google Scholar]
- Aggeletopoulou, I.; Konstantakis, C.; Assimakopoulos, S.F.; Triantos, C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb. Pathog. 2019, 137, 103774. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef]
- Fong, W.N.; Li, Q.; Yu, J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.E.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Strack, C.; Behrens, G.; Sag, S.; Mohr, M.; Zeller, J.; Lahmann, C.; Hubauer, U.; Loew, T.; Maier, L.; Fischer, M.; Baessler, A. Gender differences in cardiometabolic health and disease in a cross-sectional observational obesity study. Biol. Sex. Differ. 2022, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrin 2021, 61. [Google Scholar]
- Diviccaro, S.; Giatti, S.; Borgo, F.; Falvo, E.; Caruso, D.; Garcia-Segura, L.M.; Melcangi, R.C. Steroidogenic machinery in the adult rat colon. J. Steroid Biochem. 2020, 203. [Google Scholar]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.Q.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Motooka, D.; Hatano, K.; Hata, J.; Nishimoto, M.; Banno, E.; Takezawa, K.; Fukuhara, S.; Kiuchi, H.; et al. Firmicutes in Gut Microbiota Correlate with Blood Testosterone Levels in Elderly Men. World J. Mens. Health 2022, 40, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, R.Y.; Liu, L.A.; Lv, Y.Y.; Qi, X.Y.; Yuan, Z.S.; Fan, X.D.; Yu, C.X.; Guan, Q.B. Correlation Between Gut Microbiota and Testosterone in Male Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2022, 13. [Google Scholar]
- Colldén, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fändriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjögren, K.; Vandenput, L.; Ohlsson, C. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. -Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef] [PubMed]
- EU622779.1, G. Uncultured bacterium clone H-243(3) 16S ribosomal RNA gene, complete sequence. Available online: (accessed on.
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Hanada, K.; Minami, Y.; Kitakaze, T.; Ogata, Y.; Tokumoto, H.; Sato, T.; Kato, S.; Inui, H.; Yamaji, R. Role of gut microbiota in sex- and diet-dependent metabolic disorders that lead to early mortality of androgen receptor-deficient male mice. Am. J. Physiol-Endoc M. 2020, 318, E525–E537. [Google Scholar] [CrossRef] [PubMed]
- Hases, L.; Stepanauskaite, L.; Birgersson, M.; Brusselaers, N.; Schuppe-Koistinen, I.; Archer, A.; Engstrand, L.; Williams, C. High-fat diet and estrogen modulate the gut microbiota in a sex-dependent manner in mice. Commun. Biol. 2023, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Guo, Y.; Zhang, S.R.; Chen, Z.H.; Wu, K.Q.; Liu, Q.; Liu, K.J.; Wen, L.Z.; Wei, Y.L.; Wang, B.; Chen, D.F. Fecal Microbiota Transplantation Can Alleviate Gastrointestinal Transit in Rats with High-Fat Diet-Induced Obesity via Regulation of Serotonin Biosynthesis. Biomed. Res. Int. 2018, 2018. [Google Scholar]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.C.; Widmer, A.; Baccigalupi, L.; Ricca, E.; Iossa, S. Rescue of Fructose-Induced Metabolic Syndrome by Antibiotics or Faecal Transplantation in a Rat Model of Obesity. PLoS ONE 2015, 10, e0134893. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Yue, L.Q.; Katz, A.; Quon, M.J. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am. J. Physiol-Endoc M. 2003, 284, E804–E812. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Jansson, J.K.; Knight, R. The Earth Microbiome project: successes and aspirations. Bmc Biol. 2014, 12. [Google Scholar]
- Andrews. FastQC: a quality control tool for high throughput sequence data. Available online: (accessed on.
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011 2011, 17, 3. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wright. Using DECIPHER v2.0 to Analyze Big Biological Sequence Data in R. The R. J. 2016, 8, 352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




