1. Introduction
Catalytic chemical processes are essential to many industries, from pharmaceuticals and materials to energy production. However, the environmental impact and sustainability challenges associated with these processes have become increasingly apparent. Conventional liquid acids and bases, commonly used as catalysts, raise concerns because they are toxic, generate hazardous waste, and consume large amounts of energy. Thus, the demand for sustainable and environmentally friendly chemical processes has propelled the exploration of effective and green alternatives to conventional liquid acids and bases in catalytic transformation[
1,
2,
3,
4]. To tackle these challenges, there has been a significant amount of focus on the green catalytic transformation process. Green catalysis aims to develop clean and sustainable chemical processes that replace or minimize the use of hazardous liquid acids and bases with more eco-friendly alternatives. These alternatives ought to have comparable or superior catalytic performance while reducing their adverse impact on the environment. Green acid-base catalytic transformation is crucial in promoting chemical processes that are sustainable and environmentally friendly. Green acid-base catalytic transformation significantly enables the chemical industry to become more sustainable by focusing on sustainability, minimizing environmental impact, enhancing energy efficiency, and utilizing renewable resources. Green acid-base catalytic transformation offers a more sustainable, efficient process that addresses environmental concerns[
5,
6,
7,
8].
As heterogeneous catalysts, solid acid-base catalysts offer a promising solution for eco-friendly industrial catalysis. Compared to liquid catalysts, solid acid-base catalysts offer stronger stability, easy separation from reaction mixtures, and recyclability. Solid acid-base catalysts, unlike conventional homogeneous ones, provide localized acidic and base sites for catalytic reactions that enhance reaction rates and selectivity, making them essential for eco-friendly processes. In addition, using solid acid-base catalysts promotes sustainable and efficient chemical catalytic transformations, leading to a greener chemical industry[
9,
10]. Among various solid acid-base catalysts, layered double hydroxides (LDHs) have distinctive properties, adjustable acid-base properties, and elevated thermal stability, making them promising for solid acid-base catalysis. LDHs have a unique structure consisting of positively charged metal hydroxide nanosheets, typically with divalent metal cations like Mg
2+, Zn
2+, or Ni
2+, with anions and water molecules between the sheets. Due to this structure, LDHs exhibit dual acid-base properties, which enable LDHs to catalyze a wide range of acid-base catalytic reactions such as esterification, transesterification, aldol condensation, and biomass conversion. The catalytic performance, selectivity, and stability of LDHs can be enhanced by adjusting their composition, structure, and interlayer anions to manipulate their acid-base properties, which makes them a promising option for environmentally friendly acid-base catalytic transformation[
11,
12].
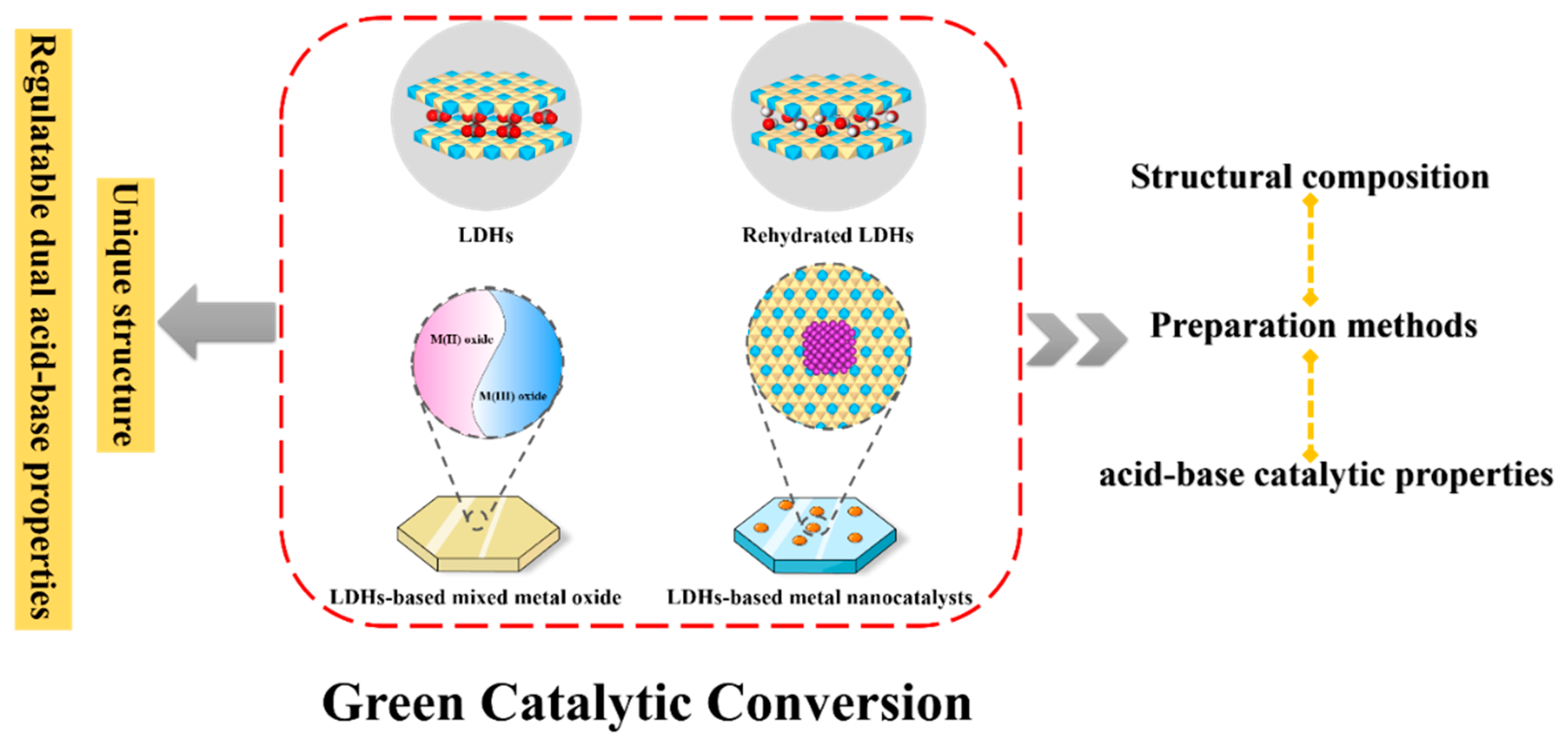
This article aims to review the application of LDHs and their derivatives as solid acid-base catalysts in green catalytic transformation (
Scheme 1). Introducing the structural composition, preparation methods, and acid-base catalytic properties of LDHs-based catalysts aims to clarify their unique properties and application potential for green catalytic transformation. This contribution reviews the application of LDHs and their derivatives as solid acid-base catalysts in acid-base green catalytic conversion, particularly the research progress of LDHs and rehydrated LDHs, LDHs-based metal nanocatalysts, and LDHs-based mixed metal oxide catalysts. The challenges and prospects of LDHs-based catalysts as green and sustainable catalysts are summarized and proposed.
2. Chemical composition, preparation, and acid-base properties of LDHs
2.1. Chemical composition
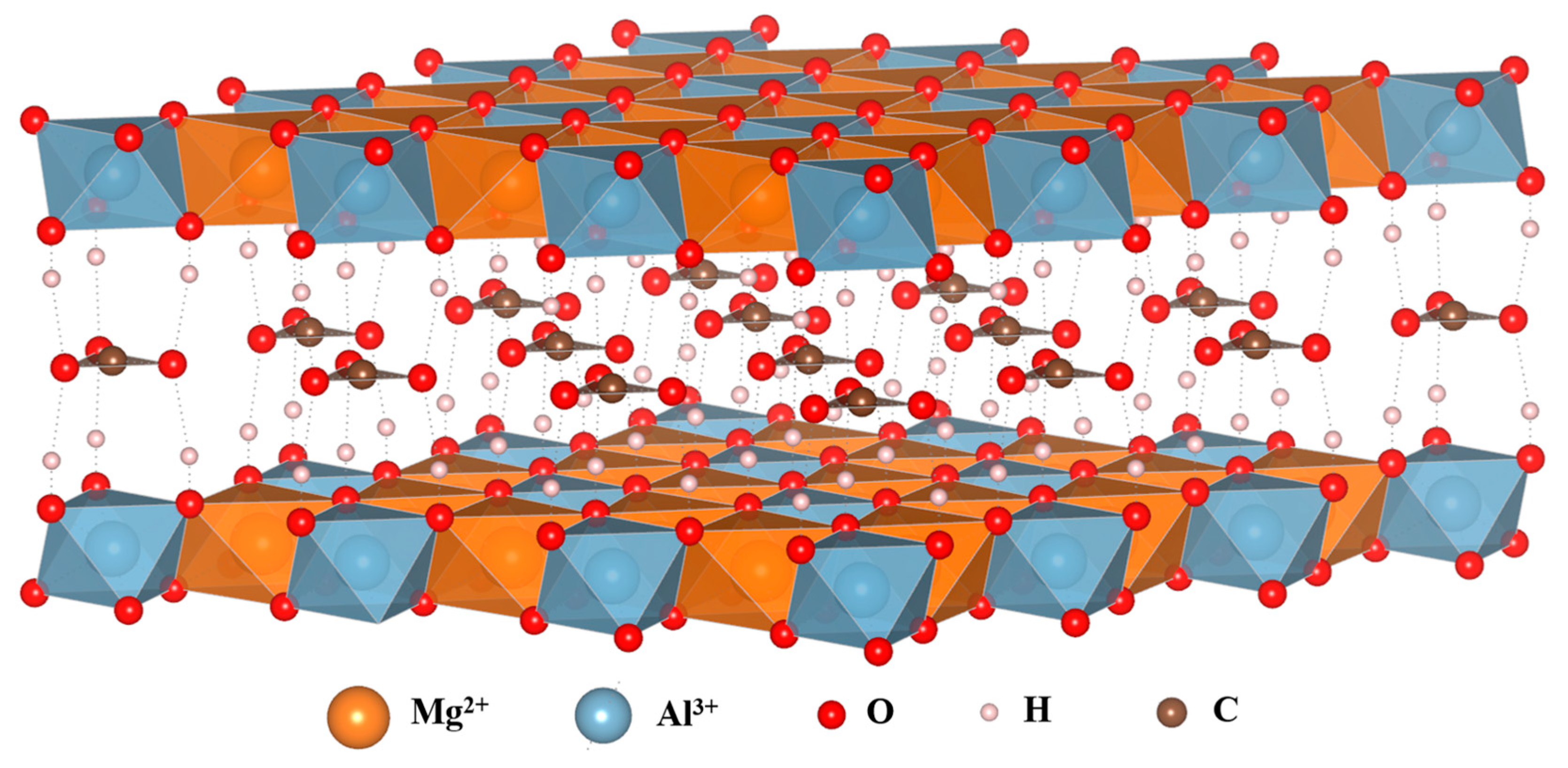
Layered double hydroxides (LDHs) are inorganic materials with positively charged layers of brucite Mg(OH)
2 and interlayer anions to balance the charge. The chemical composition of LDHs can be described as [M(II)
1-xM(III)
x(OH)
2]
x+ (A
n-)
x/n·mH
2O. M(II) and M(III) could be divalent and trivalent metal cations, such as Cu
2+, Mg
2+, Co
2+, Zn
2+, Al
3+, Fe
3+, and Mn
3+. x represents the molar ratio of M
2+/(M
2+ + M
3+), and its value greatly influences the composition and structure of hydrotalcite materials. A
n- could be organic or inorganic anions such as Cl
-, NO
3-, CO
32-, and CH
3COO
-. m represents the number of water molecules. The LDHs comprise a positively charged metal hydroxide layer with intercalated anions and water molecules. The metal cations are octahedrally coordinated by six hydroxide ions, which are tightly packed to form a positively charged metal hydroxide layer. The metal hydroxide layer is typically balanced by the presence of exchangeable interlayer anions[
13,
14,
15].
Figure 1 shows the layered structure of MgAl (2:1) LDHs. In LDHs, -OH groups on lamellae provide Brønsted acid-base sites, and metal cations in the lattice create Lewis acid-base sites. LDHs’ catalytic activity is due to these acid-base sites, which enable them to perform well in green catalytic reactions, such as condensation, hydrogenation, and biomass conversion[
16,
17]. In addition, doping LDHs with transition metal cations introduces additional Lewis acid-base sites that enhance their acid-base and catalytic activities, thus improving their catalytic performance for redox reactions during acid-base catalytic transformations[
18].
2.2. Preparation methods
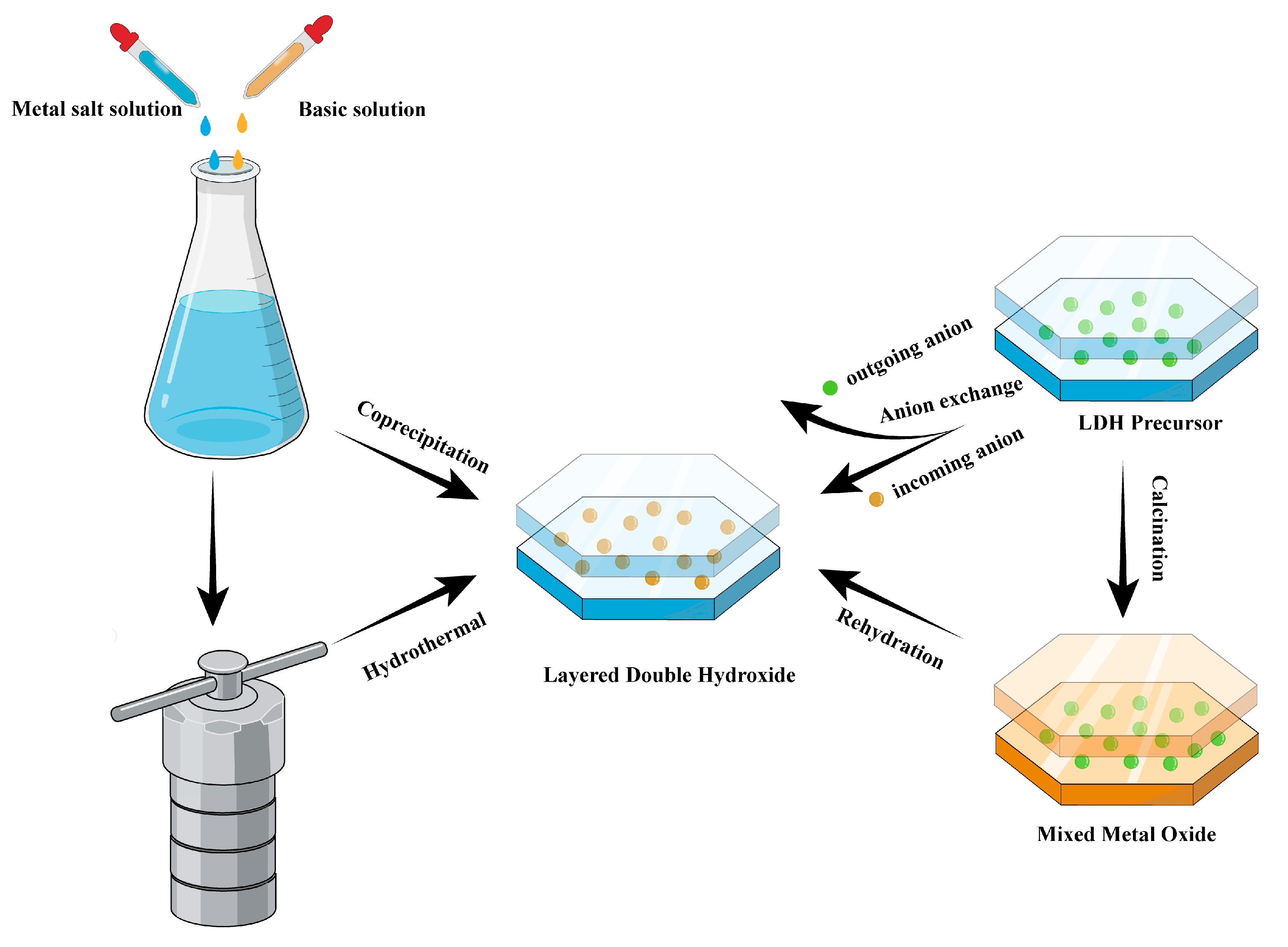
Four commonly used methods for preparing LDHs are illustrated schematically in
Figure 2. Firstly, the coprecipitation method involves the simultaneous precipitation of aqueous solutions containing metal salts under controlled pH conditions. Secondly, the ion exchange method replaces the initial interlayer anions and intercalates the desired anions into the pre-synthesized LDHs. Thirdly, the hydrothermal method synthesizes LDHs under high temperature and pressure conditions in an aqueous medium. Lastly, the rehydration method is the structural reconstruction after calcination.
The coprecipitation method is commonly used to synthesize LDHs due to its simplicity and easy scalability. Conventional coprecipitation synthesis involves the simultaneous precipitation of metal salt solutions with suitable divalent and trivalent metal cation proportions under saturated conditions by adjusting the pH of the solutions[
19]. When the hexahydrate metal complexes condense in an aqueous solution, they form uniformly distributed metal cations and interlayer anions, resulting in a hydromagnesite-like layer[
20]. This method enables a more efficient and straightforward synthesis of LDHs and their composites. The particle size, composition, and incorporation of interlayer anions can be controlled by adjusting pH, reactant concentration, and reaction temperature. Using the traditional coprecipitation method, Panda and colleagues synthesized multiple MgAl LDHs by systematically varying the molar concentration of cations, aging time, and pH. Mixing magnesium and aluminum nitrate solutions with sodium carbonate solution and maintaining the pH at different values using sodium hydroxide resulted in MgAl LDHs with different growth rates[
21]. Seftel et al. used a coprecipitation method to prepare various ZnAl LDHs with different Zn/Al ratios, and the photocatalytic activity was evaluated for the degradation of the methyl-orange dye. The photocatalytic activity increases with the increase of the cationic ratio and the calcination temperature[
22].
The anion exchange method is also referred to as indirect synthesis. Typically, the precursor of LDHs is immersed in a solution with the desired anion in an inert environment. The ion exchange process takes place, leading to the formation of new LDHs that possess different interlayer anions. The ion exchange procedure relies on the electrostatic force, as the hydroxide layer carries a positive charge, and the interlayer area contains an abundance of anions[
23]. This method provides precise control over interlayer anion composition and offers an alternative synthetic route for difficult-to-synthesize LDHs due to large anion size. Zou et al. exchanged CO
32- with OH
- in an alkaline solution to produce modified NiCo-OH LDHs, which was used as nanoarrays electrode and showed improved cycling stability and rate capacity[
24]. Das et al. used an indirect intercalation method to intercalate heteropoly acids (molybdophosphoric acid and tungstophosphoric acid) into Zn–Al hydrotalcite-like compounds to increase the acid sites on the catalysts’ surface. The intercalated catalysts showed improved catalytic activity when used in the liquid phase esterification of acetic acid and n-butanol[
25].
The hydrothermal method involves mixing metal salt solution, alkali solution, and interlayer anions in a stainless steel closed reactor. This mixture is then heated under self-generated pressure, which promotes the nucleation and growth of LDH crystals. Urea is usually added in hydrothermal processes to improve crystallinity and form stable ultra-thin structures[
26]. This method necessitates specialized equipment and a longer reaction time than other methods. However, this process enables the creation of well-crystallized LDHs with controllable particle size and interlayer spacing and enhanced catalytic activity. Ultrathin Ni-Al LDH nanosheets were prepared by simple hydrothermal method using urea as an auxiliary agent. Ni-Al LDH nanosheets exhibited an ultrathin structure, high surface area, and excellent electrochemical properties as supercapacitor electrodes[
27]. Jiang et al. fabricated NiCo LDHs loaded on carbon fiber under hydrothermal conditions using carbon fibers as the substrate material and hexamethylenetetramine (HMT) as the hydrolyzing agent[
28].
The reconstruction and rehydration method is also known as the memory effect method. Typically, LDHs are calcined at 400-500°C to obtain the corresponding composite metal oxides. Then, composite metal oxides are regenerated by mixing with water or other anionic solutions and rehydrating under an inert atmosphere to restore the original structure of LDHs. The selection of calcination temperature is crucial, as high temperatures can affect the structural recovery of LDHs[
29]. Dubnova et al. converted ZnAl LDHs into composite metal oxides by heat treatment at 400°C and then structurally reconstructed them in distilled water under an N
2 atmosphere. The reconstructed LDH catalysts exhibit high crystallinity and numerous base sites, resulting in remarkable catalytic activity toward furfural aldol condensation reactions[
30].
In comparison, the coprecipitation method is the most widely used and provides higher yields, and the anion exchange method favors the synthesis of LDHs with specific and large-size anions. The hydrothermal method produces higher-quality LDH crystals, while the rehydration method results in lower-quality crystallization. Thus, the selection of a suitable synthesis method will vary based on the catalyst’s particular demands and the intended application of the catalyst.
2.3. Acid-base properties
Solid acid-base catalysts are widely used in many green acid-base catalytic reactions, and their high acid-base activity is essential for achieving superior selectivity and conversion rates. Therefore, it is necessary to approach rational design from the perspective of acid-base properties. LDHs exhibit Lewis and Brønsted acid-base properties due to their unique structures and compositions. LDHs and their derived catalysts can be applied in various green catalytic reactions by adjusting the intensity and concentration of their acid and base sites on the catalysts’ surface.
Differences in acidity and alkalinity of LDHs arise from different parts of their structure. Lewis acidity and alkalinity of LDHs are attributed to divalent metal cations located in their metal hydroxide layers. These metal cations, including Mg
2+, Zn
2+, and Ni
2+, have partially filled d orbitals that can accept electron pairs. Lewis acid-base sites in LDHs facilitate acid-base catalyzed reactions by interacting with electron-rich species, such as lone-pair electrons or π-bonding of reactant molecules. Lewis acid-base sites enhance the catalytic activity of LDHs by facilitating bond cleavage, isomerization, and other transformations. The selectivity of catalytic reactions can be influenced by the Lewis acidity and basicity of LDHs, which provide specific binding sites and control reaction pathways. For example, stronger Lewis acid-base sites can interact preferentially with certain functional groups in reactants, leading to specific product formation. Therefore, the Lewis acidity and alkalinity of LDHs can be adjusted to enhance selectivity for the desired product. Maria et al. discovered that the amount of acid-base sites in MgAl LDHs can be tailored by adjusting the Al
3+ content. The decrease in Al
3+ content and the corresponding increase of Mg
2+ led to the increase in the base sites and the simultaneous decrease in acid sites[
31].
The Brønsted acidity and alkalinity of LDHs arise from the hydroxide ions (OH-) in the metal hydroxide layer and interlayer anions. The catalytic activity of LDHs in proton transfer reactions is enhanced by Brønsted acid-base sites, which are crucial for hydrolysis, esterification, and dehydration. The Brønsted acidity and alkalinity of LDHs can control reaction selectivity by affecting the protonation of specific functional groups and by the reaction kinetics relying on acidity and alkalinity.
Different reactants may show different affinity for Brønsted acid-base sites, leading to selective activation and subsequent transformation. Selectivity for specific products can be modulated by tailoring Brønsted acidity and alkalinity of LDHs. Carolina et al. replaced the carbonate anions in the interlayer of ZnAl LDHs and MgAl LDHs by tungstate anion (WO
42-) and molybdate anion (MoO
42-) as intercalation anions. The resulting modified LDH catalysts were used to catalyze the oxidation reaction of cyclohexanes and exhibited higher catalytic performances than the initial LDHs. The intercalation of WO
42- and MoO
42- enhanced the alkalinity of the original LDHs, resulting in a substantial improvement in the selectivity of cyclohexanone and cyclohexanol production[
32].
The coexistence of both Lewis and Brønsted acid-base sites in LDHs exhibits complementary acid-base properties, enabling them to catalyze various green acid-base catalytic reactions with different mechanisms. The Brønsted acid-base properties of LDHs can also be adjusted by changing the metal ion composition. MgAl LDHs were modified by introducing a rare earth element, Y, and subsequently calcined to produce MgAl mixed oxides, which exhibited relatively well-developed small flake morphology with high surface area and pore volume, exposing more base sites on the catalyst surface and improving the catalytic activity for the aldol condensation reaction[
33].
3. Application of LDH-based catalysts in the green acid-base catalytic transformation
LDHs along with rehydrated LDHs, LDHs-based metal nanocatalysts, and LDHs-based mixed metal oxide catalysts are considered promising catalysts due to their unique structures, excellent stability, and tunable acid-base catalytic properties.
3.1. LDH catalysts
3.1.1. Original LDH catalysts
LDHs are a class of two-dimensional inorganic nanomaterials that are widely applied as catalysts in various acid-base catalytic reactions. Phenol hydroxylation is an essential industrial reaction. Using traditional homogeneous catalysts like Fe
2+ and Co
2+ usually requires harsh reaction conditions, making separation and recovery difficult[
34,
35].
Therefore, as solid acid-base catalysts, LDHs are environmentally friendly and used under moderate reaction conditions, showing significant advantages. Jiang et al. prepared a series of CuZnFeAl-LDH catalysts by a coprecipitation method for phenol hydroxylation. The electron transfer from oxygen vacancies to Cu
2+ on the LDHs surface generates Cu
+, promoting the formation of hydroxyl radicals and enhancing catalytic activity. CuZnFeAl-LDH with 15% copper content (15/CuZnFeAl-LDH) exhibited the optimal conversion of phenol (66.9%) and selectivity of benzene diol (71.3%), which is mainly attributed to the synergistic effect between Cu
+ and oxygen vacancies promoted by acid-base ratio. Moreover, 15/CuZnFeAl-LDH presented stable recyclability under mild conditions (60°C, 1.0 MPa) while also being eco-friendly and energy efficient[
36].
In addition, LDHs can be used directly as solid acid-base catalysts for the catalytic conversion of biomass resources, that is, transformation to high-value-added chemicals and biofuels from sugar, lignocellulosic materials, and other biomass feedstocks. In recent years, there has been significant interest in the electrocatalytic oxidation of aldehydes derived from biomass into valuable acids using LDHs as catalysts. LDHs, with a thin nano lamellar structure, provide a large number of catalytic active sites. Meanwhile, the metal valence state of the cations in LDHs varies by changing the electron layer, which exhibits excellent acid-base catalytic performance. Liu and colleagues studied the electrocatalytic oxidation of biomass-derived aldehydes using ultrathin NiV LDHs for efficient catalysis[
37]. They discovered that ultrathin NiV LDHs with a size of 2.6 nm can expose a larger surface area with rich metal active sites, which facilitates the adsorption of aldehydes on the catalyst surface and the generation of hydroxyl radicals, resulting in superior electrocatalytic performance. The generated hydroxyl radicals can favorably promote the conversion of aldehyde to carboxyl groups and generally apply to different biomass-derived benzylic aldehydes and furan aldehydes.
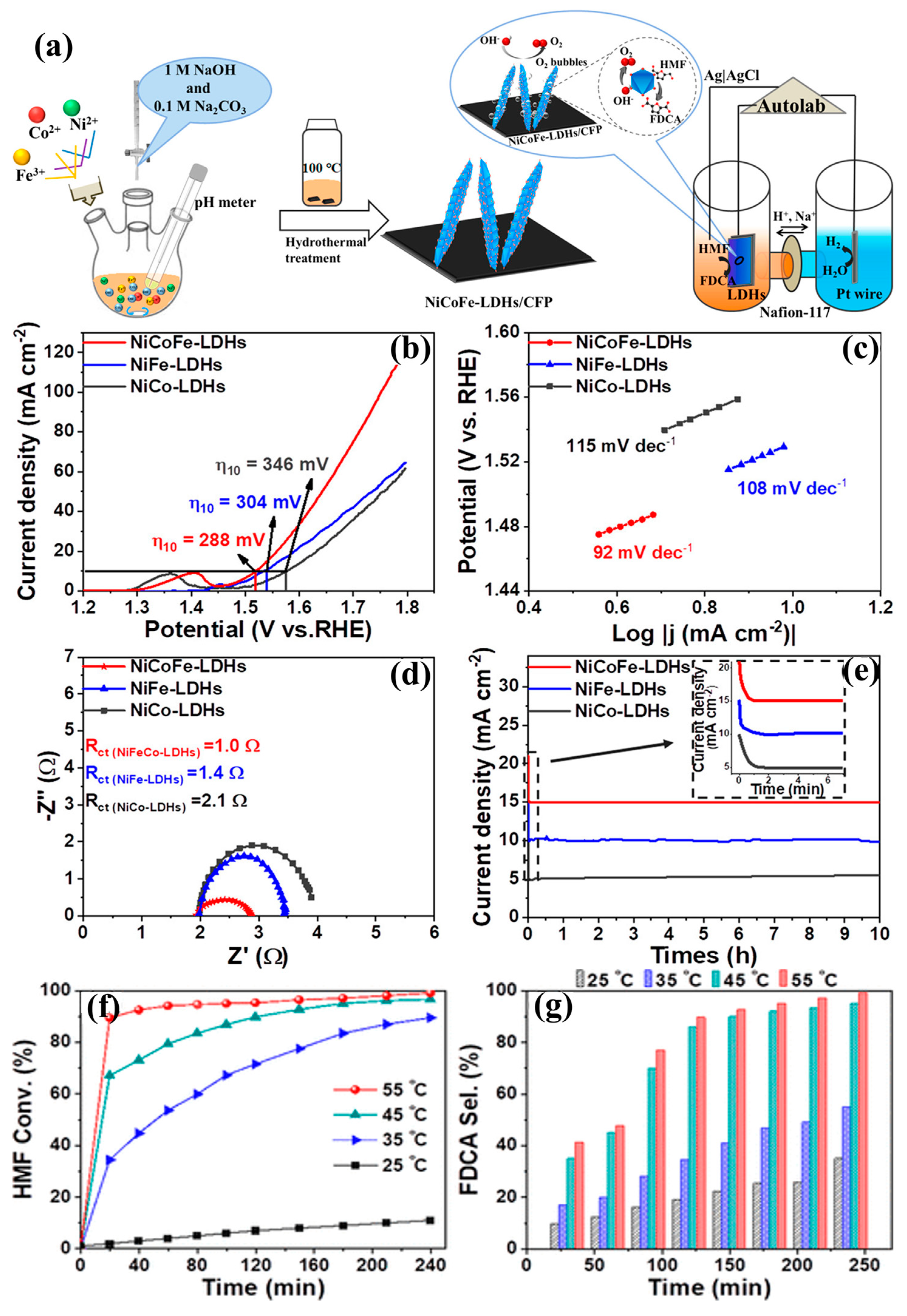
Zhang et al. synthesized trimetallic NiCoFe LDHs with a one-step controllable synthesis for both an efficient oxygen evolution reaction (OER) and the highly selective oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) into value-added 2,5-furan-dicarboxylic acid (FDCA), which is shown in
Figure 3[
38]. In the OER study, the NiCoFe LDHs showed superior performance compared to previously reported LDH catalysts. They exhibited the lowest necessary overpotential (288 mV), a much smaller charge transfer resistance (1.0 Ω), and a larger Cdl value of 2.62 mF cm
−2, indicating low charge transfer resistance and fast catalytic kinetics of oxygen evolution. It was discovered that the introduction of Fe
3+ led to increased active sites and enhanced OER activity of NiCoFe-LDHs compared to NiCo-LDHs. NiCoFe LDHs exhibited excellent performance, with up to 95% HMF conversion and 84.9% FDCA selectivity in 1 hour during the oxidation reaction of HMF. HMF oxidation is more likely to occur at lower potentials due to the rich active metal sites on the thin layer of NiCoFe LDHs. Additionally, the introduced Fe
3+ leads to electronic synergistic effects with Ni
2+ and Co
2+, enhancing the electrocatalytic oxidation of HMF. This study provides an effective method for converting biomass-derived chemicals into value-added products without using noble metal-containing catalysts.
In addition, LDHs catalysts are widely used in thermal catalysis to convert biomass derivatives into value-added products. Wang et al. investigated the catalytic transfer hydrogenation of furfural to furfuryl alcohol by NiFe LDHs using 2-propanol as a hydrogen donor[
39]. LDHs with a Ni/Fe molar ratio of 3:1 demonstrated the best catalytic activity, which was attributable to the synergistic effect of acidic and base sites. At 140°C for 5 h, it converted 97.0% of furfural and yielded 90.2% furfuryl alcohol. First, 2-propanol was absorbed on NiFe LDHs and dissociated into alkoxide and protons by interacting with acid sites (attributing to Ni
2+ and Fe
3+) and base sites (attributing to OH
-). Then, the acidic site may activate the carbonyl group of furfural and form a transitional state with six links, leading to the production of free furfuryl alcohol and acetone through hydrogen transfer. Moreover, this catalyst applies to catalytic hydrogenation reactions of various aldehydes and ketones.
In addition to catalyzing the conversion of biomass-derived aldehydes, LDHs are also widely utilized as solid acid-base catalysts for transforming biomass-derived sugars. A series of MgAl LDHs catalysts were synthesized by Ye et al. and applied to the conversion of glucose/food waste to methyl lactate (MLA) without homogeneous alkalines[
40]. MgAl LDHs with Mg/Al (5:1) showed the largest interlayer distance and the highest density of basic site, resulting in superior catalytic activity. Due to the in-situ reconstruction, the reused MgAl LDHs exhibited higher specific surface area and larger interlayer space, gradually increasing catalytic activity during the recycling test.
The alkalinity of LDHs can be regulated by changing the ratio of metal ions and modifying the interlayer anions. Therefore, LDHs can be designed for the cascade conversion of glucose. However, LDHs catalysts are not commonly used to directly convert glucose to lactic acid because the acidic environment can deactivate the catalyst. Most current research on LDHs catalysts has focused on glucose isomerization for fructose production[
41,
42,
43]. Yu et al. investigated the performance of LDHs as solid-base catalysts for the thermochemical isomerization of biomass-derived glucose to fructose[
41]. The MgAl LDHs were synthesized via conventional urea hydrolysis and coprecipitation methods, followed by aqueous miscible organic solvent treatment. It was found that the treated LDHs exposed more active sites due to their high porosity, and the fructose yield increased with the increasing crystallite size of LDHs. This study presents a methodology to establish the relationship between the structure and catalytic performance of LDHs in thermochemistry. It proposes a practical approach for the tunable structural design of LDHs as solid-base catalysts.This work provides a methodology for establishing the structure-catalytic performance relationship of LDHs in thermochemistry. It elucidates a practical idea for the tunable structural design of LDHs as solid base catalysts.
3.1.2. Rehydrated LDH catalysts
The composite metal oxides obtained by calcination LDHs at a specific temperature possess a sound structure and memory effect. The original structure of LDHs can be restored by doping the corresponding anions (such as OH
-) between the layers of the composite metal oxides. The rehydrated LDHs with numerous Brønsted acid-base sites allow for effective regulation of the density and intensity of base sites, resulting in a notable enhancement of the catalytic performance of the original LDHs catalysts in alkali-catalyzed reactions[
44]. Due to the distinct structural defects generated in the reconstruction process, Rehydrated LDHs can act as carriers by loading metal atoms or clusters onto the cationic vacancies on the surface. The catalytic performance of rehydrated LDHs can be enhanced by modifying their surface structure and creating strong metal-carrier connections through the local electron-transfer effect[
45,
46]. The aldehyde-alcohol condensation reaction is a classic and essential organic synthesis reaction that can produce numerous value-added chemicals for industrial use. One example of such reactions is the condensation of isobutyraldehyde (IBD) and formaldehyde (FA), an industrially significant alkali-catalyzed reaction. Rehydrated LDHs have been widely discussed as solid-base catalysts for aldehyde-alcohol condensation reactions due to environmental pollution and equipment corrosion caused by homogeneous alkali catalysts such as NaOH and KOH[
47,
48].
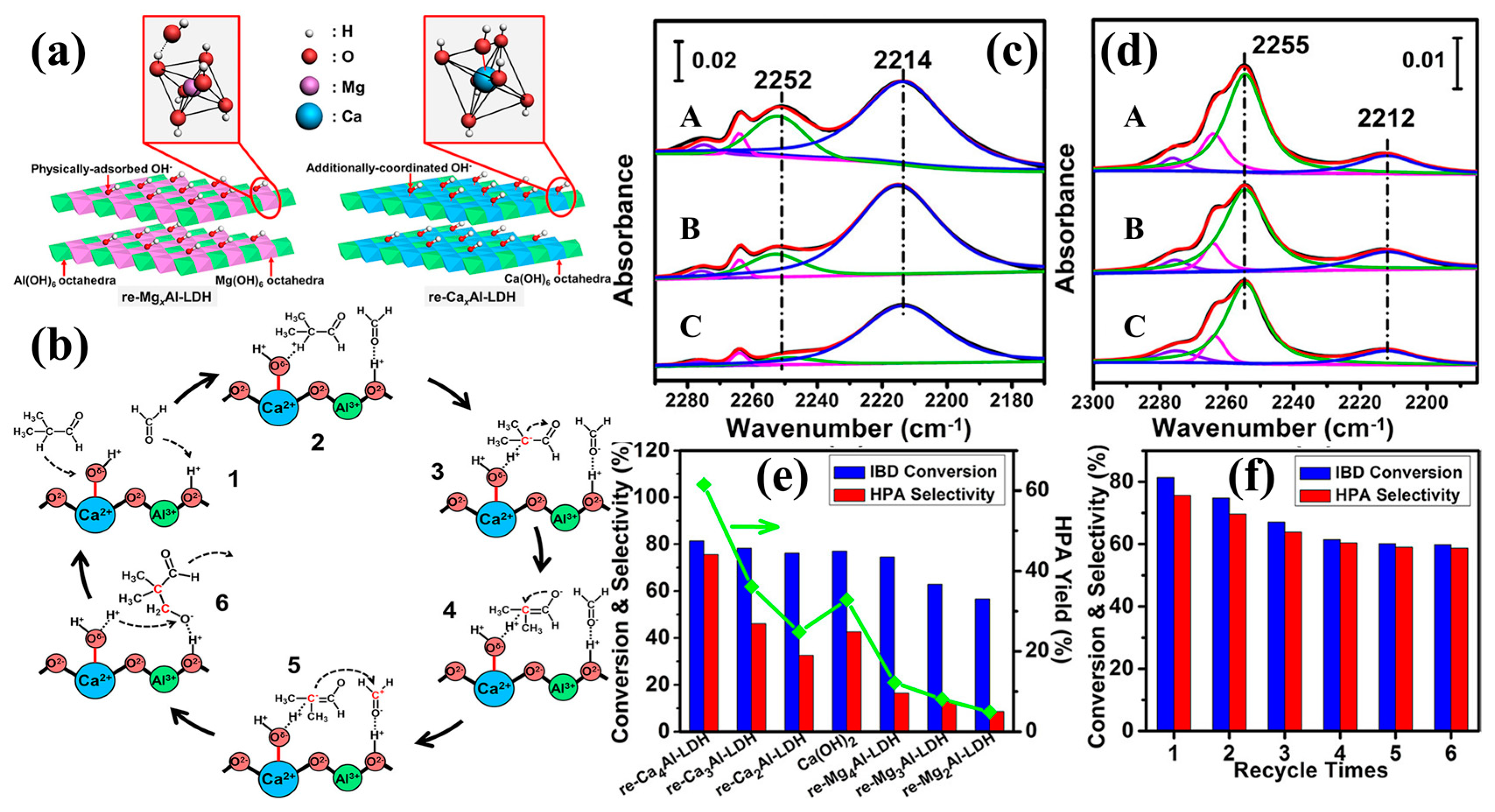
Bing et al. synthesized two rehydrated LDHs catalysts (re-Ca
xAl-LDH and re-Mg
xAl-LDH) and used them for aldol condensation reactions of IBD and FA[
49]. The relevant schematic is presented in
Figure 4. The re-Ca
xAl-LDH host layer was found to contain a distorted Ca(OH)
2 octahedron and has additional Ca−OH coordination, which provides weak Brønsted base sites. On the contrary, re-Mg
xAl-LDH consists of an octahedral structure formed by Mg(OH)
2 and surface-adsorbed hydroxyl group via non-covalent interaction, which provides medium Brønsted base sites. The Ca/Al molar ratio in the LDH precursor can be adjusted to increase the concentration of weak Brønsted base sites for re-Ca
xAl-LDH. The optimized re-Ca
4Al-LDH showed excellent catalytic performance in producing hydroxypivaldehyde (HPA) through the condensation of IBD and FA, with a 61.5% HPA yield. This result is significantly higher than that of re-Mg
xAl-LDH and other solid base catalysts and is comparable to the catalytic results of liquid bases.
To further improve the fine structure of weak Brønsted alkaline sites, Bing et al. prepared rehydrated CaMnAl-LDH (re-Ca
4Mn
xAl-LDH) as a solid base catalyst utilizing Mn as a promoter[
50]. The catalytic performance of the aldol condensation reaction was enhanced by modulating the activity of weak Brønsted base sites by altering the electronic structure of Ca-O-Al with Mn. Compared to re-Ca
4Al-LDH, re-Ca
4Mn
xAl-LDH displayed highly exposed Ca
2+ s-orbital and strengthened coordination between Ca
2+ with 7-fold OH
−, providing weakened Brønsted base sites. The optimized re-Ca
4Mn
xAl-LDH displayed high catalytic performance in the IBD and FA condensation, resulting in the formation of HPA with a yield of 70.3%.
Regarding the isomerization of biomass-derived sugars, the process typically requires catalysis from base sites as well. Kwon et al. investigated the alkaline and catalytic properties of MgAl LDHs prepared by calcination and rehydration as solid base catalysts for the isomerization of glucose to fructose[
51]. It was found that the hydroxyl groups were released from the interlayers of the LDHs precursors under calcination at a specific temperature, giving composite metal oxides. The alkaline properties of the catalysts rely on the calcination temperature. Weak base sites were generally obtained when the LDHs precursors were calcinated at low temperatures. In contrast, strong base sites were obtained when the LDHs precursors were calcinated at relatively high temperatures. Remarkably, the rehydrated MgAl LDHs exhibit both weak and strong base sites, and their base properties could be systematically tuned by heat treatment and rehydration. Therefore, the rehydrated MgAl LDHs can effectively isomerize glucose to fructose because of their appropriate base and structure properties.
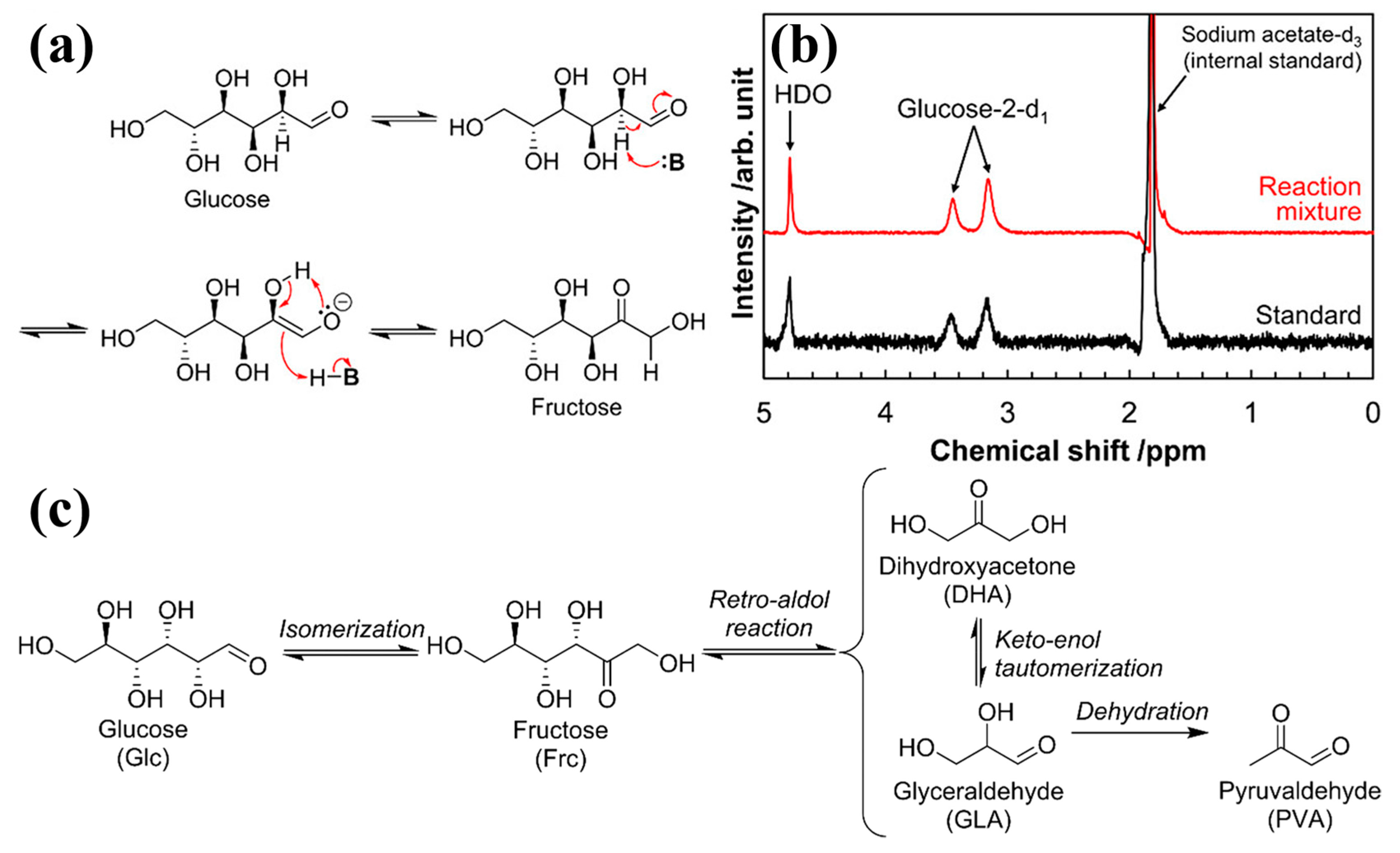
Yabushita et al. studied the effective isomerization of glucose to fructose in ethanol solvent catalyzed by rehydrated MgAl LDHs, giving up to 56% yield with a high selectivity of 80%[
52]. The reaction process and mechanism are shown in
Figure 5. The rehydrated MgAl LDHs were obtained by calcination and subsequent reconstruction of the original layered structure via the memory effect. The mechanistic study demonstrates that the base sites are responsible for the catalysis of glucose-to-fructose isomerization. The rehydrated MgAl LDHs exhibit the highest catalytic performance due to their high density of base sites. An active and selective catalyst for large-scale fructose production as a renewable feedstock could be developed by precisely controlling the strength and density of base sites.
3.2. LDHs-based metal nanocatalysts
It is well known that LDHs can be converted into highly-dispersed active metal nanoparticles by in situ reduction in the H
2 atmosphere and anchored to a mixed metal oxide matrix, giving supported metal catalysts. The most attractive characteristic of this structure topotactic transformation process is the facile tuning over metal-support interactions, such as electronic metal-support interaction and metal/support acid-base interaction. The quantity and strength of the acid-base structure of supports can be efficiently modified by precisely controlling the topotactic transformation parameters of LDH precursors, such as reduction atmosphere, temperature, and heating rate. Moreover, the reduction temperature has a significant impact on the size and catalytic performance of metal nanoparticles. Increasing the reduction temperature leads to more metal nanoparticles and larger particle sizes while also altering their catalytic performance[
53].
Cui et al. reported the synthesis of Cu-based nanoparticle catalysts by calcining CuMgAl LDHs precursors to obtain mixed metal oxides, followed by the reduction treatment process[
54]. The resulting Cu-based nanoparticle catalysts (Cu/MMO) exhibited high dispersibility of 6-9 nm Cu nanoparticles and configurable acid-base sites on the support (MgO and Al
2O
3). The Cu-based nanoparticle catalysts were applied for low-temperature hydrogenation of dimethyl oxalate (DMO) to ethylene glycol, and the optimal catalyst Cu/MMO-S3 resulted in an excellent catalytic performance of 98.2% conversion and selectivity of 96.1%. The results indicated that the Lewis acid sites (Al
3+) and medium-strong base sites (Mg
2+−O
2− pair) of supports act as active sites for the adsorption of polarized C=O/C−O group in the DMO molecule, while H
2 undergoes dissociation adsorption on the Cu
0 site. The unique ternary synergistic catalysis of Cu and acid-base sites makes a predominant contribution to the remarkable catalytic performance toward DMO hydrogenation to ethylene glycol.
This LDHs-derived metal nanocatalyst is also widely used in hydrogenolysis and hydrogenation reactions of biomass-derived aldehydes. As a building block, furfural (FF) offers a promising and rich platform for lignocellulosic biofuels and value-added chemicals. It stands out as a bridge connecting biomass resources and the chemical industry. It is well known that the C=O bond in furfural molecules can interact with the base sites on the catalyst surface to promote the catalytic conversion performance[
55,
56]. The LDHs-based metal nanocatalysts possess abundant acid sites, base sites, and metal active sites on the surface, which provide high catalytic performance for furfural hydrogenation. Yang et al. reported three non-precious intermetallic compounds (Ni
3Sn
1, Ni
3Sn
2, and Ni
3Sn
4) derived from LDHs precursors[
57]. These compounds showed a highly uniform dispersion of metal nanoparticles, and they exhibited surprisingly improved catalytic performance toward furfural selective hydrogenation (C=O) to furfuryl alcohol. Notably, Ni
3Sn
2 nanocatalysts showed the best catalytic performance with 100% conversion of FF and 99% selectivity of furfuryl alcohol at 100 °C under 2 MPa H
2. The research results verify that the electron transfer from Sn to Ni promoted the activation adsorption of C=O bonds on Ni top site while inhibiting the adsorption of C=C bonds.
Zhu and colleagues synthesized nano-twin Cu particles with rich defects via topotactic transformation of LDH precursors. These particles effectively enhanced the target activation of C-O and C=O bonds, facilitating the conversion of furfural to cyclopentanone[
58]. Compared with regular spherical Cu nanoparticles, nano-twin Cu particles showed higher conversion kinetics with close to 100% furfural conversion and up to 92% selectivity of cyclopentanone. Producing cyclopentanone (CPO) starts with the hydrogenation of C=O on metal sites, which results in furfuryl alcohol (FA). FA then undergoes a rearrangement on acid sites, forming 4-hydroxy-2-cyclopentenone (HCP). The C-OH in HCP is then selectively hydro-deoxygenated and hydrogenated to C=C on metal sites, resulting in CPO. Therefore, the multi-stepped surface defects of nano-twin Cu particles provided abundant acid sites and metal sites, which promoted the selective hydrogenation conversion of FF to CPO.
5-hydroxymethylfurfural (HMF) is also a particularly promising biomass-based intermediate due to its wide resource of fructose, glucose, and cellulose. Also, because of its versatile reactive functional groups to convert into value-added chemicals by selective hydrogenation, notably 2,5-dimethylfuran (DMF) and 2,5-bis(hydroxymethyl)furan (DHMF)[
59,
60]. Similar to the catalytic hydrogenation of furfuryl, the LDHs-based metal catalysts can effectively catalyze the hydrodeoxygenation of HMF.
Wang et al. constructed a highly efficient and selective Cu/ZnO-Al
2O
3 catalyst via in situ reduction of a CuZnAl-LDHs precursor[
61]. They studied the impact of Cu
0/Cu
+ species on the formation of various intermediates and their synergistic promoting effect on HMF hydrodeoxygenation. The formation of different intermediates depends on the Cu
+/Cu
0 ratio. Cu/ZnO-Al
2O
3 with a high Cu
+/Cu
0 ratio showed higher DMF selectivity (90.1%) at 100% HMF conversion than Cu/MgO-Al
2O
3. During the conversion process, Cu
0 was preferable to adsorb the C=O bond and the hydrogen molecule, while the Cu
+ site selectively absorbed and activated the C−O bond in the hydroxymethyl group. With the synergy of dual active sites, the Cu/ZnO-Al
2O
3 catalyst showed high activity and selectivity to DMF.
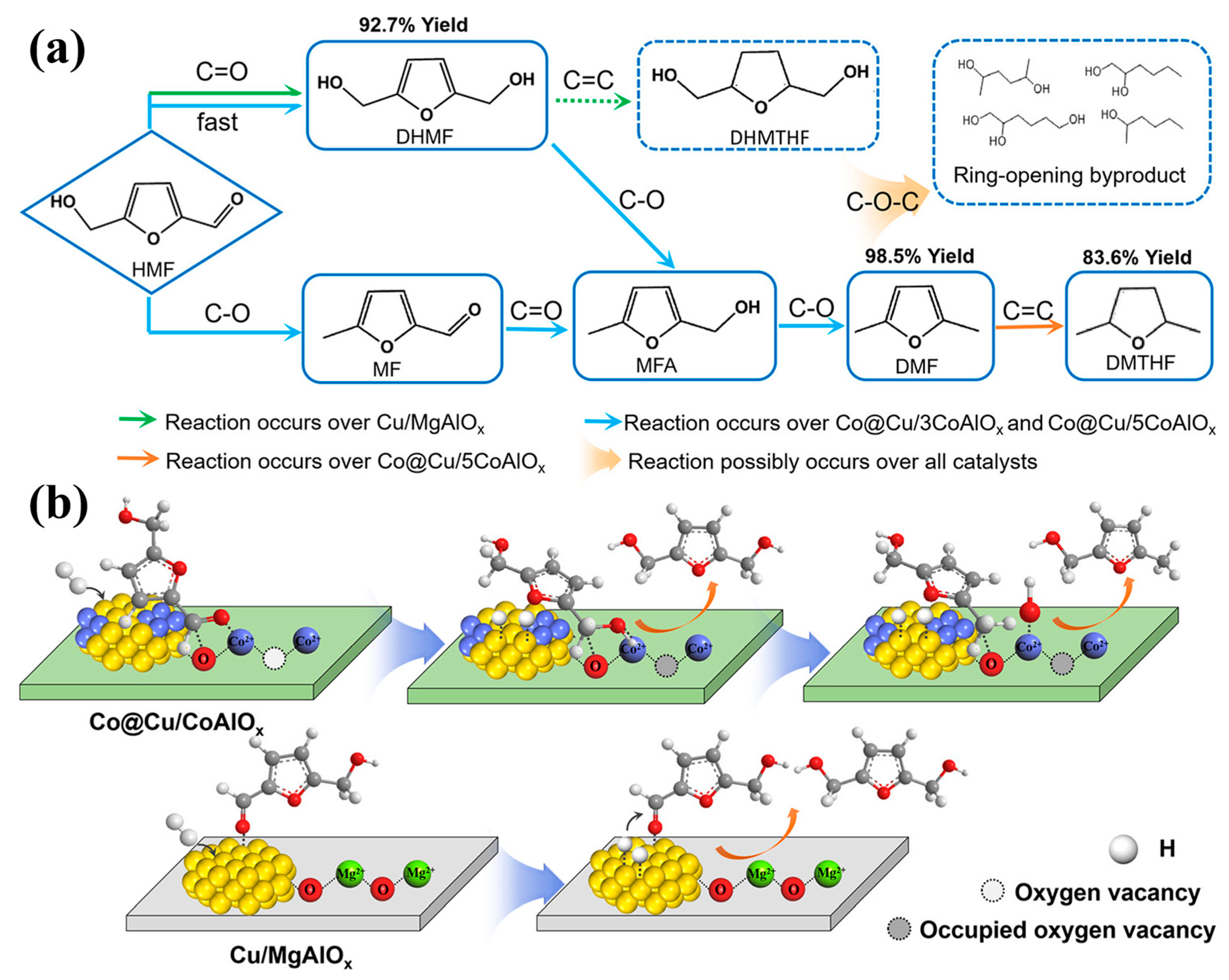
Wang et al. fabricated different Cu-based catalysts with multiple interfaces derived from LDHs to control the reaction pathway and product selectivity in the hydrogenation of HMF[
62]. It was found that the Cu/Co ratio at the metal nanoparticle interface significantly affected the hydrogenation of C=O bonds in HMF. The reaction pathways for hydrogenation of HMF on different catalysts are illustrated in
Figure 6a. When Cu/MgAlO
x with no Co elements is used as a catalyst, a hydrogenation reaction is only carried out on the C=O bond, mainly producing 2,5-bis (hydroxymethyl) furan (DHMF). Co@Cu/
3CoAlO
x catalyzes both C=O bond hydrogenation and C-OH bond hydrogenolysis, mainly producing 2,5-dimethylfuran (DMF), whereas Co@Cu/
5CoAlO
x produces DMF together with the over-hydrogenated product (DMTHF). The possible mechanism of HMF hydrogenation is illustrated in
Figure 6b. The C=O bond in HMF interacts with the Co metal site in Co@Cu/CoAlO
x, and the resulting intermediate is then hydrogenated by Cu nanoparticles to form a C-OH group. Subsequently, the saturated C-OH groups undergo cleavage over the same sites, producing 5-methylfurfuryl alcohol (MFA), ultimately yielding DMF. For Cu/MgAlO
x, the C=O bond interacts with the Cu metal site, leading to exclusive DHMF production.
Zhe et al. developed well-distributed Co-based catalysts through the in situ H
2 reduction of LDHs and applied them for efficient selective hydrogenation of HMF to DMF[
63]. The results showed that the Co metal nanoparticles uniformly distributed on the metal oxide matrix and demonstrated strong synergistic interaction with the metal oxides, therefore exhibiting superior reactivity under mild conditions. The well-distributed Co-metal active sites on the composite metal oxide support promoted hydrogenation in this reaction. The Co-based catalysts show high activity on the hydrogenation of C−O and C=O, making them promising for biomass utilization.
3.3. LDHs-based mixed metal oxide catalysts
LDHs-derived mixed metal oxides are obtained from calcination at various temperatures of an LDHs precursor, possessing high surface area, phase purity, basic surface properties, and structural stability[
64]. Moreover, LDH-derived mixed metal oxides containing various metal cations exhibit their original acid-basic properties of the MgAl system and reduction-oxidation properties as catalysts depending on the other metal species incorporated[
65]. LDHs-based mixed metal oxides are rich in Lewis acid-base sites, making them commonly used in acid-base catalysis due to their high metal dispersion and porosity. It is possible to manipulate the crystal structure of mixed metal oxides by adjusting the calcination conditions of the LDH precursors. This regulation affects the density and intensity of their acid-base sites and, ultimately, their catalytic properties[
66,
67]. LDH-based mixed metal oxides can act as carriers for depositing metal nanoparticles onto laminates via calcination, which produces metal-based catalysts derived from LDHs, exhibiting better catalytic properties[
68].
Yan et al. synthesized acid-base mixed metal oxides (MgZrAl-MMO) via the calcination transformation of MgZrAl-LDH precursors. The obtained MgZrAl-MMO catalysts are evaluated by the ethanolysis of urea reaction to produce diethyl carbonate (DEC)[
69]. It has been discovered that both the weak acid sites and medium strength base sites contribute to the overall yield of DEC, indicating an acid-base synergistic catalysis in this reaction. Specifically, the medium-strength Lewis base sites (Zr
4+-O
2-, Mg
2+-O
2-) activate ethanol, while weak Lewis acid sites (mostly ZrO
2) activate urea and intermediate ethyl carbamate.
The Mg/Al mixed metal oxide catalysts were prepared by J. Kuljiraseth et al and used as acid-base catalysts for the esterification of benzoic acid with 2-ethylhexanol. After being calcinated at 500°C, the Mg/Al mixed metal oxides retained their clay structure and possess both acid and base sites. The total density of acid and base sites decreased with the increase of the Mg/Al ratio. Furthermore, the strength of the acid and base sites varied depending on their phase compositions and coordination number. The activity of calcined LDHs catalysts was tested for the esterification of benzoic acid with 2-ethylhexanol to produce 2-ethylhexyl benzoate. The acid-base sites of the catalysts enhanced significantly the conversion of benzoic acid[
70].
Recently, there has been much interest in converting biomass-derived glycerol to value-added oligo glycerol through catalytic etherification using acid and base sites of catalysts[
71]. Elena et al. examined the calcined MgAl and CaAl LDHs with different acid-base properties used as catalysts for the one-pot etherification reaction of glycerol towards short-chain polyglycerols[
72]. The results reveal that MgAl mixed metal oxides possess robustly base sites, whereas CaAl mixed metal oxides exhibit relatively high acidity. The acidity and basicity of the mixed metal oxide catalysts affect the catalytic results. Catalysts with higher acidity showed higher conversion, whereas catalysts with lower acidity resulted in lower conversion. Moreover, stronger base sites and lower acidity favored short-chain polyglycerols, and higher calcination temperature favored a higher degree of polymerization.
Pyrolysis is an efficient method of using low-value resources to create high-value products and fuels. Acid-base synergistic catalysis is especially effective for pyrolysis production of desirable chemicals[
73]. In recent years, acid-base bifunctional catalysts derived from LDHs, have garnered significant interest due to their distinct structures and acid-base properties. Pyrolysis reactions with acid-base bifunctional catalysts produce high-value products while minimizing waste. The researchers, Yang et al., prepared a series of MgAl mixed metal oxide catalysts with varying acidity and alkalinity[
74]. They achieved this by adjusting the Mg/Al ratio in the precursor of MgAl LDHs. Then, they studied the catalytic ability to upgrade low-rank coal resource pyrolysis through an acid-base synergistic effect. The results showed that as the Mg/Al ratio increased in the MgAl mixed metal oxides, the base sites increased while the quantity of acid sites decreased. The acid sites in the MgAl mixed metal oxides facilitated the cracking of heavy components and decreased the production of heavy tar. The introduction of basicity sites in the MgAl mixed metal oxides enhanced the resistance to carbon deposition. After upgrading, the CO
2 yield and the content of light aromatic hydrocarbons in tar increased, which can be attributed to the acid-base synergistic effect of the MgAl mixed metal oxides.
LDHs-based mixed metal oxides have also been applied as acid-base bifunctional catalysts in hydrogen production via auto-thermal reforming of biomass-derived acetic acid[
75]. Ni-based catalysts (Ni/ CaO-Al
2O
3) originating from CaAl LDHs were synthesized by the direct calcination of CaAl LDHs, which are prepared by the coprecipitation method. Ni nanoparticles were anchored in the framework of CaO- Al
2O
3 support, resulting in a strong interaction between Ni metal active sites and the acid-base sites on the support. The interaction among Ni, CaO, and Ca
12Al
14O
33 effectively enhanced the catalytic performance of the reaction and was responsible for thermal stability, resistance to oxidation of Ni
0 species, and inhibiting coke deposition.
4. Conclusions and perspectives
LDHs and their derivatives are widely used as solid acid-base catalysts in green catalytic transformations due to their unique structure and properties. LDH-based catalysts exhibit both Lewis and Brønsted acid-base sites, allowing for a wide range of acid-base catalytic reactions in biomass conversion. The dual acid-base nature enables substrate activation and conversion, increasing catalytic activity and selectivity. LDHs-based acid-base catalysts also possess adjustable acid-base properties, which can be modified through ion doping, anion exchange, and structural alterations. There are opportunities available to improve the catalytic performance of specific substrates and achieve desired target products. LDH-based acid-base catalysts typically demonstrate high surface area and porosity, providing adequate active sites for catalytic reactions and facilitating the efficient diffusion of reactants and products. In addition, LDH-based acid-base catalysts exhibit thermal and chemical stability, ensuring stable and sustained catalytic performance under the harsh reaction conditions encountered during the catalytic conversion process.
Table 1 summarizes some of the LDH-based acid-base catalysts outlined in this paper, along with their corresponding green acid-base catalytic process conditions.
Despite their effectiveness, LDH-based acid-base catalysts still encounter several challenges during the transformation process. For the full potential of LDHs-based catalysts in acid-base green conversion applications to be realized, further research and development are necessary.
The primary issue is catalyst deactivation. During the conversion of biomass-derived substrates, tar and coke residues can accumulate on the catalysts’ surface, causing deactivation. These residues obstruct the active sites, preventing reactant contact with catalytic active sites and inhibiting catalytic activity. Researchers are currently exploring strategies, such as regeneration and modification treatments, to improve the fouling resistance of LDHs-based catalysts.
It is essential to pay attention to the adaptability of specific substrates. Different substrates have unique chemical compositions and structural complexities. The catalytic performance of LDHs-based catalysts may vary depending on the specific substrate. Therefore, further optimization of the catalyst composition and acid-base properties is necessary to accommodate the unique properties of different substrates. It is crucial to optimize LDHs-based catalysts for specific substrates to achieve superior selectivity and conversion. The optimization involves tailoring the LDHs-based catalysts to match the unique characteristics of the substrates in terms of composition, morphology, and acid-base properties. In addition, it is important to understand the interactions between LDHs-based catalysts and substrates to design catalysts for specific green catalytic conversion reactions.
Maximizing the catalytic activity and selectivity of LDHs-based catalysts in acid-base green catalytic conversion reactions requires optimizing reaction conditions, such as temperature, pressure, and substrate-to-catalyst ratio. Changes in reaction conditions can significantly impact catalytic performance, conversion efficiency, and product selectivity. Further studies are needed to determine the optimal reaction parameters for different acid-base catalytic conversions using LDHs-based catalysts, considering the specific characteristics of LDHs-based catalysts.
The precise control of composition, morphology, and acid-base properties of LDHs-based catalysts presents another challenge. Further optimization and development of synthesis methods are required to ensure reproducibility, scalability, and cost-effectiveness. Developing efficient synthetic routes and understanding the structure-property relationship are crucial for the practical application of LDHs-based catalysts in green catalytic conversion processes.
An in-depth understanding of the reaction mechanisms that occur during green acid-base catalytic conversion over LDHs-based catalysts is essential for the rational design and optimization of catalysts. Mechanistic studies can lead to the development of more efficient and selective LDH-based catalysts by providing insights into active sites, reaction pathways, and intermediate species. Elucidating the underlying mechanisms and guiding catalyst design strategies can be accomplished using techniques such as in situ spectroscopy, kinetic modeling, and computational simulation.
Collaborative efforts among researchers in catalysis, materials science, and acid-base catalytic conversion can overcome these challenges and limitations. LDHs-based solid acid-base catalysts can be optimized and tailored for specific substrates and target products, enabling efficient and sustainable chemical production from renewable resources.
Author Contributions
Conceptualization, X.L.Y. and S.H.; formal analysis, X.L.Y. and L.S.C.; investigation, X.L.Y. and L.S.C.; writing—original draft preparation, X.L.Y. and L.S.C.; resources, S.H.; writing—review and editing, S.H. and G.J.Z.; supervision, S.H. and G.J.Z.; project administration, S.H. and G.J.Z.; funding acquisition, G.J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The R&D Program of Beijing Municipal Education Commission (KZ202210011015).
Data Availability Statement
The corresponding author can provide the data from this study upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vafaeezadeh, M.; Alinezhad, H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. Journal of Molecular Liquids 2016, 218, 95–105. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Fattahi, A. A study on the catalytic activity and theoretical modeling of a novel dual acidic mesoporous silica. RSC Advances 2014, 4. [Google Scholar] [CrossRef]
- Manríquez-Ramírez, M.; Gómez, R.; Hernández-Cortez, J.G.; Zúñiga-Moreno, A.; Reza-San Germán, C.M.; Flores-Valle, S.O. Advances in the transesterification of triglycerides to biodiesel using MgO–NaOH, MgO–KOH and MgO–CeO2 as solid basic catalysts. Catalysis Today 2013, 212, 23–30. [Google Scholar] [CrossRef]
- Suwannakarn, K.; Lotero, E.; Goodwin, J.G. Solid bronsted acid catalysis in the gas-phase esterification of acetic acid. INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH 2007, 46, 7050–7056. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.-L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Applied Catalysis B: Environmental 2014, 160-161, 730–741. [Google Scholar] [CrossRef]
- Patial, S.; Raizada, P.; Aslam Parwaz Khan, A.; Singh, A.; Van Le, Q.; Huy Nguyen, V.; Selvasembian, R.; Mustansar Hussain, C.; Singh, P. Emerging new-generation covalent organic frameworks composites as green catalysts: design, synthesis and solar to fuel production. Chemical Engineering Journal 2022, 433. [Google Scholar] [CrossRef]
- Liu, K.-G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coordination Chemistry Reviews 2021, 436. [Google Scholar] [CrossRef]
- Yadav, G.; Yadav, N.; Ahmaruzzaman, M. Microwave-assisted synthesis of biodiesel by a green carbon-based heterogeneous catalyst derived from areca nut husk by one-pot hydrothermal carbonization. Sci Rep 2022, 12, 21455. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Synthesis and acid catalysis of cellulose-derived carbon-based solid acid. Solid State Sciences 2010, 12, 1029–1034. [Google Scholar] [CrossRef]
- Elmekawy, A.A.; Shiju, N.R.; Rothenberg, G.; Brown, D.R. Environmentally Benign Bifunctional Solid Acid and Base Catalysts. Industrial & Engineering Chemistry Research 2014, 53, 18722–18728. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, X.; Liu, G.; Li, Y.; Zhu, T.; Xin, Y.; Wang, Q. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NO(x) with NH(3). J Hazard Mater 2020, 400, 123260. [Google Scholar] [CrossRef]
- Hu, S.-L.; Liang, S.; Mo, L.-Z.; Su, H.-H.; Huang, J.-S.; Zhang, P.-J.; Qin, J.-N. Conversion of biomass-derived monosaccharide into furfural over Cr–Mg-LDO@bagasse catalysts. Sustainable Chemistry and Pharmacy 2023, 32. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, K.; Li, B.; Shim, H.; Huang, Y. Key Considerations on the Industrial Application of Lignocellulosic Biomass Pyrolysis toward Carbon Neutrality. Engineering 2023. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, S.; Ke, L.; Wu, Q.; Zhang, Q.; Cui, X.; Dai, A.; Xu, C.; Cobb, K.; Liu, Y.; et al. Research progress on pyrolysis of nitrogen-containing biomass for fuels, materials, and chemicals production. Sci Total Environ 2023, 872, 162214. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Kumar, R.; Shukla, P.; Sharma, P.; Kulabhi, A. Biochar from agricultural biomass: Current status and future scope. Materials Today: Proceedings 2023. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy & Environment 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Li, C.; Zhang, C. Recent Progress of Hydrogenation and Hydrogenolysis Catalysts Derived from Layered Double Hydroxides. Catalysts 2022, 12. [Google Scholar] [CrossRef]
- Zhu, J.; Li, T.; Wang, S.; Chen, Y.; Ge, F.; Xu, Y. Lattice-distortion active sites of Ni-doped CuMgFe LDH for benzotraizole degradation. Journal of Environmental Chemical Engineering 2022, 10. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Applied Surface Science 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. Journal of Materials Chemistry A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Panda, H.S.; Srivastava, R.; Bahadur, D. Synthesis and <i>in situ</i> mechanism of nuclei growth of layered double hydroxides. BULLETIN OF MATERIALS SCIENCE 2011, 34, 1599–1604. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Witte, K.; Tendeloo, G.; Cool, P.; Vansant, E.F. Zn-Al layered double hydroxides: Synthesis, characterization and photocatalytic application. MICROPOROUS AND MESOPOROUS MATERIALS 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Morel-Desrosiers, N.; Pisson, J.; Israëli, Y.; Taviot-Guého, C.; Besse, J.-P.; Morel, J.-P. Intercalation of dicarboxylate anions into a Zn–Al–Cl layered double hydroxide: microcalorimetric determination of the enthalpies of anion exchange. J. Mater. Chem. 2003, 13, 2582–2585. [Google Scholar] [CrossRef]
- Zou, W.; Guo, W.; Liu, X.; Luo, Y.; Ye, Q.; Xu, X.; Wang, F. Anion Exchange of Ni-Co Layered Double Hydroxide (LDH) Nanoarrays for a High-Capacitance Supercapacitor Electrode: A Comparison of Alkali Anion Exchange and Sulfuration. Chemistry 2018, 24, 19309–19316. [Google Scholar] [CrossRef]
- Das, J.; Parida, K.M. Heteropoly acid intercalated Zn/Al HTlc as efficient catalyst for esterification of acetic acid using n-butanol. Journal of Molecular Catalysis A: Chemical 2007, 264, 248–254. [Google Scholar] [CrossRef]
- Wei, Y.; Li, F.; Liu, L. Liquid exfoliation of Zn–Al layered double hydroxide using NaOH/urea aqueous solution at low temperature. RSC Advances 2014, 4. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Su, D.; Tang, Z.; Zhang, J.; Liu, Y.; Yuan, A.; Kong, Q. Ultrathin Ni-Al layered double hydroxide nanosheets with enhanced supercapacitor performance. Ceramics International 2017, 43, 14395–14400. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X. Carbon fiber/Ni-Co layered double hydroxide@NiMoO4/graphene oxide sandwich structure flexible electrode materials: Facile synthesis and high supercapacitor performance. Journal of Alloys and Compounds 2019, 794, 13–20. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg-Al and Zn-Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef]
- Dubnova, L.; Danhel, R.; Meinhardova, V.; Korolova, V.; Smolakova, L.; Kondratowicz, T.; Kikhtyanin, O.; Capek, L. Reconstruction of the ZnAl Mixed Oxides Into the Layered Double Hydroxide Catalysts Active in the Aldol Condensation of Furfural: The Role of ZnO Particles. Front Chem 2021, 9, 803764. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Kapustin, A.E.; Panchenko, V.N.; Butenko, E.O.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Synthetic and natural materials with the brucite-like layers as high active catalyst for synthesis of 1-methoxy-2-propanol from methanol and propylene oxide. Journal of Molecular Catalysis A: Chemical 2016, 423, 22–30. [Google Scholar] [CrossRef]
- Terzi, C.M.; dos Santos, E.H.; Carvalho, C.; Prevot, V.; Wypych, F.; Forano, C.; Nakagaki, S. MgAl and ZnAl layered double hydroxides modified with molybdate and tungstate anions as catalysts for oxidation of cyclohexane. Catalysis Today 2023, 422. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, G.; Guo, Y.; Guo, Y.; Gong, X.-Q. Effect of One-Pot Rehydration Process on Surface Basicity and Catalytic Activity of MgyAl1-aREEaOx Catalyst for Aldol Condensation of Citral and Acetone. ACS Sustainable Chemistry & Engineering 2016, 4, 1591–1601. [Google Scholar] [CrossRef]
- Pithakratanayothin, S.; Tongsri, R.; Chaisuwan, T.; Wongkasemjit, S. Influences of M–Sn intermetallics (M = Ni, Cu) prepared by mechanical alloying on phenol hydroxylation. Catalysis Science & Technology 2017, 7, 5413–5421. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Konnerth, H.; Yeh, J.-Y.; Prechtl, M.H.G.; Wen, C.-Y.; Wu, K.C.W. De novo synthesis of Cr-embedded MOF-199 and derived porous CuO/CuCr2O4 composites for enhanced phenol hydroxylation. Green Chemistry 2019, 21, 1889–1894. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, W.; Liang, J.; Shen, J.; Fu, X.; He, H.; Yan, S.; Ren, X. Enhanced catalytic phenol hydroxylation by CuZnFeAl layered double hydroxides: synergistic effects of Cu+ and oxygen vacancies. New Journal of Chemistry 2021, 45, 179–188. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Z.; Liu, Y.; Zhang, M.; Wang, Y.; Wan, Y.; Yan, K. Efficient electrooxidation of biomass-derived aldehydes over ultrathin NiV-layered double hydroxides films. Journal of Energy Chemistry 2023, 78, 412–421. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, B.; Chen, Z.; Xu, H.; Yan, K. Trimetallic NiCoFe-Layered Double Hydroxides Nanosheets Efficient for Oxygen Evolution and Highly Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Catalysis 2020, 10, 5179–5189. [Google Scholar] [CrossRef]
- Wang, T.; Hu, A.; Wang, H.; Xia, Y. Catalytic transfer hydrogenation of furfural into furfuryl alcohol over Ni–Fe-layered double hydroxide catalysts. Journal of the Chinese Chemical Society 2019, 66, 1610–1618. [Google Scholar] [CrossRef]
- Ye, X.; Shi, X.; Xu, H.; Feng, Y.; Jin, B.; Duan, P. Enhanced catalytic activity of layered double hydroxides via in-situ reconstruction for conversion of glucose/food waste to methyl lactate in biorefinery. Sci Total Environ 2022, 829, 154540. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Hanif, A.; Tsang, D.C.W.; Shang, J.; Su, Z.; Song, H.; Ok, Y.S.; Poon, C.S. Tuneable functionalities in layered double hydroxide catalysts for thermochemical conversion of biomass-derived glucose to fructose. Chemical Engineering Journal 2020, 383. [Google Scholar] [CrossRef]
- Upare, P.P.; Chamas, A.; Lee, J.H.; Kim, J.C.; Kwak, S.K.; Hwang, Y.K.; Hwang, D.W. Highly Efficient Hydrotalcite/1-Butanol Catalytic System for the Production of the High-Yield Fructose Crystal from Glucose. ACS Catalysis 2019, 10, 1388–1396. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Zhang, X.; Wei, M. Supported Ag Catalysts on Mg–Al Oxides toward Oxidant-Free Dehydrogenation Reaction of Benzyl Alcohol. Industrial & Engineering Chemistry Research 2018, 57, 15606–15612. [Google Scholar] [CrossRef]
- Valente, J.S.; Pfeiffer, H.; Lima, E.; Prince, J.; Flores, J. Cyanoethylation of alcohols by activated Mg–Al layered double hydroxides: Influence of rehydration conditions and Mg/Al molar ratio on Brönsted basicity. Journal of Catalysis 2011, 279, 196–204. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Tišler, Z.; Velvarská, R.; Kubička, D. Reconstructed Mg-Al hydrotalcites prepared by using different rehydration and drying time: Physico-chemical properties and catalytic performance in aldol condensation. Applied Catalysis A: General 2017, 536, 85–96. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Zhao, X.; Lei, N.; Zhang, T. Selective aldol condensation of biomass-derived levulinic acid and furfural in aqueous-phase over MgO and ZnO. Green Chemistry 2016, 18, 3430–3438. [Google Scholar] [CrossRef]
- Lee, R.; Vanderveen, J.R.; Champagne, P.; Jessop, P.G. CO2-Catalysed aldol condensation of 5-hydroxymethylfurfural and acetone to a jet fuel precursor. Green Chemistry 2016, 18, 5118–5121. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation. ACS Catalysis 2017, 8, 656–664. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Zheng, L.; Rao, D.; Yang, Y.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. A CaMnAl-hydrotalcite solid basic catalyst toward the aldol condensation reaction with a comparable level to liquid alkali catalysts. Green Chemistry 2018, 20, 3071–3080. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, J.Y.; An, S.; Yang, I.; Jung, J.C. Tuning the base properties of Mg–Al hydrotalcite catalysts using their memory effect. Journal of Energy Chemistry 2020, 46, 229–236. [Google Scholar] [CrossRef]
- Yabushita, M.; Shibayama, N.; Nakajima, K.; Fukuoka, A. Selective Glucose-to-Fructose Isomerization in Ethanol Catalyzed by Hydrotalcites. ACS Catalysis 2019, 9, 2101–2109. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, K.; Shi, Z.; Wan, C.; Pan, J.; Li, D.; Au, C.; Jiang, L. Influence of reduction temperature on Ni particle size and catalytic performance of Ni/Mg(Al)O catalyst for CO2 reforming of CH4. International Journal of Hydrogen Energy 2020, 45, 2794–2807. [Google Scholar] [CrossRef]
- Cui, G.; Meng, X.; Zhang, X.; Wang, W.; Xu, S.; Ye, Y.; Tang, K.; Wang, W.; Zhu, J.; Wei, M.; et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid−base sites. Applied Catalysis B: Environmental 2019, 248, 394–404. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem Rev 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy & Environmental Science 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Chen, Y.; Liu, W.; Feng, H.; Wang, B.; Zhang, X.; Wei, M. The selective hydrogenation of furfural over intermetallic compounds with outstanding catalytic performance. Green Chemistry 2019, 21, 5352–5362. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Ma, X.; An, Z.; Guo, S.; Shu, X.; Song, H.; Xiang, X.; He, J. A gradient reduction strategy to produce defects-rich nano-twin Cu particles for targeting activation of carbon-carbon or carbon-oxygen in furfural conversion. Journal of Catalysis 2020, 389, 78–86. [Google Scholar] [CrossRef]
- Kazi, F.K.; Patel, A.D.; Serrano-Ruiz, J.C.; Dumesic, J.A.; Anex, R.P. Techno-economic analysis of dimethylfuran (DMF) and hydroxymethylfurfural (HMF) production from pure fructose in catalytic processes. Chemical Engineering Journal 2011, 169, 329–338. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chemistry 2008, 10. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Feng, J.; Fornasiero, P.; He, Y.; Li, D. Insight into the Effect of Dual Active Cu0/Cu+ Sites in a Cu/ZnO-Al2O3 Catalyst on 5-Hydroxylmethylfurfural Hydrodeoxygenation. ACS Sustainable Chemistry & Engineering 2020, 8, 15288–15298. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial Structure-Determined Reaction Pathway and Selectivity for 5-(Hydroxymethyl)furfural Hydrogenation over Cu-Based Catalysts. ACS Catalysis 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- An, Z.; Wang, W.; Dong, S.; He, J. Well-distributed cobalt-based catalysts derived from layered double hydroxides for efficient selective hydrogenation of 5-hydroxymethyfurfural to 2,5-methylfuran. Catalysis Today 2019, 319, 128–138. [Google Scholar] [CrossRef]
- Gao, D.L.; Lin, W.W.; Lin, Q.J.; Dai, F.F.; Xue, Y.X.; Chen, J.H.; Liu, Y.X.; Huang, Y.; Yang, Q. Remarkable adsorption capacity of Cu2+-doped ZnAl layered double hydroxides and the calcined materials toward phosphate. Journal of Environmental Chemical Engineering 2023, 11. [Google Scholar] [CrossRef]
- Takehira, K. Recent development of layered double hydroxide-derived catalysts − Rehydration, reconstitution, and supporting, aiming at commercial application −. Applied Clay Science 2017, 136, 112–141. [Google Scholar] [CrossRef]
- Teruel, L.; Bouizi, Y.; Atienzar, P.; Fornes, V.; Garcia, H. Hydrotalcites of zinc and titanium as precursors of finely dispersed mixed oxide semiconductors for dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 154–159. [Google Scholar] [CrossRef]
- Xia, S.; Shao, M.; Zhou, X.; Pan, G.; Ni, Z. Theoretical and experimental investigation into photoelectrocatalytic oxidation and reduction property of ZnFeTi mixed metal oxides. Journal of Molecular Catalysis A: Chemical 2015, 406, 127–136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, Y.; Feng, J.; Wu, T.; Li, D. Highly efficient PdAg catalyst using a reducible Mg-Ti mixed oxide for selective hydrogenation of acetylene: Role of acidic and basic sites. Journal of Catalysis 2017, 348, 135–145. [Google Scholar] [CrossRef]
- Yan, T.; Bing, W.; Xu, M.; Li, Y.; Yang, Y.; Cui, G.; Yang, L.; Wei, M. Acid-base sites synergistic catalysis over Mg-Zr-Al mixed metal oxide toward synthesis of diethyl carbonate. RSC Adv 2018, 8, 4695–4702. [Google Scholar] [CrossRef]
- Kuljiraseth, J.; Wangriya, A.; Malones, J.M.C.; Klysubun, W.; Jitkarnka, S. Synthesis and characterization of AMO LDH-derived mixed oxides with various Mg/Al ratios as acid–basic catalysts for esterification of benzoic acid with 2-ethylhexanol. Applied Catalysis B: Environmental 2019, 243, 415–427. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Towards the Sustainable Production of Higher-Molecular-Weight Polyglycerol. Macromolecular Chemistry and Physics 2011, 212, 1284–1293. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of acid–base properties of calcined MgAl and CaAl layered double hydroxides on the catalytic glycerol etherification to short-chain polyglycerols. Chemical Engineering Journal 2015, 264, 547–556. [Google Scholar] [CrossRef]
- Wei, B.; Jin, L.; Wang, D.; Shi, H.; Hu, H. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, C.; Chen, Z.; Wang, D.; Wang, D.; Ibrahim Sharif, M.; Li, J.; Yu, J.; Gao, S. The effect of acid-base synergistic catalysis on upgrading of volatiles from coal pyrolysis over Mg-Al composite oxides. Fuel 2022, 321. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.; Jia, X.; Chen, B.; An, S.; Xie, X.; Huang, L. Ca–Al layered double hydroxides-derived Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. International Journal of Hydrogen Energy 2019, 44, 20007–20016. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).